Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
56 results about "Urothelial carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
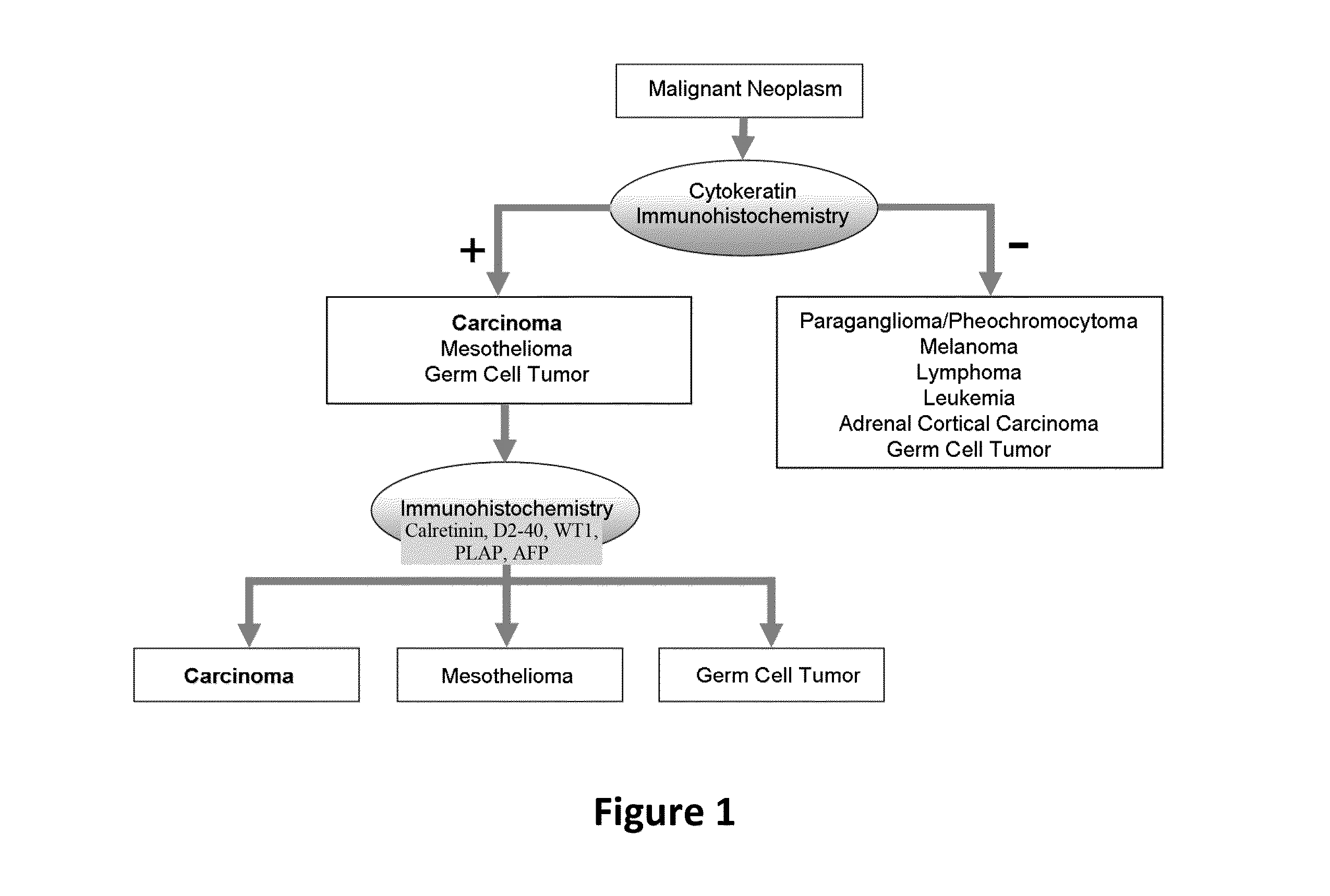
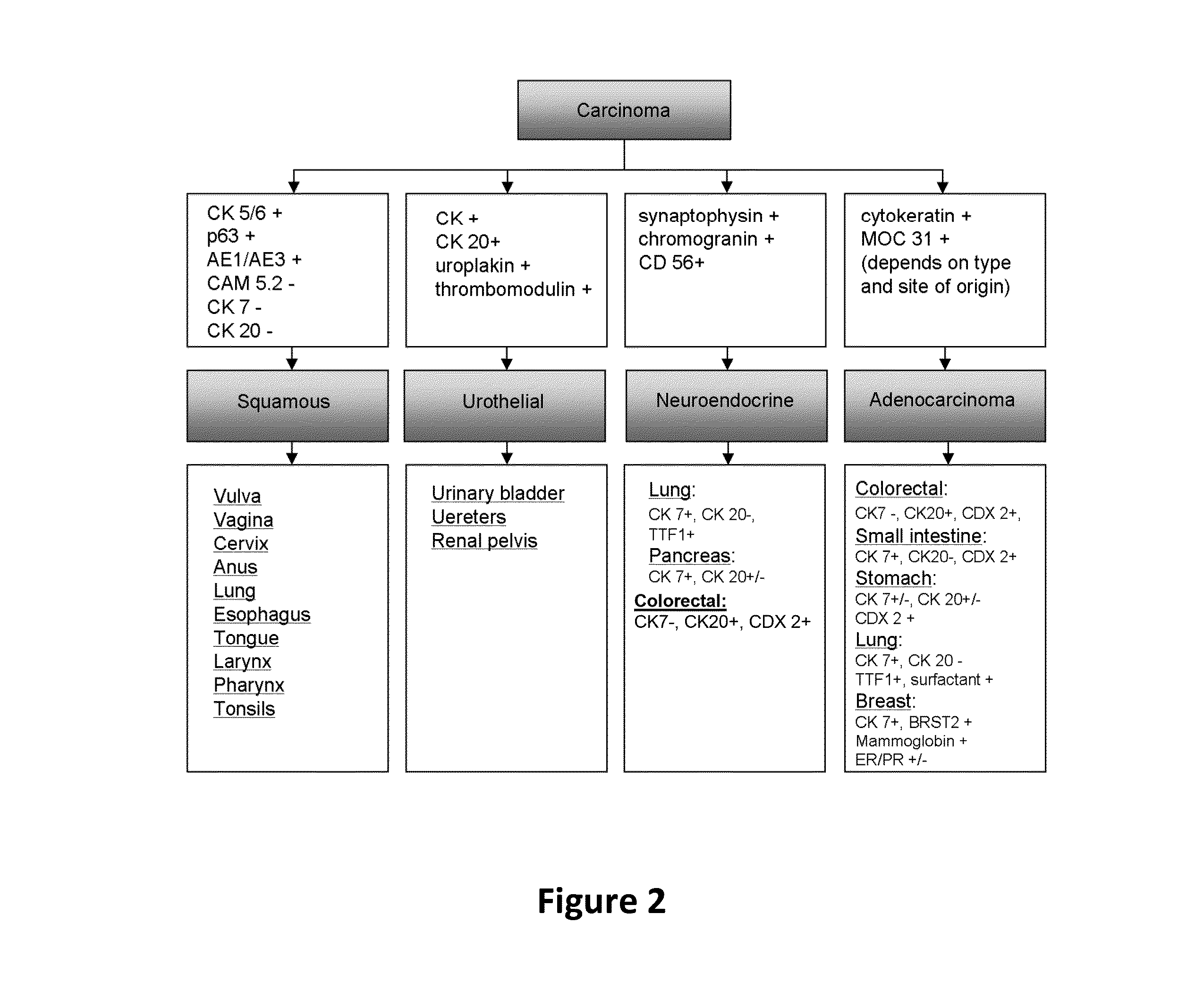
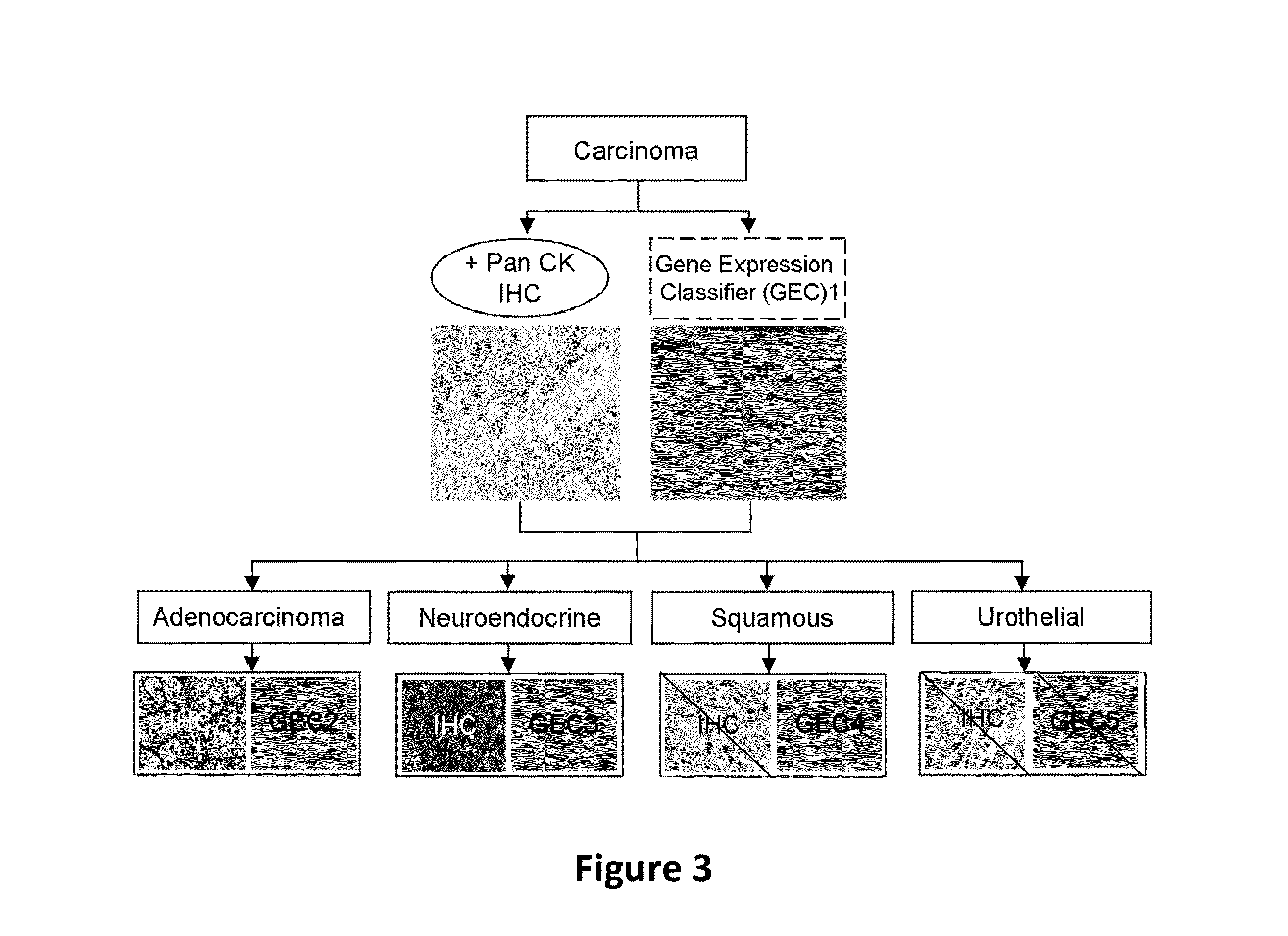
Hybrid model for the classification of carcinoma subtypes
ActiveUS20130172203A1Effective treatmentMicrobiological testing/measurementLibrary screeningSquamous CarcinomasPrimary sites
A two-tiered classification system that can be integrated with the current algorithm used by pathologists for identification of the site of origin for ‘malignancy with unknown primary ’ is presented. In use, morphology, immunohistochemical (IHC) studies, and microarry-based top tier gene expression classifiers first subclassify cytokeratin positive carcinomas into adenocarcinoma, squamous cell carcinoma, neuroendocrine carcinoma and urothelial carcinoma. Subsequently, organ-specific IHC-markers, if available, are used in conjunction with microarray-based second tier gene expression classifiers to assign the primary site of origin to the sample. This new hybrid approach combines IHC with a hierarchy of quantitative gene expression based classifiers into an algorithmic method that can assist pathologists to further refine and support their decision making process.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
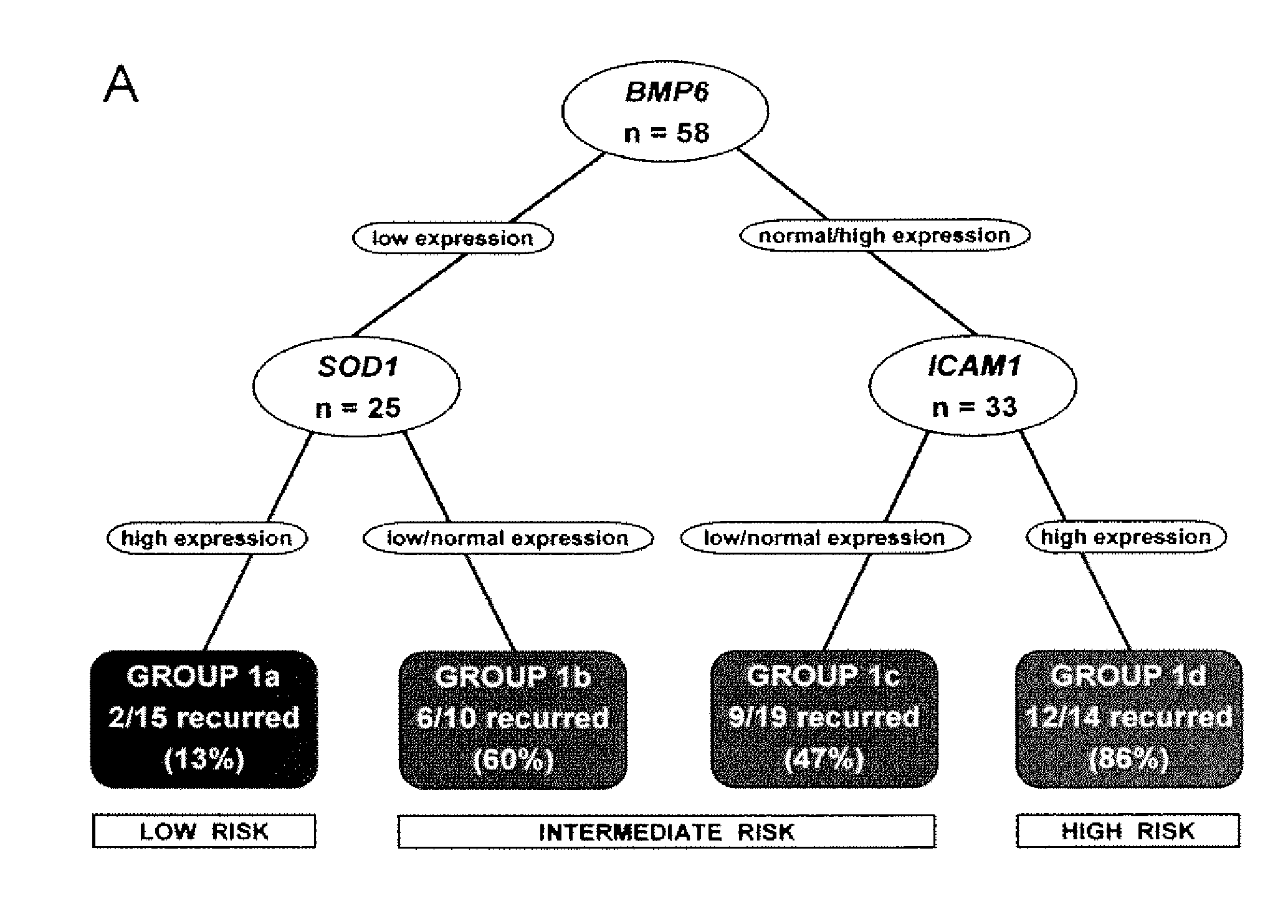
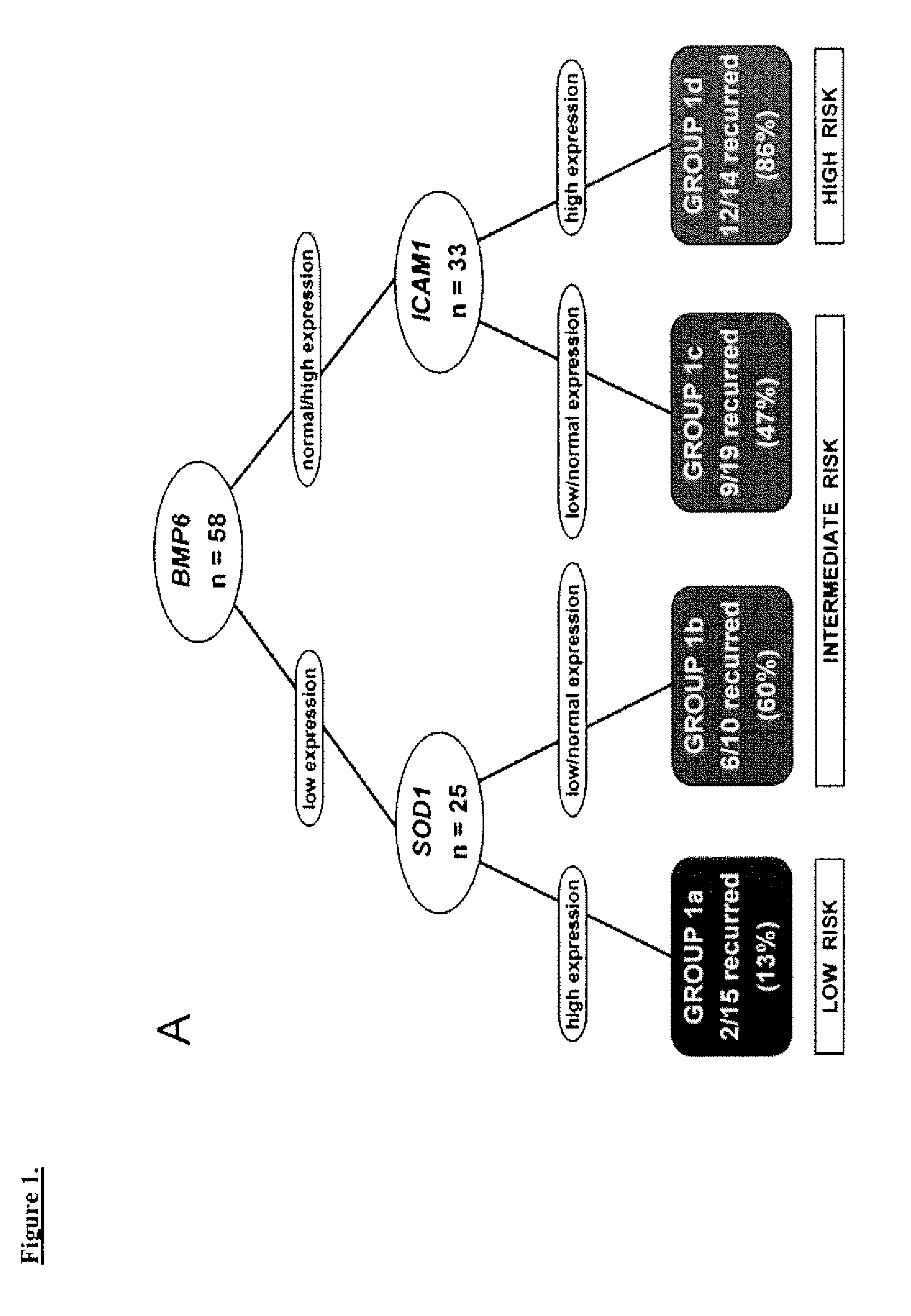
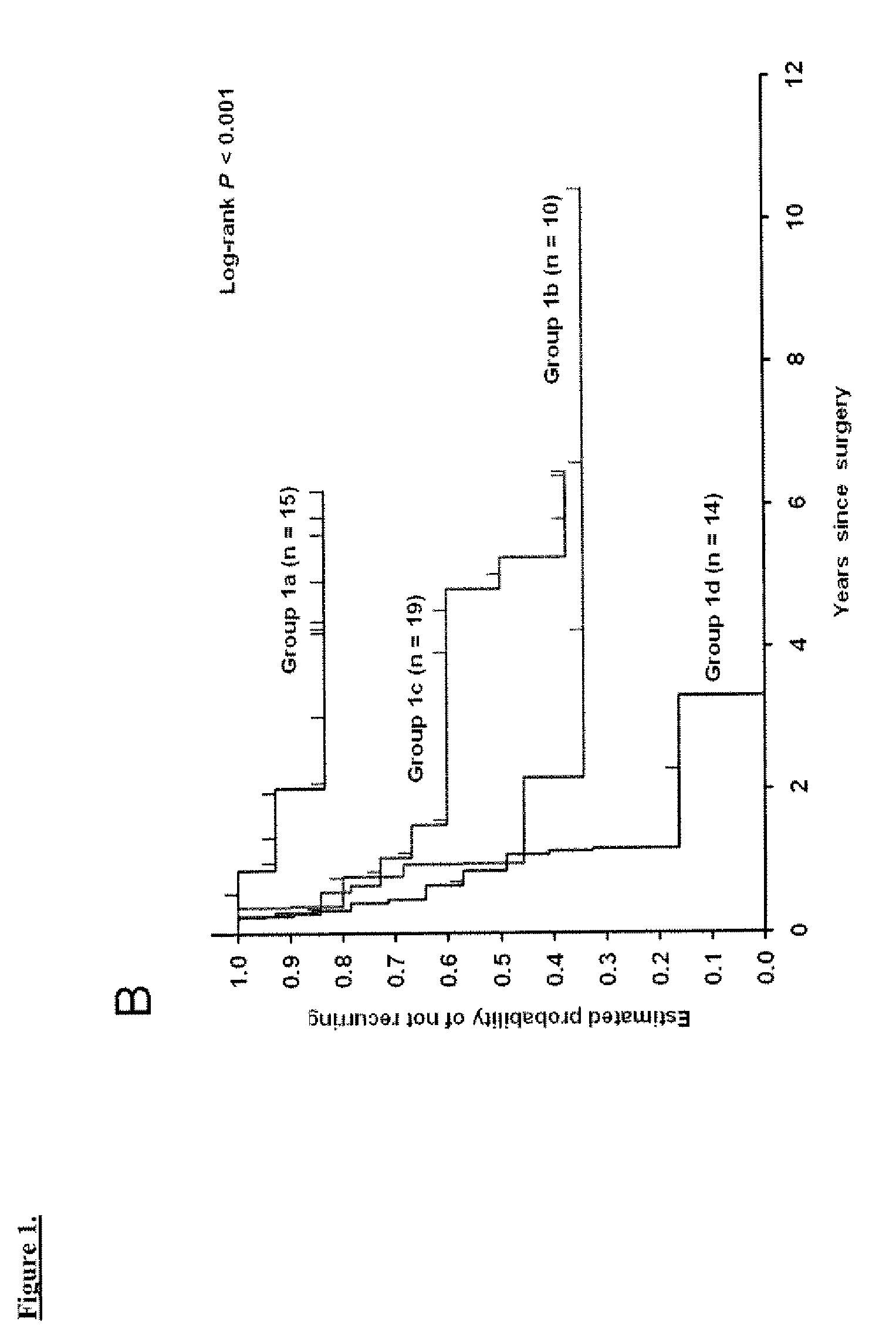
Prognostic panel for urinary bladder cancer
The present invention relates to methods of prognosing urothelial carcinoma. In one embodiment, the present invention provides a method of prognosing urothelial carcinoma by determining expression levels of JUN, MAP2K6, STAT3 and / or ICAM1. In another embodiment, the present invention provides an single prognostic panel made up of eight gene markers. In another embodiment, the present invention provides a single prognostic panel made up of eleven gene markers.
Owner:UNIV OF SOUTHERN CALIFORNIA
Urine exfoliated tumor cell micro-fluidic chip detection technology aiming at urothelium carcinoma
PendingCN106867867AImprove detection accuracyGet rid of dependenceBioreactor/fermenter combinationsBiological substance pretreatmentsStainingCell trapping
The invention belongs to the field of biological fluid detection in vitro, and concretely relates to a urine exfoliated tumor cell micro-fluidic chip detection technology aiming at urothelium carcinoma. The micro-fluidic chip comprises a sample inlet, a cell capturing area and a sample outlet which are connected sequentially, wherein the cell capturing area is provided with a plurality of cell sorting devices; each cell sorting device is formed by three columnar bulges arranged in an arc shape; a gap exists between every two columnar bulges; an arc-shaped opening is used as a liquid flow inlet; gaps at two sides of each middle columnar bulge are used as liquid flow outlets which are symmetrically distributed. According to the urine exfoliated tumor cell micro-fluidic chip detection technology provided by the invention, various cells in urine are separated and captured, multiple downstream cytology staining methods can be compatible for realizing cell recognition, the detection accuracy of urine exfoliated tumor cells in human urine is improved, the dependency on subjective experiences of pathology doctors in a conventional method is avoided, and the whole process is noninvasive. In addition, a lossless cell capturing and recovering manner lays a foundation on downstream molecular biology analysis.
Owner:ZHEJIANG UNIV +1
Immunomonotherapy for urothelial carcinoma
PendingUS20210040213A1Reduce delay progressionImprove toleranceOrganic active ingredientsInorganic active ingredientsAntiendomysial antibodiesImmunotherapy
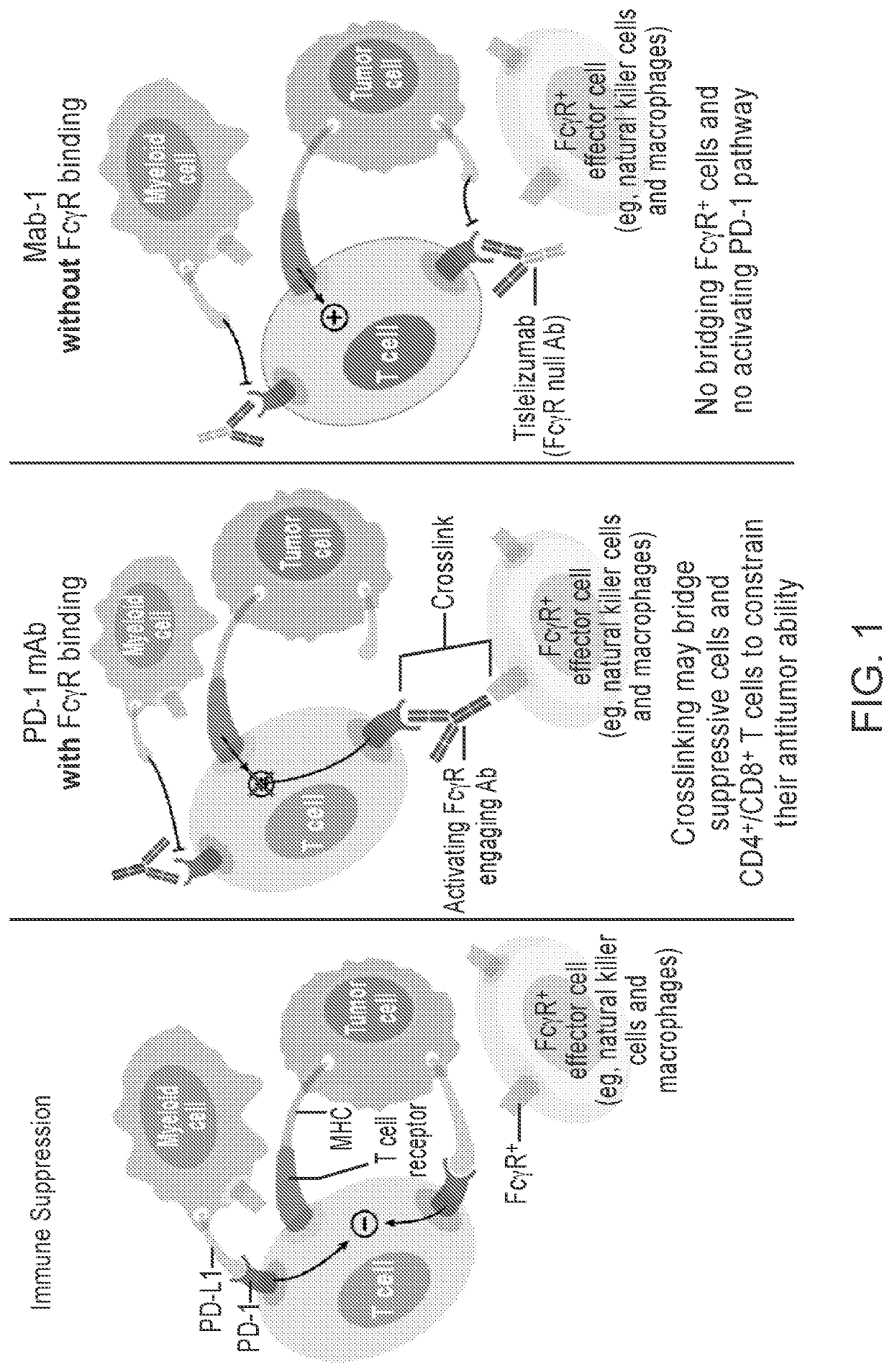
Disclosed herein is a method for immunotherapy of a patient with urothelial carcinoma (UC) comprising administering to the patient an anti-PD-1 antibody reduces FcγR binding thus reducing antibody-dependent phagocytosis.
Owner:BEIGENE
Kit and method for detecting urothelial cancer
InactiveUS7910316B2Easy and highly detectionEasy diagnosisMicrobiological testing/measurementBiological testingNucleic Acid ProbesBiology
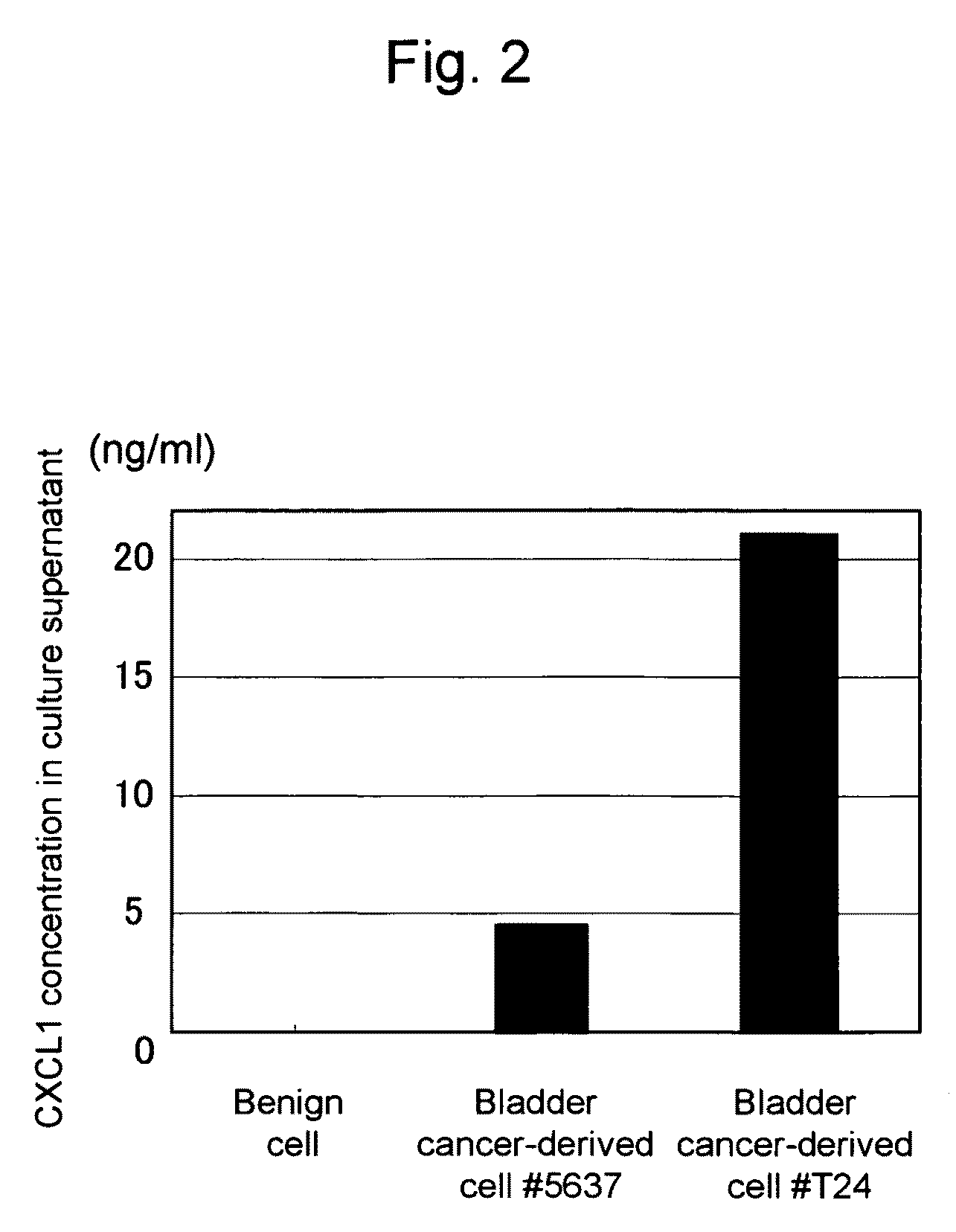
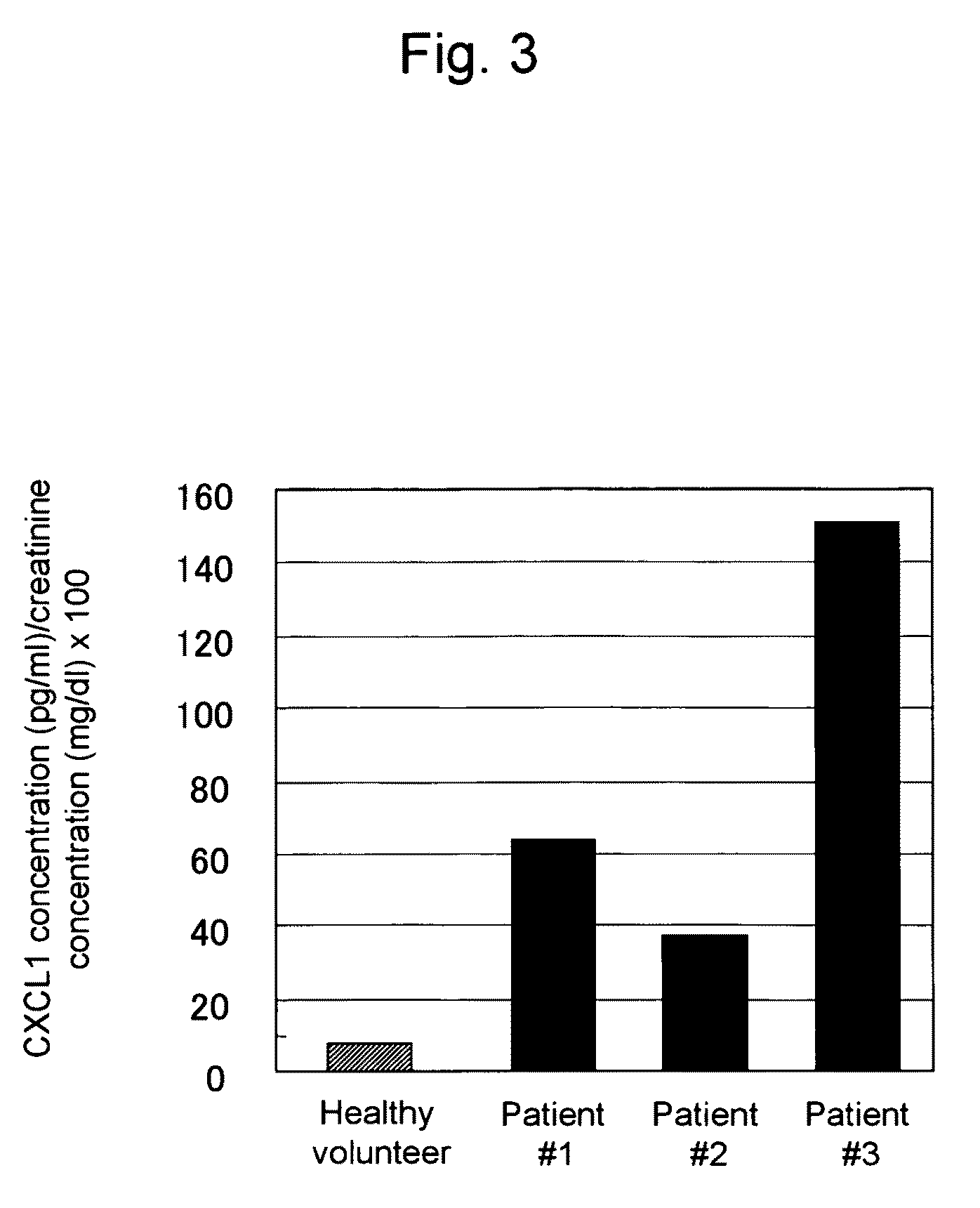
This invention relates to a method for detecting in vitro a urothelial cancer, comprising measuring CXCL1 protein, or expression of a gene encoding the protein, in a biological sample from a subject, and to a kit for diagnosing a urothelial cancer comprising an antibody or nucleic acid probe, which is capable of binding specifically to the CXCL1 protein or a gene encoding the protein, respectively.
Owner:TORAY IND INC +1
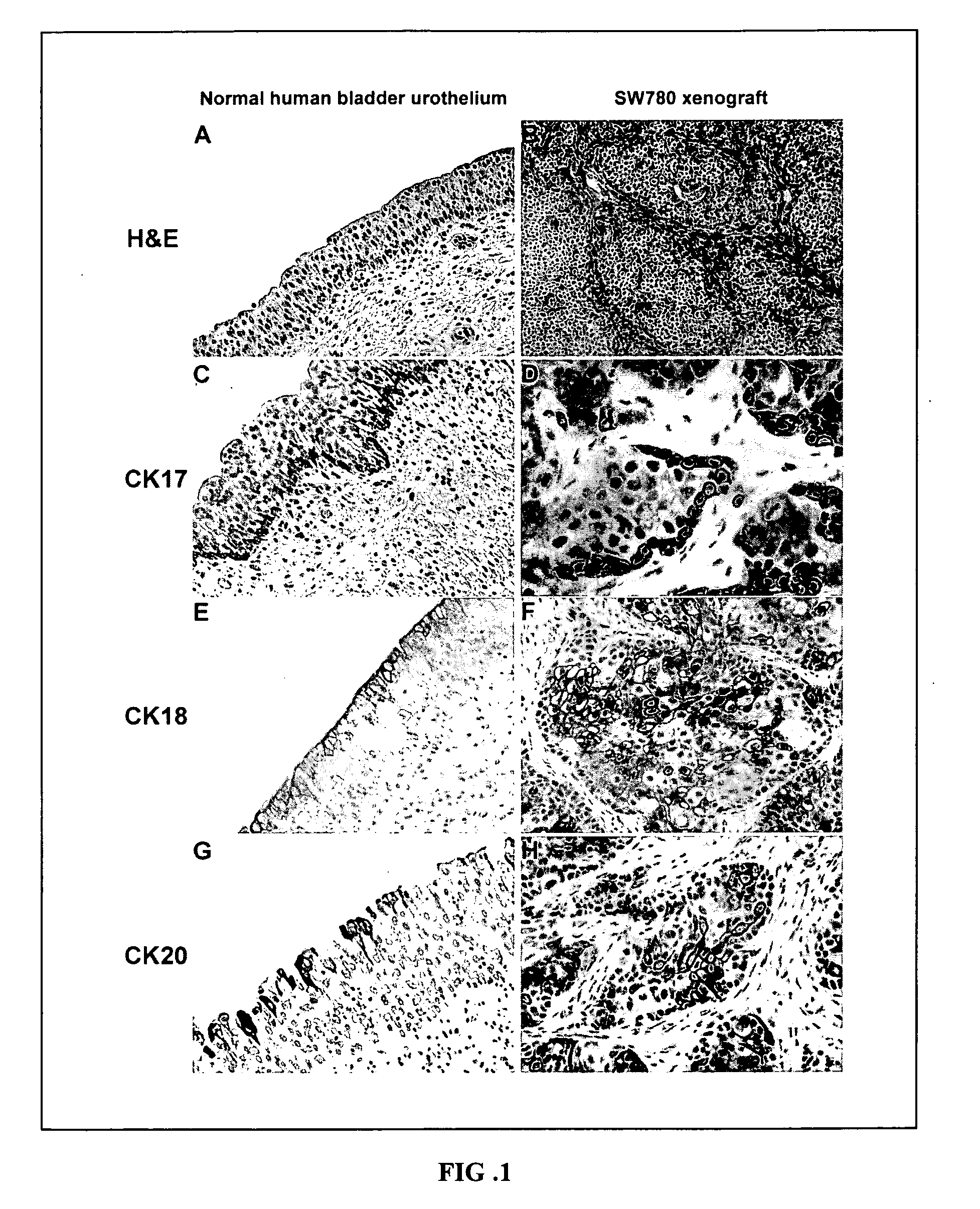
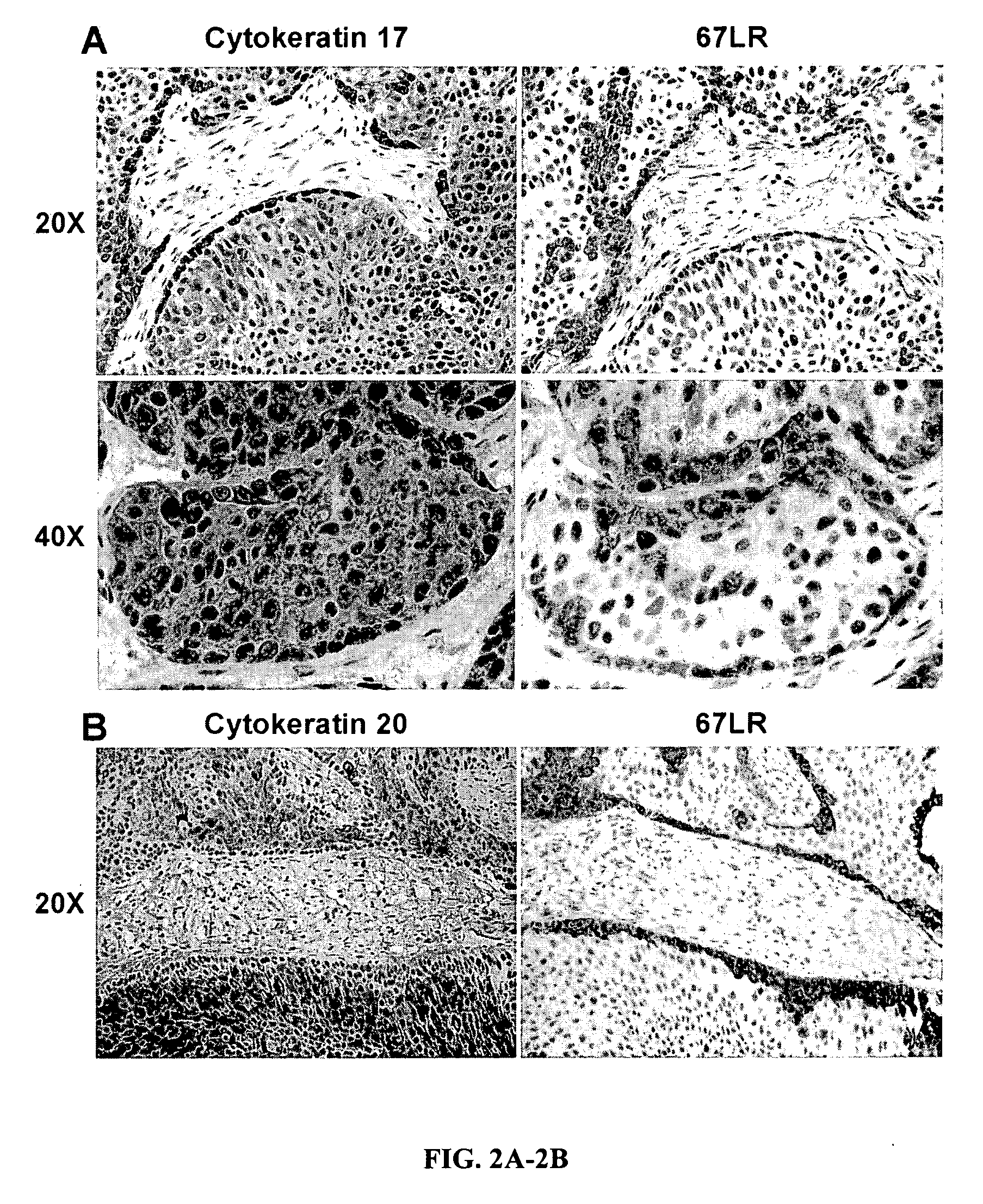
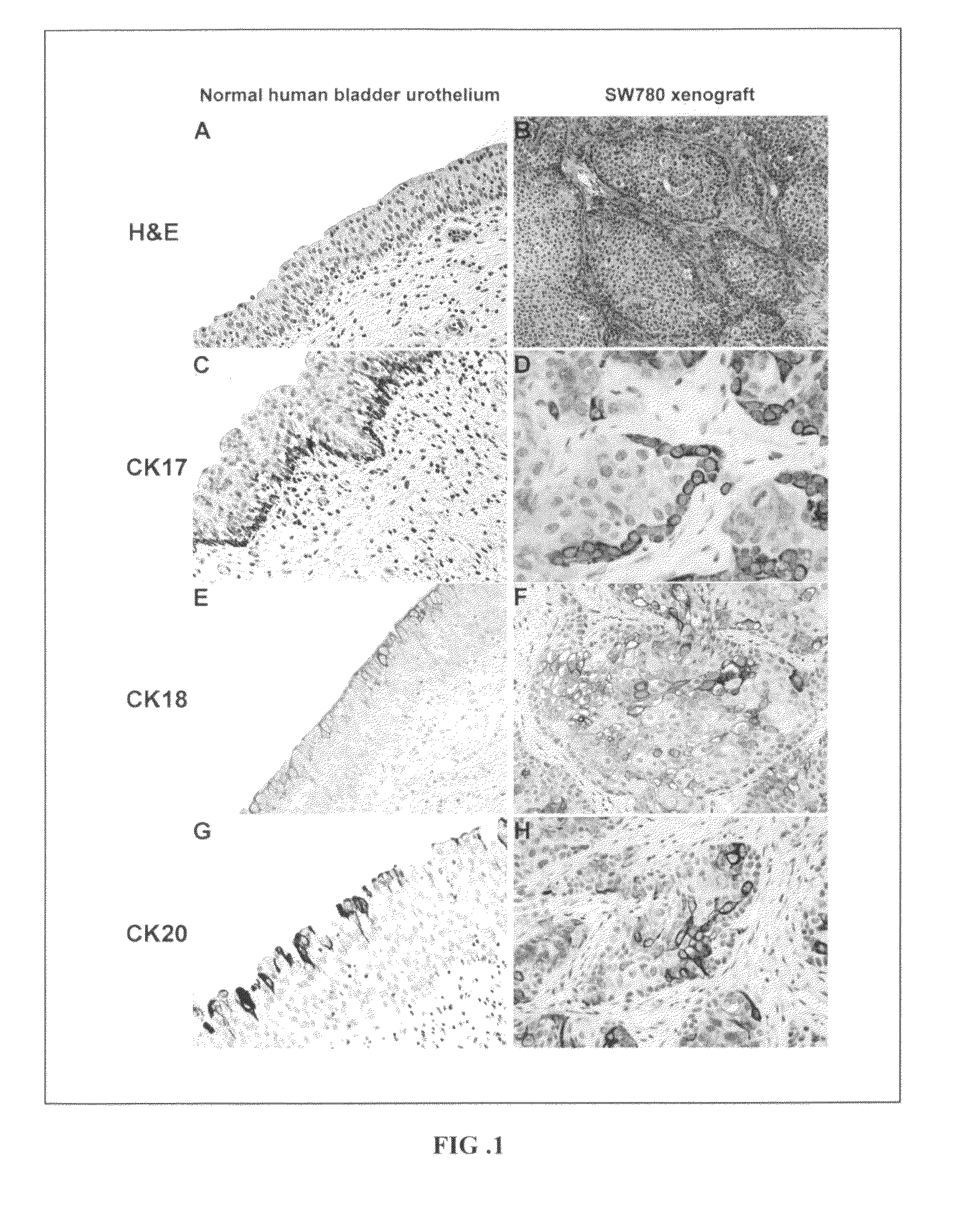
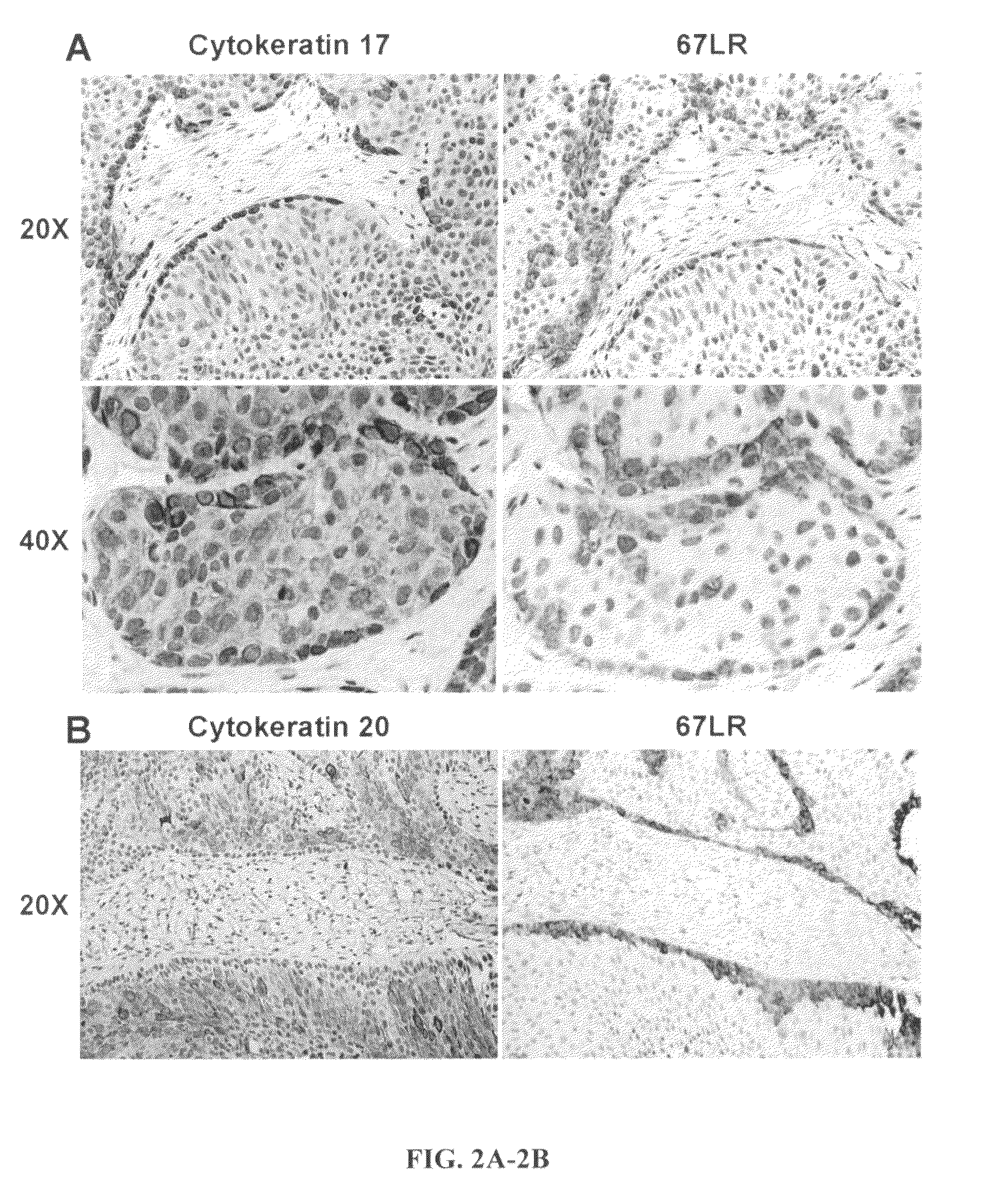
Urothelial differentiation of urothelial carcinoma: a bladder cancer stem cell model
ActiveUS20090047212A1Low toxicityGood curative effectUltrasonic/sonic/infrasonic diagnosticsGeneral/multifunctional contrast agentsCancer cellRegimen
The present invention provides a method of treating cancer in a patient (e.g., a human patient) in need thereof, the method comprising administering a therapeutically effective regimen, the regimen comprising administering to a patient in need thereof a compound that targets 67 laminin receptor (67LR). In a particular embodiment, the compound that targets 67LR is an antibody or antibody fragment. In particular, the present invention provides a method of treating cancer comprising administering to a patient in need thereof an antibody that binds to 67LR. The present invention also provides a method of treating cancer comprising administering to a patient in need thereof an antibody conjugate, wherein the antibody conjugate comprises an antibody that binds to 67LR linked to a therapeutic agent, a protein toxin, a cytotoxic agent or other moiety. The present invention provides pharmaceutical compositions for the treatment of cancer comprising an antibody that binds to 67LR in an amount effective to reduce cancer stem cells and / or cancer cells in a patient. The invention also provides for means to detect and monitor cancer stem cells based on their expression of 67LR.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
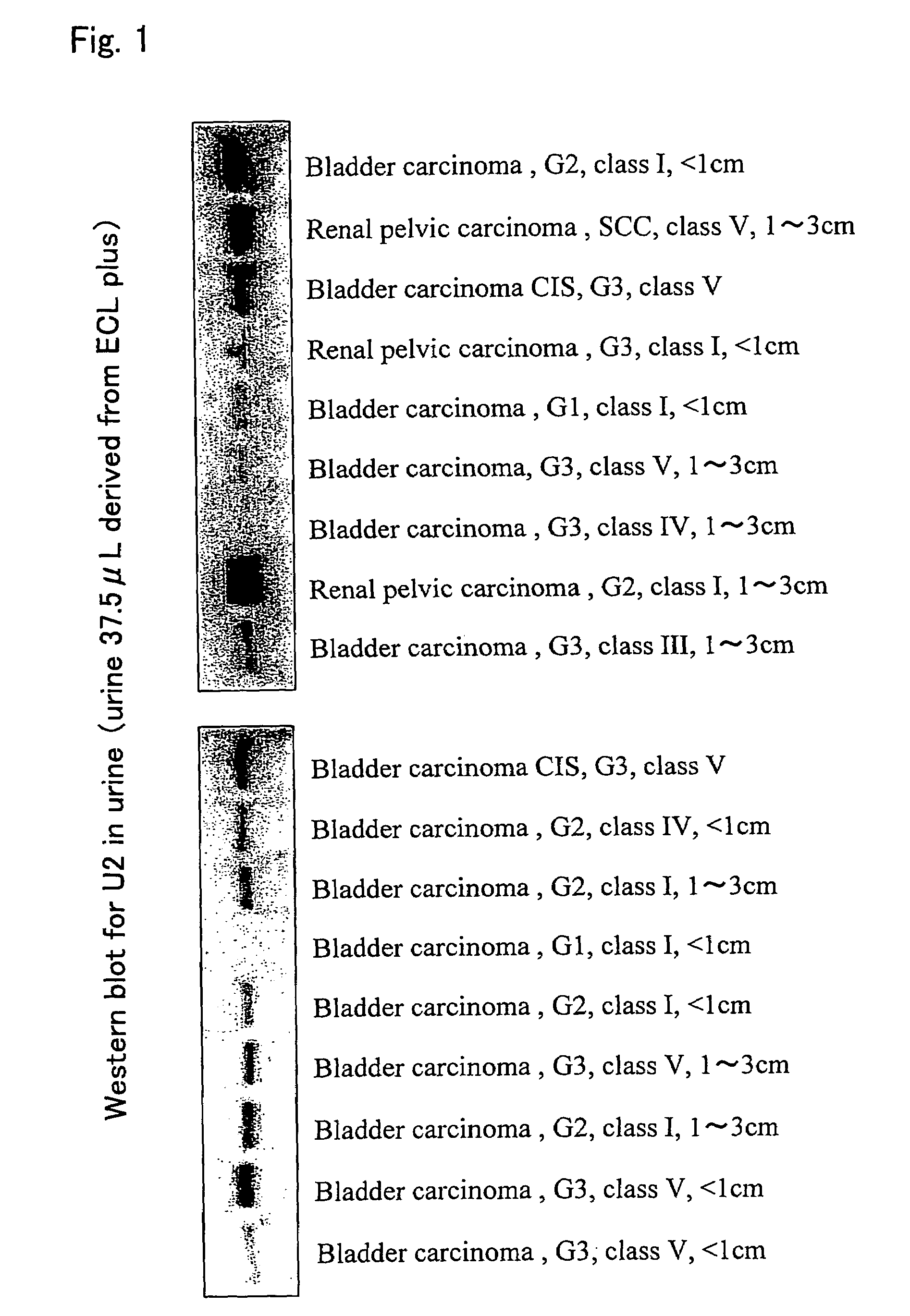
Tumor marker for urothelial carcinoma
InactiveUS7323312B2Efficient detectionImprove fusion efficiencyMicrobiological testing/measurementAbsorbent padsCalreticulinTumor marker
This invention relates to a method for detecting urothelial carcinoma using an antibody that specifically reacts with a calreticulin protein or a fragment thereof and immunoassaying a calreticulin protein in a sample.
Owner:TSS BIOTECH
Prognostic panel for urinary bladder cancer
ActiveUS20090246790A1Great likelihoodSugar derivativesMicrobiological testing/measurementBladder cancerMAP2K6
The present invention relates to methods of prognosing urothelial carcinoma. In one embodiment, the present invention provides a method of prognosing urothelial carcinoma by determining expression levels of JUN, MAP2K6, STAT3 and / or ICAM1. In another embodiment, the present invention provides an single prognostic panel made up of eight gene markers. In another embodiment, the present invention provides a single prognostic panel made up of eleven gene markers.
Owner:UNIV OF SOUTHERN CALIFORNIA
Human urothelial carcinoma specific antibody and application thereof
ActiveCN103592439AImmunoglobulins against cytokines/lymphokines/interferonsMicroorganism based processesEpitheliumAntiendomysial antibodies
The invention provides a new human urothelial carcinoma tumor marker-human tumor necrosis factor receptor superfamily TNFRSF25 and a hybridoma cell generating a monoclonal antibody resisting the tumor marker and a monoclonal antibody DTUC1 secreted thereby, wherein the monoclonal antibody DTUC1 is secreted by the hybridoma with collection number CGMCC No.8118. The monoclonal antibody DTUC1 is in positive reaction with the human urothelial carcinoma tissue and urothelial carcinoma cell lines such as 5637, UMUC3, SW780, J82, T24 and RT4, and is not in cross reaction with the human normal urothelium tissue and other non-urothelial carcinoma cells; according to mass spectrometric detection, the identified antigen is TNFRSF25. The invention also provides an in-vitro diagnosis kit comprising the monoclonal antibody DTUC1 and a method for detecting the content of tumor markers in urine by use of the monoclonal antibody DTUC1.
Owner:中科健兰(北京)医学研究院
Methods of treating urothelial carcinoma using an Anti-pd-1 antibody
InactiveUS20190315865A1Inhibitory activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCTLA4 ProteinAntigen binding
This disclosure provides a method for treating a subject afflicted with a urothelial carcinoma or cancer derived therefrom, which method comprises administering to the subject an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity or the combination of (a) an antibody or an antigen-binding portion thereof that specifically binds to a PD-1 receptor and inhibits PD-1 activity; and (b) an antibody or an antigen-binding portion thereof that specifically binds to a Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) and inhibits CTLA-4 activity.
Owner:BRISTOL MYERS SQUIBB CO
Application of bladder urothelium carcinoma detection combined marker
InactiveCN112877441AImprove detection efficiencyImprove detection accuracyMicrobiological testing/measurementDNA/RNA fragmentationARID1ACore gene
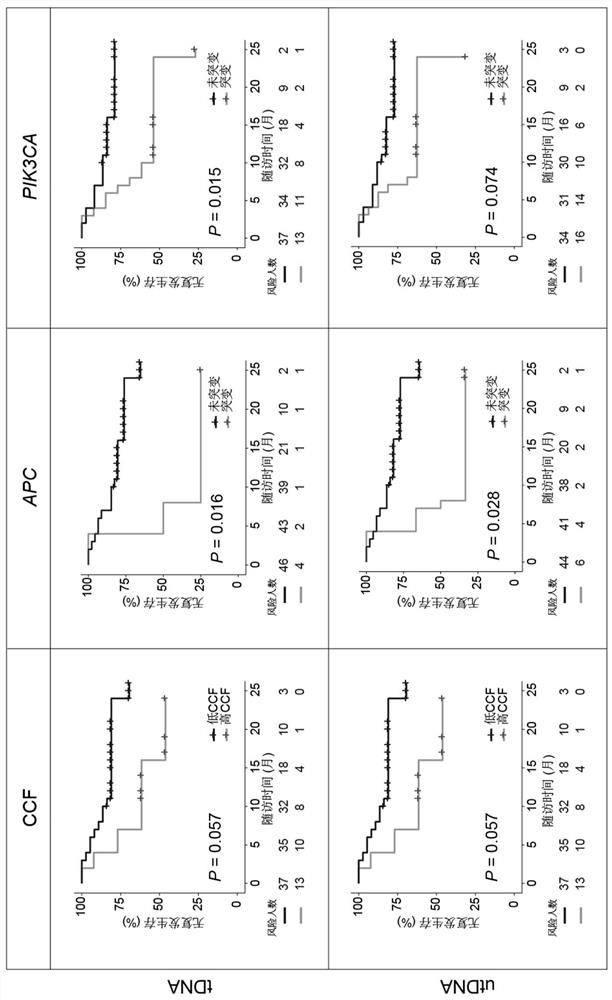
The invention discloses application of a bladder urothelium carcinoma detection combined marker, and belongs to the technical field of molecular medicine. The bladder urothelium carcinoma detection combined marker comprises the following gene combinations: TERT, TP53, FGFR3, PIK3CA and ARID1A. According to the invention, it is found for the first time that a combination of five genes of TERT, TP53, FGFR3, PIK3CA and ARID1A can accurately diagnose bladder urothelial carcinoma. Compared with an original method, the method has the advantages that the five core gene detection combinations reduce the sequencing workload by 40 times, and good diagnostic performance is maintained.
Owner:苏州仁端生物医药科技有限公司
Urothelial differentiation of urothelial carcinoma: a bladder cancer stem cell model
ActiveUS8784772B2Enhance or improve the prophylactic effect(s) of another therapyShorten the durationUltrasonic/sonic/infrasonic diagnosticsGeneral/multifunctional contrast agentsCancer cellRegimen
The present invention provides a method of treating cancer in a patient (e.g., a human patient) in need thereof, the method comprising administering a therapeutically effective regimen, the regimen comprising administering to a patient in need thereof a compound that targets 67 laminin receptor (67LR). In a particular embodiment, the compound that targets 67LR is an antibody or antibody fragment. In particular, the present invention provides a method of treating cancer comprising administering to a patient in need thereof an antibody that binds to 67LR. The present invention also provides a method of treating cancer comprising administering to a patient in need thereof an antibody conjugate, wherein the antibody conjugate comprises an antibody that binds to 67LR linked to a therapeutic agent, a protein toxin, a cytotoxic agent or other moiety. The present invention provides pharmaceutical compositions for the treatment of cancer comprising an antibody that binds to 67LR in an amount effective to reduce cancer stem cells and / or cancer cells in a patient. The invention also provides for means to detect and monitor cancer stem cells based on their expression of 67LR.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Gene combination for human tumor grading and application of gene combination
PendingCN113604571AImprove reliabilityIncrease credibilityMicrobiological testing/measurementParanasal Sinus CarcinomaHuman tumor
The invention relates to the field of tumor grading detection, in particular to a gene combination for human tumor grading and application of the gene combination. The gene combination for human tumor grading is composed of a gene set A, a gene fragment set B and a gene fragment set C. The gene combination for human tumor grading is obtained from high-throughput sequencing data of actual urinary tract epithelial cancer cases of Peking University First Hospital through specific pairing clustering analysis, the data from the real data have higher reliability and credibility, and malignant degree grading and prognosis prediction can be accurately carried out on urinary tract epithelial cancer and pan-cancer.
Owner:PEKING UNIV FIRST HOSPITAL
Anti-pd-l1 antibody treatment of bladder cancer
InactiveUS20190359715A1Effective treatmentLower Level RequirementsPharmaceutical delivery mechanismImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAbnormal tissue growth
Provided are methods of treating bladder cancer (e.g., urothelial carcinoma, UC) in a subject having bladder cancer, e.g., UC, with an effective dose regimen of an anti-PD-L1 antibody, e.g., durvalumab, or an antigen binding fragment thereof. Also provided are methods in which an anti-PD-L1 antibody is used in combination with another immunotherapeutic agent, e.g., tremelimumab to treat a bladder cancer, e.g., UC, in a subject having bladder cancer. In some cases, the subject undergoing treatment is identified as having a bladder cancer or tumor that is PD-L1-low / neg, or PD-L1-high. Methods are also provided in which anti-PD-L1 antibody treatment of bladder cancer is used following a standard of care or first-line therapy in subjects who have progressed following such therapies or who have relapsed after a prior treatment regimen.
Owner:MEDIMMUNE LLC
Methods of detection and treatment of urothelial cancer
InactiveUS20200181709A1High expressionSymptoms improvedMicrobiological testing/measurementAmide active ingredientsDiseaseRegimen
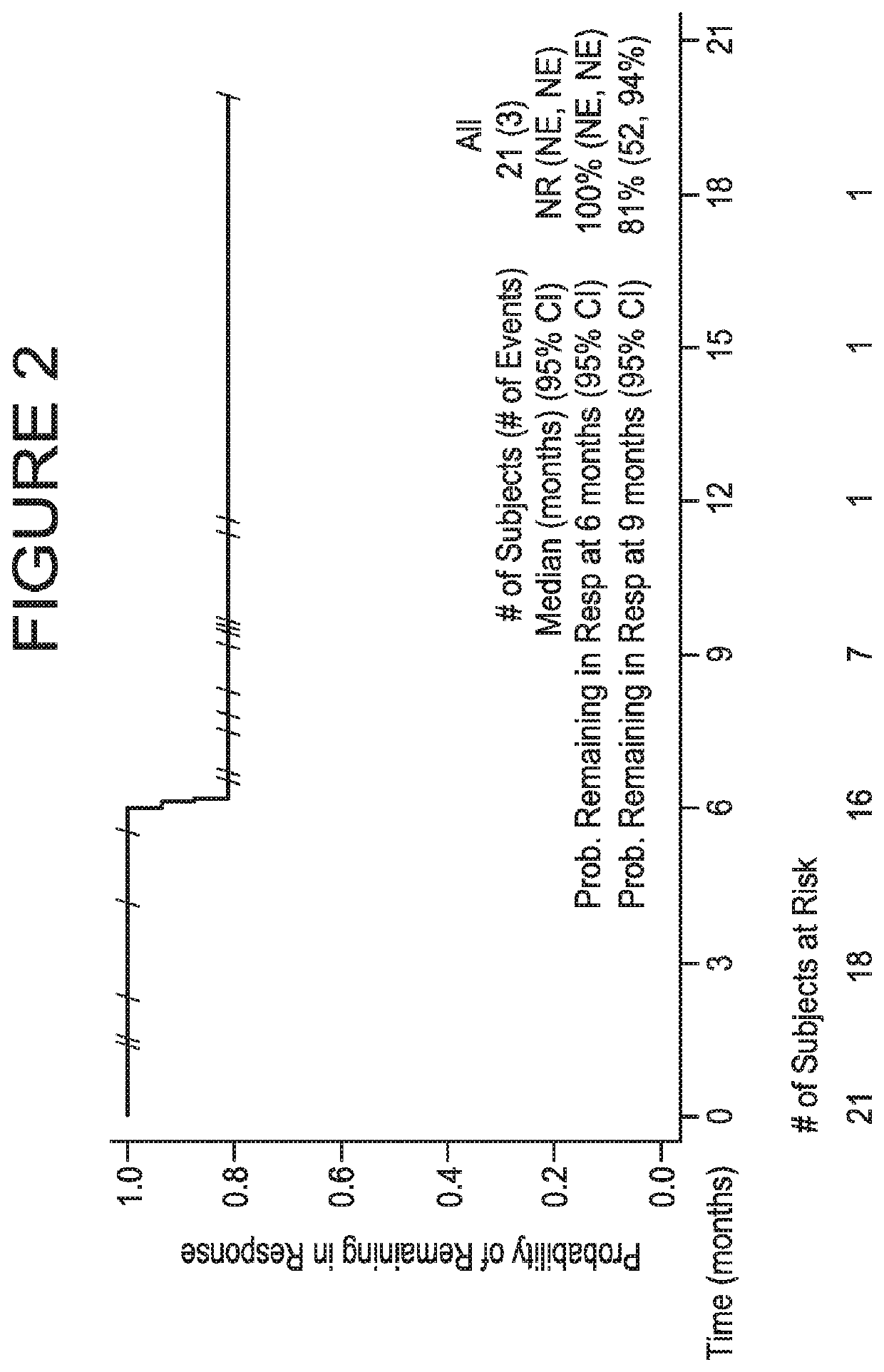
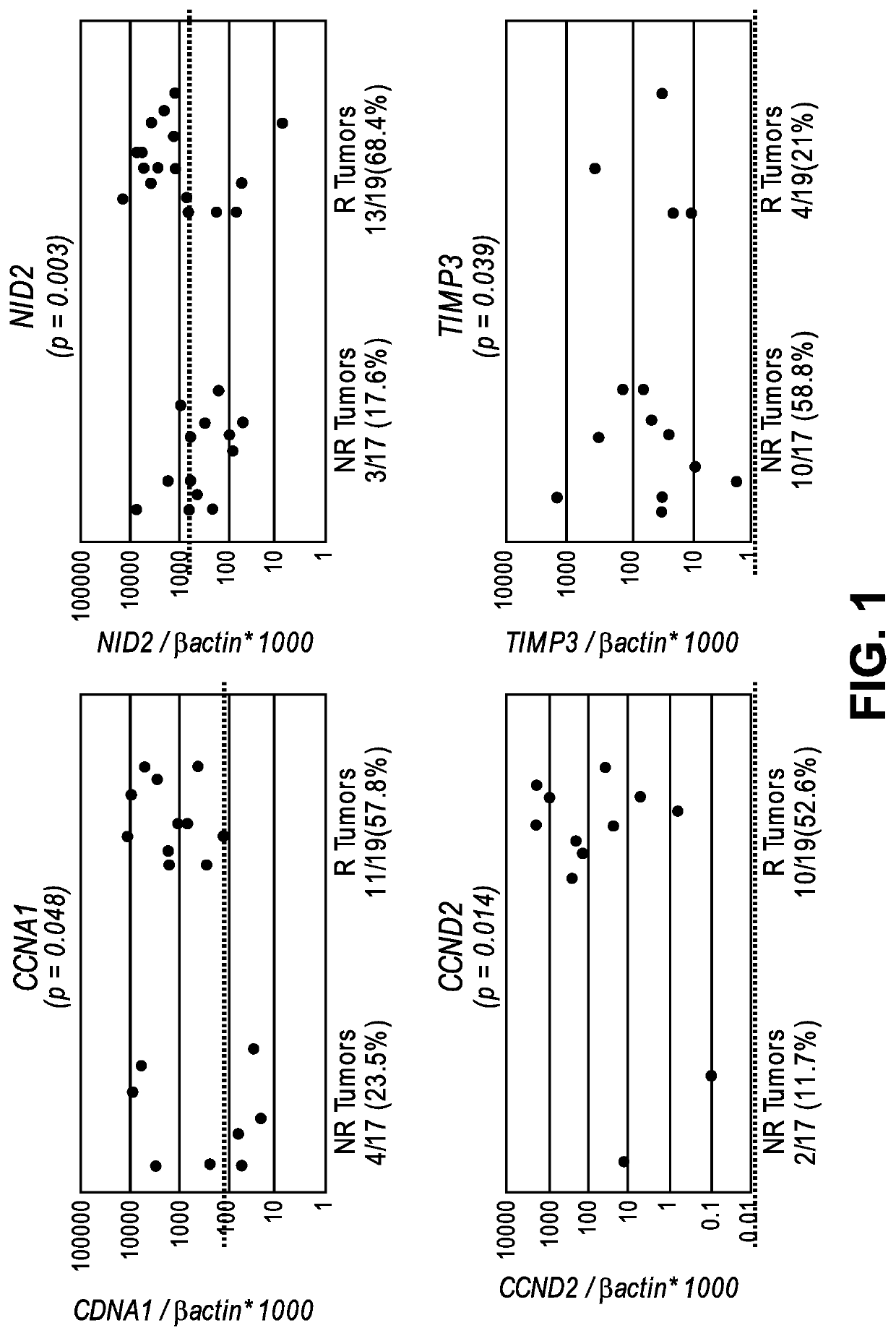
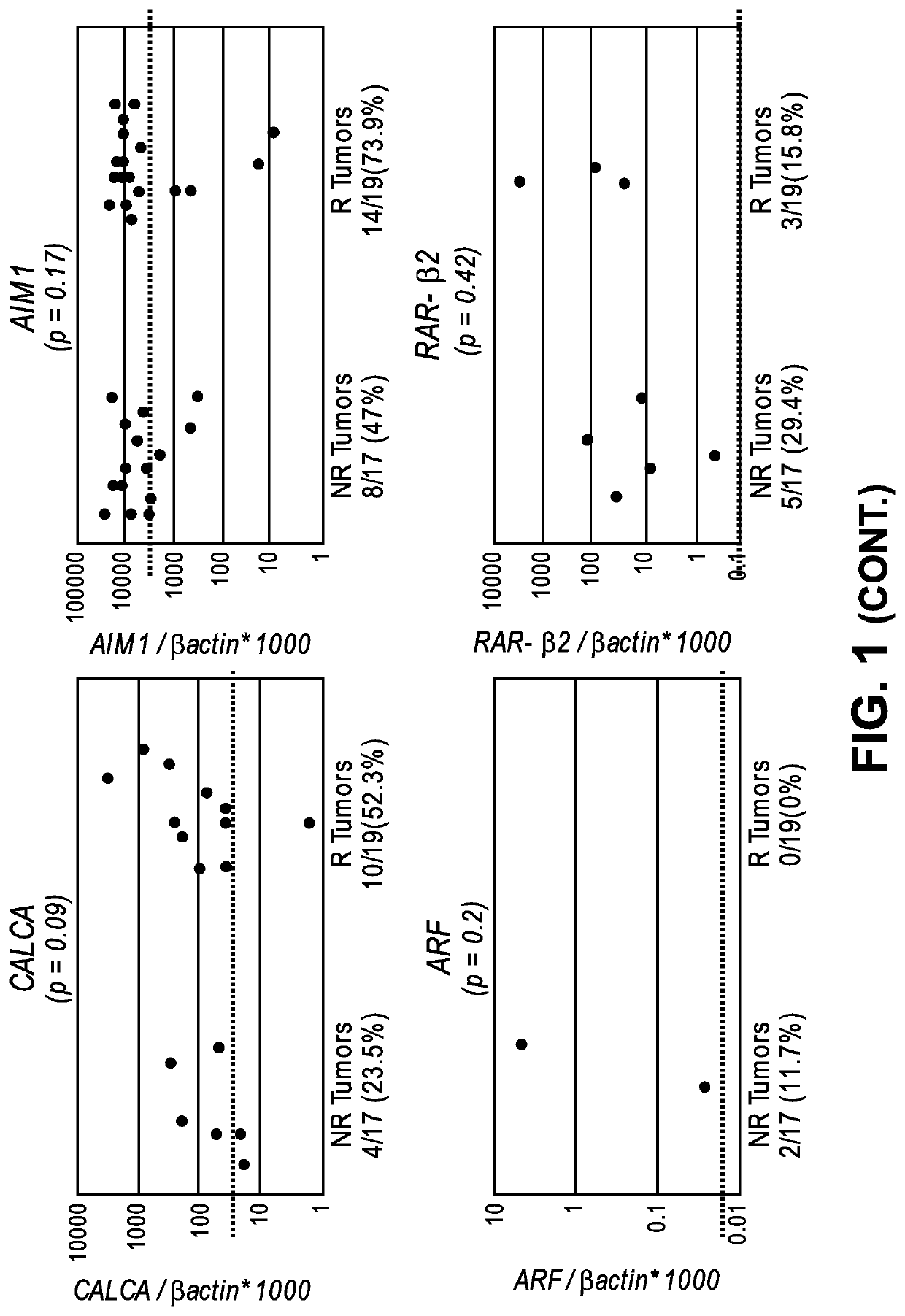
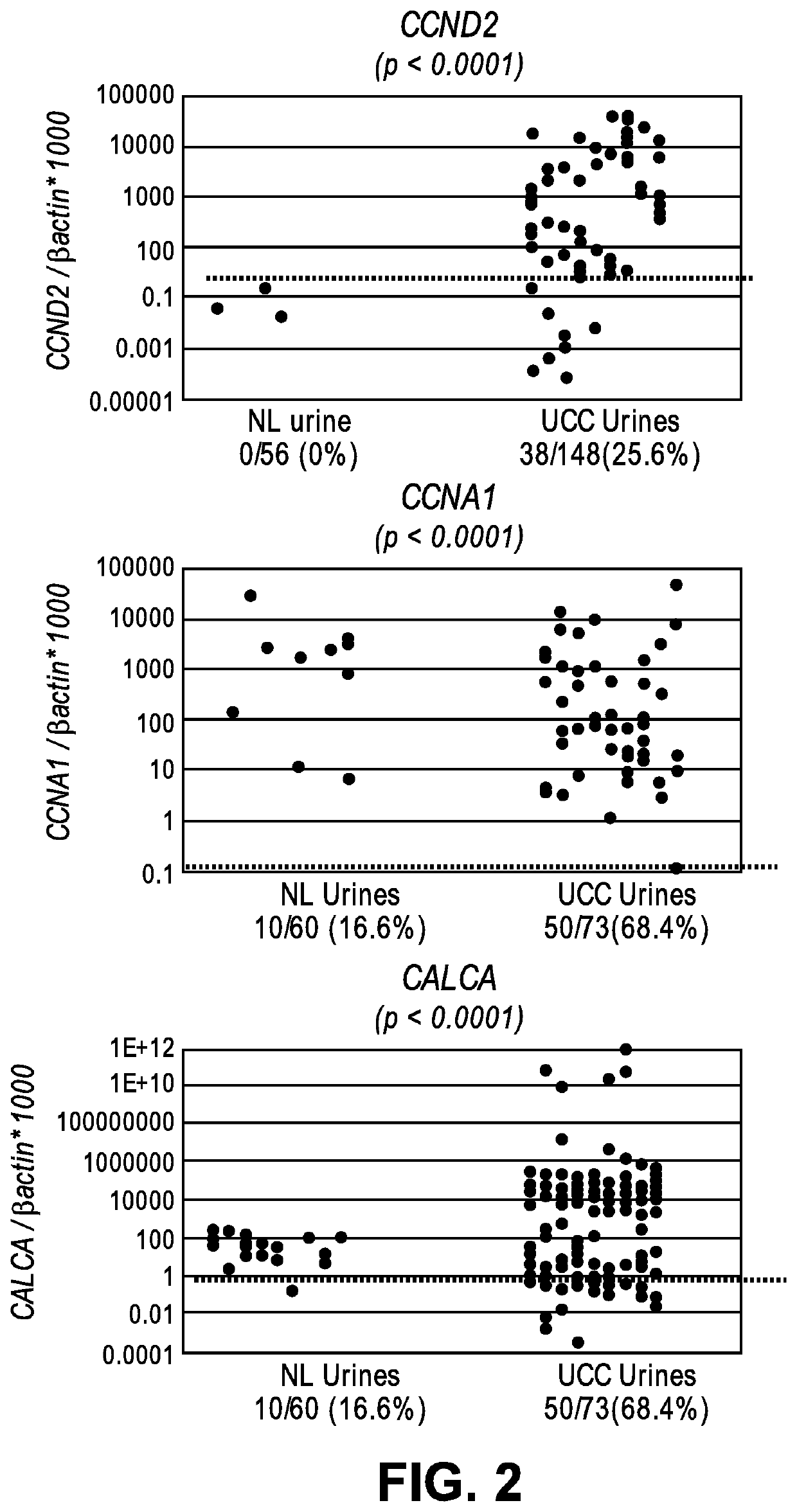
The invention provides methods for detecting a cellular proliferative disorder (e.g., urothelial cancer) in a subject by assessing the methylation status of the CCND2, CCNA1 or CALCA promoter in a nucleic acid sample. The methods of the invention are useful for diagnostic, prognostic as well therapeutic regimen predictions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
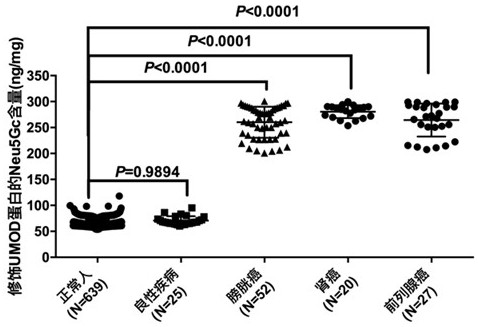
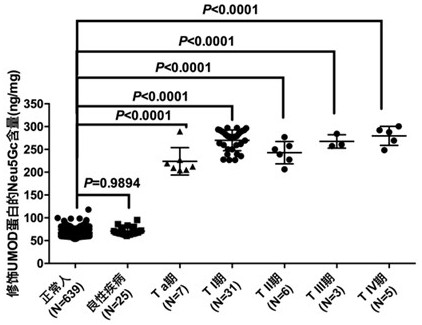
Kit for detecting urothelial carcinoma by identifying UMOD-modified Neu5Gc in urine based on LIP
ActiveCN112763713AEasy to operateHigh sensitivityBiological testingAgainst vector-borne diseasesPolyesterCellulose
The invention discloses a kit for detecting urothelial carcinoma by identifying UMOD-modified Neu5Gc in urine based on LIP, and the kit comprises a capture reagent, a detection buffer solution and test paper, wherein the capture reagent is biotinylated coupled lamprey immune protein LIP; the detection buffer solution is prepared from 50mM of Tris-base, 0.15M of NaCl, 0.01% of Tween-20 and 7.5% of BSA; the test paper is provided with a bottom plate, a sample adding pad, a fluorescent microsphere coupling release pad, a nitrocellulose membrane and a water absorption pad are sequentially and fixedly connected to the bottom plate from left to right in a step shape, and the fluorescent microsphere coupling release pad is prepared by spraying coupling liquid of europium fluorescent nanoparticles, streptavidin SA and rabbit IgG on polyester fibers; the nitrocellulose membrane is sequentially provided with a detection band formed by UMOD monoclonal antibodies and a reference band formed by goat anti-rabbit IgG from left to right.
Owner:LIAONING NORMAL UNIVERSITY
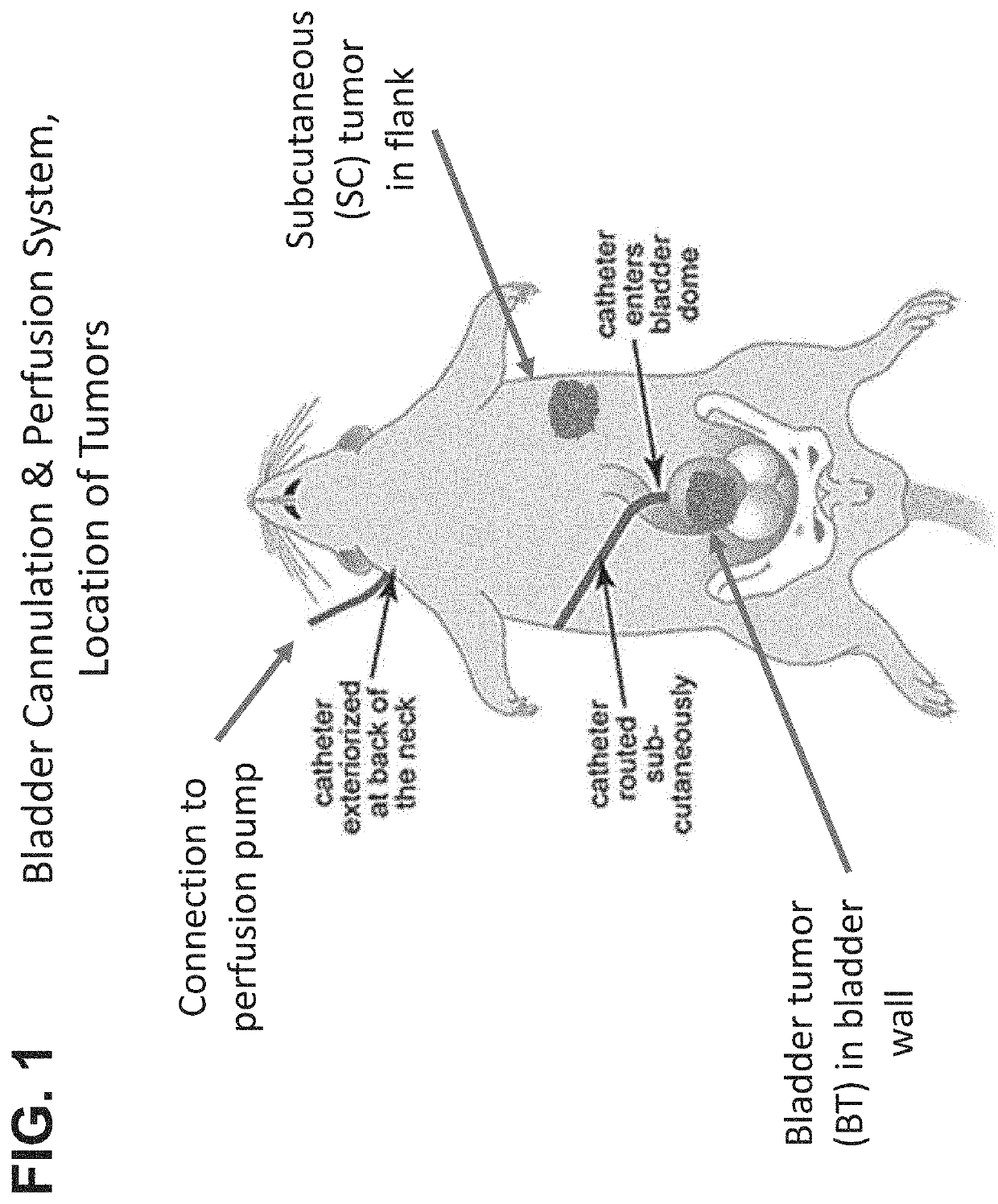
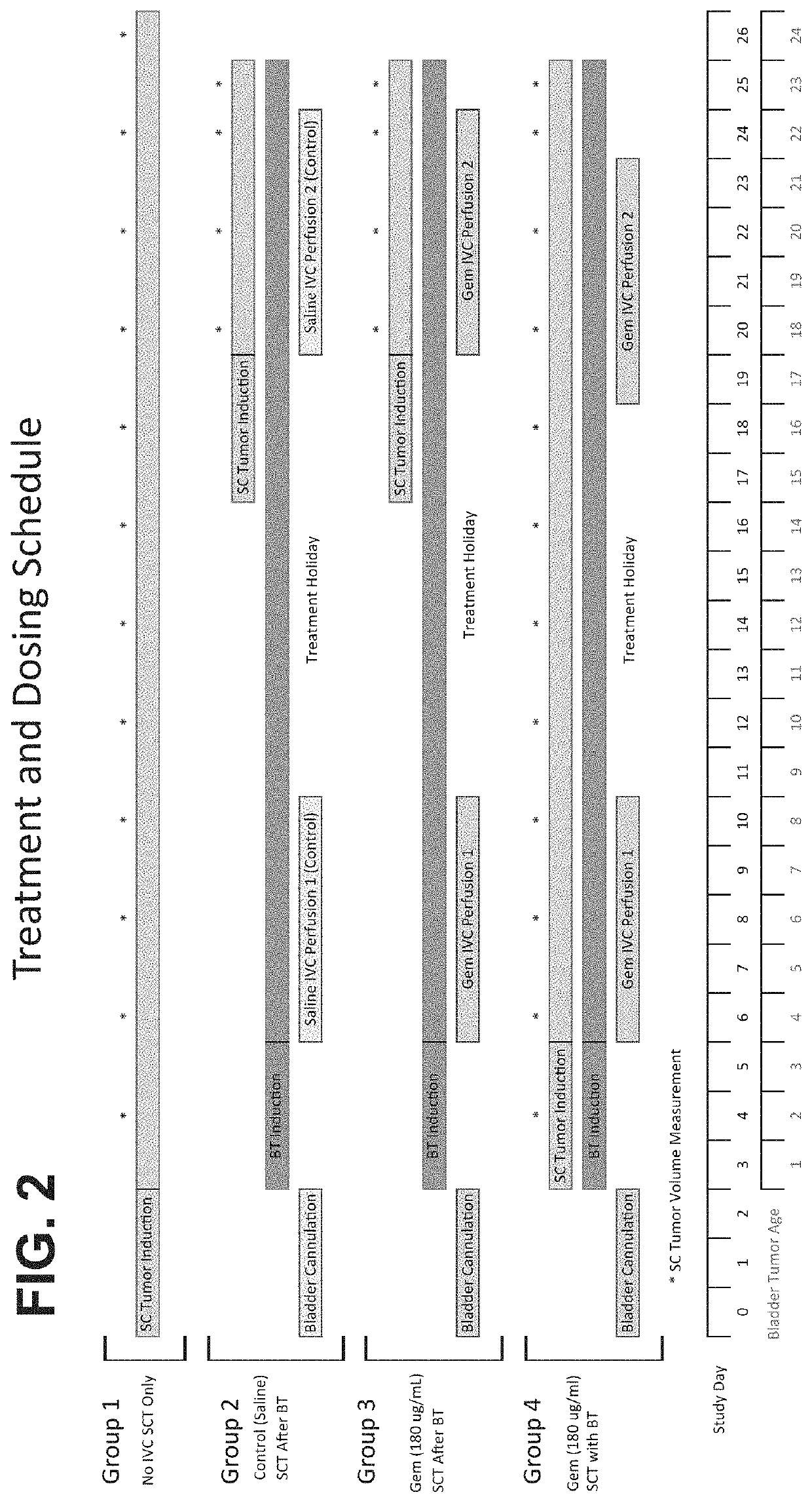
Methods of treating tumor metastasis
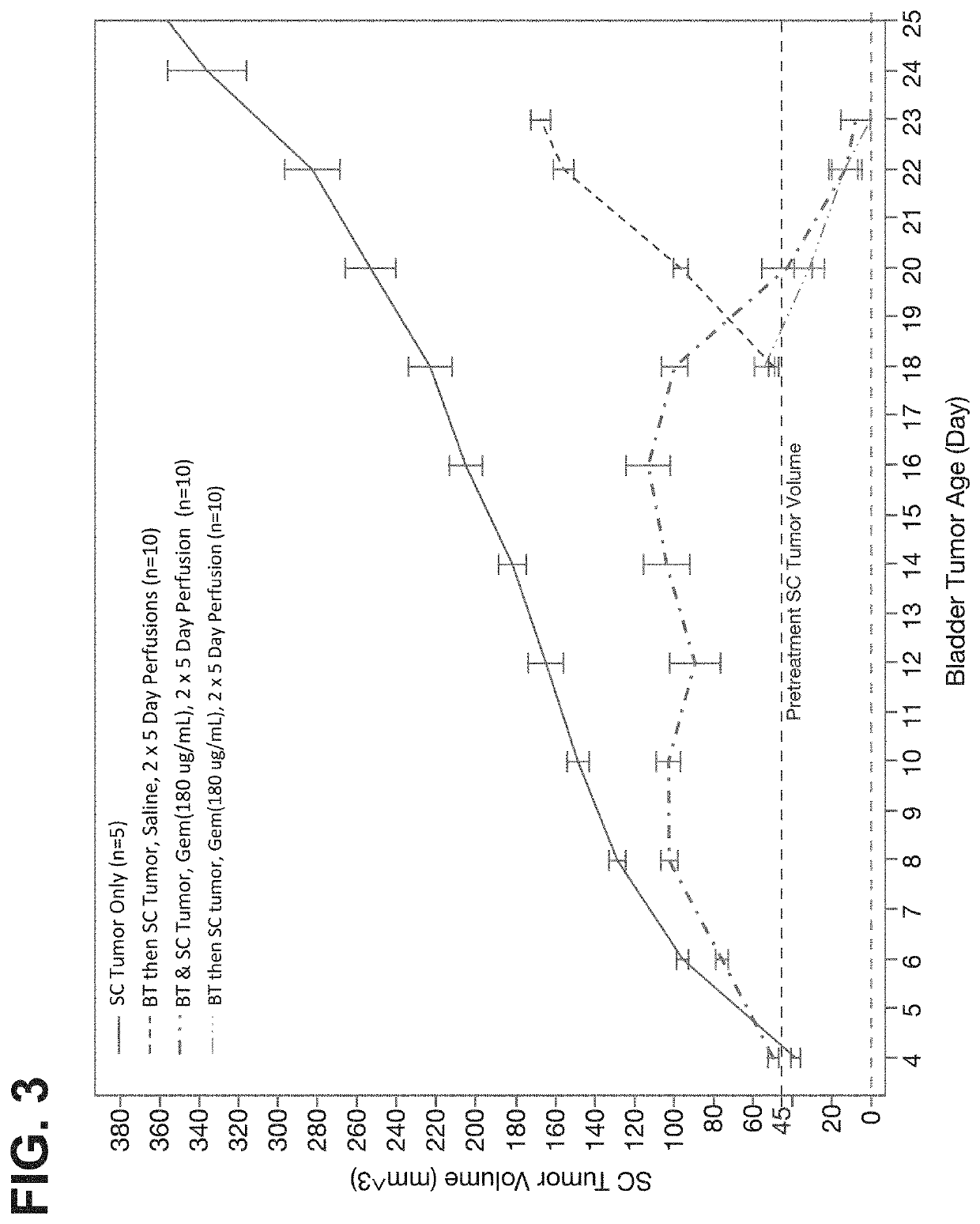
The present invention provides methods for treating or suppressing tumor metastasis at a site distinct from the bladder in an individual having a urothelial carcinoma of lower tract, comprising locally delivering to the bladder an effective amount of a chemotherapeutic agent (such as gemcitabine), wherein the chemotherapeutic agent is delivered continuously to the bladder for a sustained period of time (such as at least 24 hours).
Owner:TARIS BIOMEDICAL
FISH probe and kit for identifying peripheral blood circulation tumor cells (CTCs) of urothelial carcinoma patient
InactiveCN105986033AAccurate countAccurate identificationMicrobiological testing/measurementDiagnostic SpecificityWilms' tumor
The invention relates to a FISH probe and kit for identifying peripheral blood circulation tumor cells (CTCs) of a urothelial carcinoma patient, belonging to the field of biomedicine. The FISH probe for identifying peripheral blood CTCs of a urothelial carcinoma patient contains a chromosome 3 centromere and a chromosome 7 centromere. The kit contains the probe. By utilizing the genetic characteristics of urothelial carcinoma in combination with the diagnosis specificity of the chromosome 3 and chromosome 7, the FISH probe is utilized to label the peripheral blood enriched CTCs, thereby implementing accurate counting and identification on the urothelial carcinoma CTCs, and lowering the unnecessary waste of time, human resources and cost.
Owner:广州迈景基因医学科技有限公司 +1
Marker combination and kit for detecting tumor cells in body fluid sample
PendingCN113552367AHigh sensitivityImprove featuresBiological material analysisBiological testingEpitheliumLysis
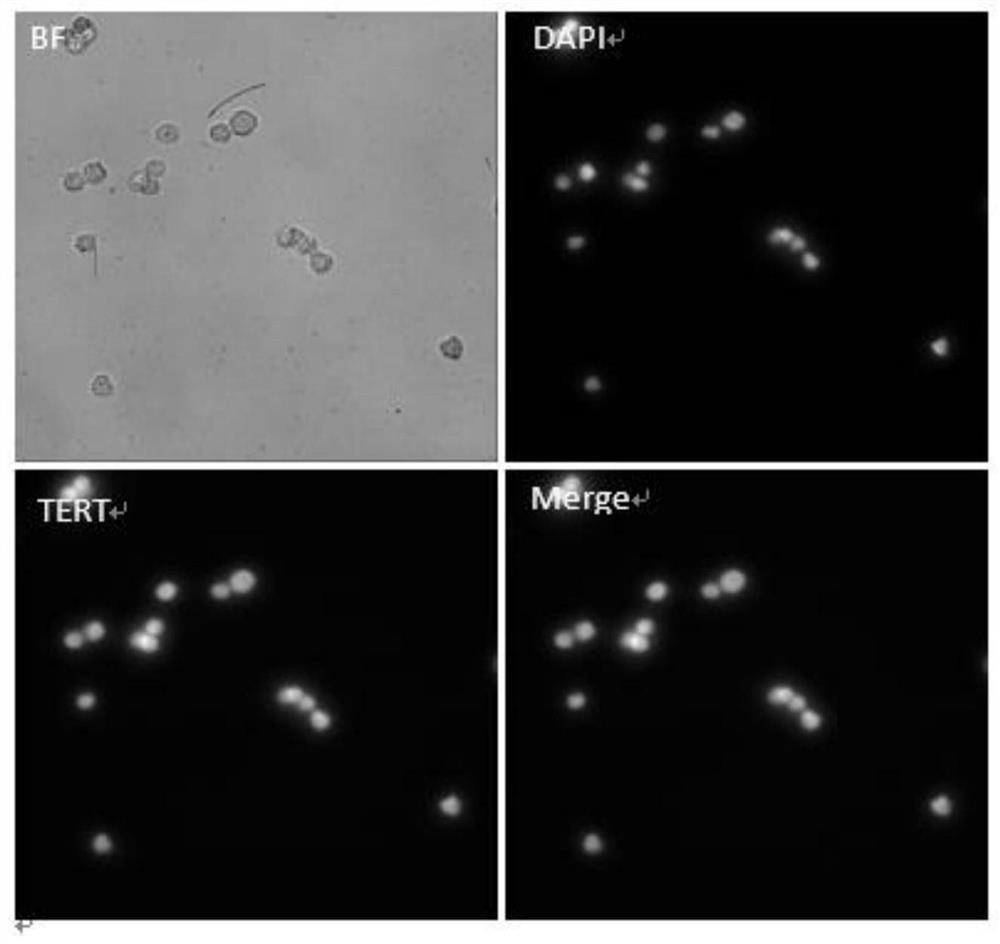
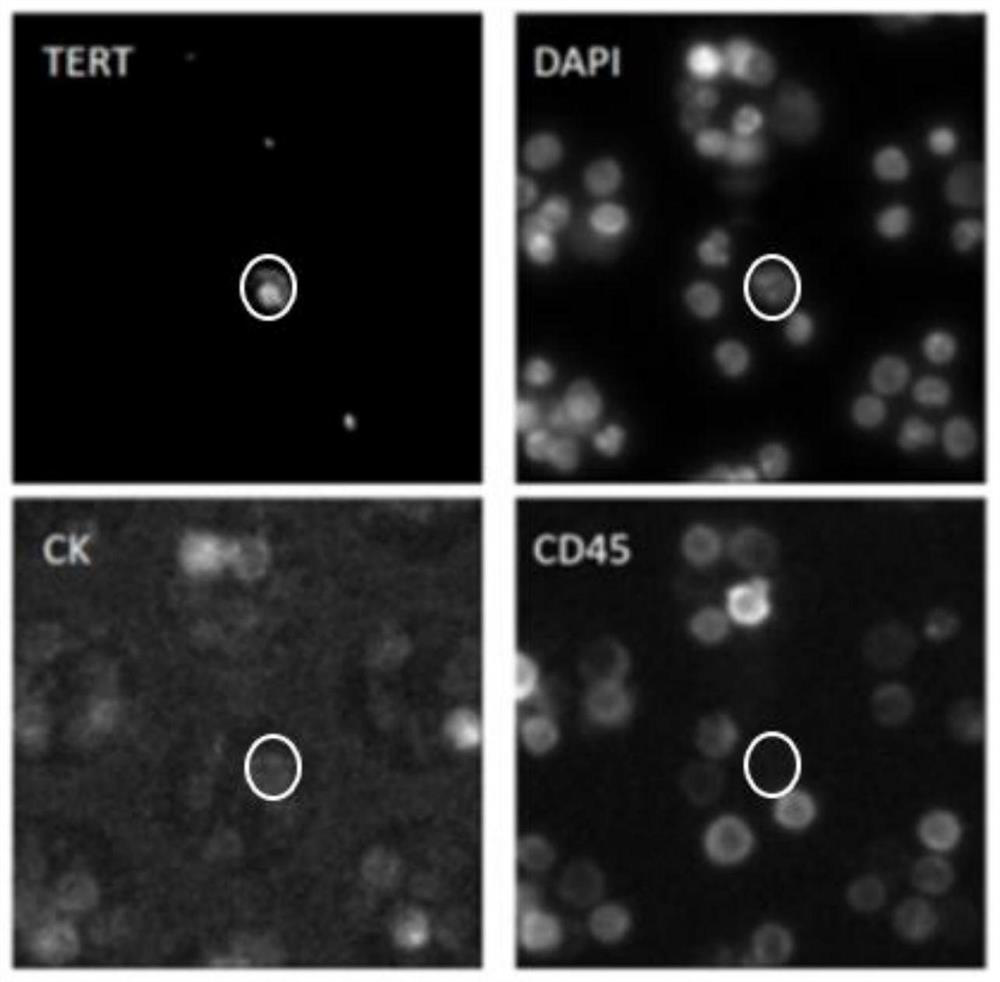
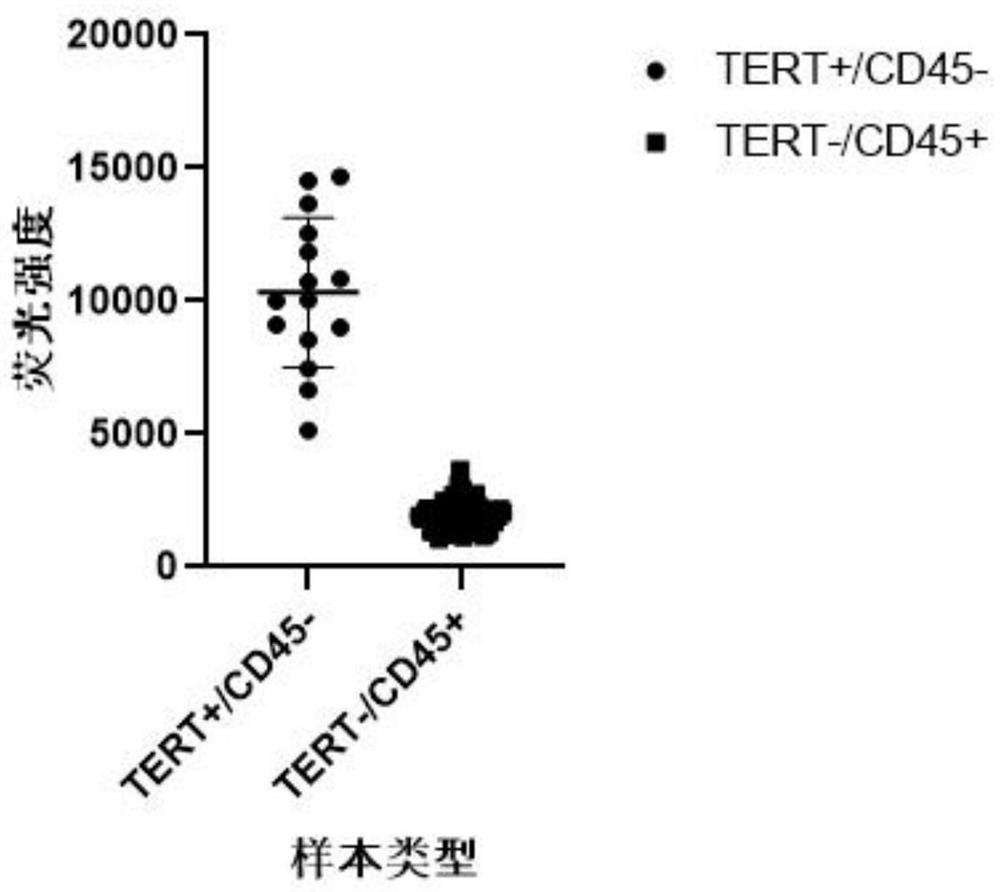
The invention discloses a marker combination and a kit for detecting tumor cells in a body fluid sample. The marker combination comprises TERT and CD45, and further comprises CK and a cell nucleus dye. The marker combination can be used for detecting tumor cells in a body fluid sample. The kit provided by the invention comprises the target composition, a red blood cell lysis reagent and a target cell enrichment reagent. The invention provides a group of novel tumor cell recognition markers TERT+ / CK+ / CD45- / DAPI+, wherein TERT is telomerase reverse transcriptase. The method has the advantages that firstly, the detection sensitivity and specificity can be obviously improved; d ansecondly, tumor cells derived from epithelium can be detected. For a patient with urothelial carcinoma, tumor cells and normal cells cannot be distinguished by using a traditional EpCAM+ / CK+ / CD45- / DAPI+ detection method, and the tumor cells and the normal cells can be obviously distinguished by using the TERT+ / CK+ / CD45- / DAPI+ detection method.
Owner:3D BIOMEDICINE SCI & TECH CO LTD
Method for culturing upper urinary tract urothelial cell carcinoma organoids
InactiveCN113736736ARetain heterogeneityReliable clinical dataArtificial cell constructsTumor/cancer cellsDigestion TreatmentUrothelial Cell
The invention discloses a method for culturing upper urinary tract urothelial cell carcinoma organoids. The method comprises the following steps: placing small upper urinary tract urothelial cell carcinoma tissue blocks in an inhibitor containing type II collagenase for first digestion treatment to obtain a first digest; placing the first digest in a pancreatin substitute containing an inhibitor, and performing second digestion treatment to obtain a second digest; sequentially performing first neutralization treatment, filtering treatment and centrifugal treatment on the second digest to obtain single cell precipitate; placing the single cell precipitate in a mixed substrate of a preset culture medium and substrate glue for resuspension treatment to obtain a gel suspension containing all cells; and culturing the gel suspension according to a preset scheme to obtain the upper urinary tract urothelial cell carcinoma organoids. According to the scheme, reliable clinical data can be provided for use of chemotherapeutic drugs and personalized treatment of upper urinary tract urothelial cell tumors.
Owner:深圳明澳生物科技有限公司
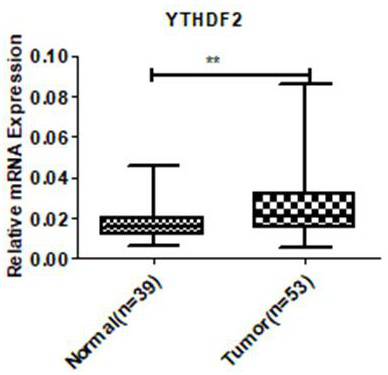
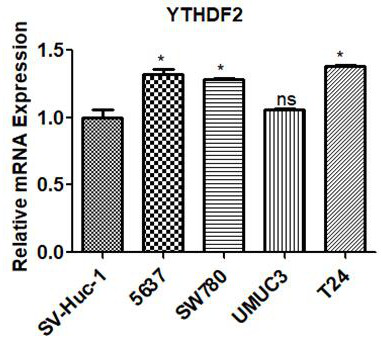
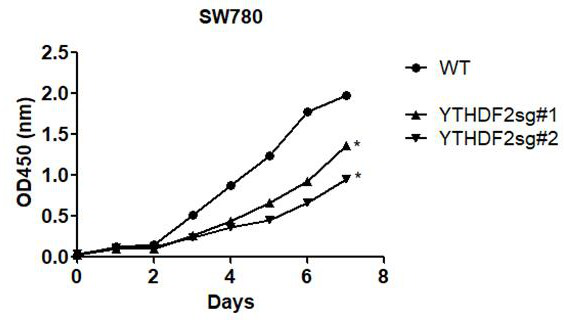
Application of ythdf2 gene in diagnosis, prevention and treatment of urothelial carcinoma
ActiveCN109680064BPromote proliferationImprove mobilityMicrobiological testing/measurementPharmaceutical drugGene Expression Inhibitor
The invention discloses the application of YTHDF2 gene in the diagnosis, prevention and treatment of urothelial carcinoma. On the one hand, the present invention discloses the application of a reagent for detecting YTHDF2 gene in preparing products for diagnosing urothelial carcinoma, and on the other hand discloses the application of an inhibitor of YTHDF2 gene expression in preparing a medicament for treating or preventing urothelial carcinoma. The present invention confirms the role of YTHDF2 in urothelial cancer for the first time, YTHDF2 is up-regulated in urothelial cancer tissue, and promotes the proliferation and migration of urothelial cancer cells, YTHDF2 can be used as a clinical diagnosis of urothelial cancer and potential targets for therapy.
Owner:SHENZHEN LUOHU PEOPLELS HOSPITAL
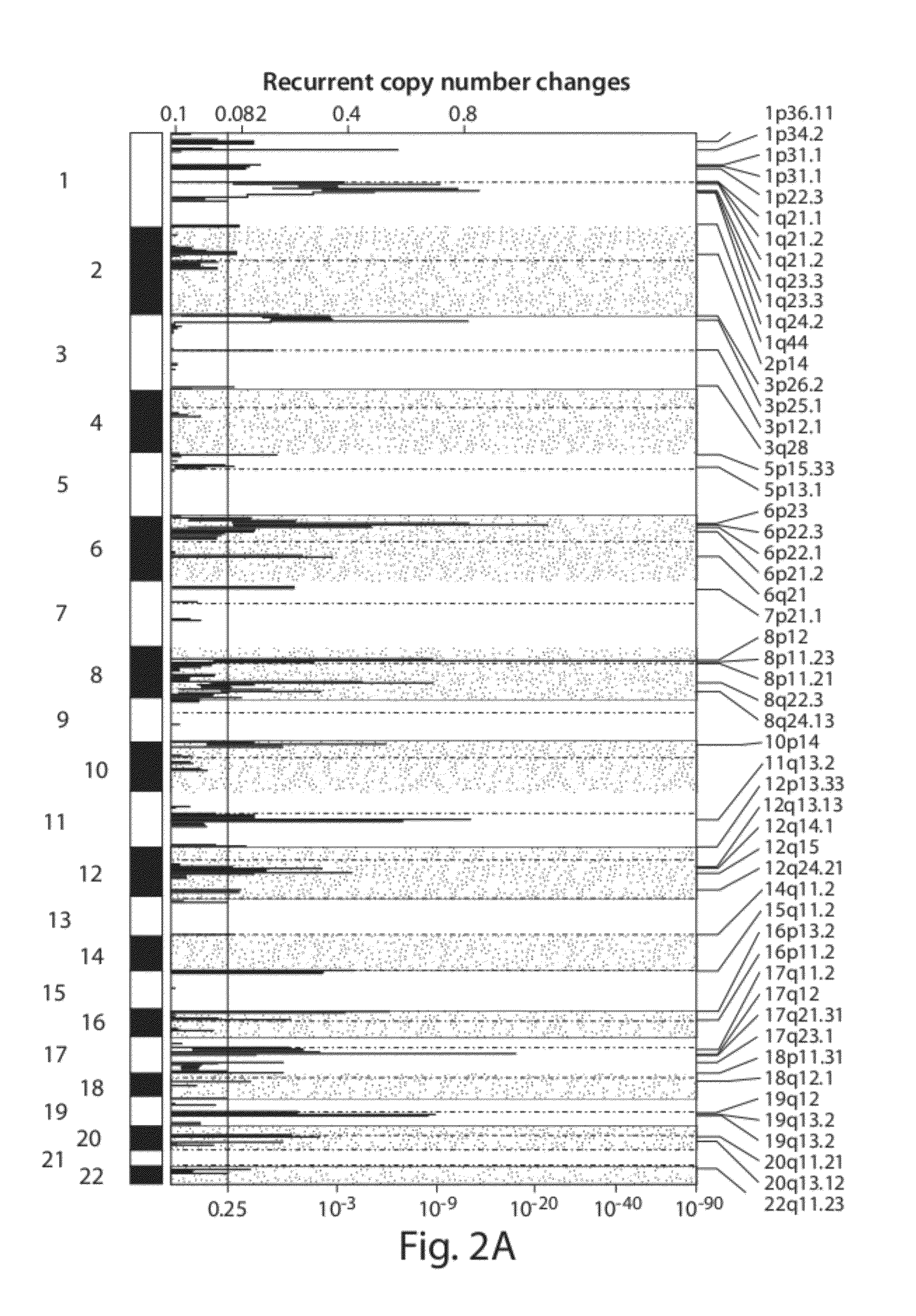
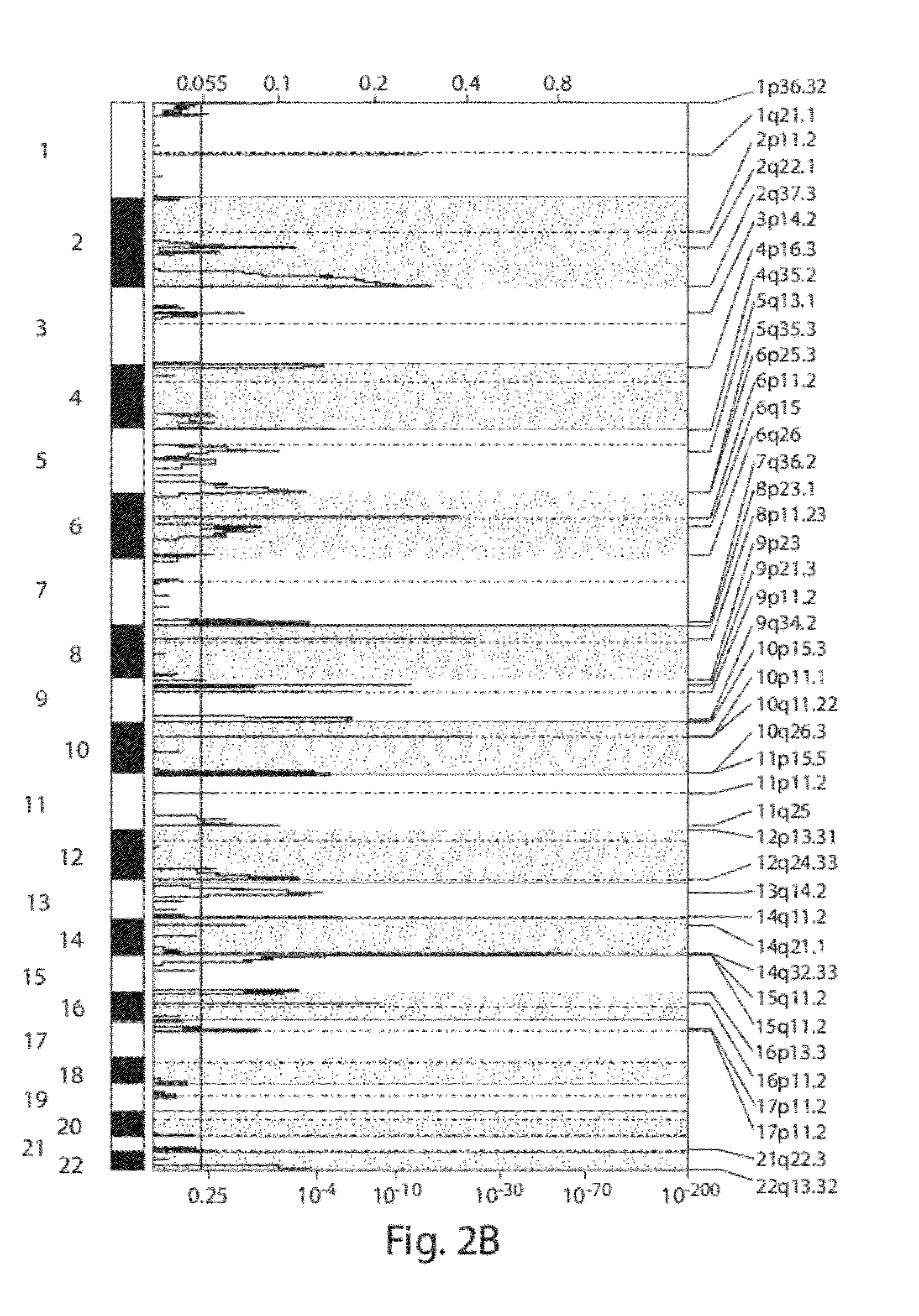
Chromosome copy number gain as a biomarker of urothelial carcinoma lethality
ActiveUS20120295807A1Improved prognosisHigh risk of developingMicrobiological testing/measurementLibrary screeningFavorable prognosisTissue sample
Diagnostic assays for medically classifying cancer patients are provided. The method comprises assessing a tissue sample of the patient for the presence of a copy number gain of chromosome regions 1q23.3 and / or 1q21.2. Copy number gain of chromosome regions 1q23.3 and / or 1q21.2 is indicative of a less favorable prognosis as compared to the prognosis if there was no copy number gain in the same regions.
Owner:DANA FARBER CANCER INST INC
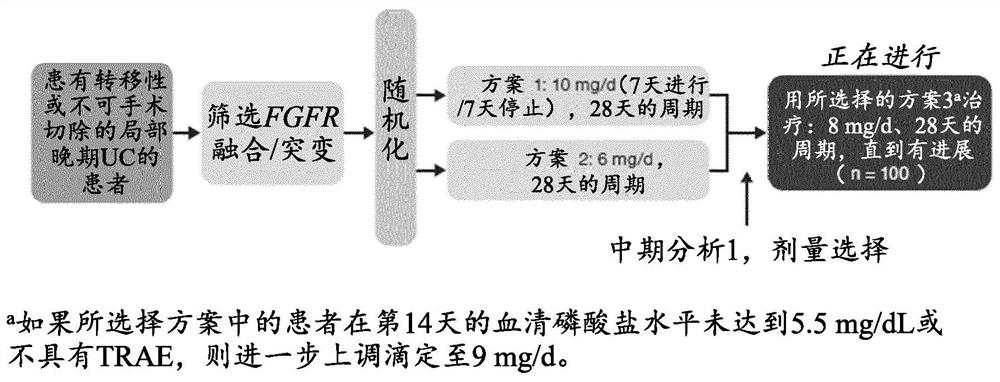
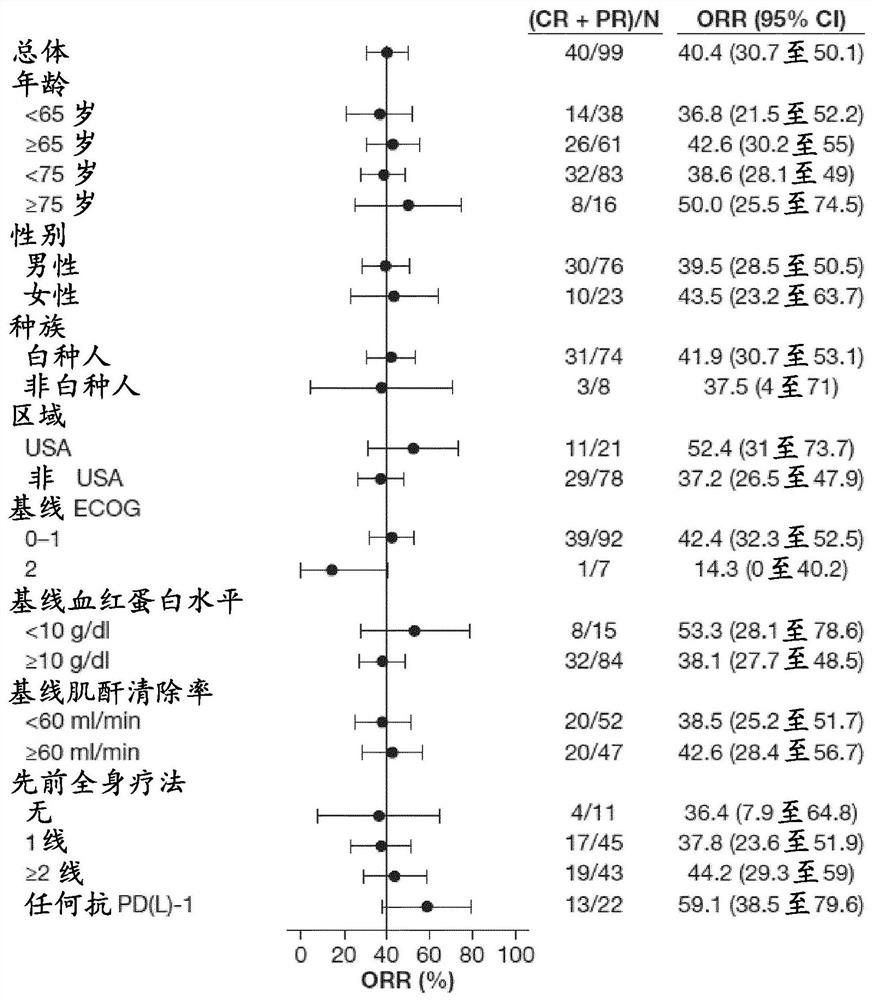
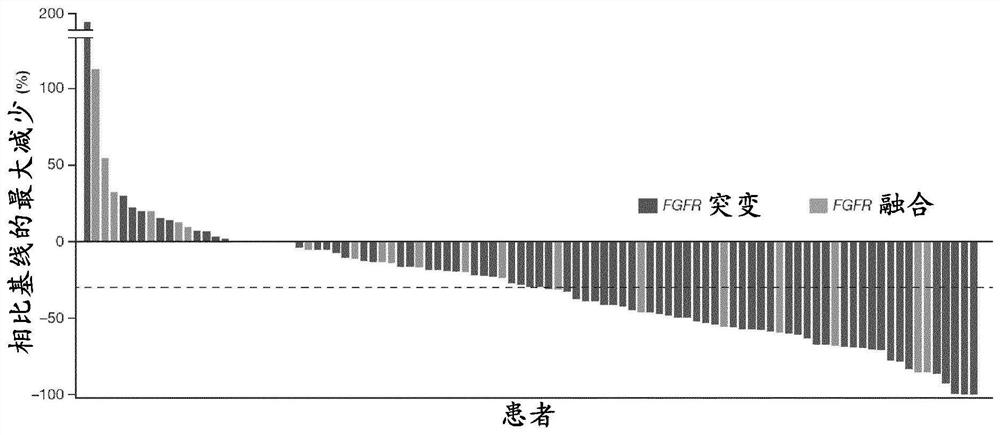
FGFR tyrosine kinase inhibitors for the treatment of urothelial carcinoma
PendingCN113645975AOrganic active ingredientsMicrobiological testing/measurementApproved drugTyrosine-kinase inhibitor
Described herein methods of treating urothelial carcinoma with an approved drug product containing a fibroblast growth factor receptor (FGFR) inhibitor. Also described herein are methods of selling or offering for sale an approved drug product containing a fibroblast growth factor receptor (FGFR) inhibitor.
Owner:JANSSEN PHARMA NV
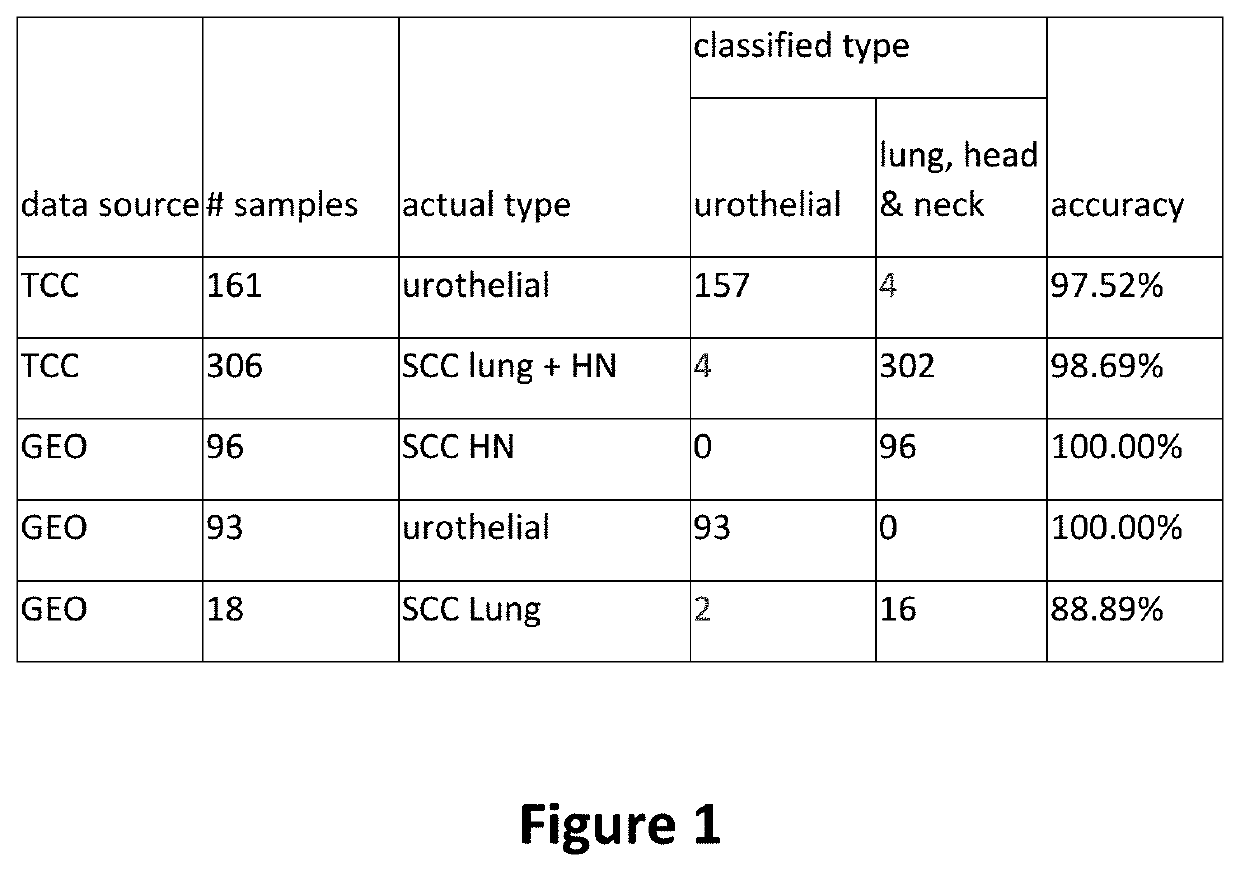
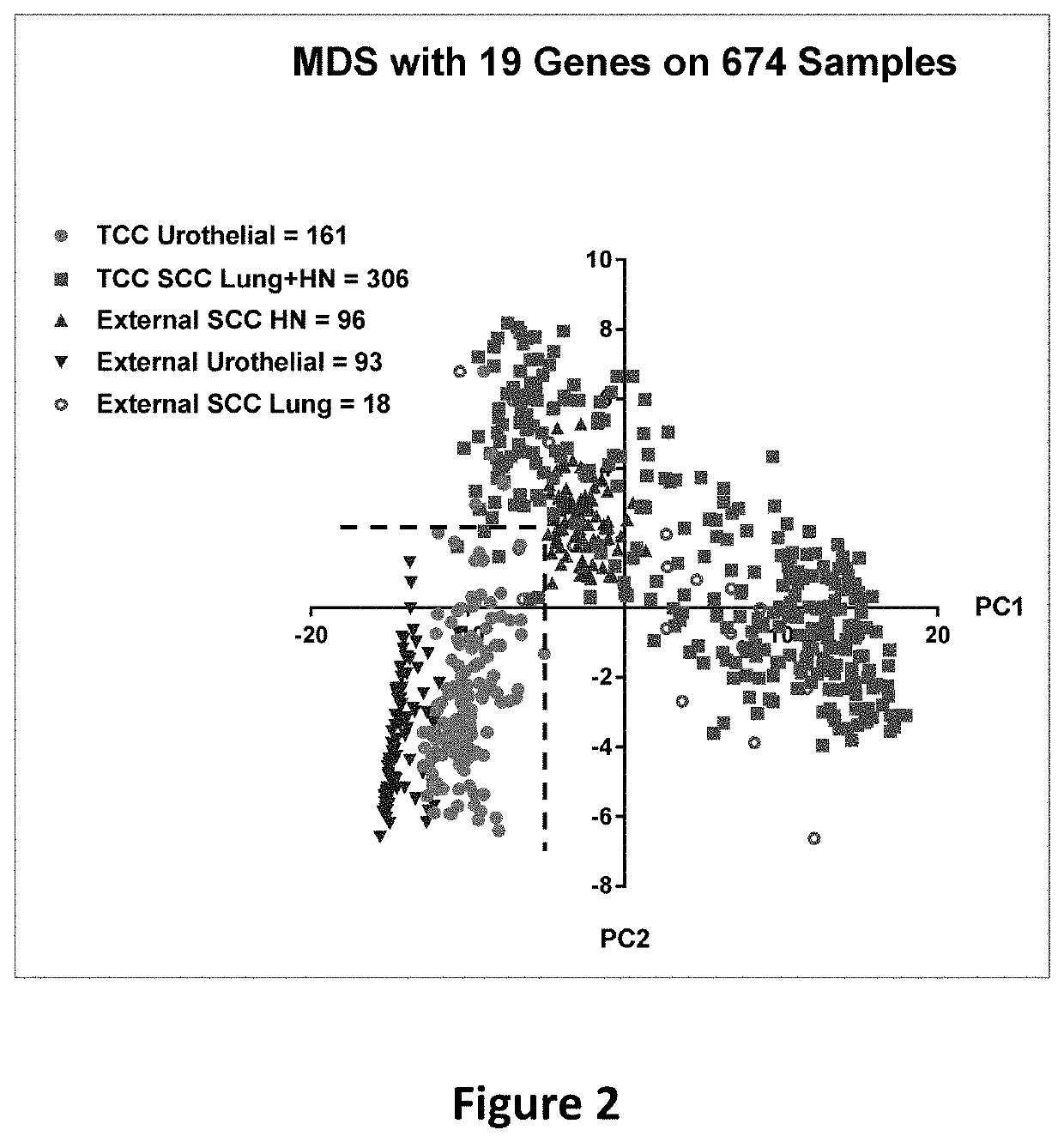
Method of distinguishing urothelial carcinoma from lung and head and neck squamous cell carcinoma
ActiveUS20200190603A1Improve accuracyInorganic active ingredientsMicrobiological testing/measurementSquamous CarcinomasSquamous Cell Cancers
A method of distinguishing between urothelial carcinoma and squamous cell carcinoma of head and neck and lung primaries is presented. A 19-gene signature was developed which differentiates between metastatic urothelial carcinoma and squamous cell carcinoma in a metastatic site when the primary site is either known or unknown.
Owner:H LEE MOFFITT CANCER CENT & RES INST INC
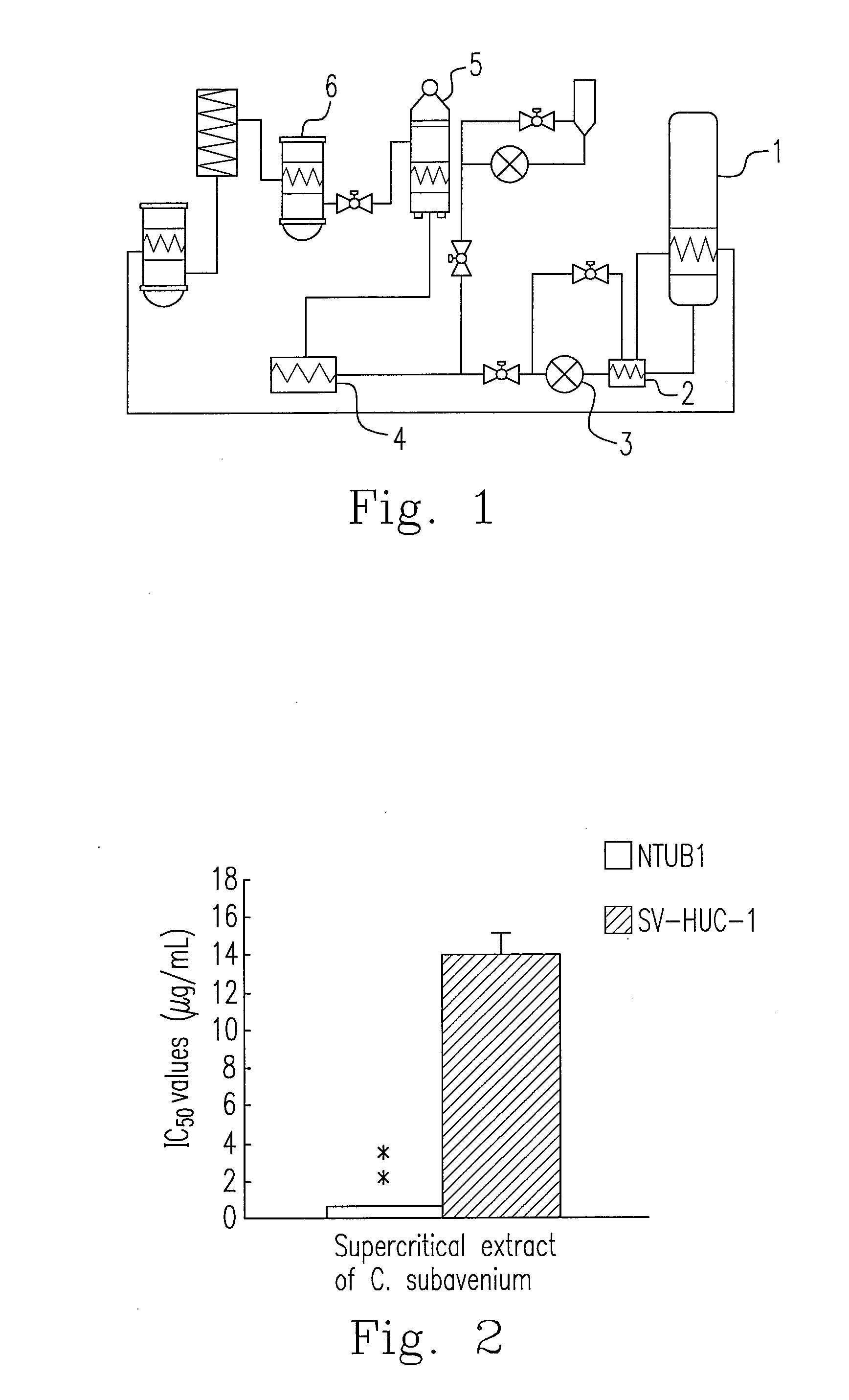
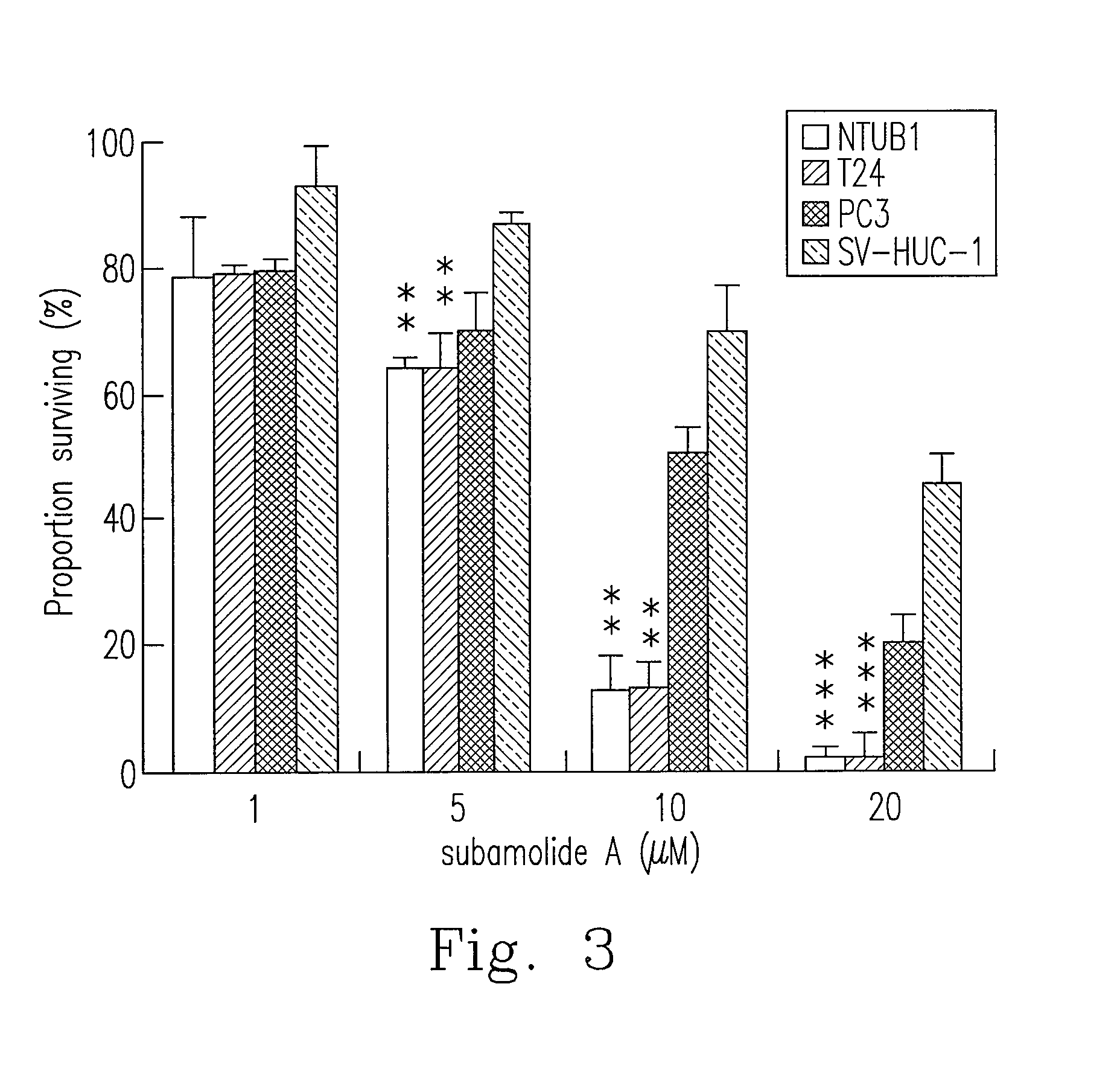
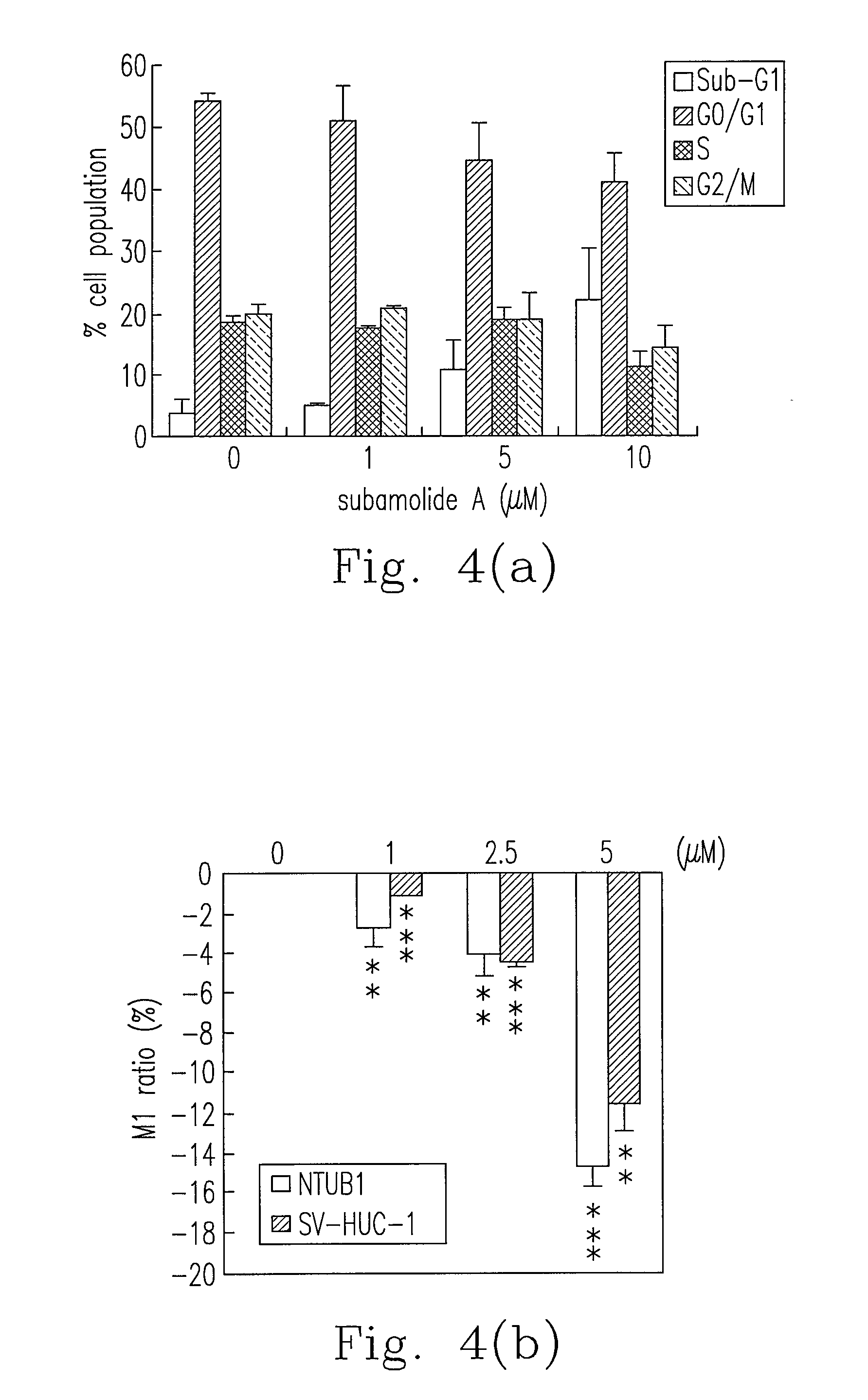
Anti-human urothelial carcinoma of supercritical carbon dioxide extract of cinnamomum subavenium, and the preparation process and uses
InactiveUS20130122110A1Reduce usageReduce energy consumptionBiocideHeavy metal active ingredientsCisplatinBULK ACTIVE INGREDIENT
What is disclosed in the invention is a preparation method of a supercritical Cinnamomum subavenium extract, which is made from the material, the dried stem of C. subavenium. The extract is obtained by extracting C. subavenium which is pulverized as particles with supercritical carbon dioxide fluid. The C. subavenium extract or its active ingredient, subamolide A, can be used to inhibit the growth of human urothelial carcinoma cell lines. In addition, the C. subavenium extract (or subamolide A) is able to synergistically inhibit the growth of human urothelial carcinoma cell lines with cisplatin (CDDP) or gemcitabine (Gem). Therefore, the C. subavenium extract (or subamolide A) can be an anticancer drug alone, or forms a pharmaceutical composition with CDDP (or Gem) to treat with cancers in respect of urinary system.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
Therapy for metastatic urothelial cancer with the antibody-drug conjugate, sacituzumab govitecan (immu-132)
PendingUS20210393617A1Receive treatment wellUnexpected synergistic effectInorganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAntigen binding
The present invention relates to therapeutic ADCs comprising SN-38 attached to an anti-Trop-2 antibody or antigen-binding antibody fragment. The ADC may be administered at a dosage of between 4 mg / kg and 18 mg / kg, preferably 4, 6, 8, 9, 10, 12, 16 or 18 mg / kg, most preferably 8 to 10 mg / kg. When administered at specified dosages and schedules, the ADC can reduce solid tumors in size, reduce or eliminate metastases and is effective to treat cancers resistant to standard therapies, such as radiation therapy, chemotherapy or immunotherapy. Preferably, the ADC is administered in combination with one or more other therapeutic agents, such as a PARP inhibitor, a microtubule inhibitor, a Bruton kinase inhibitor or a PI3K inhibitor. Most preferably, the ADC is of use for treating a Trop-2 expressing cancer, such as metastatic urothelial cancer.
Owner:IMMUNOMEDICS INC
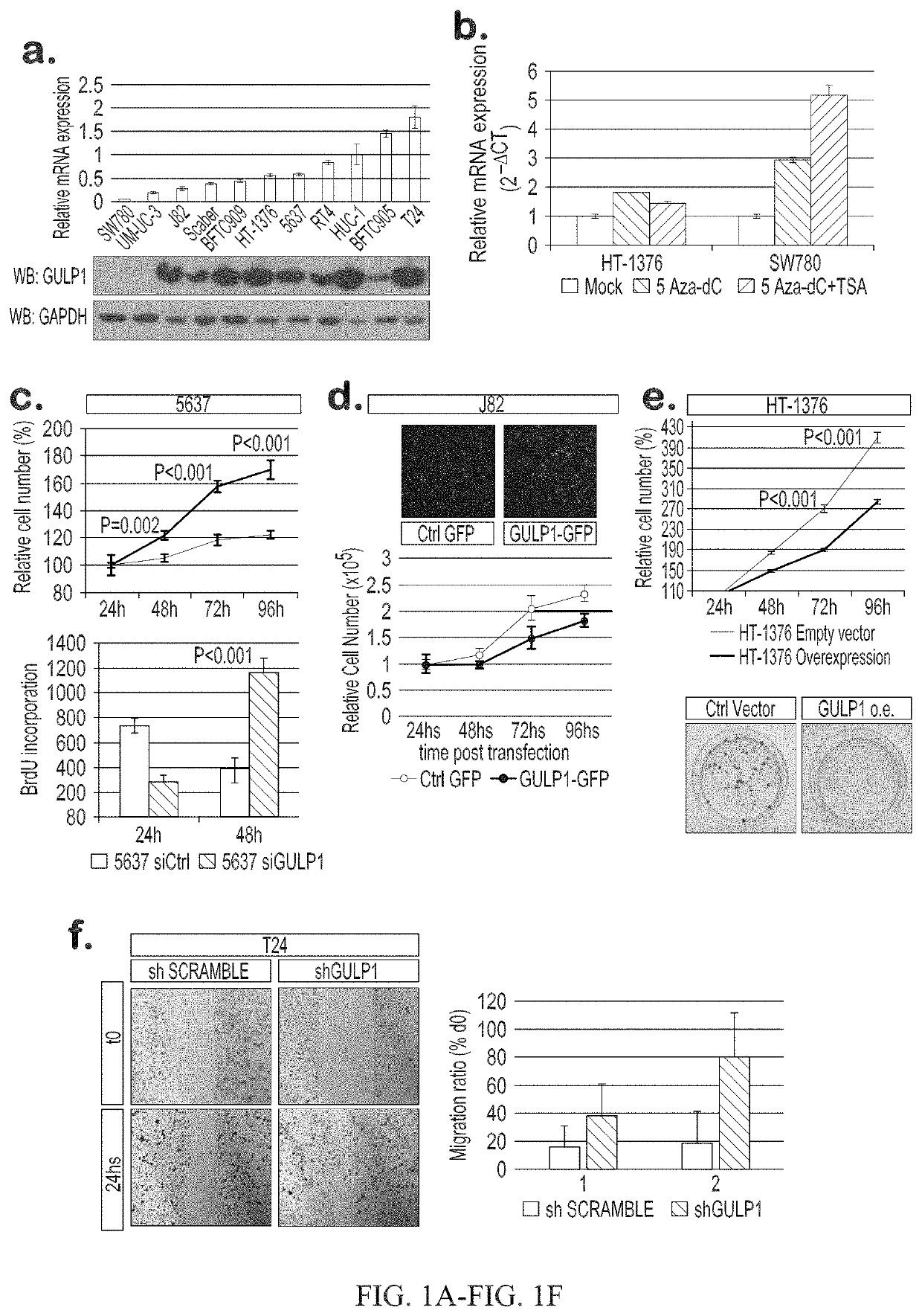
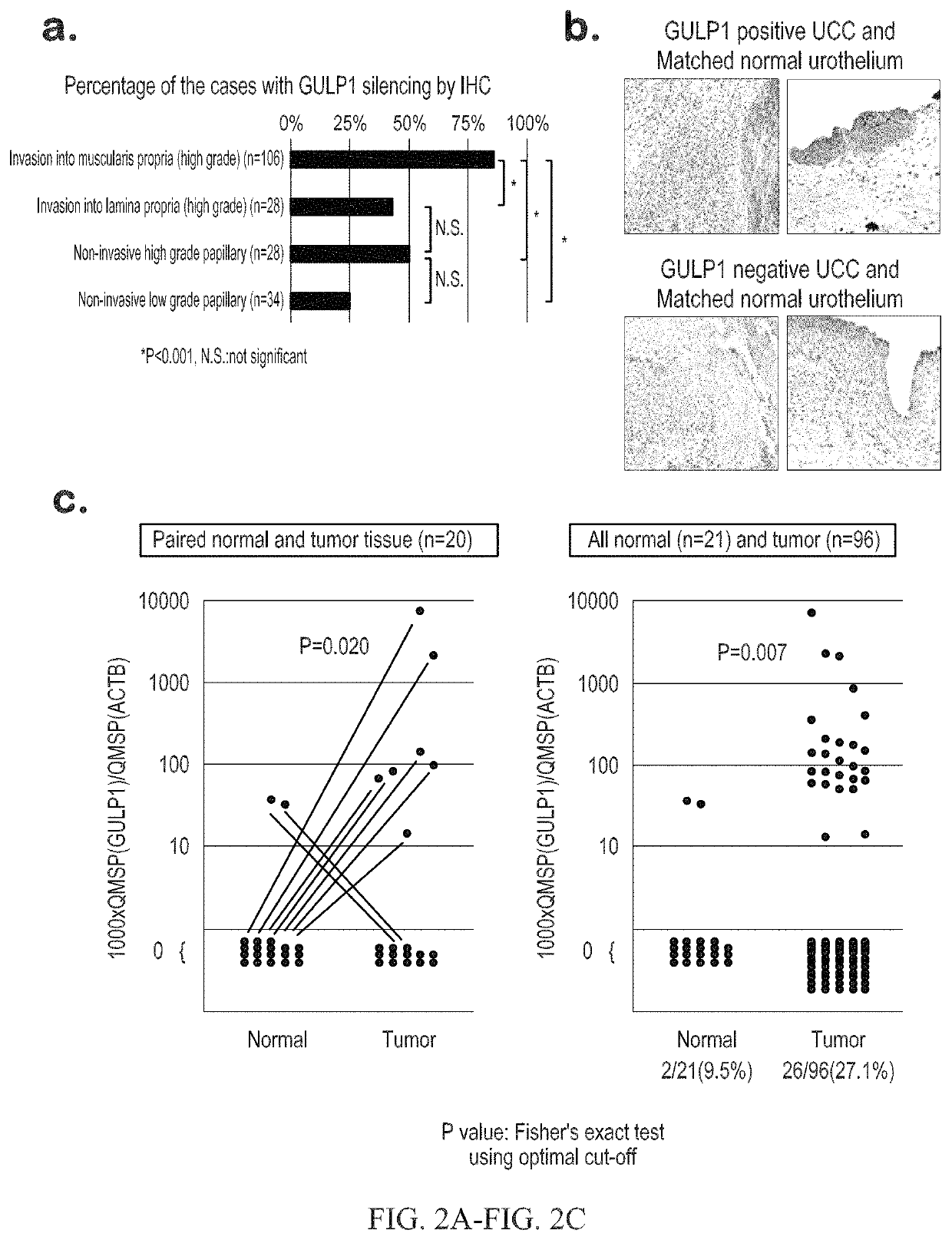
Urothelial cancer and methods of detection and targeted therapy
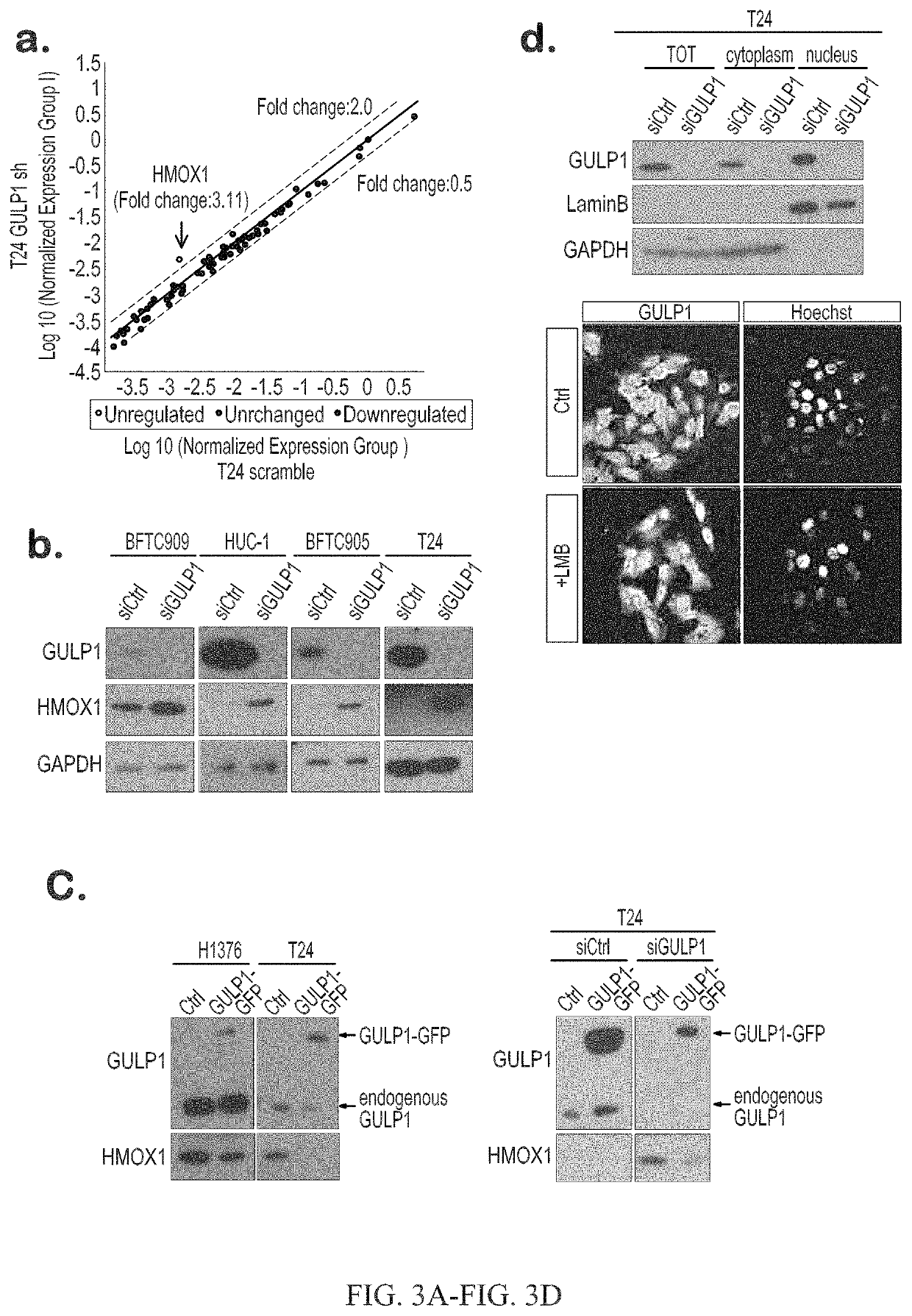
ActiveUS10941450B2Symptoms improvedHigh expressionOrganic active ingredientsMicrobiological testing/measurementDiseaseRegimen
The invention provides methods for detecting a cellular proliferative disorder (e.g., urothelial cancer) in a subject by assessing the methylation status of the GULP1 promoter in a nucleic acid sample. The methods of the invention are useful for diagnostic, prognostic as well as therapeutic regimen predictions.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods of treating urothelial carcinoma using an Anti-pd-1 antibody
PendingUS20220213191A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCellular antigensAntiendomysial antibodies
This disclosure provides a method for treating a subject afflicted with a urothelial carcinoma or cancer derived therefrom, which method comprises administering to the subject an antibody or an antigen-binding portion thereof that specifically binds to a Programmed Death-1 (PD-1) receptor and inhibits PD-1 activity or the combination of (a) an antibody or an antigen-binding portion thereof that specifically binds to a PD-1 receptor and inhibits PD-1 activity; and (b) an antibody or an antigen-binding portion thereof that specifically binds to a Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) and inhibits CTLA-4 activity.
Owner:BRISTOL MYERS SQUIBB CO
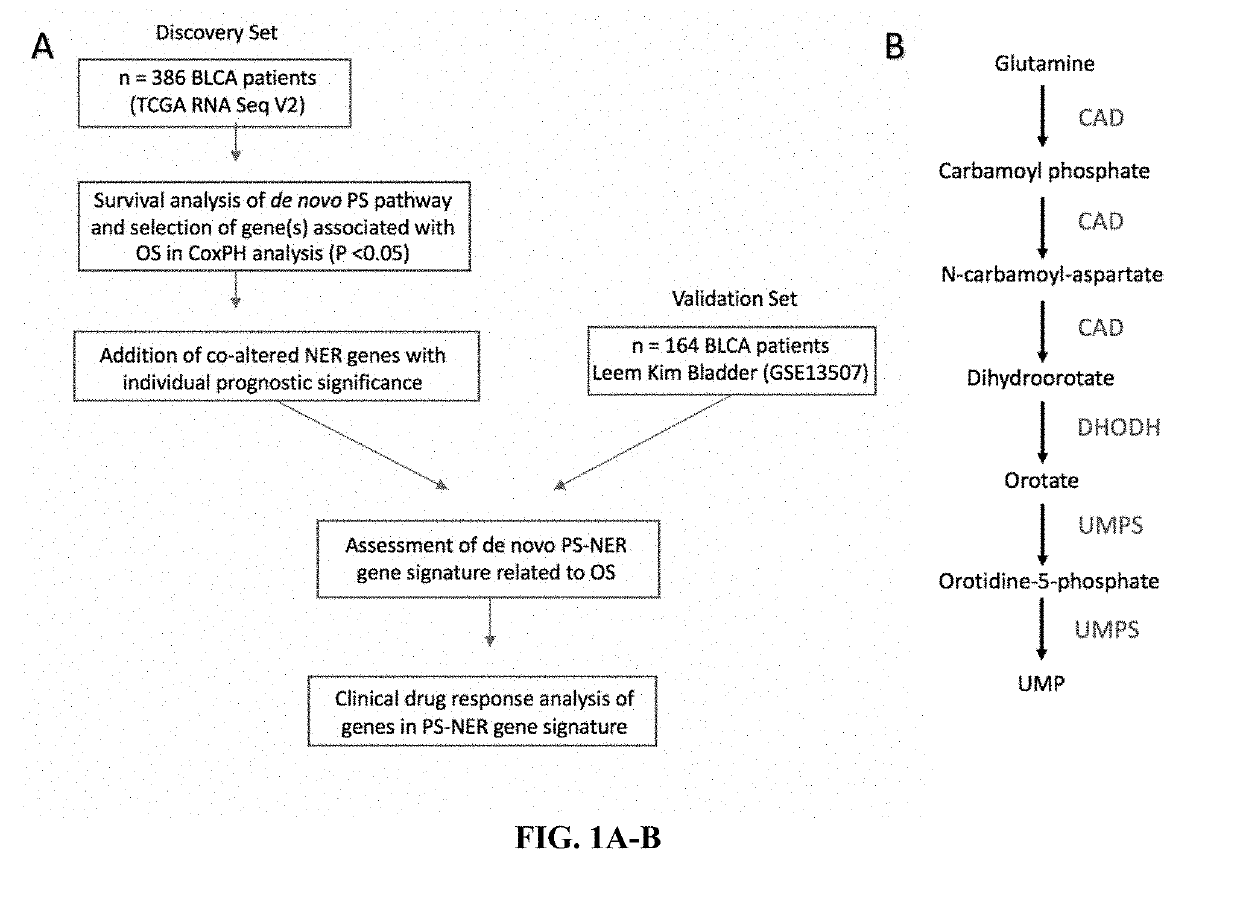
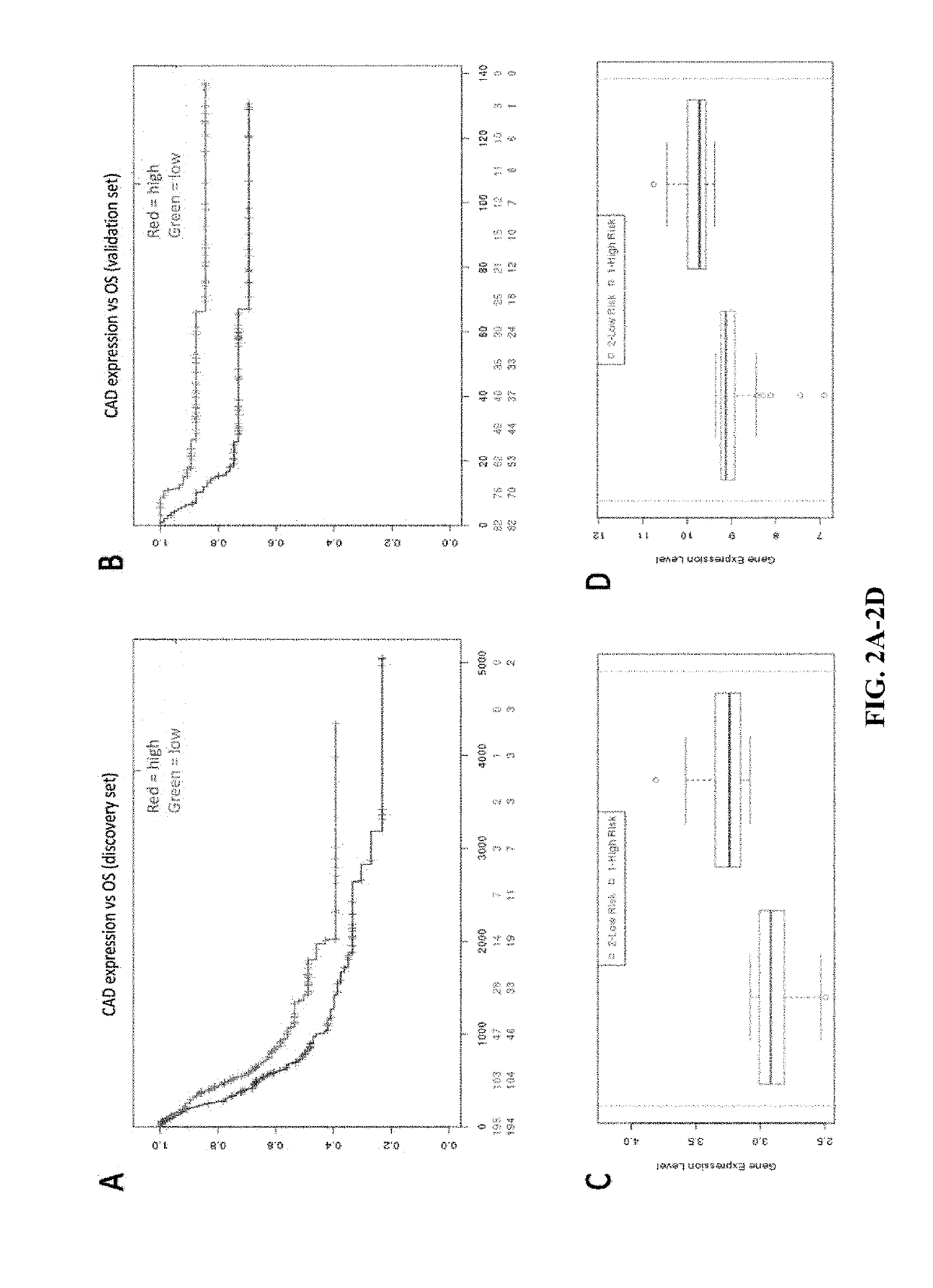
Biomarkers in de novo pyrimidine synthesis pathways and chemoresistance
InactiveUS20190233900A1Predict responsiveness to chemotherapyPredict to survival rateInorganic active ingredientsMicrobiological testing/measurementCisplatin based chemotherapyNucleotide Excision Repair Pathway
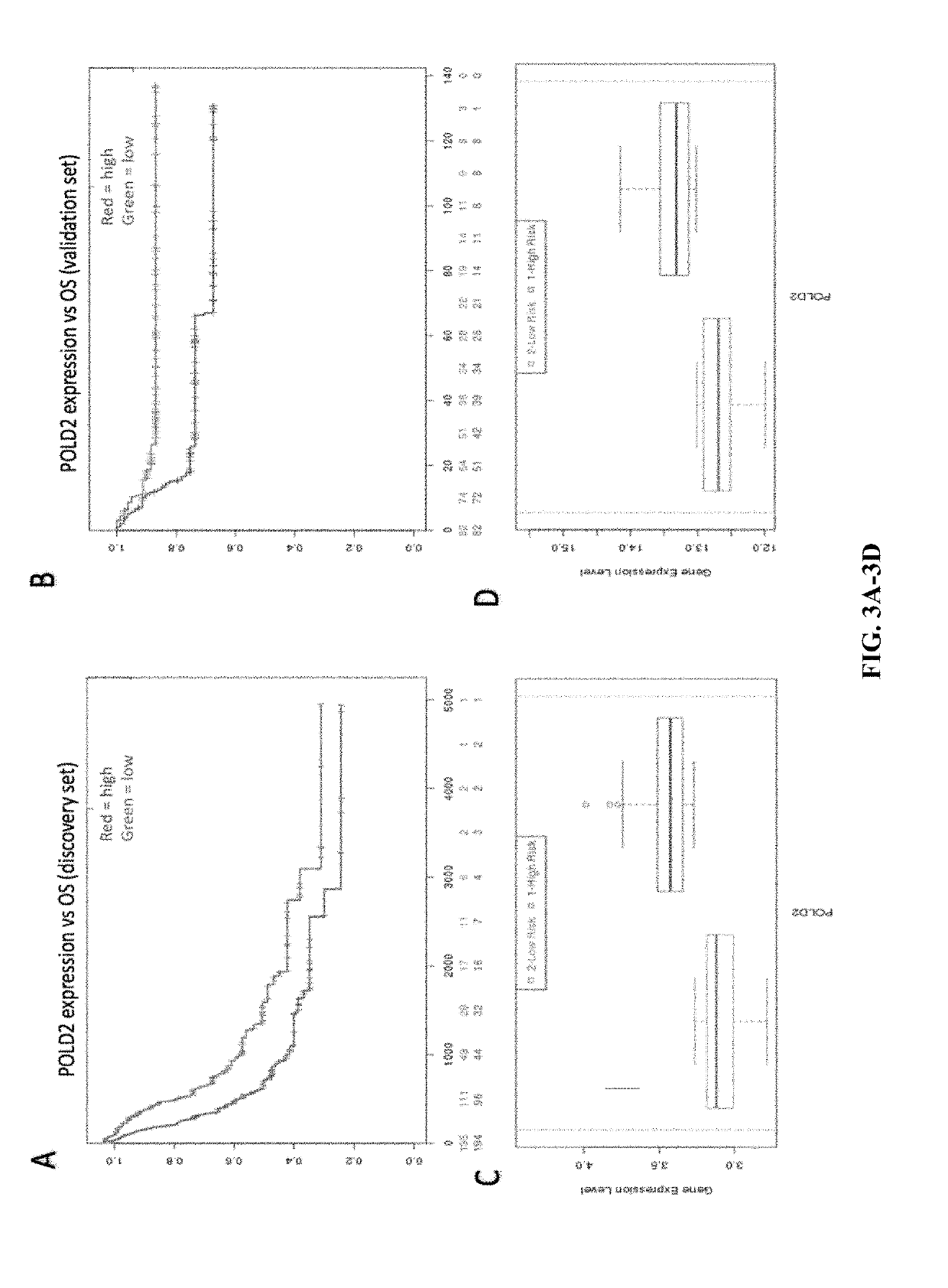
Compositions, methods, and uses of de novo pyrimidine synthesis pathway element, CAD, and optionally a second gene in a nucleotide excision repair pathway, POLD2 in determining a predicted survival rate, predicted responsiveness to a cisplatin-based chemotherapy of a patient diagnosed with bladder urothelial carcinoma are provided.
Owner:NANTOMICS LLC +1
FGFR tyrosine kinase inhibitors for the treatment of urothelial carcinoma
PendingCN113645974AOrganic active ingredientsMicrobiological testing/measurementTyrosine-kinase inhibitorTyrosine
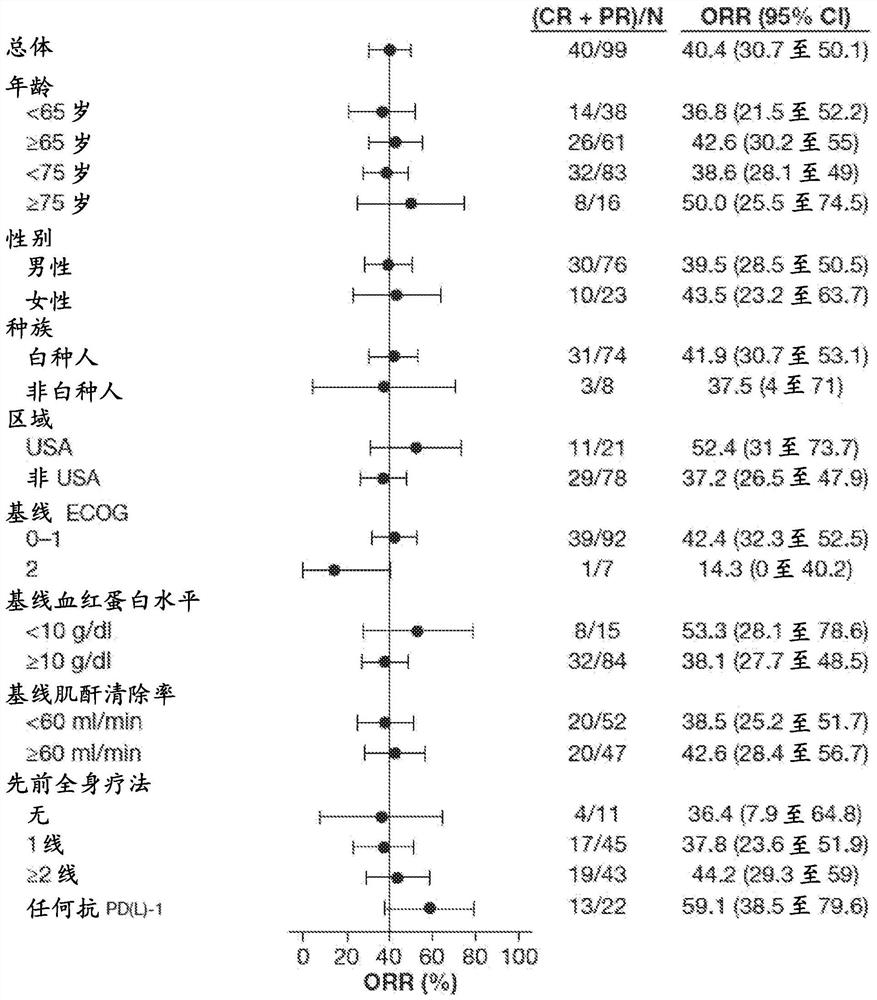
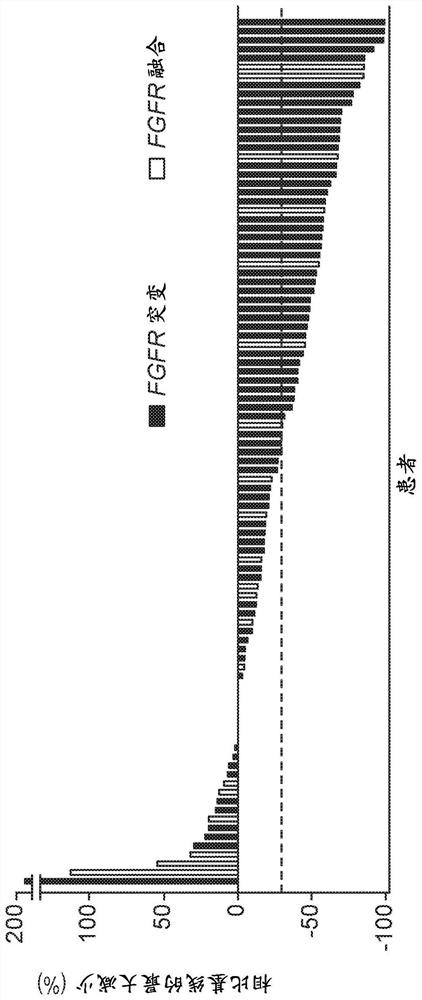
Described here are methods of treating urothelial carcinoma in a patient comprising evaluating a biological sample from the patient for the presence of at least two fibroblast growth factor receptor (FGFR) genetic alterations and treating the patient with an FGFR inhibitor. Also described herein are methods of treating urothelial carcinoma in a patient harboring at least two fibroblast growth factor receptor (FGFR) genetic alterations comprising administering a FGFR inhibitor.
Owner:JANSSEN PHARMA NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com