FGFR tyrosine kinase inhibitors for the treatment of urothelial carcinoma
A technique for urothelial carcinoma, FGFR2, applied in the field of FGFR tyrosine kinase inhibitors for the treatment of urothelial carcinoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0234] Example 1: Phase 2, multicenter, open-label study (NCT 02365597)
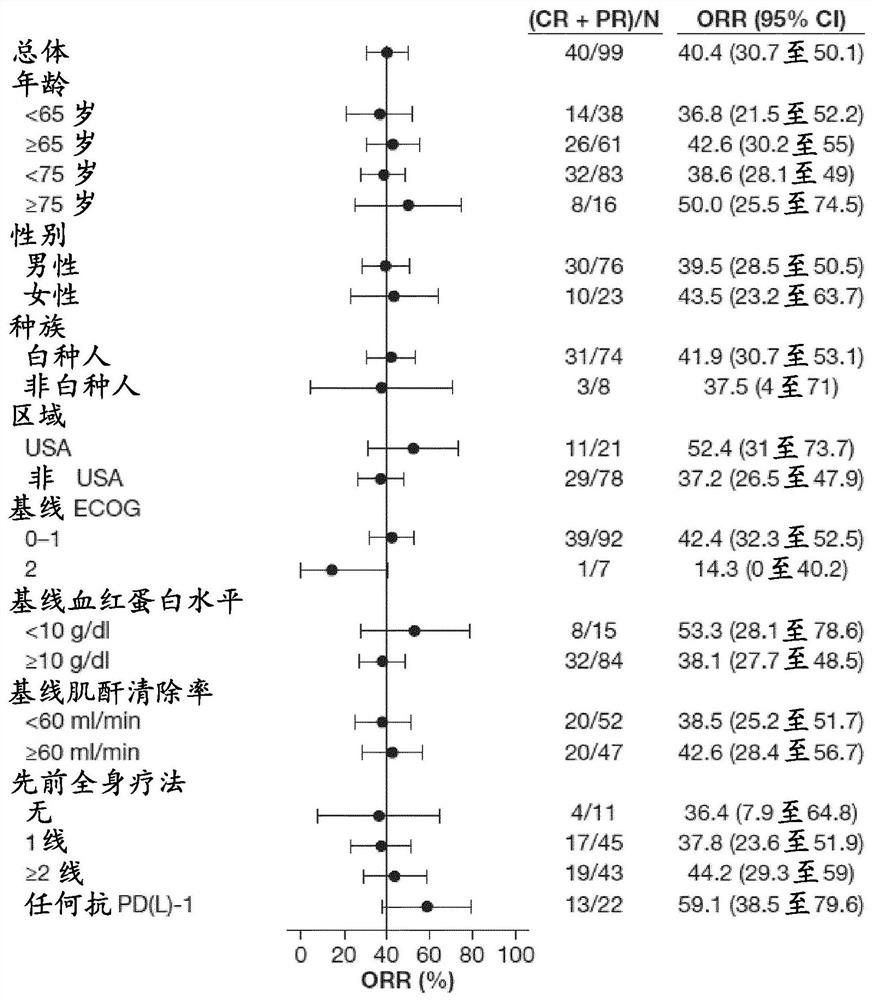
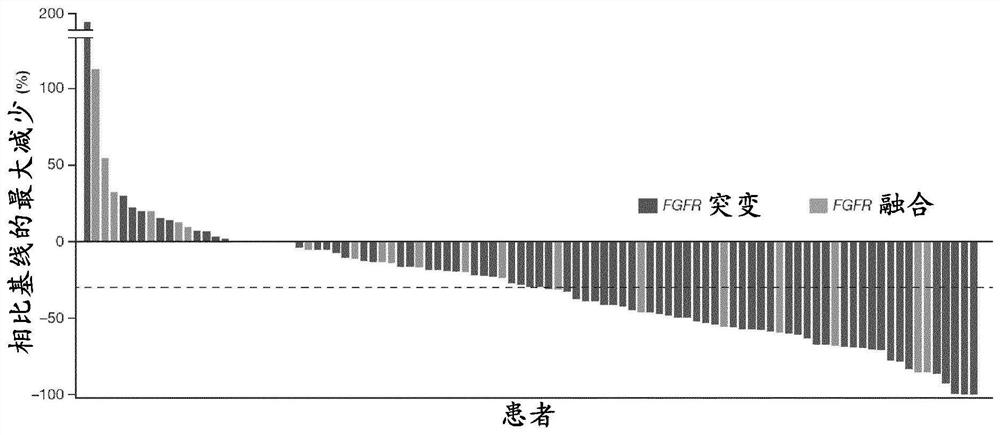
[0235] A phase 2, multicenter, open-label study was conducted to evaluate erdafitinib in subjects with metastatic or unresectable urothelial carcinoma carrying a selective FGFR genetic alteration (FGFR translocation or mutation) efficacy and safety.
[0236] The study included a screening phase (molecular screening at any time prior to the first dose and study screening within 30 days of the first dose), a treatment phase, and a post-treatment follow-up phase. The treatment period included the period from the first dose to the end-of-treatment follow-up. The follow-up period was extended until the subject died, withdrew consent, was lost to follow-up, or the end of the study, whichever came first.
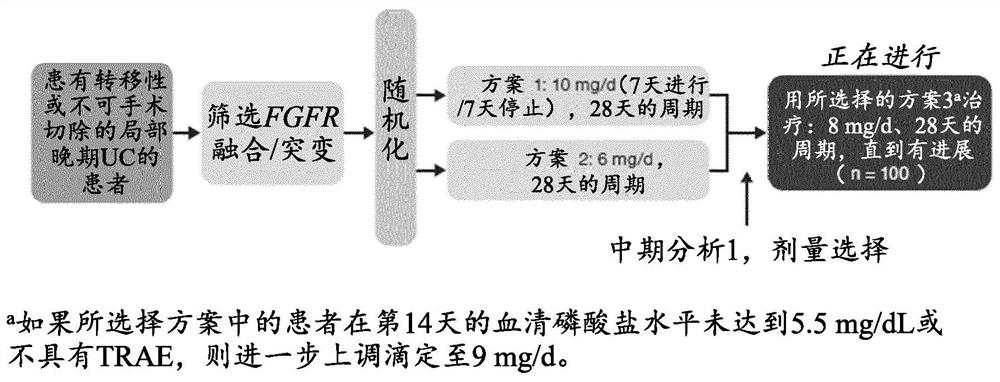
[0237] Study treatments were administered in 28-day cycles. Prior to interim analysis 1, there were 2 treatment options. Patients were randomly assigned to the following 2 regimens in a 1:1 to 28-day cycl...
example 2
[0343] Example 2: Pharmacodynamics and Pharmacokinetics
[0344] Pharmacodynamics
[0345] Cardiac Electrophysiology
[0346] Erdafitinib had no major effect on the QTc interval (ie, >20 ms) based on the assessment of the QTc interval in an open-label, dose-escalation, and dose-expansion study in 187 patients with cancer.
[0347] serum phosphate
[0348] Erdafitinib increased serum phosphate levels due to FGFR inhibition. Erdafitinib should be increased to the maximum recommended dose early in the cycle and administered continuously daily to achieve a target serum phosphate level of 5.5-7.0 mg / dL
[0349] Drugs that increase serum phosphate levels (such as potassium phosphate supplements, vitamin D supplements, antacids, phosphate-containing enemas or laxatives, and drugs known to contain phosphate drug as an excipient), unless there is no substitute. To manage elevated phosphate, the use of phosphate binders is permitted. Avoid concomitant use of agents that alter ...
example 3
[0370] Example 3: Drug Interactions
[0371] Effect of other drugs on erdafitinib
[0372] 1. Strong CYP2C9 or CYP3A4 inhibitors
[0373] clinical impact
[0374] Coadministration of erdafitinib with strong CYP2C9 or CYP3A4 inhibitors increased erdafitinib plasma concentrations;
[0375] Increased plasma concentrations of erdafitinib may lead to increased drug-related toxicity.
[0376] clinical management
[0377] Alternative therapies other than strong CYP2C9 or CYP3A4 inhibitors were considered during erdafitinib treatment.
[0378] • If coadministration with strong CYP2C9 or CYP3A4 inhibitors is unavoidable, closely monitor for adverse reactions and consider dose modification accordingly. If strong inhibitors are discontinued, the dose of erdafitinib can be increased in the absence of drug-related toxicity.
[0379] 2. Strong CYP2C9 or CYP3A4 inducers
[0380] clinical impact
[0381] Coadministration of erdafitinib with strong CYP2C9 or CYP3A4 inducers can signifi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com