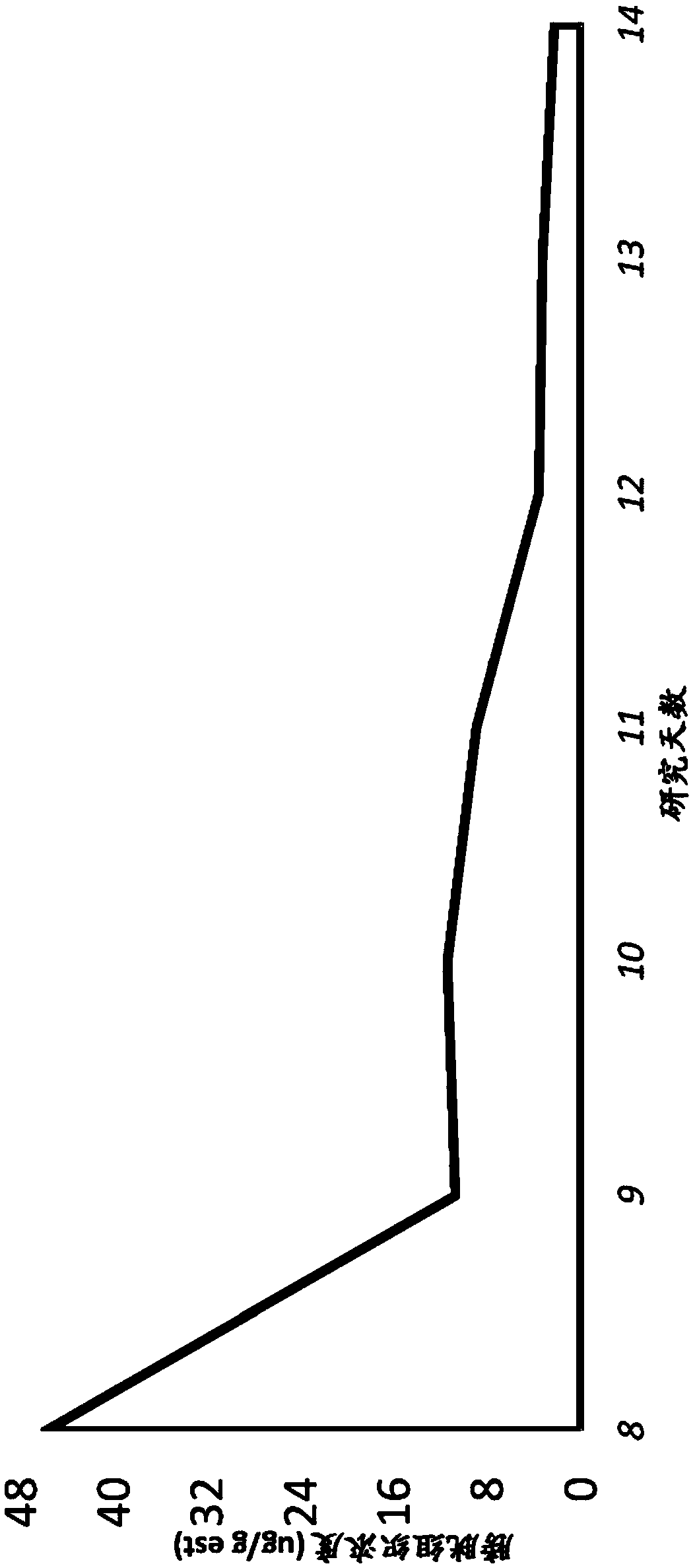
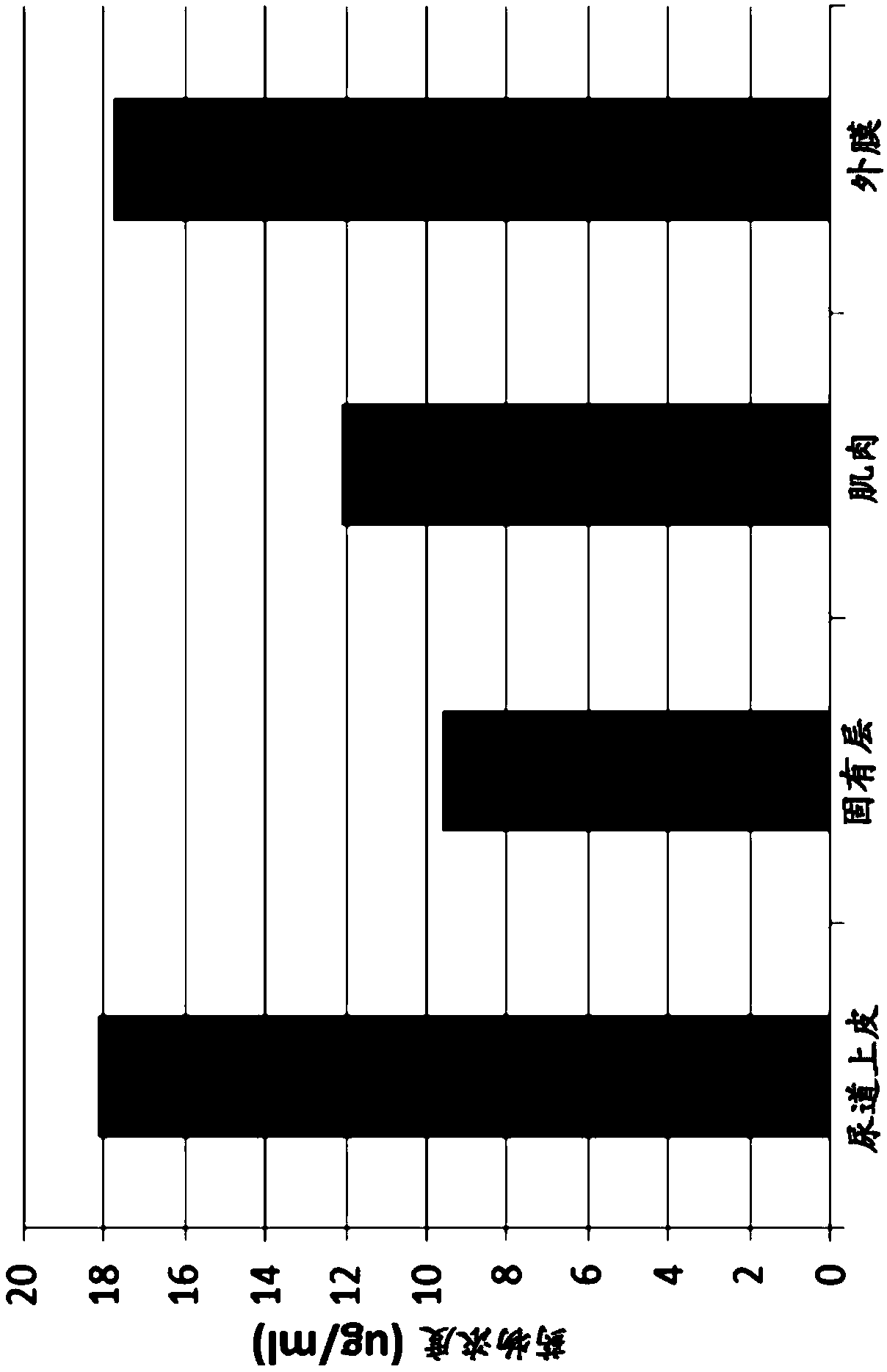
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Urothelial cancer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Urothelial cancers encompass carcinomas of the bladder, ureters, and renal pelvis, which occur at a ratio of 50:3:1, respectively. Cancer of the urothelium is a multifocal process. Patients with cancer of the upper urinary tract have a 30% to 50% chance of developing cancer of the bladder at some point in their lives.
Detection and treatment of renal cancer
The present invention relates to compositions and methods for cancer therapies and diagnostics, including but not limited to, cancer markers. In particular, the present invention provides cancer markers associated with specific cancers and diagnostic assays for the detection of such markers as indicative of the presence of kidney and urothelial cancers.
Owner:NORTHWESTERN UNIV
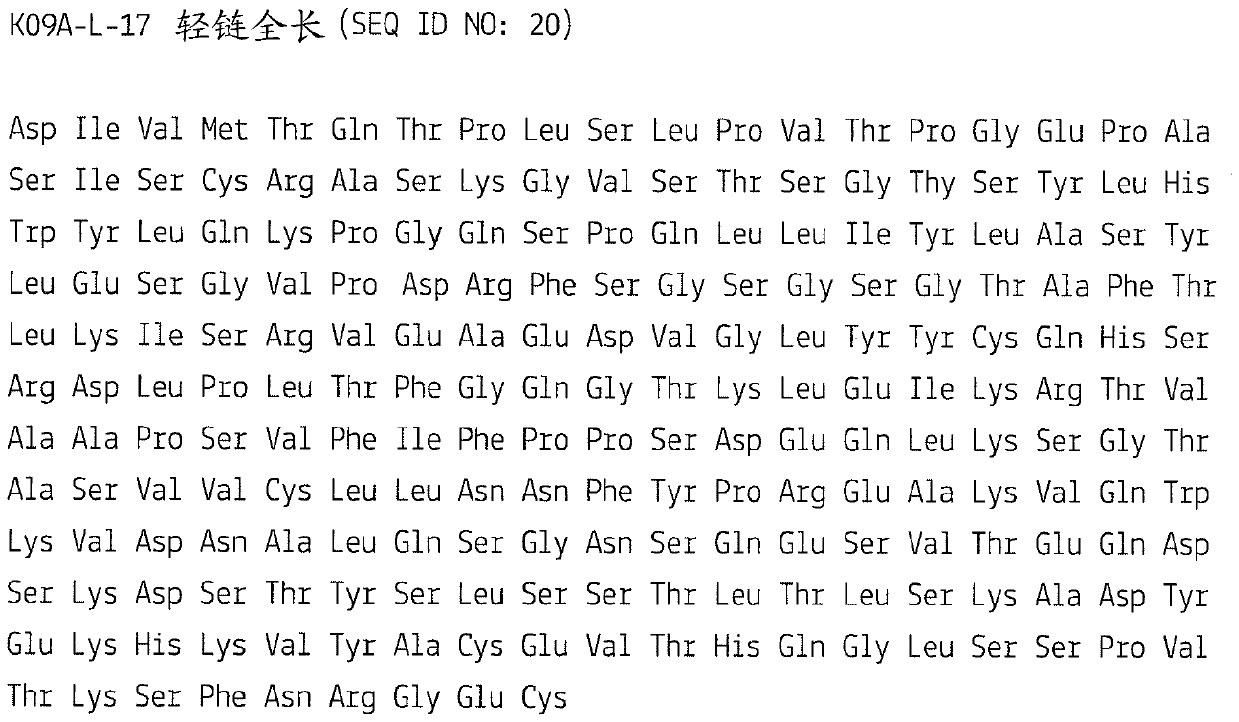
Kit and method for detecting urothelial cancer
InactiveUS7910316B2Easy and highly detectionEasy diagnosisMicrobiological testing/measurementBiological testingNucleic Acid ProbesBiology
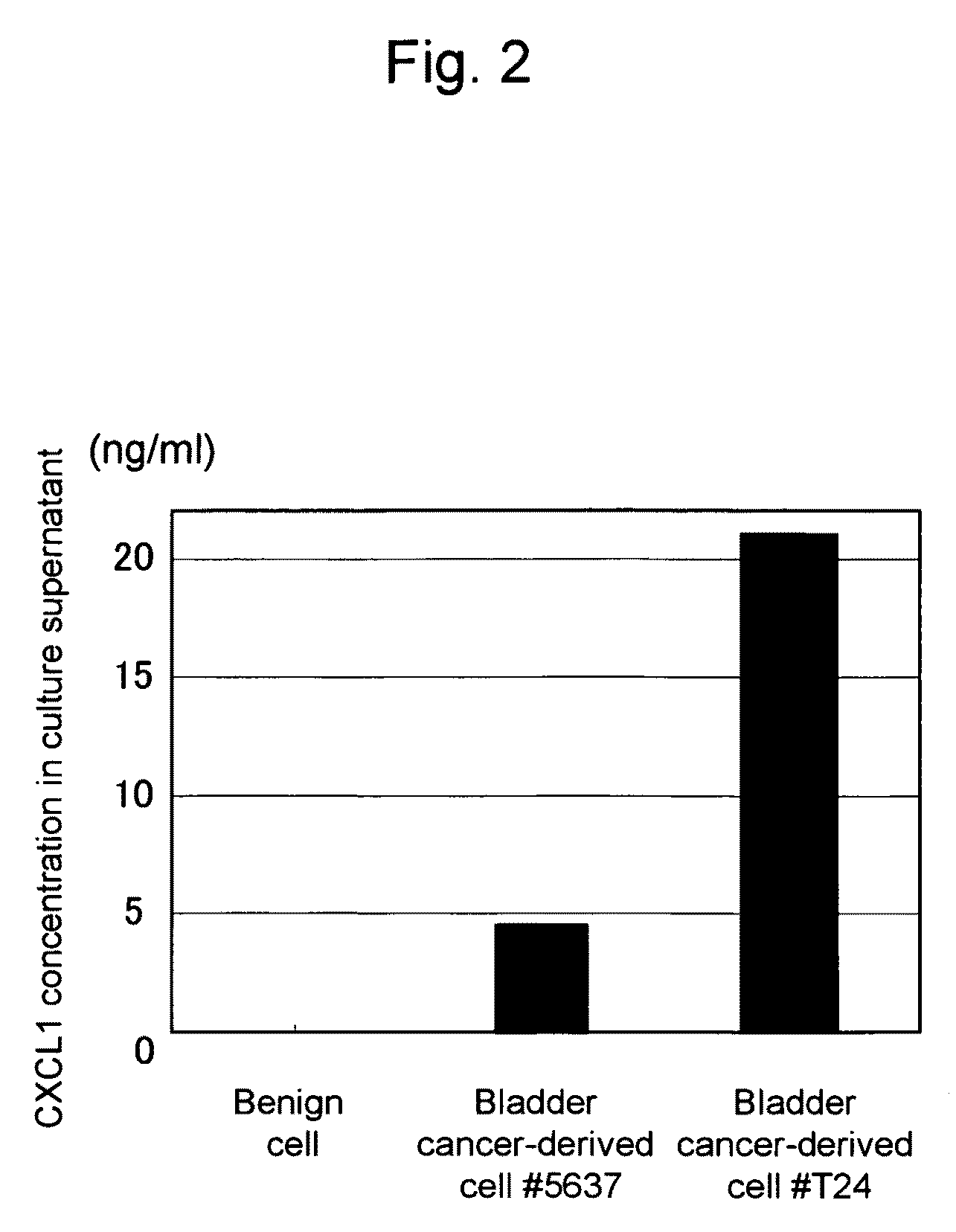
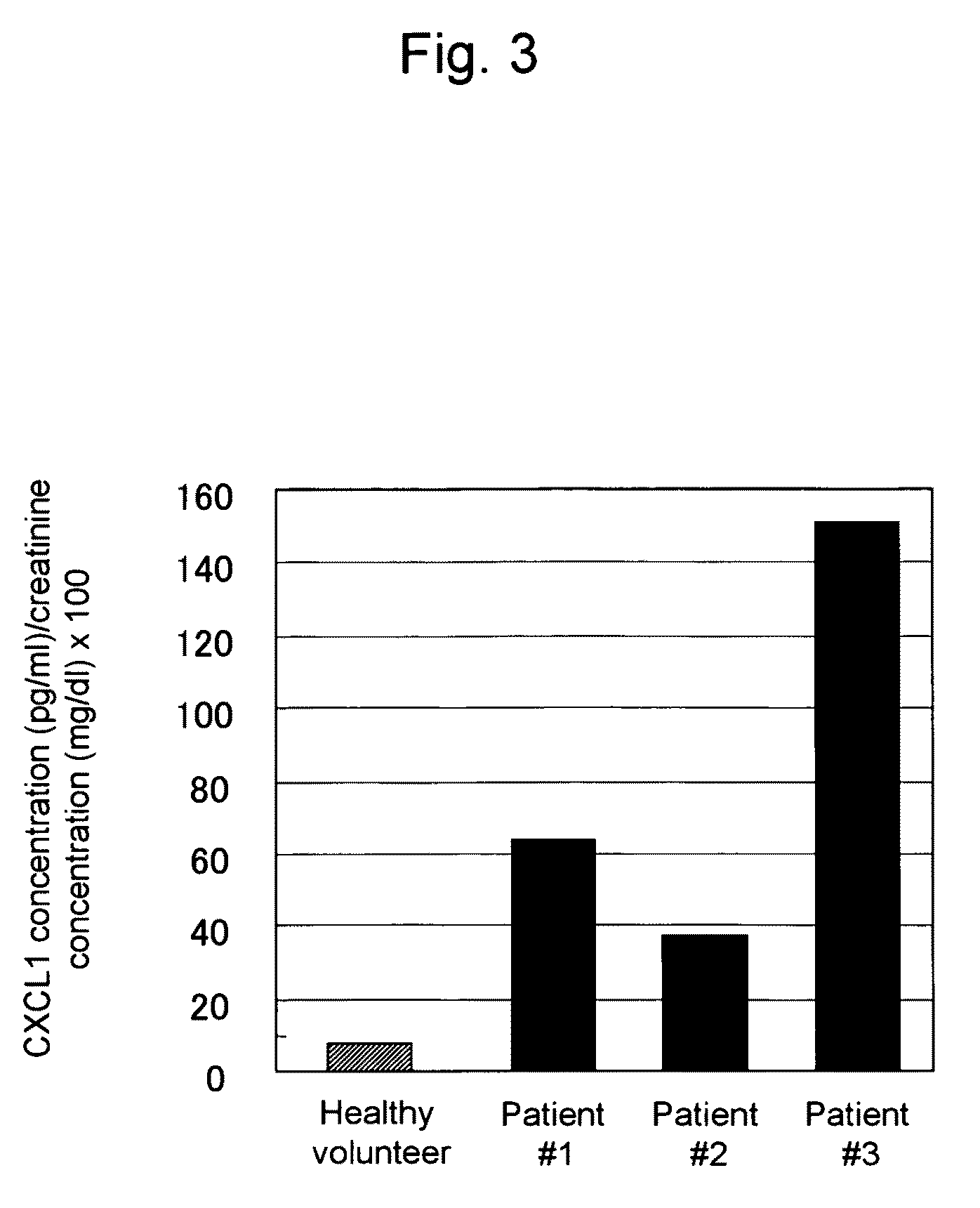
This invention relates to a method for detecting in vitro a urothelial cancer, comprising measuring CXCL1 protein, or expression of a gene encoding the protein, in a biological sample from a subject, and to a kit for diagnosing a urothelial cancer comprising an antibody or nucleic acid probe, which is capable of binding specifically to the CXCL1 protein or a gene encoding the protein, respectively.
Owner:TORAY IND INC +1
Semaphorin 3A as a diagnostic marker for urothelial cancer
ActiveUS10139412B2Simple and reliable processSensitive highDisease diagnosisBiological testingSemaphorinCancer treatment
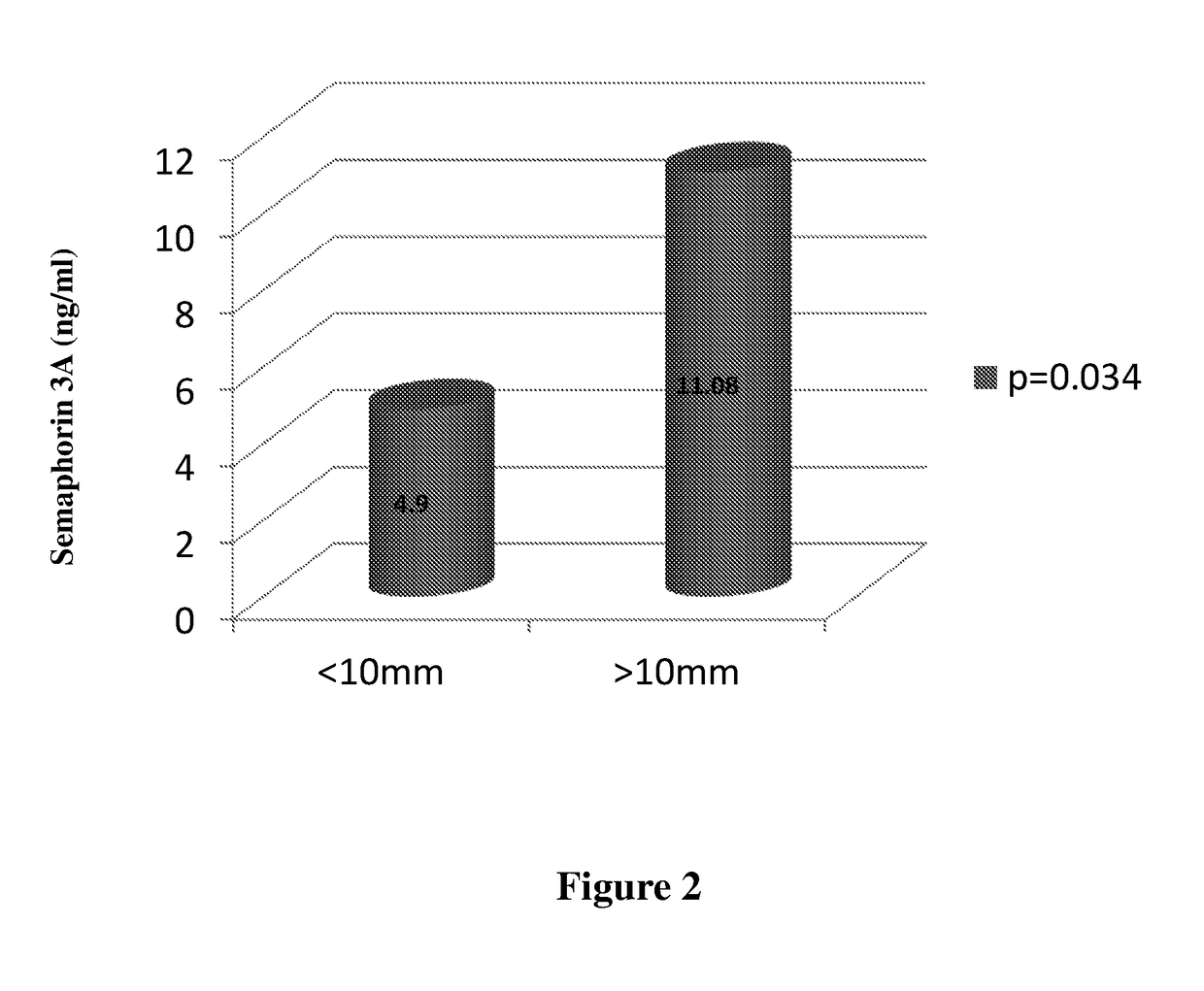
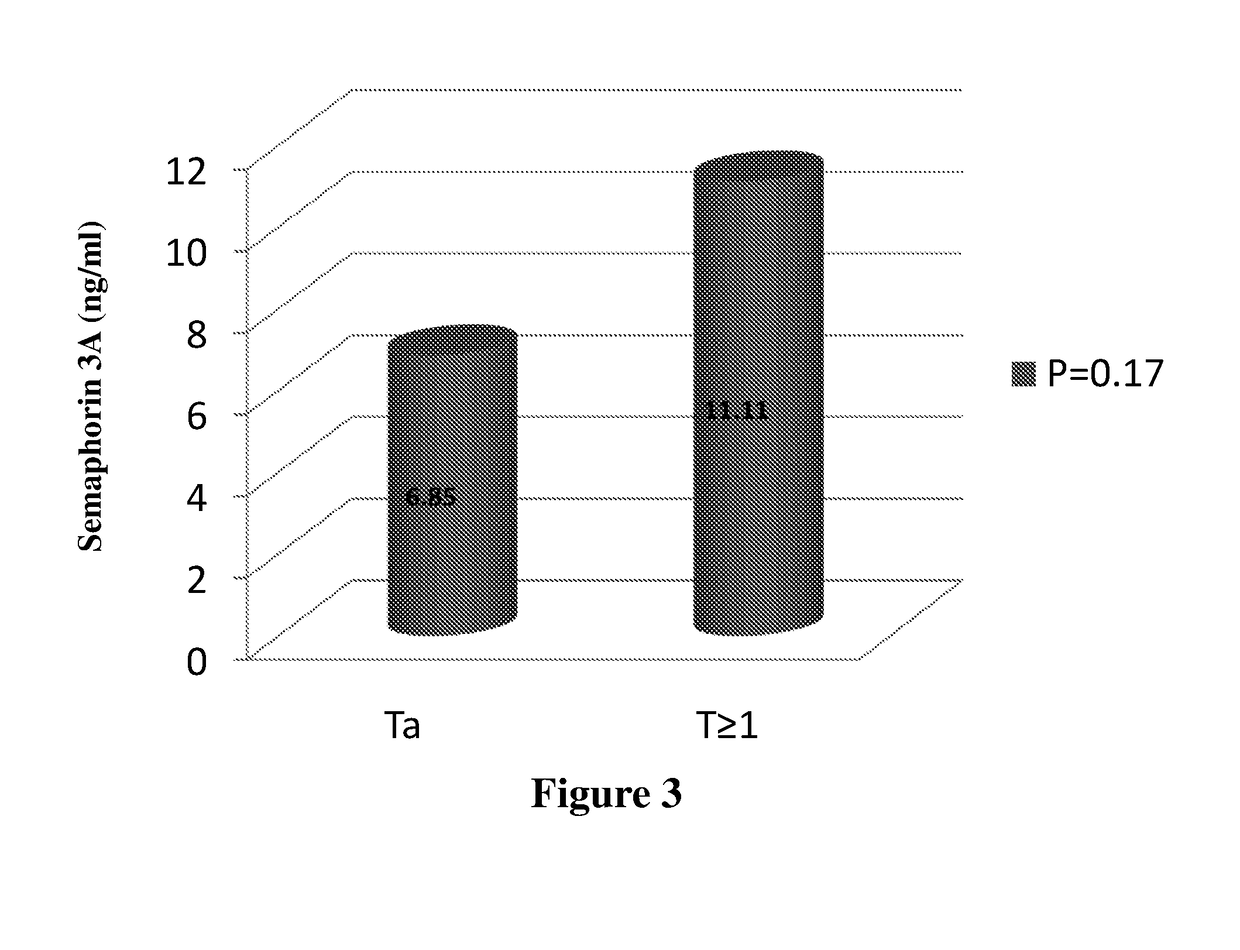
The present invention is directed to methods and kits useful for diagnosis and / or prognosis of urothelial cancer in a subject. The present invention further relates to methods of assessing severity of cancer and methods of determining efficacy of a treatment for cancer. The methods and kits of the invention comprise determining the levels of semaphorin 3A in a biological sample of a subject.
Owner:MEDICAL RES & DEV FUND FOR HEALTH SERVICES BNAI ZION MEDICAL CENT +1
Triaging of patients having asymptomatic hematuria using genotypic and phenotypic biomarkers
New methods for identifying patents with hematuria who are at low risk of having urothelial cancer (UC) include combining selected phendtypic variables with levels of genotypic expression into a new metric, the "G+P INDEX." The G+P INDEX combines age, sex, smoking history, presence of hematuria, and frequency of hematuria with genotypic expression of the genetic markers, MDK, CDC2, HOXA13, IGFBP5, and optionally IL8Rb, then determining of the G+P INDEX value obtained for a patient is within one of three groups, either: (1) at High Risk of UC, (2) at Risk of UC, or (3) at Low Risk of UC.
Owner:PACIFIC EDGE
Diagnosis and prognosis of cancer based on telomere length as measured on cytological specimens
InactiveUS20090162839A1Overcome subjectivityImprove the level ofMicrobiological testing/measurementCytologyLung cancer
The present invention concerns a quantitative in situ assessment of mean telomere length, particularly in relation to nuclear area, for the diagnosis and / or prognosis of cancer. In particular aspects, the methods and compositions regard diagnosis and / or prognosis of bladder cancer, urothelial cancer, lung cancer, and lymphoma.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Treatment of advanced her2 expressing cancer
InactiveUS20200237910A1High response rateImprove responseAntibody ingredientsImmunoglobulinsProstate cancerBiliary tract
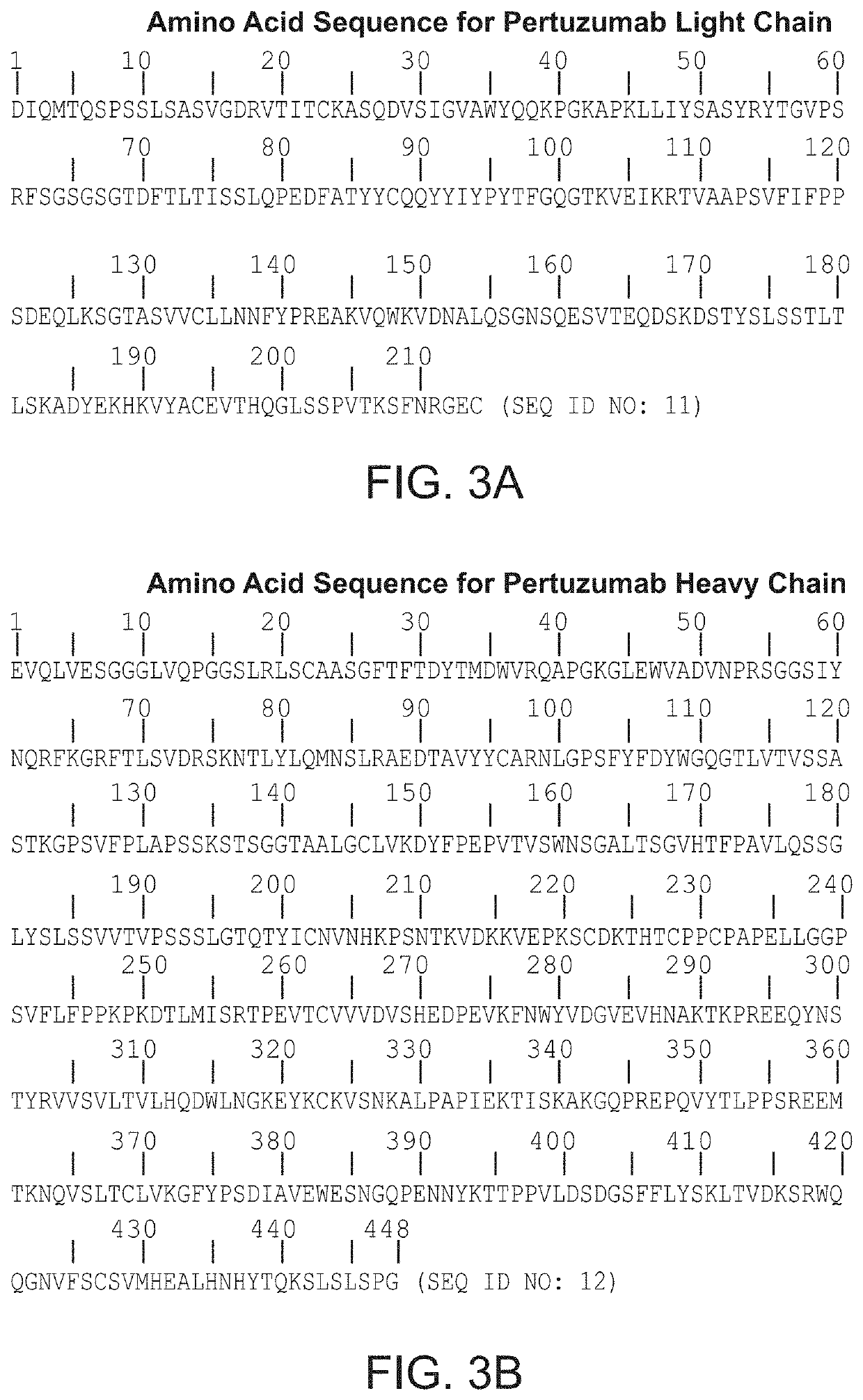
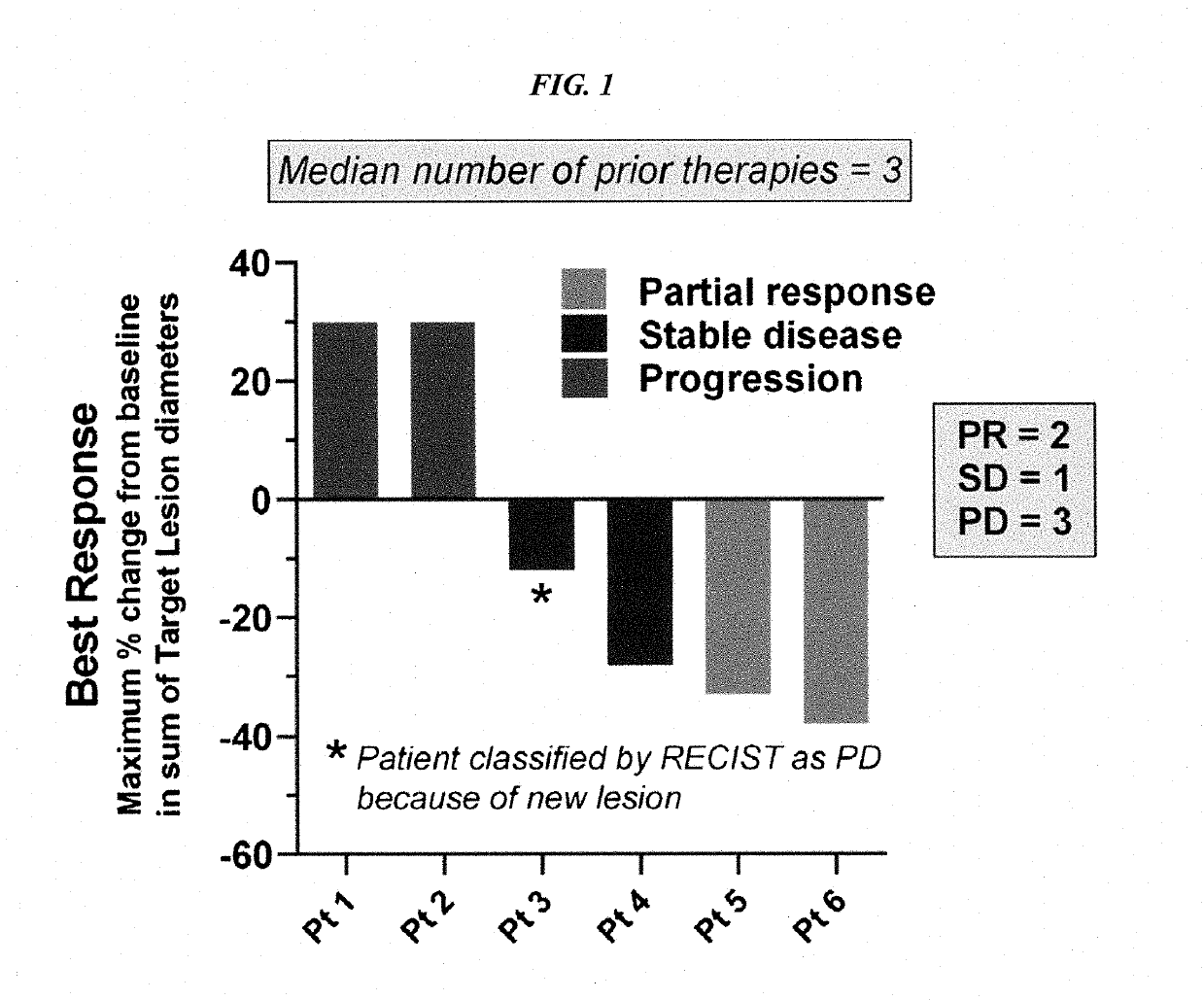
Methods for the treatment of patients with HER2-positive, HER2-amplified and / or HER2-mutated advanced cancer by administration of pertuzumab plus trastuzumab are disclosed. In one aspect, the cancer is advanced HER2-positive, HER2-amplified and / or HER2-mutated colorectal, biliary, bladder, urothelial, salivary, lung, pancreatic, ovarian, prostate, or skin cancer. In another aspect, the cancer is HER2-positive, HER2-amplified and / or HER2-mutated colorectal, biliary, bladder, urothelial, salivary, lung, pancreatic, ovarian, prostate, or skin cancer that is refractory to one or more other treatment regimens.
Owner:GENENTECH INC
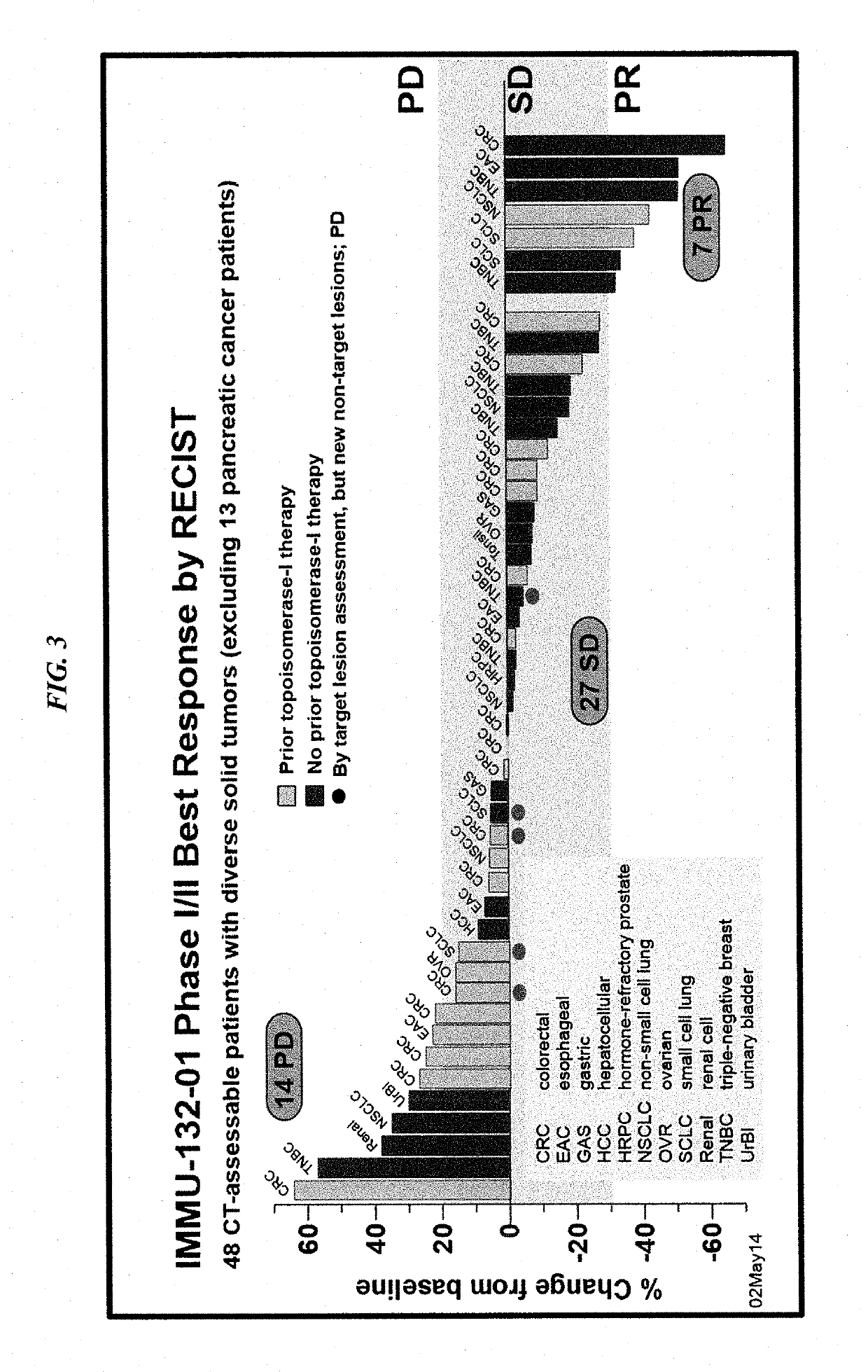
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (IMMU-132)
ActiveUS10413539B2Reducing certain severe side effectsReceive treatment wellImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsLymphatic SpreadAntibody fragments
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
Cancer marker and utilization thereof
InactiveUS9857375B2Diagnose urothelial cancer simplyImprove accuracyOrganic active ingredientsMicrobiological testing/measurementSquamous cancerBacteriuria
The present invention provides a novel cancer marker that is useful in the diagnosis of urothelial cancer. The present invention uses ubiquilin 2 as a cancer marker for urothelial cancer (renal pelvis cancer, ureteral cancer, and bladder cancer). Detection of ubiquilin 2 in a urine sample allows easy and accurate diagnosis of a possibility of urothelial cancer. The present invention is also applicable to the diagnosis of squamous cancer (esophageal cancer, cervical cancer, etc.).
Owner:NARA MEDICAL UNIVERSITY
Formulations for treating bladder cancer
Compositions and methods for making and using proliposomal and liposomal formulations of chemotherapeutic agents are disclosed. The proliposomal and liposomal formulations of chemotherapeutics, as well as medicaments and dosage forms that include such formulations, can be used with treatment regimens for bladder cancer and urothelial cancer. Hence, the formulations, medicaments, and dosage forms of the invention are suitable to treat bladder cancers by intravesical administration and to treat urothelial cancers. The formulations according to the invention include (a) a taxane (e.g., paclitaxel, docetaxel) or cisplatin, (b) a first phospholipid, dipalmitoyl phosphatidylcholine (DMPC), and (c) a second phospholipid, dimyrsityl phosphatidyl glycerol sodium (DMPG). The proliposomal formulations form liposomes upon contact with an aqueous vehicle.
Owner:TESORX PHARMA LLC +1
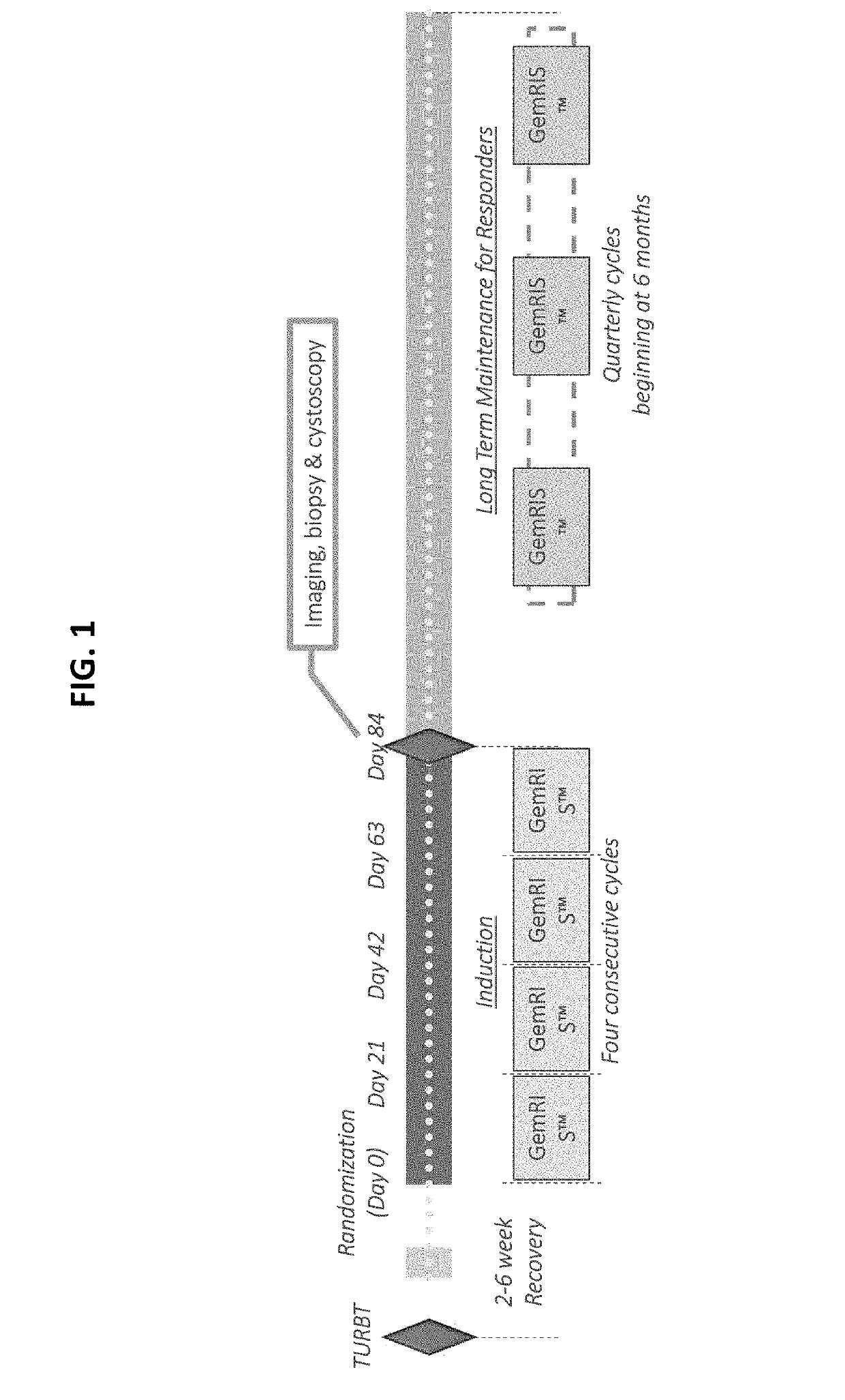
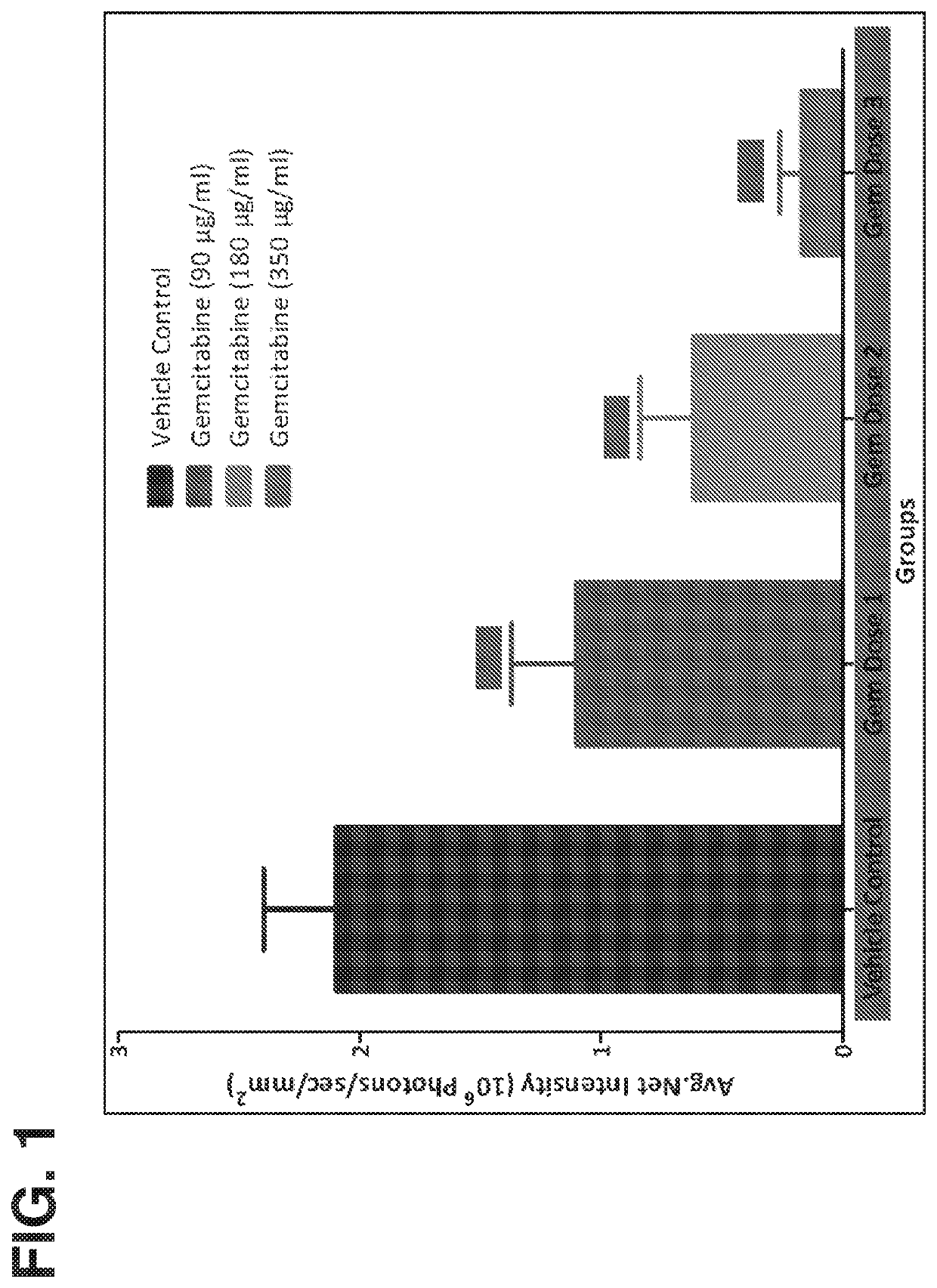
Methods of treatment and maintenance therapy for bladder cancer using gemcitbine
ActiveUS20190175637A1Good effectSymptoms improvedAntibacterial agentsOrganic active ingredientsBladder cancerMaintenance therapy
Provided are methods of treating urothelial carcinomas of the lower tract comprising administering comprising administering gemcitabine continuously and locally to the bladder of an individual in an induction therapy and / or maintenance therapy.
Owner:TARIS BIOMEDICAL
CfDNA classification method and device and application
PendingCN111833963ALower Sequencing CostsChallengingMedical data miningMicrobiological testing/measurementGenomicsParanasal Sinus Carcinoma
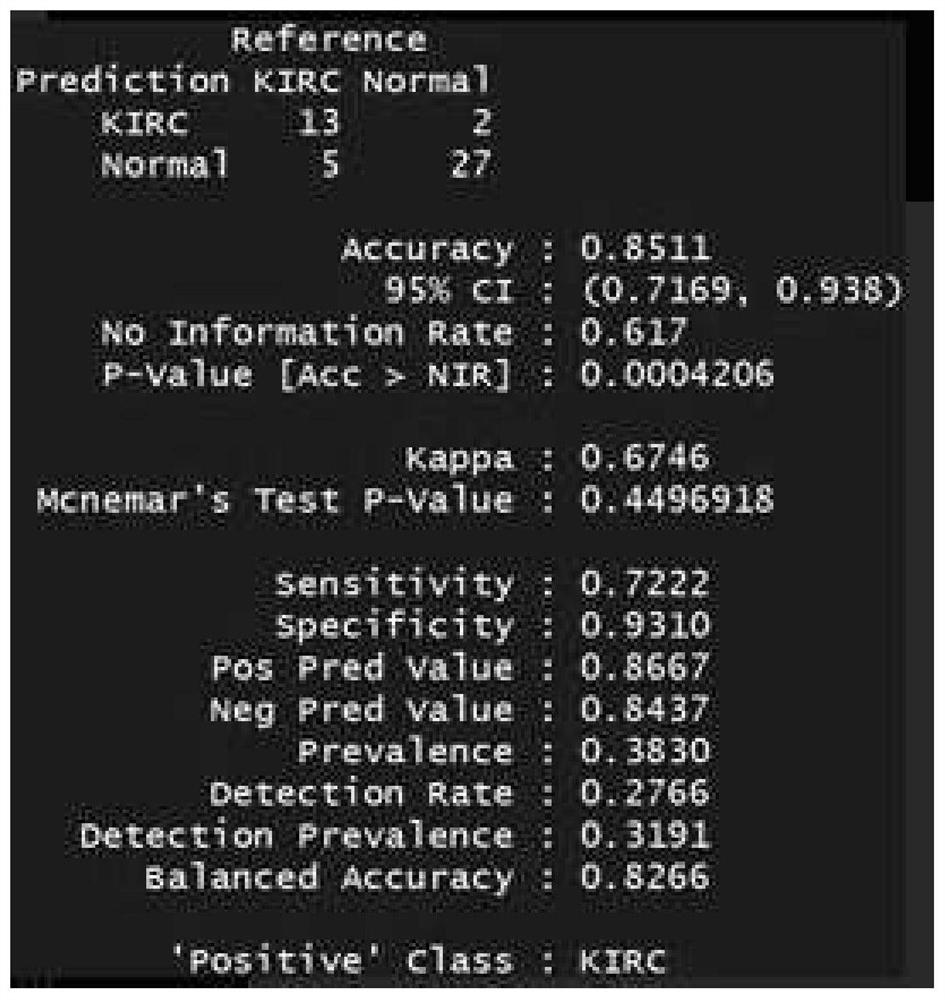
The invention belongs to the field of genomics and bioinformatics, and relates to a cfDNA classification method and device and application. The cfDNA classification method comprises the following steps: calculating copy number variation data of cfDNA in a target sample; calculating the similarity between the target cfDNA copy number variation data and the cfDNA copy number variation data of each classification label; and determining the classification to which the target cfDNA belongs by using a classifier model according to the similarity. Diagnosis of up to three urogenital system tumors canbe completed at a time, so that the method and device have high sensitivity and specificity. Particularly, the sensitivity and the specificity in the aspects of diagnosis and dynamic monitoring of the urothelial cancer are higher than those of a detection method used clinically at present.
Owner:BEIJING INST OF GENOMICS CHINESE ACAD OF SCI CHINA NAT CENT FOR BIOINFORMATION +1
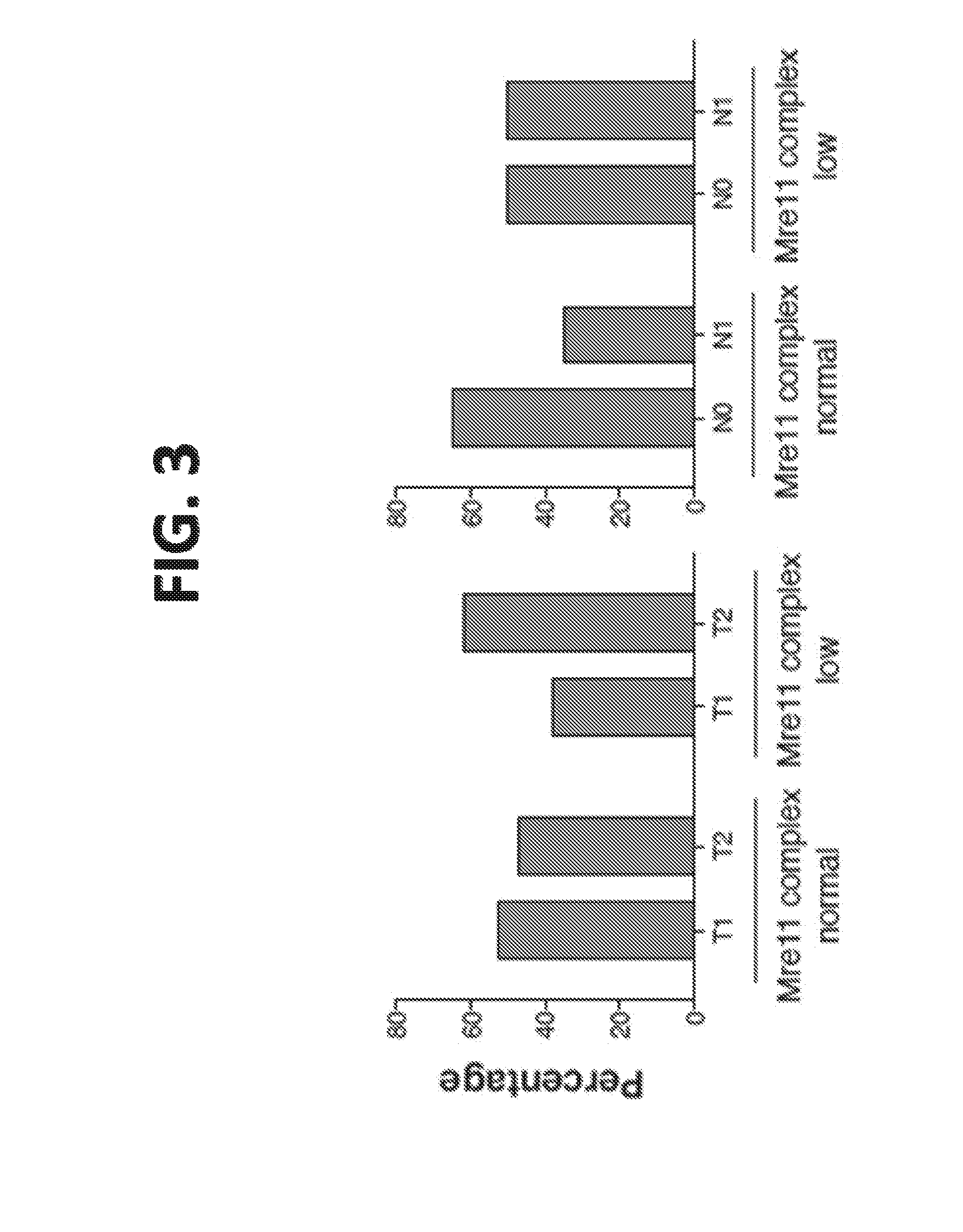
Compositions and methods for the treatment of cancers associated with a deficiency in the mre11/rad50/nbs1 DNA damage repair complex
InactiveUS20150313922A1Excessive levelHigh sensitivityHeavy metal active ingredientsBiocideCytotoxicityHormones regulation
Provided are compositions and methods for the identification and treatment of cancers exhibiting reduced MRE11 / RAD50 / NBS1 (MRN) complex formation and / or functionality as well as methods for the identification and use of cytotoxic agents, including clastogenic agents, for the treatment of cancers exhibiting reduced MRN complex formation and / or functionality. Also provided are methods for detecting and treating cancers, in particular breast cancers, such as hormone-negative breast cancers (HNBCs) and triple-negative breast cancers (TNBCs), colorectal cancers, urothelial cancers, and other cancers that exhibit reduced MRN complex formation and / or functionality and are correspondingly sensitive to growth and / or survival inhibition by one or more cytotoxic agents.
Owner:SLOAN KETTERING INST FOR CANCER RES
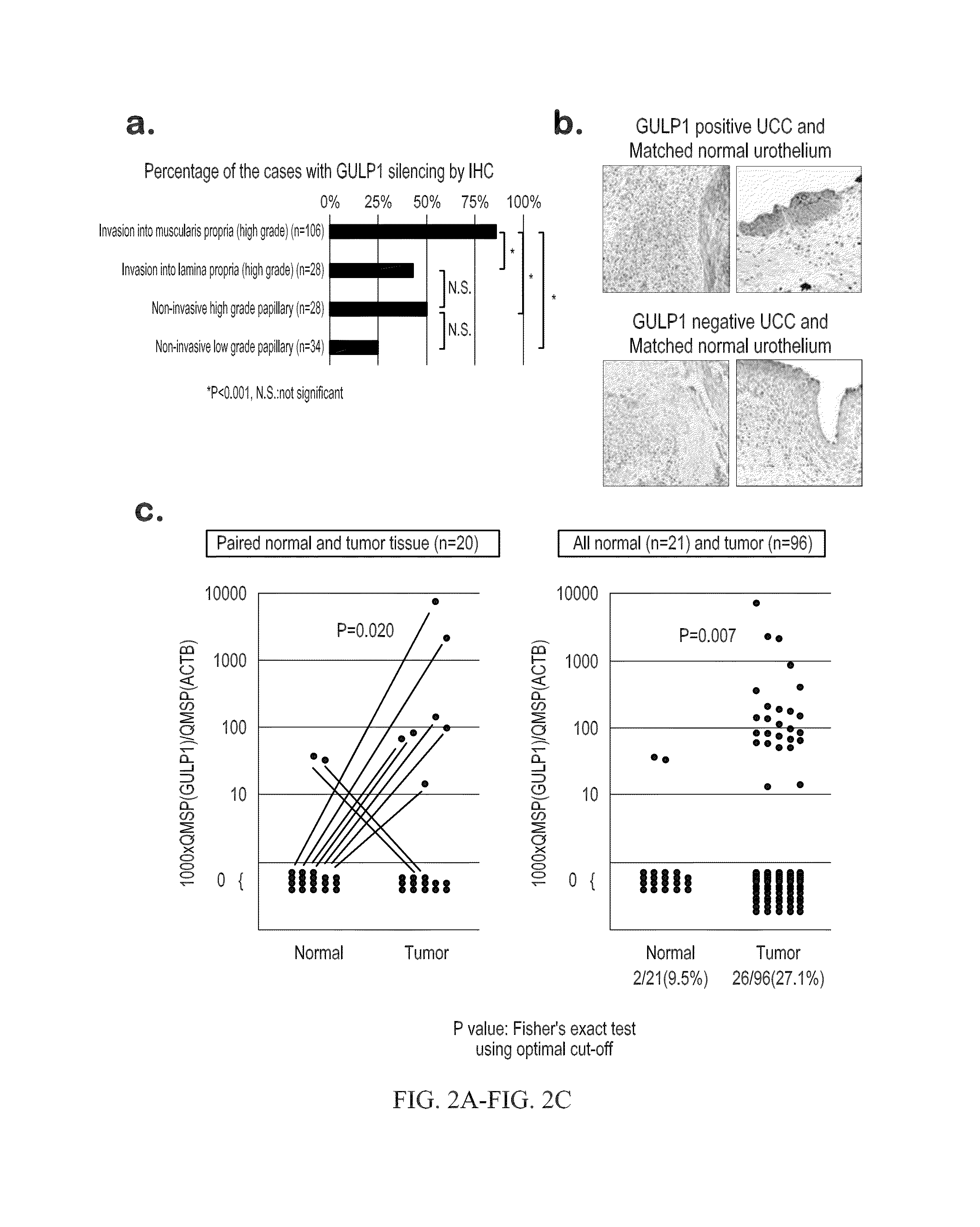
Urothelial cancer and methods of detection and targeted therapy
ActiveUS20160273051A1Symptoms improvedHigh expressionOrganic active ingredientsMicrobiological testing/measurementMolecular Targeted TherapiesTargeted therapy
The invention provides methods for detecting a cellular proliferative disorder (e.g., urothelial cancer) in a subject by assessing the methylation status of the GULP1 promoter in a nucleic acid sample. The methods of the invention are useful for diagnostic, prognostic as well as therapeutic regimen predictions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Benzyloxy aromatic ring structure compound and preparation method and application thereof
ActiveCN112028870AAvoid interactionIncrease lethalityAntibacterial agentsAntimycoticsDiseaseStage melanoma
The invention provides a benzyloxy aromatic ring structure compound represented by a general formula (I), a stereoisomer, an enantiomer or a pharmaceutically acceptable salt thereof, a preparation method thereof, a pharmaceutical composition containing the same, and uses thereof. The compound shown in the general formula (I) can be used for preparing a small-molecule inhibitor of PD1 / PDL1 interaction, and can be used for preventing and / or treating diseases related to PD1 / PDL1 interaction, especially cancers, such as non-small cell lung cancer, small cell lung cancer, melanoma, head and neck cancer, kidney cancer, bladder cancer, local advanced or metastatic urothelial cancer, breast cancer, cervical cancer, metastatic Merkel cell cancer, prostate cancer, liver cancer, intestinal cancer, stomach cancer, multiple myeloma, mantle cell lymphoma, diffuse large B cell lymphoma, liver cancer, hodgkin lymphoma, chronic lymphocytic leukemia, squamous cell carcinoma and the like.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
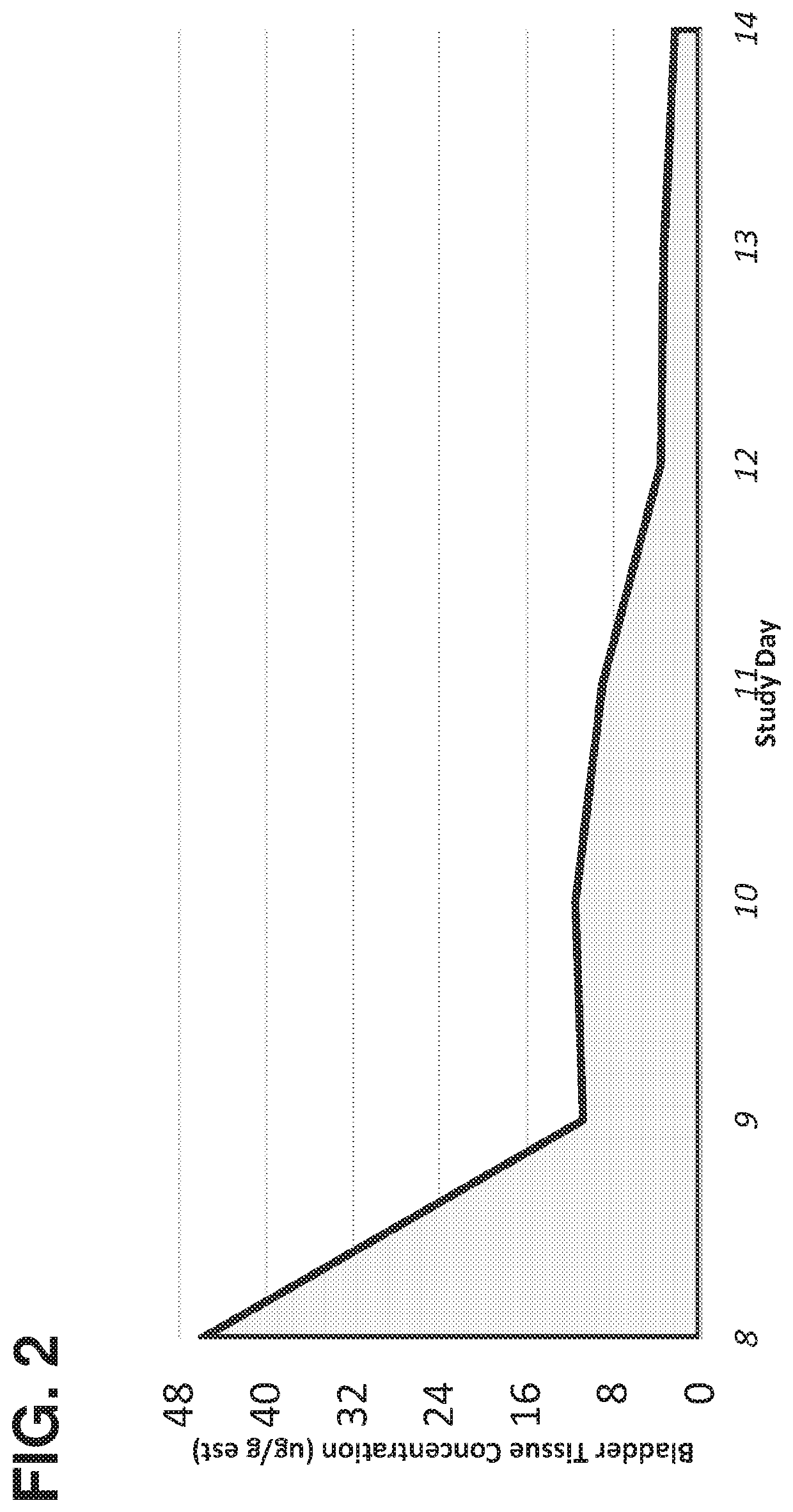
Method of treating lower tract urothelial cancer
PendingUS20190388338A1Reduce recurrenceShorten the progressOrganic active ingredientsPharmaceutical delivery mechanismImmunomodulating AgentAntineoplastic Antimetabolites
The present invention provides methods, devices, and kits related to treatment of urothelial carcinomas of the lower tract with antimetabolite (such as gemcitabine). In some aspects the methods, devices and kits relate to treatment of urothelial carcinomas of the lower tract with an antimetabolite (such as gemcitabine) and an immunomodulating agent.
Owner:TARIS BIOMEDICAL
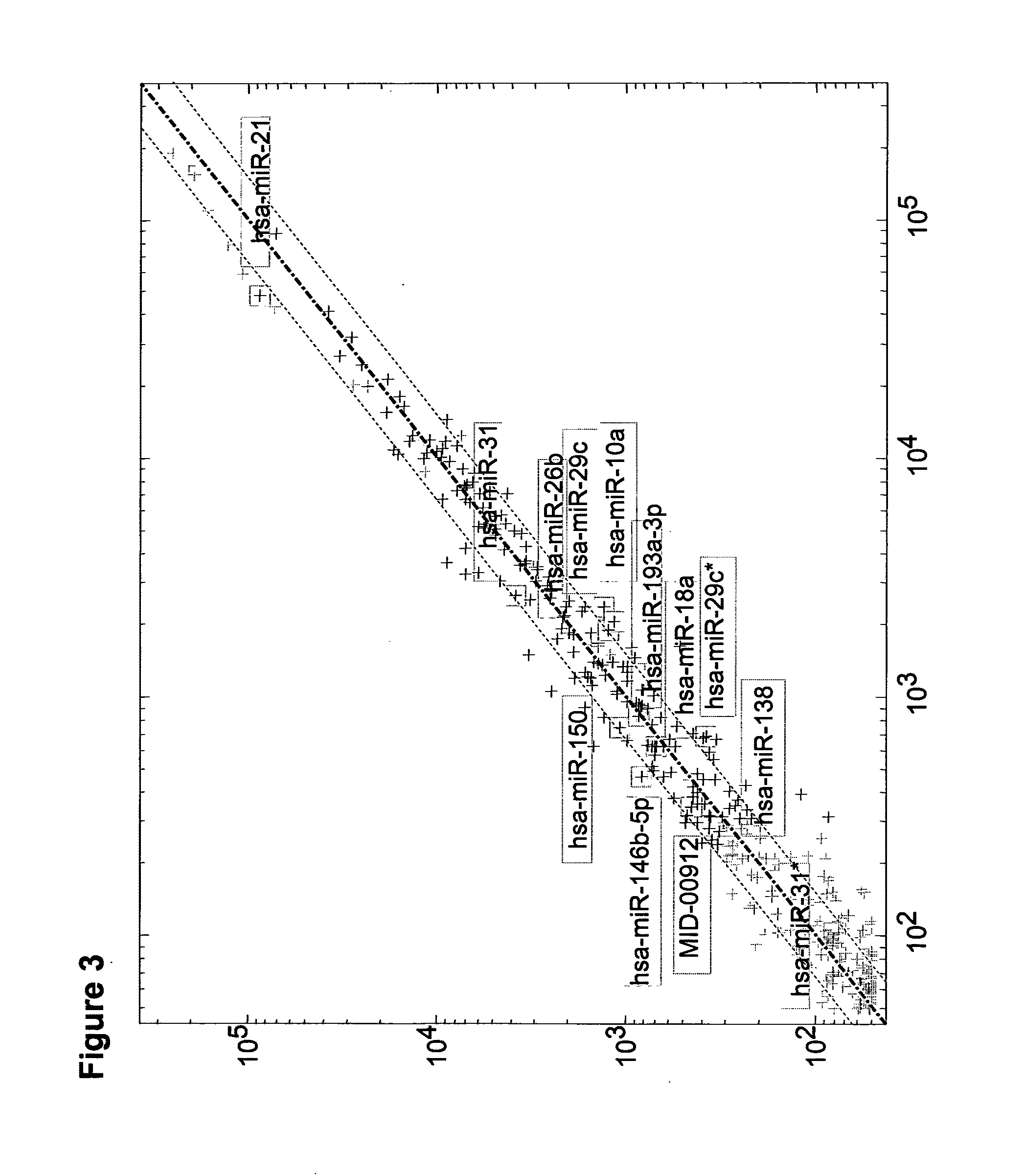
Compositions and methods for determining the prognosis of bladder urothelial cancer
Described herein are compositions and methods for the prediction of bladder cancer risk of invasiveness. The compositions are microRNA molecules associated with the prognosis of bladder cancer, as well as various nucleic acid molecules relating thereto or derived therefrom.
Owner:ROSETTA GENOMICS
Site-specific conjugation of linker drugs to antibodies and resulting ADCs
The present invention relates to antibody-drug conjugates (ADCs) wherein a linker drug is site-specifically conjugated to an antibody through an engineered cysteine, and their use as a medicament, notably for the treatment of human solid tumors and haematological malignancies, in particular breast cancer, gastric cancer, colorectal cancer, urothelial cancer, ovarian cancer, uterine cancer, lung cancer, mesothelioma, liver cancer, pancreatic cancer, prostate cancer, and leukaemia.
Owner:BYONDIS BV
Combination of a pd-1 antagonist and eribulin for treating urothelial cancer
The present disclosure describes combination therapies comprising an antagonist of Programmed Death 1 receptor (PD-1) and eribulin or a pharmaceutically acceptable salt thereof, and the use of the combination therapies for the treatment of urothelial cancer.
Owner:MERCK & CO INC +1
Method for validating existence of urinary exosome, non-invasive method for identifying urothelial cancer, and method for predicting recurrence and progression of urothelial cancer patient after treatment
ActiveUS20170350902A1High recurrenceSpeed up progressDisease diagnosisBiological testingBacteriuriaAfter treatment
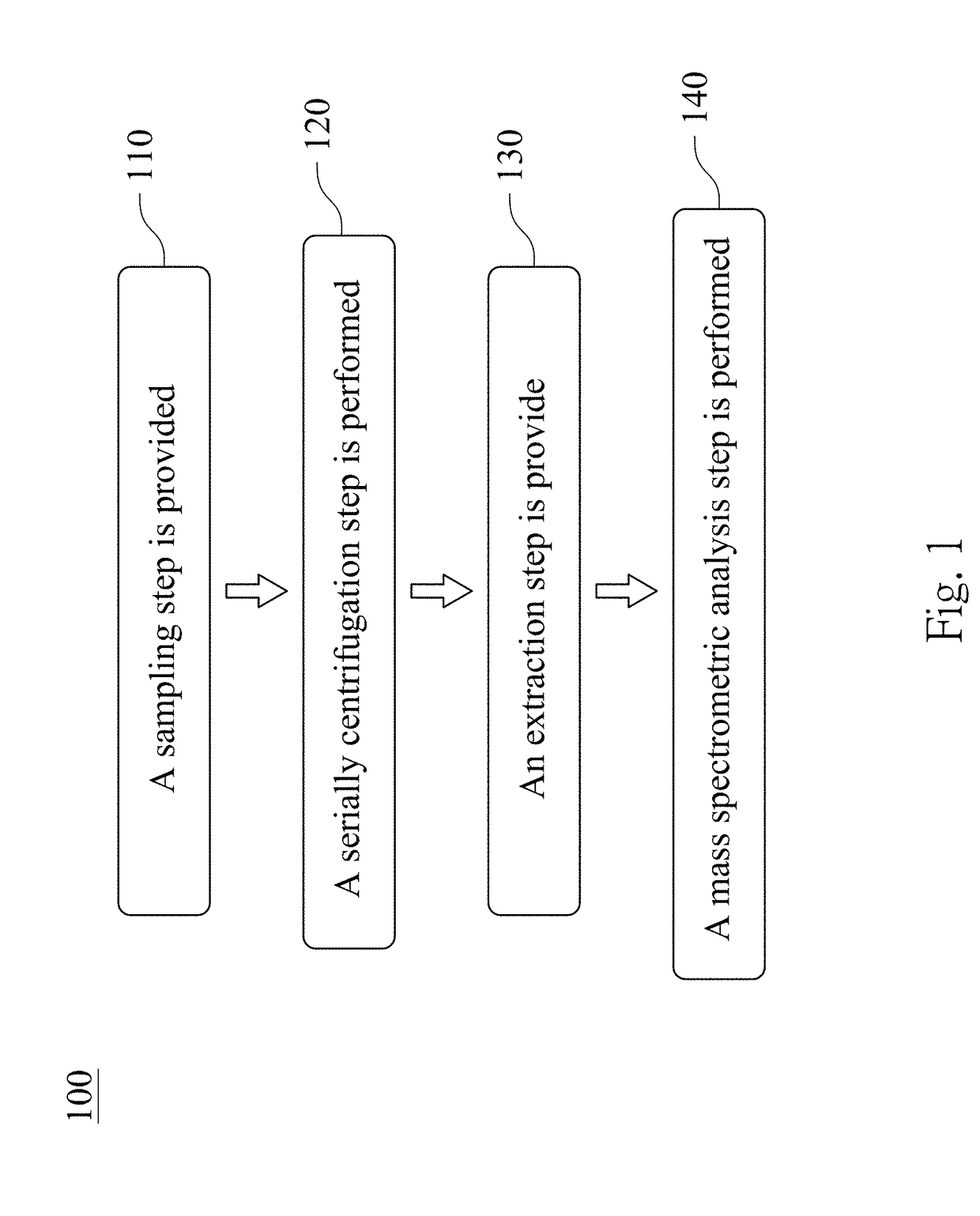
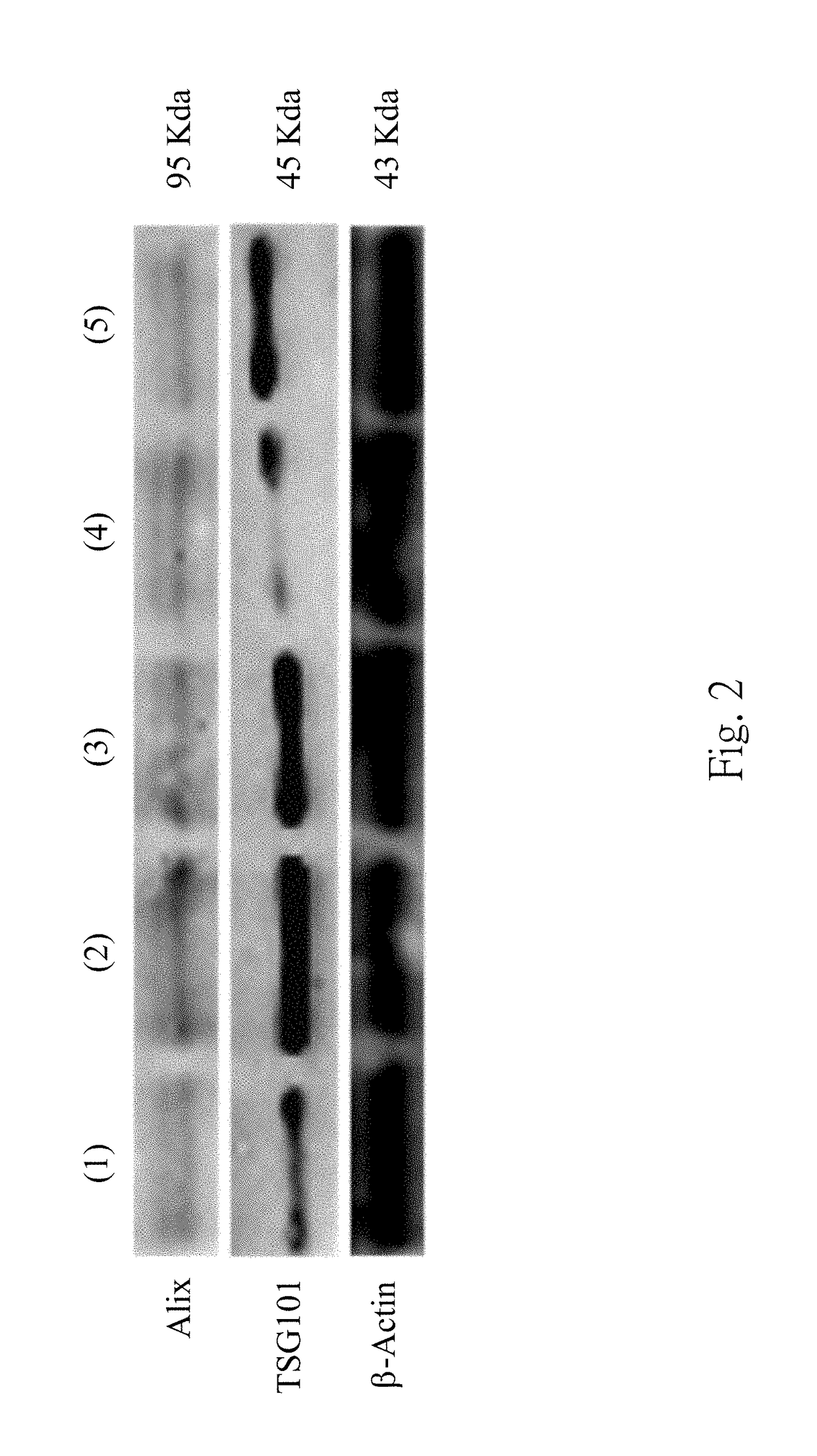
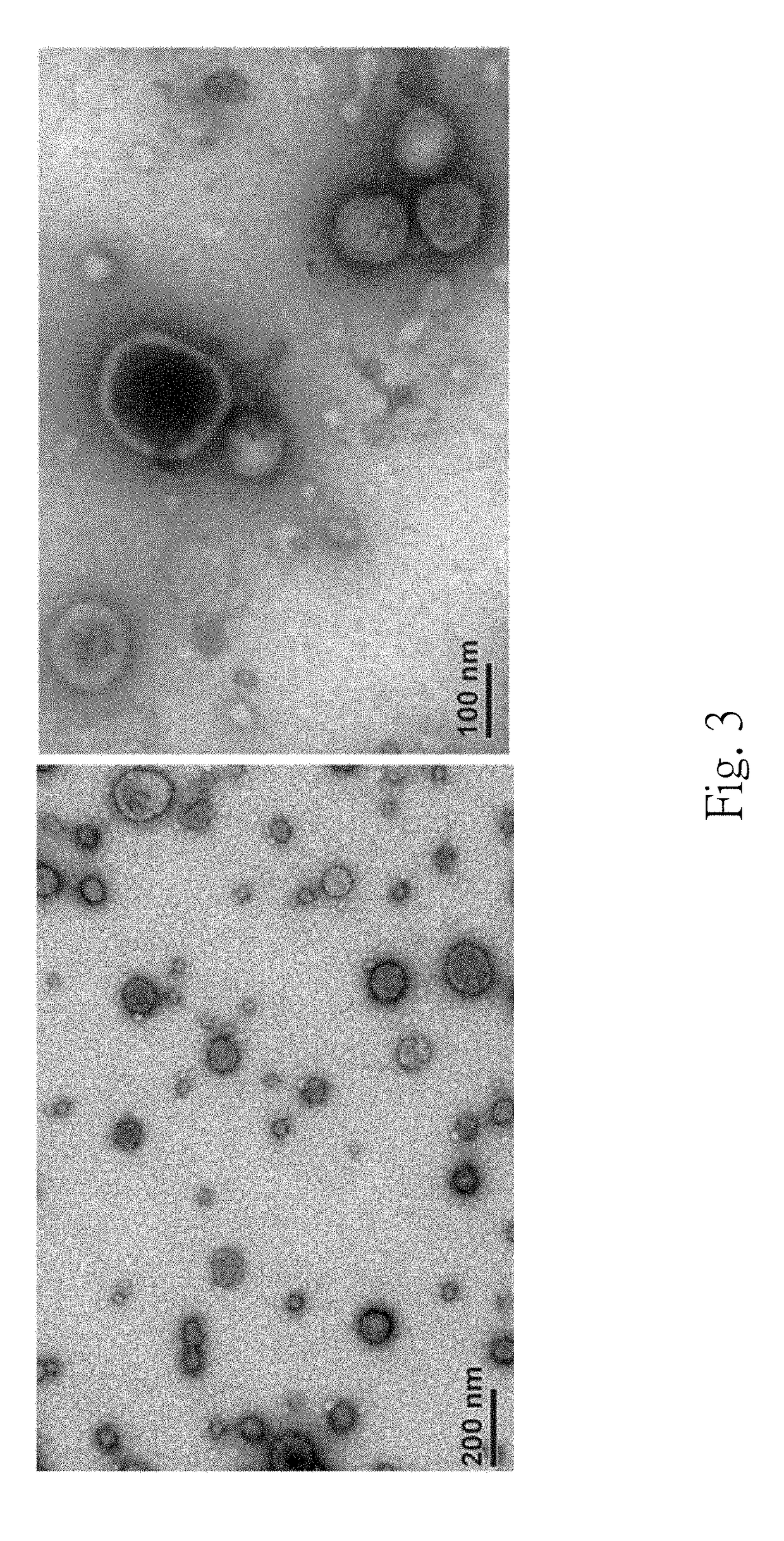
The present disclosure relates to a method for validating an existence of a urinary exosome including steps as follows. A urine sample is obtained from a subject. The urine sample is performing a serially centrifugation step to obtain a third precipitate. The third precipitate is resuspended with an extraction solvent to obtain a third mixture, and the third mixture is centrifuged to obtain a fourth supernatant. The fourth supernatant is analyzed by a mass spectrometry to detect whether there is a particular peptide therein.
Owner:CHINA MEDICAL UNIVERSITY(TW)
Methods of treatment and maintenance therapy for bladder cancer using gemcitabine
ActiveUS10792297B2Good effectSymptoms improvedAntibacterial agentsOrganic active ingredientsMaintenance therapyCancer research
Provided are methods of treating urothelial carcinomas of the lower tract comprising administering comprising administering gemcitabine continuously and locally to the bladder of an individual in an induction therapy and / or maintenance therapy.
Owner:TARIS BIOMEDICAL
Application of bacteroides fragilis and zwitterionic capsular polysaccharide thereof in preparation of medicine for preventing and treating tumors of genitourinary system
ActiveCN114344325AImprove immunityEffective colonizationOrganic active ingredientsUnknown materialsProstate cancerT cell
The invention discloses application of bacteroides fragilis and / or zwitterionic capsular polysaccharide thereof in preparation of a medicine for preventing and treating cancers of a urogenital system. The bacteroides fragilis, especially the bacteroides fragilis ZY-312 with the preservation number of CGMCC No.10685 and the zwitter-ion capsular polysaccharide A of the bacteroides fragilis ZY-312 can regulate the levels of T cells and immune factors, enhance body immunity and effectively prevent and treat kidney cancer, bladder cancer, prostate cancer, bladder urinary tract epithelial cancer, ovarian cancer, breast cancer, cervical cancer and other urogenital system tumors.
Owner:GUANGZHOU ZHIYI PHARMA INC
Method for detection of urothelial cancer
ActiveUS20130095520A1Reduce the burden onReduction of national medical expenseOrganic chemistryEnergy modified materialsAmino-Levulinic AcidFluorescence
It is to provide a method for detecting urothelial cancer simply and with high accuracy. It is a method for detecting urothelial cancer comprising administering 5-aminolevulinic acid (ALA), a derivative thereof, or a salt of these to a test subject, collecting urine from the test subject, and detecting the presence of fluorescence or amount of fluorescence in the collected urine.
Owner:SBI PHARMA CO LTD +1
Liquid Dosage Forms to Treat Cancer
ActiveUS20200268737A1Dispersion deliveryPharmaceutical non-active ingredientsRenal Cell CancersPharmaceutical drug
This invention relates to a liquid pharmaceutical composition comprising cabozantinib to treat locally advanced or metastatic solid tumors, particularly advanced urothelial cancer or renal cell carcinoma in patients in need thereof.
Owner:EXELIXIS INC
Aminopyrimidopyrazole/pyrrole derivatives and preparation method and use thereof
ActiveCN112961158BNovel structurePrevent proliferationOrganic active ingredientsOrganic chemistryPharmaceutical medicinePyrrole
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
A compound with benzyloxy aromatic ring structure, its preparation method and use
ActiveCN112028870BAvoid interactionIncrease lethalityAntibacterial agentsAntimycoticsDiseaseSquamous Carcinomas
The present invention provides a compound with benzyloxy aromatic ring structure represented by general formula (I), its stereoisomer, enantiomer or pharmaceutically acceptable salt thereof, its preparation method, and its pharmaceutical combination objects and their uses. The compound represented by the general formula (I) can be used to prepare small molecule inhibitors of PD1 / PD-L1 interaction, which can be used to prevent and / or treat diseases related to PD1 / PD-L1 interaction, especially cancer , such as, inter alia, non-small cell lung cancer, small cell lung cancer, melanoma, head and neck cancer, kidney cancer, bladder cancer, locally advanced or metastatic urothelial cancer, breast cancer, cervical cancer, metastatic Merkel cell carcinoma, prostate cancer Carcinoma, liver cancer, colon cancer, gastric cancer, multiple myeloma, mantle cell lymphoma, diffuse large B-cell lymphoma liver cancer, Hodgkin's lymphoma, chronic lymphocytic leukemia, squamous cell carcinoma and other cancers.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Formulations for treating bladder cancer
Compositions and methods for making and using proliposomal and liposomal formulations of chemotherapeutic agents are disclosed. The proliposomal and liposomal formulations of chemotherapeutics, as well as medicaments and dosage forms that include such formulations, can be used with treatment regimens for bladder cancer and urothelial cancer. Hence, the formulations, medicaments, and dosage forms of the invention are suitable to treat bladder cancers by intravesical administration and to treat urothelial cancers. The formulations according to the invention include (a) a taxane (e.g., paclitaxel, docetaxel) or cisplatin, (b) a first phospholipid, dipalmitoyl phosphatidylcholine (DMPC), and (c) a second phospholipid, dimyrsityl phosphatidyl glycerol sodium (DMPG). The proliposomal formulations form liposomes upon contact with an aqueous vehicle.
Owner:TESORX PHARMA LLC
Method For Predicting Recurrence And Progression Of Urothelial Cancer Patient After Treatment
The present disclosure relates to a method for predicting a recurrence and a progression of an urothelial cancer patient after a treatment including steps as follows. A urine sample is obtained from a subject. The urine sample is performing a serially centrifugation step to obtain a third precipitate. The third precipitate is resuspended with an extraction solvent to obtain a third mixture, and the third mixture is centrifuged to obtain a fourth supernatant. The fourth supernatant is analyzed by a mass spectrometry to detect whether there is a particular peptide therein.
Owner:CHINA MEDICAL UNIVERSITY(TW)
Diagnosis of risk of urothelial cancer
PendingUS20180011115A1Increased risk of developingIncreased riskDisease diagnosisBiological testingMedicineCvd risk
The present invention related to a method for detecting a history of exposure to a chemical(s) comprising measuring the concentration of thrombomodulin in a sample isolated from a subject, and determining whether the concentration of thrombomodulin is altered compared to control reference levels.
Owner:NORTHERN BANK LTD
Aminopyrimidopyrazole/pyrrole derivatives and preparation method and use thereof
ActiveCN112961159BNovel structurePrevent proliferationOrganic active ingredientsOrganic chemistryPharmaceutical medicineStomach cancer
The invention relates to aminopyrimidopyrazole / pyrrole derivatives, a preparation method and application thereof, and belongs to the field of medicine. The present invention provides compounds of formula I, stereoisomers thereof, and pharmaceutically acceptable salts of compounds or stereoisomers thereof. Biological experiments have proved that the compound of the present invention can not only significantly inhibit the proliferation of breast cancer, lung cancer, gastric cancer, bile duct cancer, urothelial cancer and other cancer cells, but also has a broad-spectrum anti-cancer effect. The proliferation of tumor cells also showed inhibitory effect, and it can inhibit the growth of tumors in vivo, which provides a new option for the development of anti-tumor, anti-lung and liver fibrosis drugs.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Method of treating lower tract urothelial cancer
PendingCN109475571AImprove the quality of lifeOrganic active ingredientsMedical devicesImmunomodulating AgentAntineoplastic Antimetabolites
The present invention provides methods, devices, and kits related to treatment of urothelial carcinomas of the lower tract with antimetabolite (such as gemcitabine). In some aspects the methods, devices and kits relate to treatment of urothelial carcinomas of the lower tract with an antimetabolite (such as gemcitabine) and an immunomodulating agent.
Owner:TARIS BIOMEDICAL
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com