Aminopyrimidopyrazole/pyrrole derivatives and preparation method and use thereof
An alkyl and aryl technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., to achieve the effect of inhibiting the growth of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
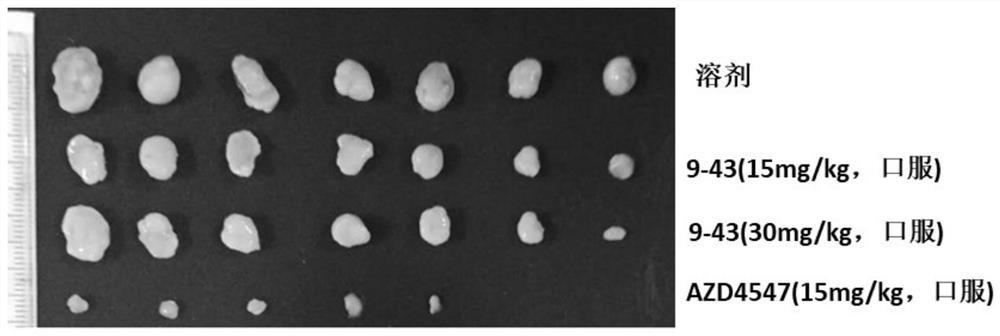
Image
Examples
Embodiment 1
[0120] Example 1 Preparation of the compound of the present invention
[0121]
[0122] Raw material 1 (1.08 g, 8 mmol) was dissolved in DMF (20 mL), N-iodosuccinimide (4.5 g, 20 mmol) was added, and the reaction was performed at 80° C. for 5 h and monitored by TLC. After the reaction was completed, the reaction was quenched with a saturated aqueous solution of hydrosulfite, the reaction solution was poured into water, a yellow solid was precipitated, the solid was filtered and dried to obtain Intermediate 2 (white solid, 98%), MS m / z (ESI) : 261.9[M+H] + .
[0123]
[0124] Intermediate 2 (2.09 g, 8 mmol) was dissolved in 150 mL of tetrahydrofuran, compound 3 (1.8 g, 9.6 mmol), triphenylphosphine (4.2 g, 16 mmol) were added, N 2 protection, stirred at 0 °C, and then added dropwise diisopropyl azodicarboxylate (3.1 mL, 16 mmol), the dropwise addition was completed, and after half an hour, the reaction was transferred to room temperature for 3 h and monitored by TLC. A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com