Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
883 results about "Fibroblast growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
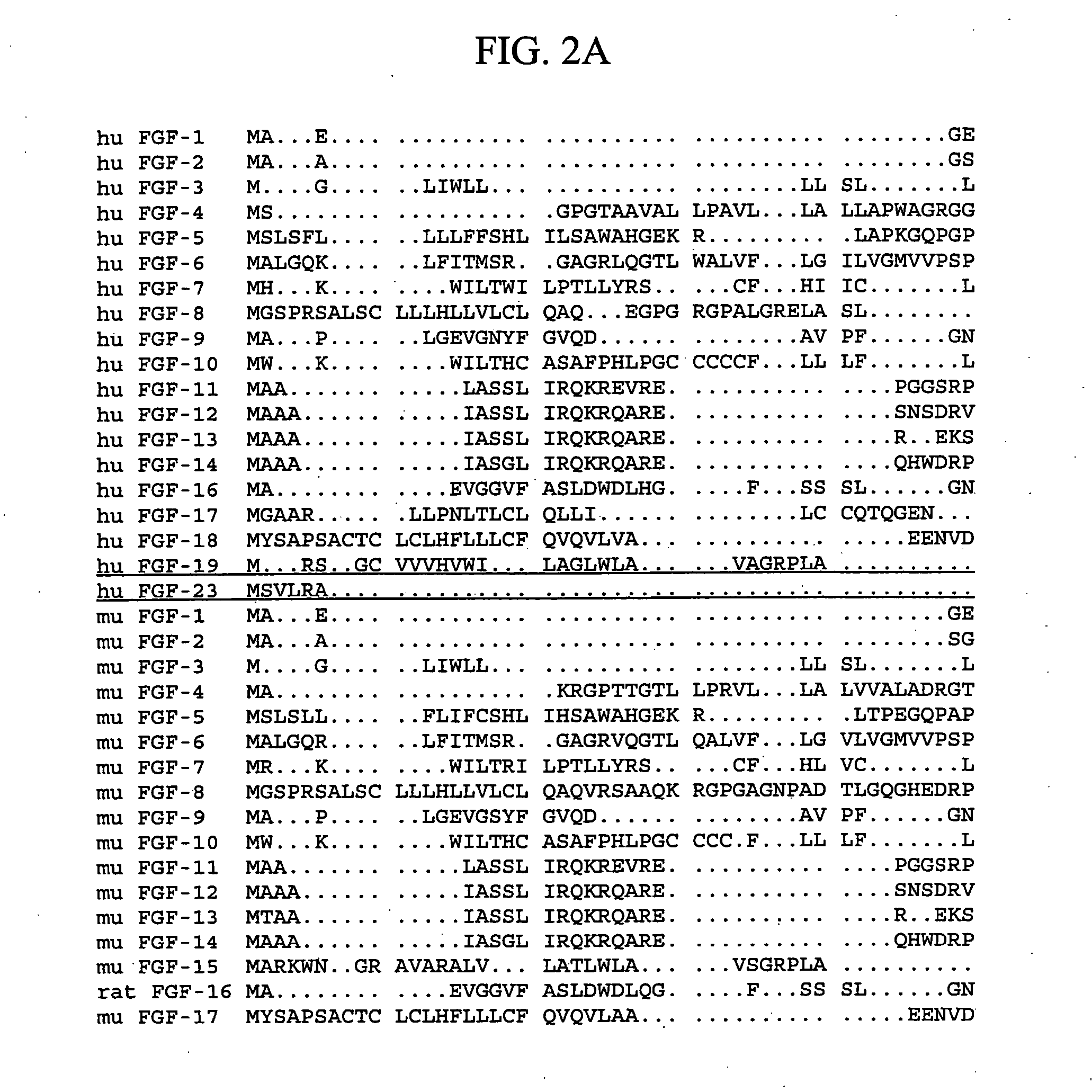
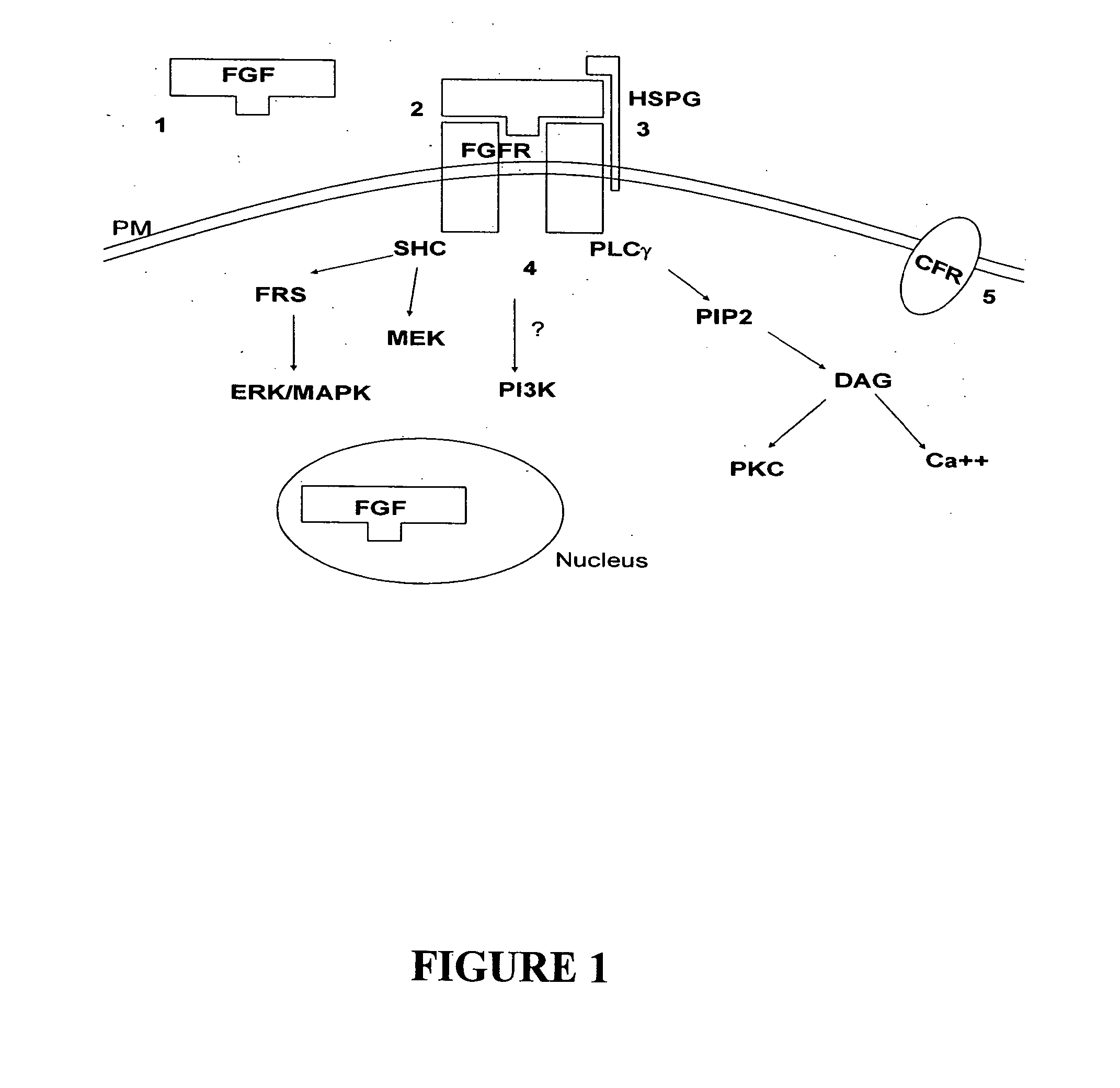
The fibroblast growth factors (FGF) are a family of cell signalling proteins that are involved in a wide variety of processes, most notably as crucial elements for normal development. Any irregularities in their function lead to a range of developmental defects. These growth factors generally act as systemic or locally circulating molecules of extracellular origin that activate cell surface receptors. A defining property of FGFs is that they bind to heparin and heparan sulfate, thus some of them are found to be sequestered in the extracellular matrix of tissues that contains heparan sulfate proteoglycans and they are released locally upon injury or tissue remodeling.
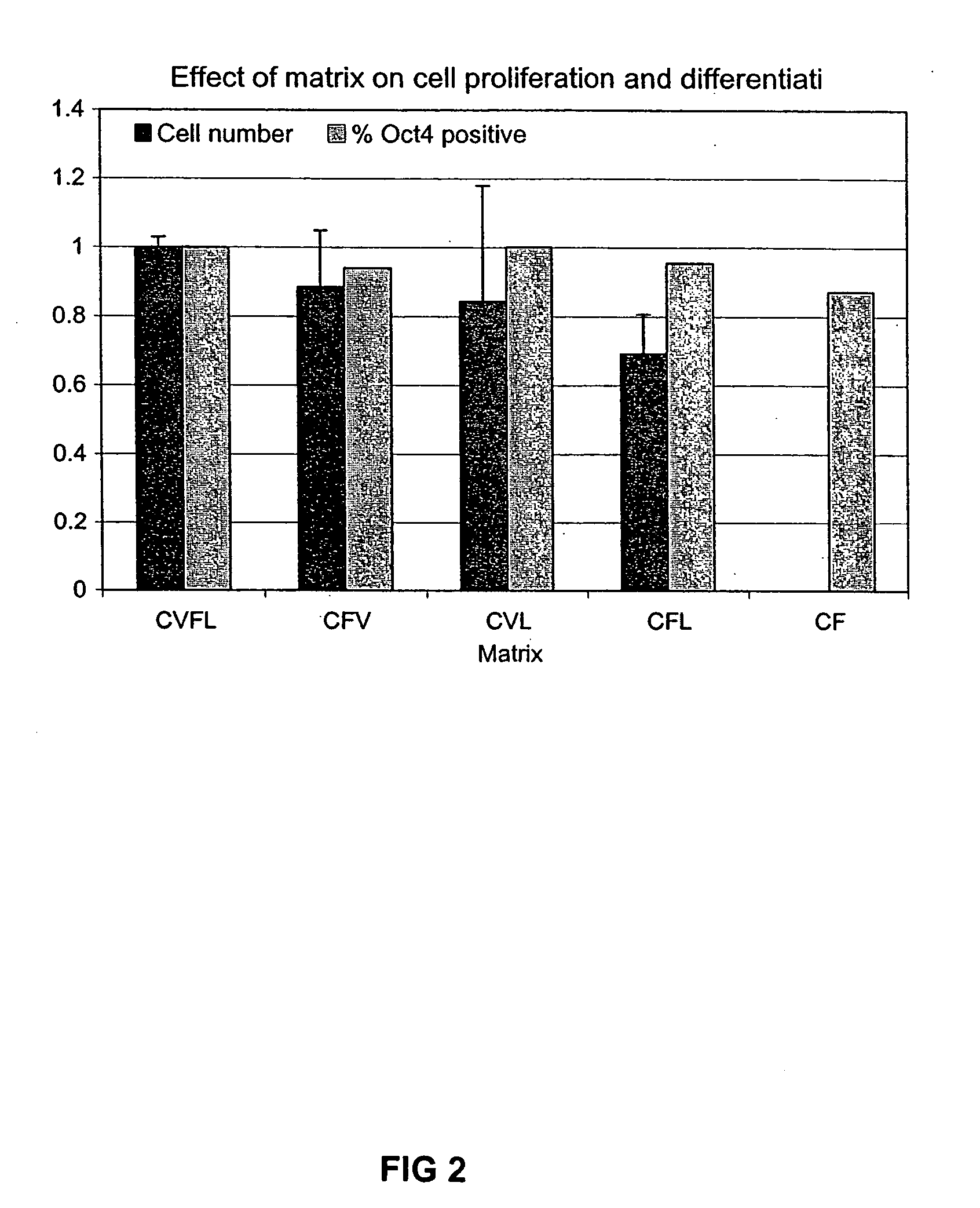
Defined media for stem cell culture
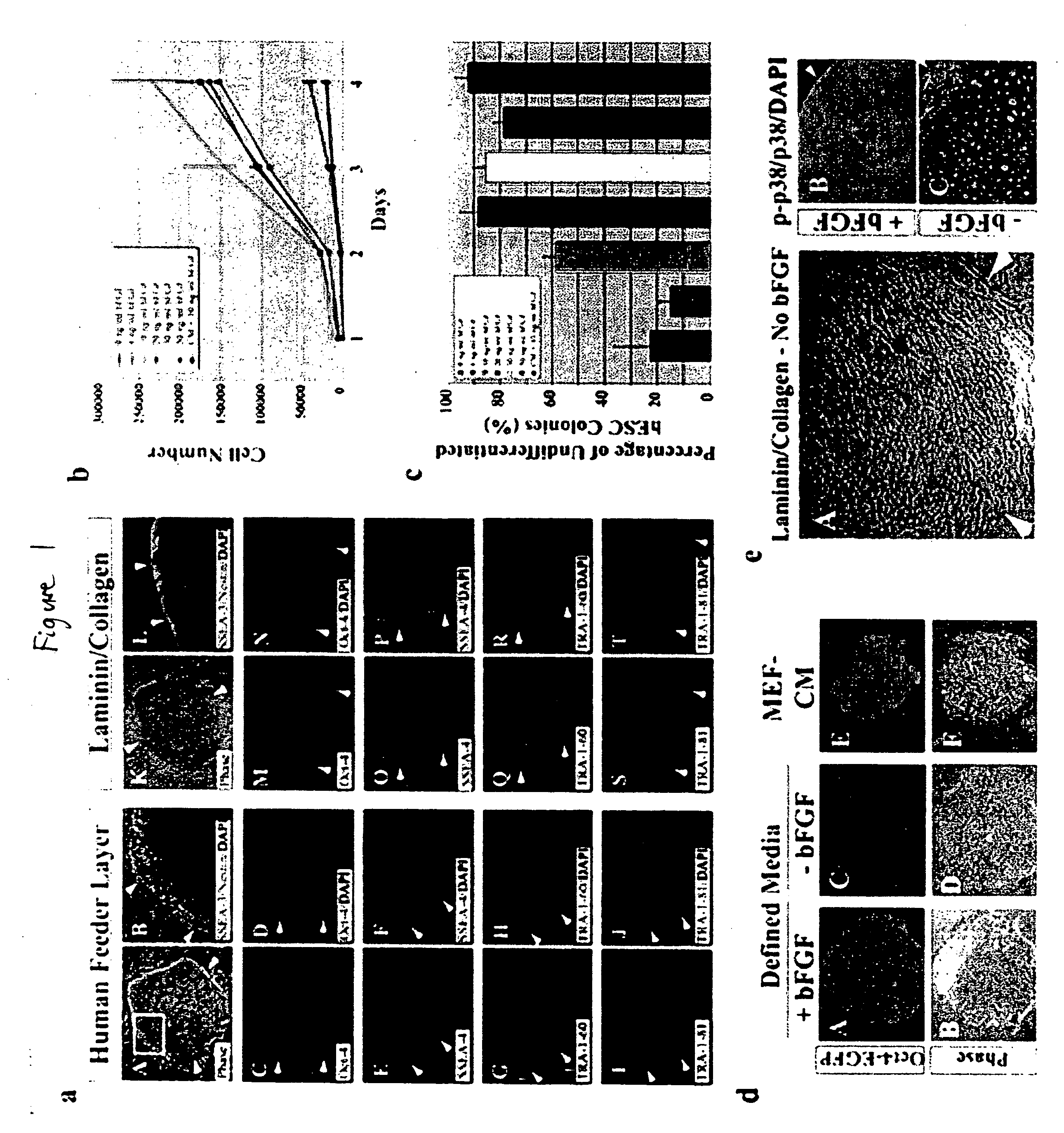
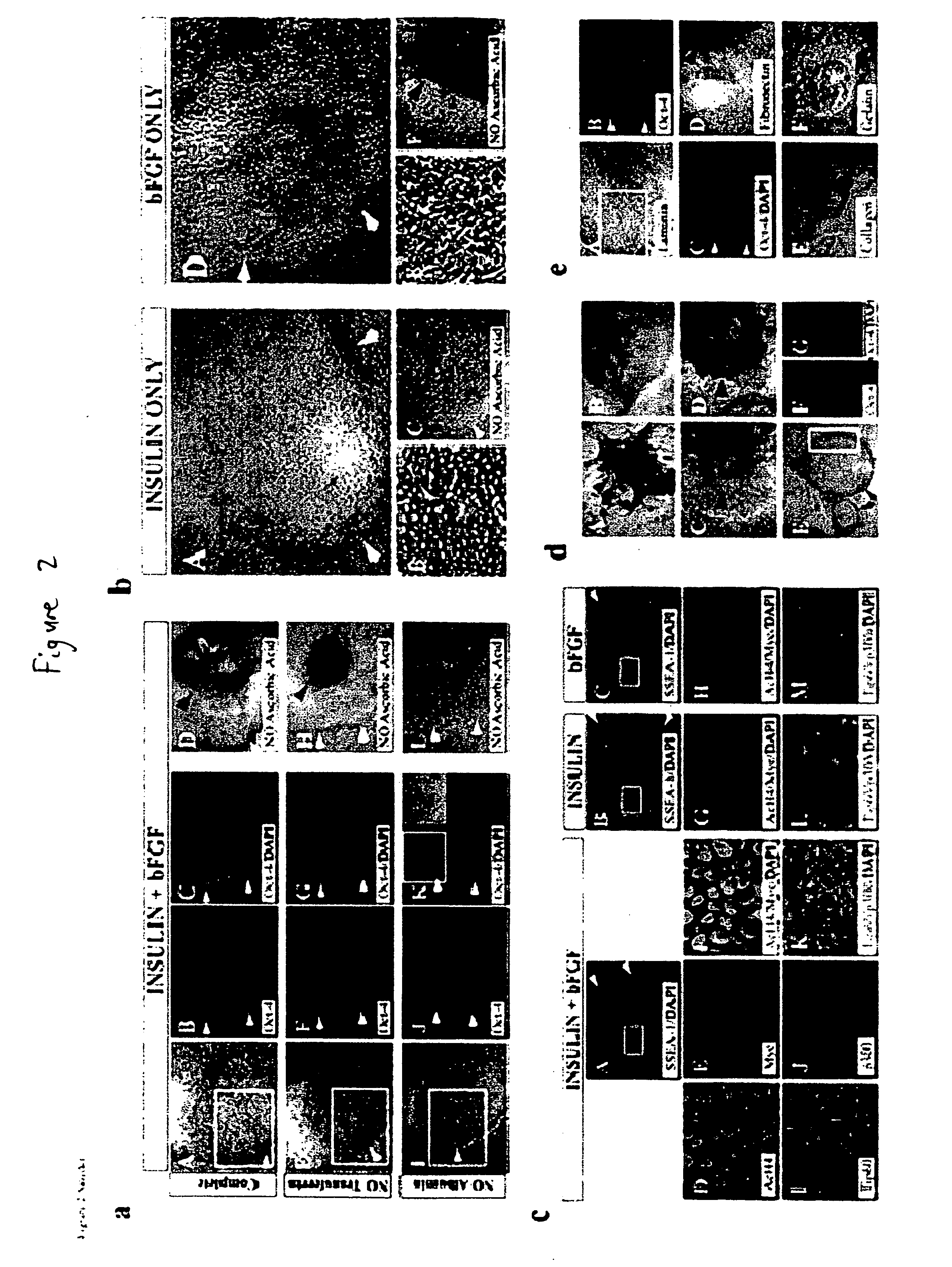
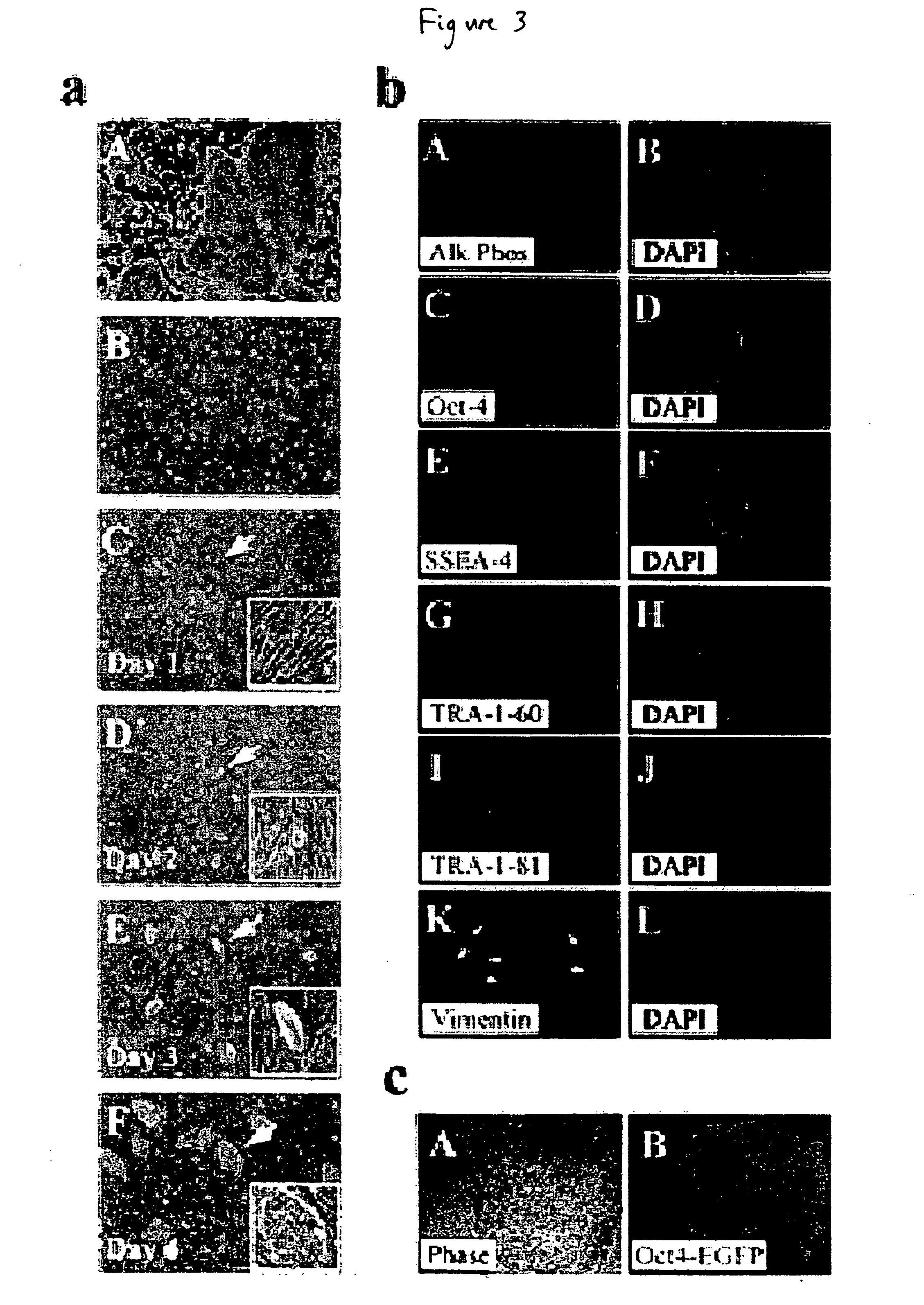
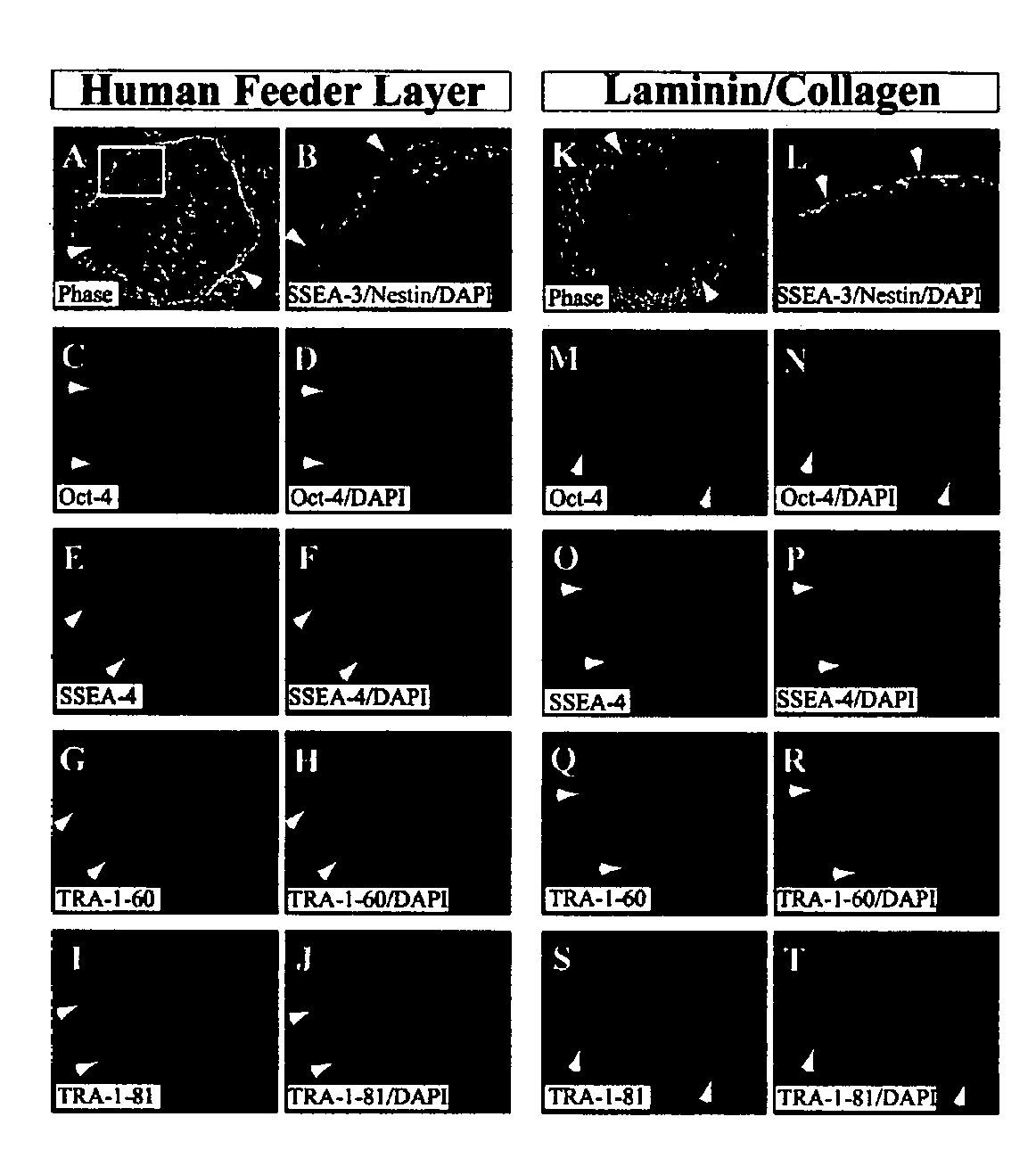
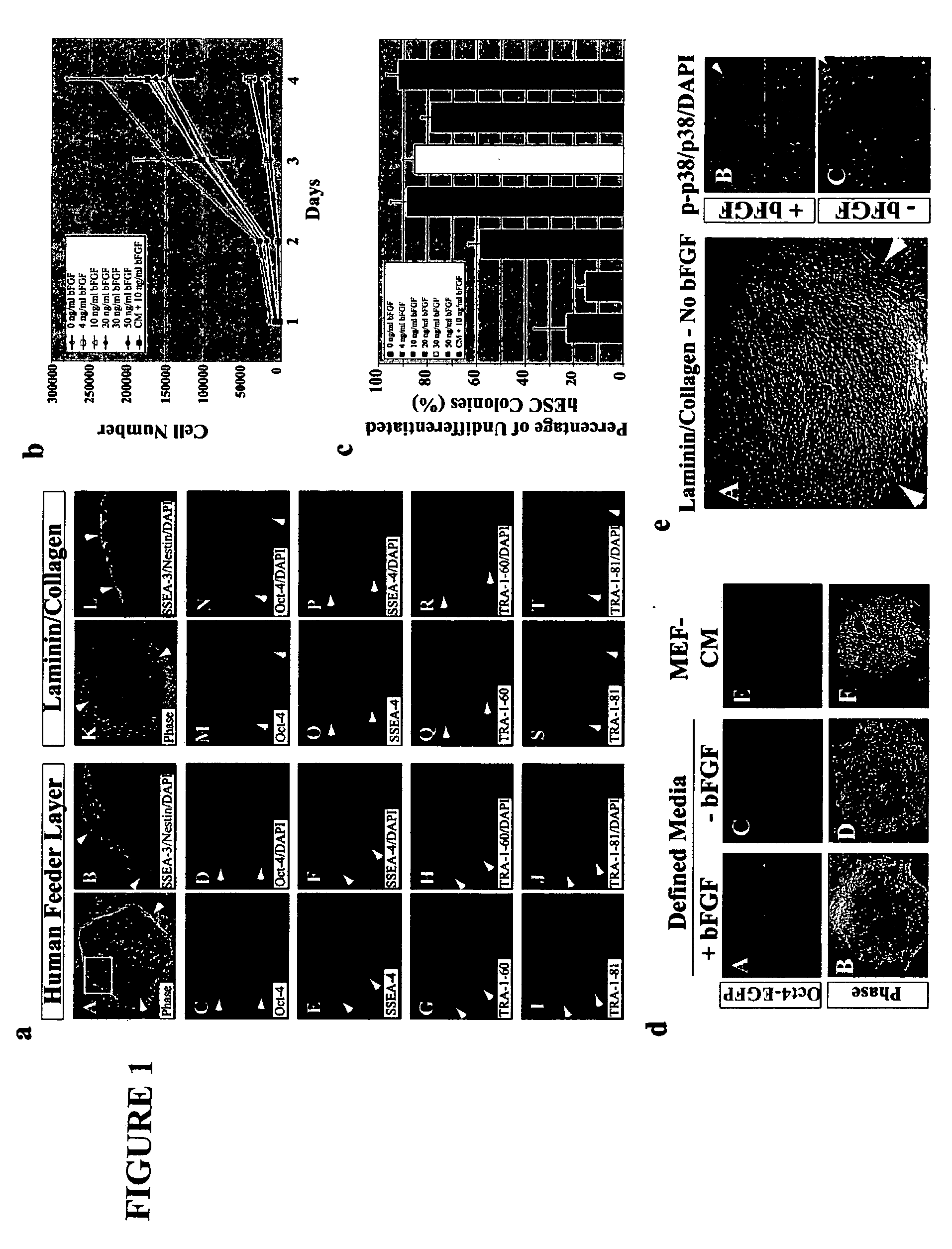
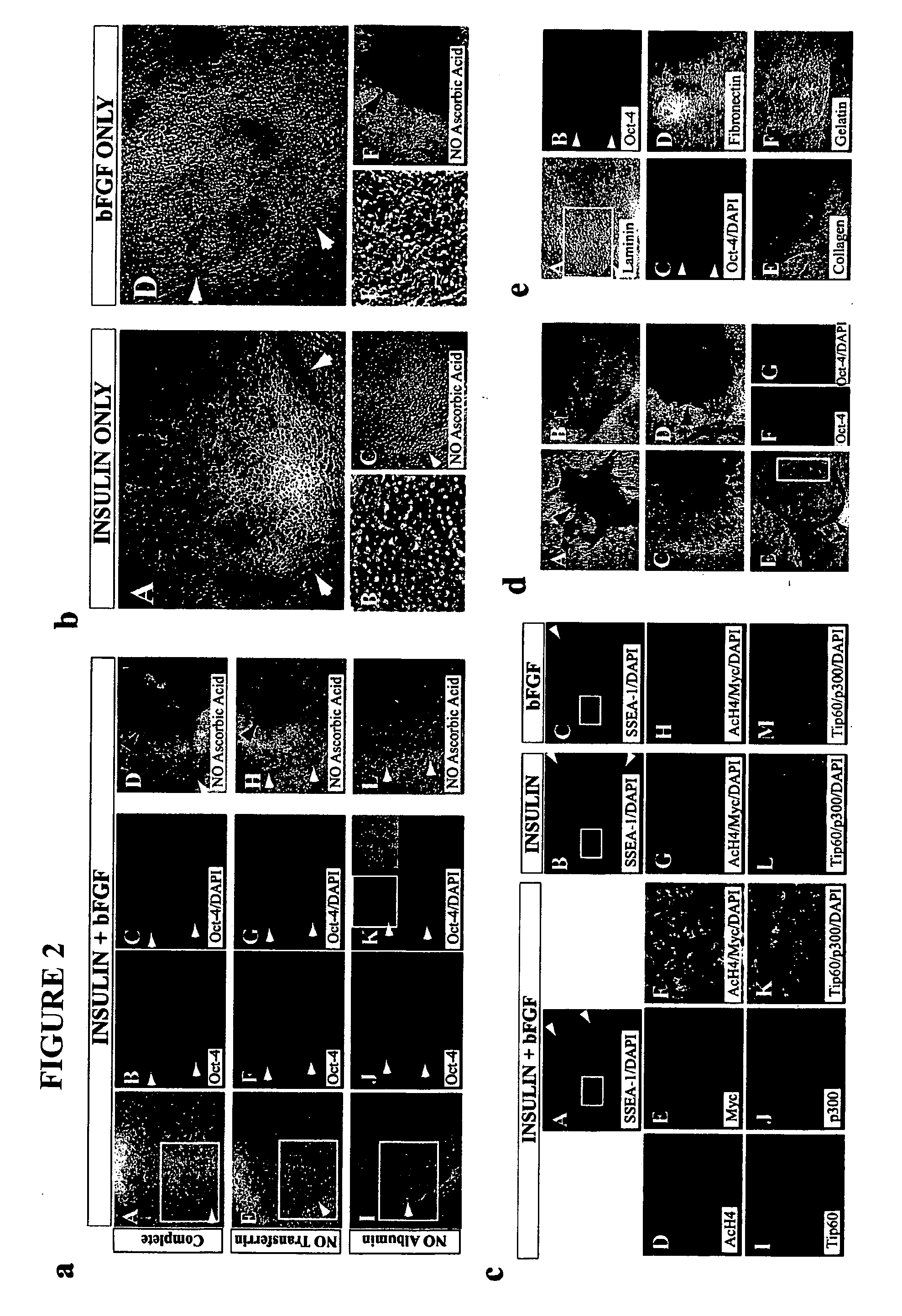
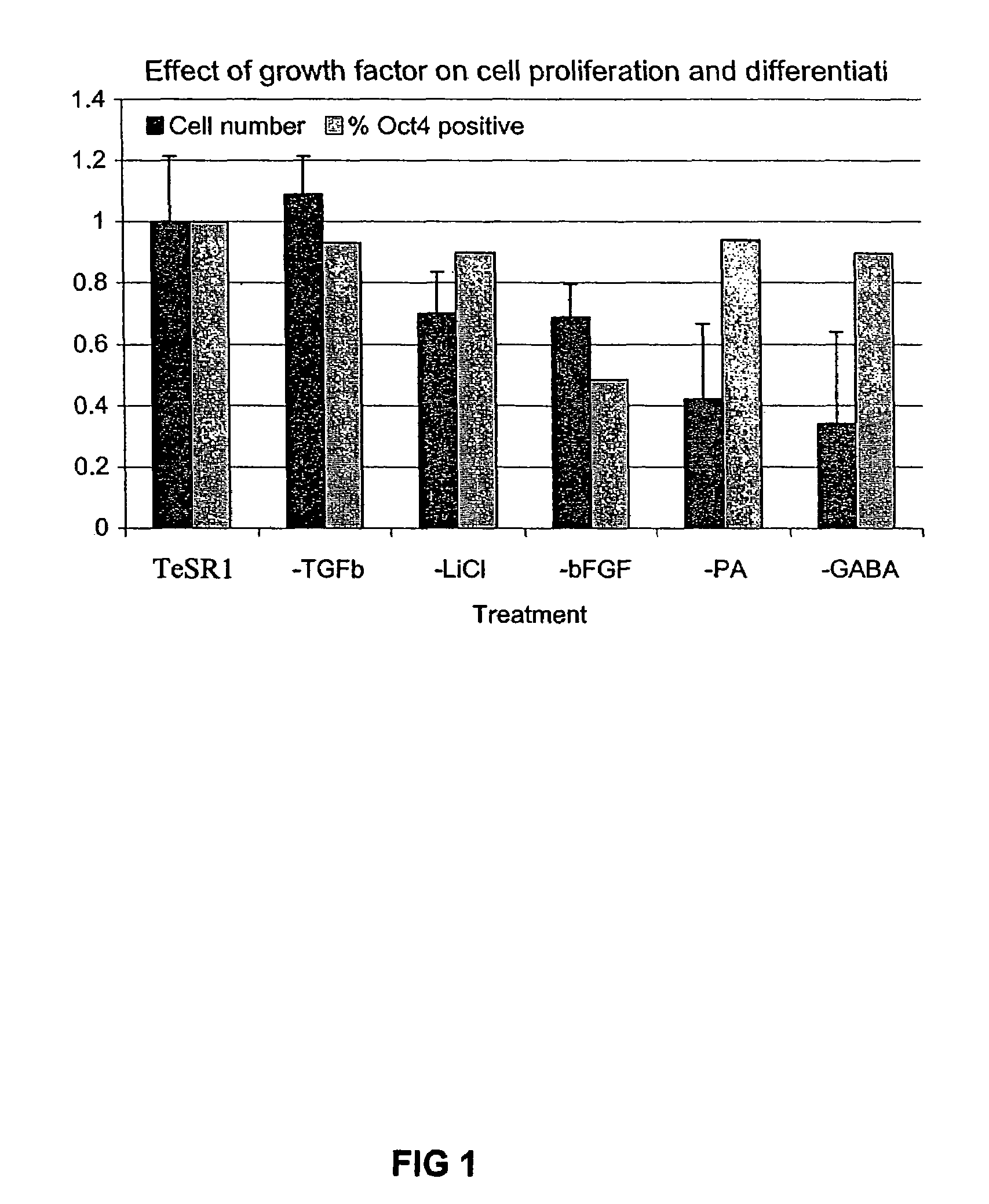
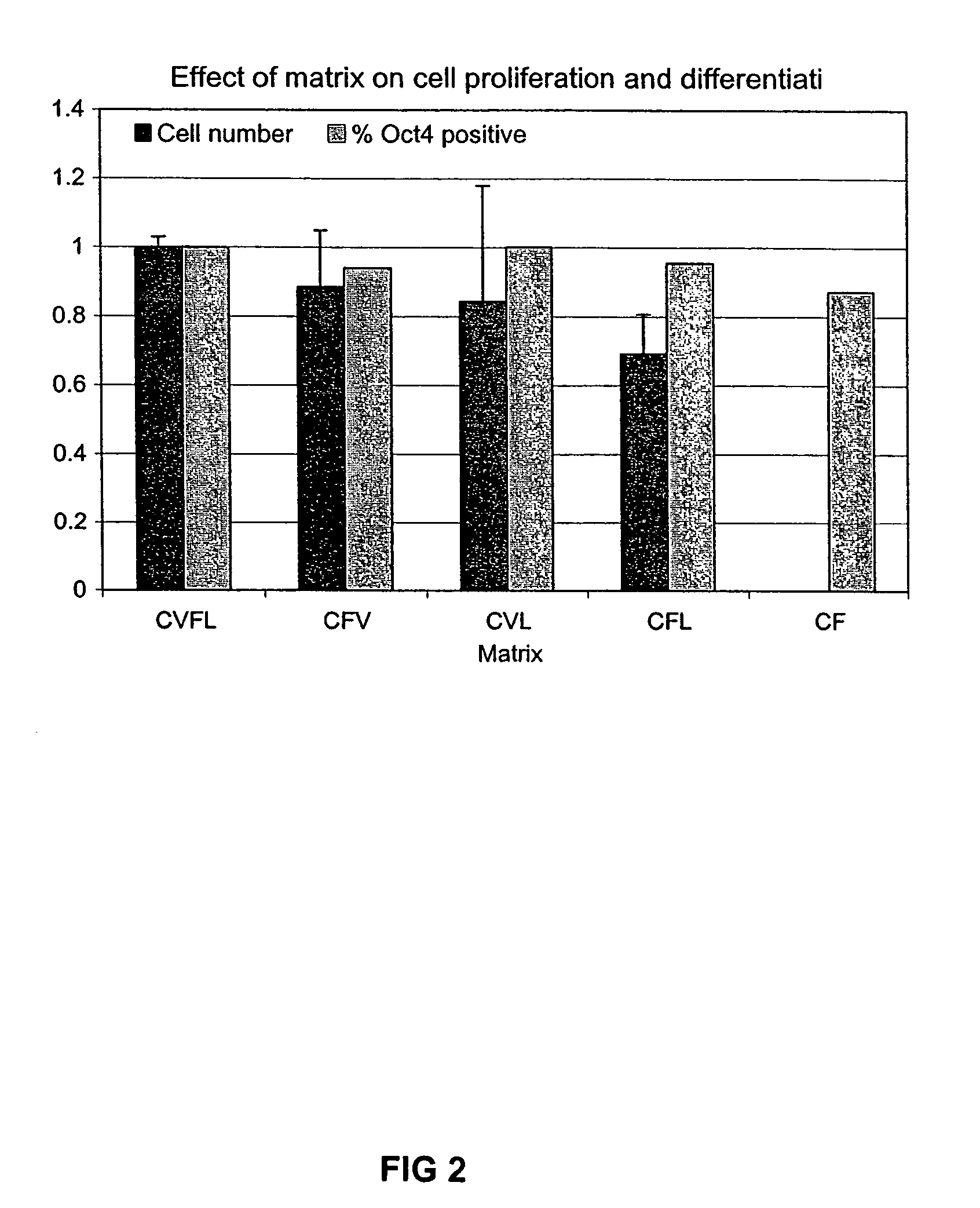
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Defined media for pluripotent stem cell culture
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Cultivation of primate embryonic stem cells
InactiveUS20050148070A1Improve cloning efficiencyAvoid variabilityOrganic active ingredientsCulture processMammalFeeder Layer
The invention relates to methods for culturing human embryonic stem cells by culturing the stem cells in an environment essentially free of mammalian fetal serum and in a stem cell culture medium including amino acids, vitamins, salts, minerals, transferring, insulin, albumin, and a fibroblast growth factor that is supplied from a source other than just a feeder layer the medium. Also disclosed are compositions capable of supporting the culture and proliferation of human embryonic stem cells without the need for feeder cells or for exposure of the medium to feeder cells.
Owner:WISCONSIN ALUMNI RES FOUND
Cultivation of primate embryonic stem cells
InactiveUS20050244962A1Maintain normalMaintaining the karyotype of the stem cellsCulture processArtificial cell constructsFeeder LayerStem cell culture
The invention relates to methods for culturing human embryonic stem cells by culturing the stem cells in an environment essentially free of mammalian fetal serum and in a stem cell culture medium including amino acids, vitamins, salts, minerals, transferring, insulin, albumin, and a fibroblast growth factor that is supplied from a source other than just a feeder layer the medium. Also disclosed are compositions capable of supporting the culture and proliferation of human embryonic stem cells without the need for feeder cells or for exposure of the medium to feeder cells.
Owner:WICELL RES INST
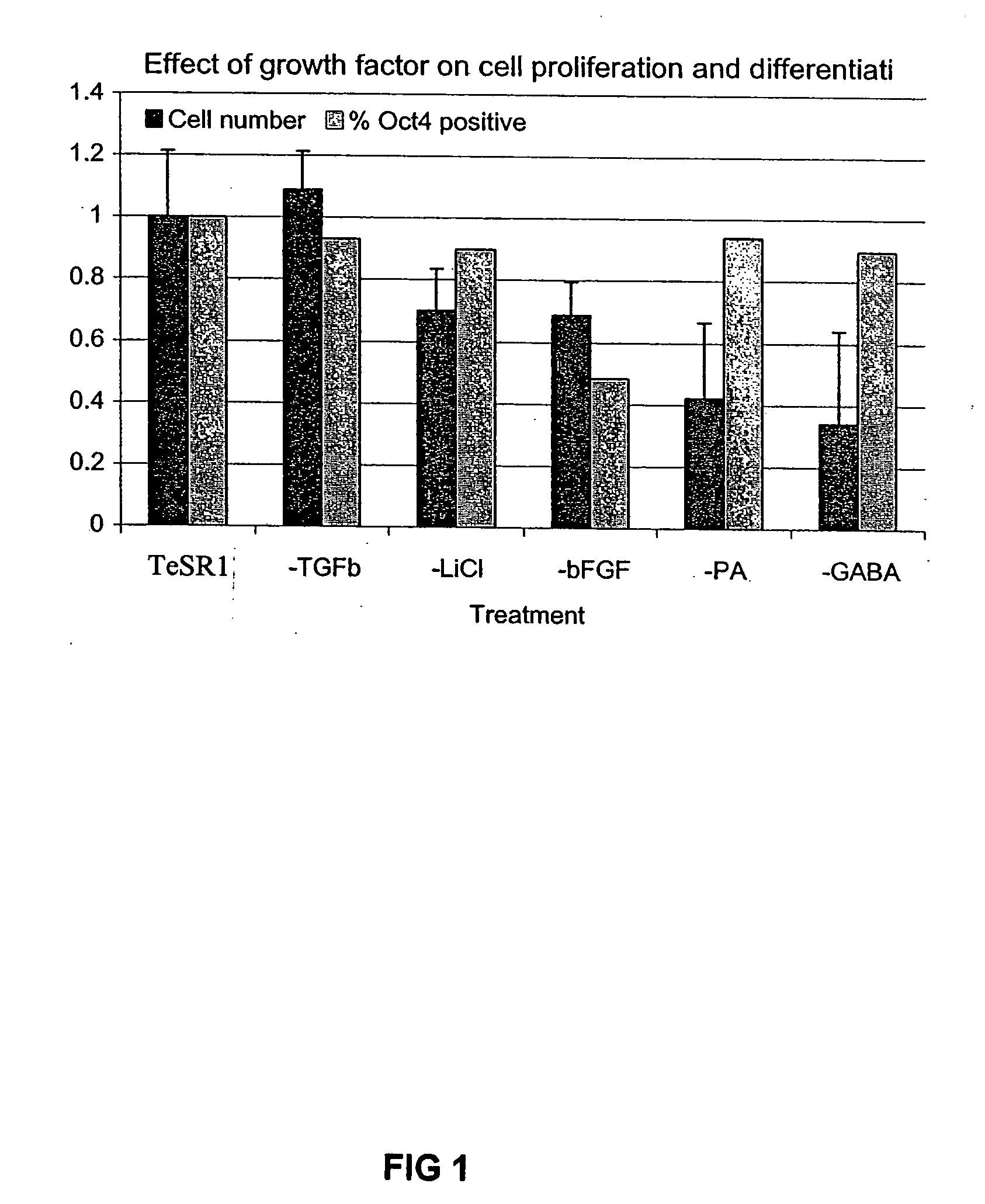
Culturing human embryonic stem cells in medium containing pipecholic acid and gamma amino butyric acid
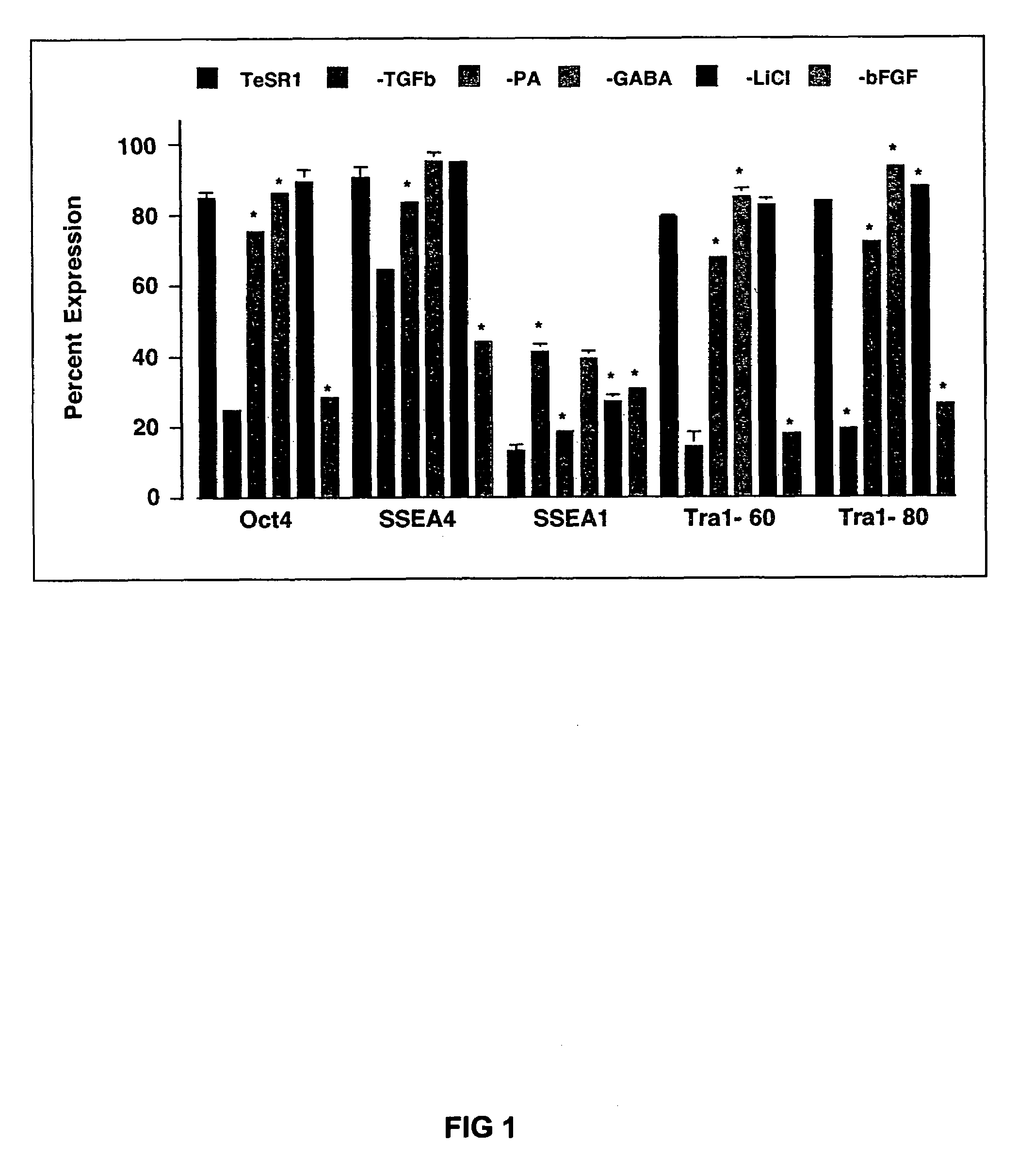
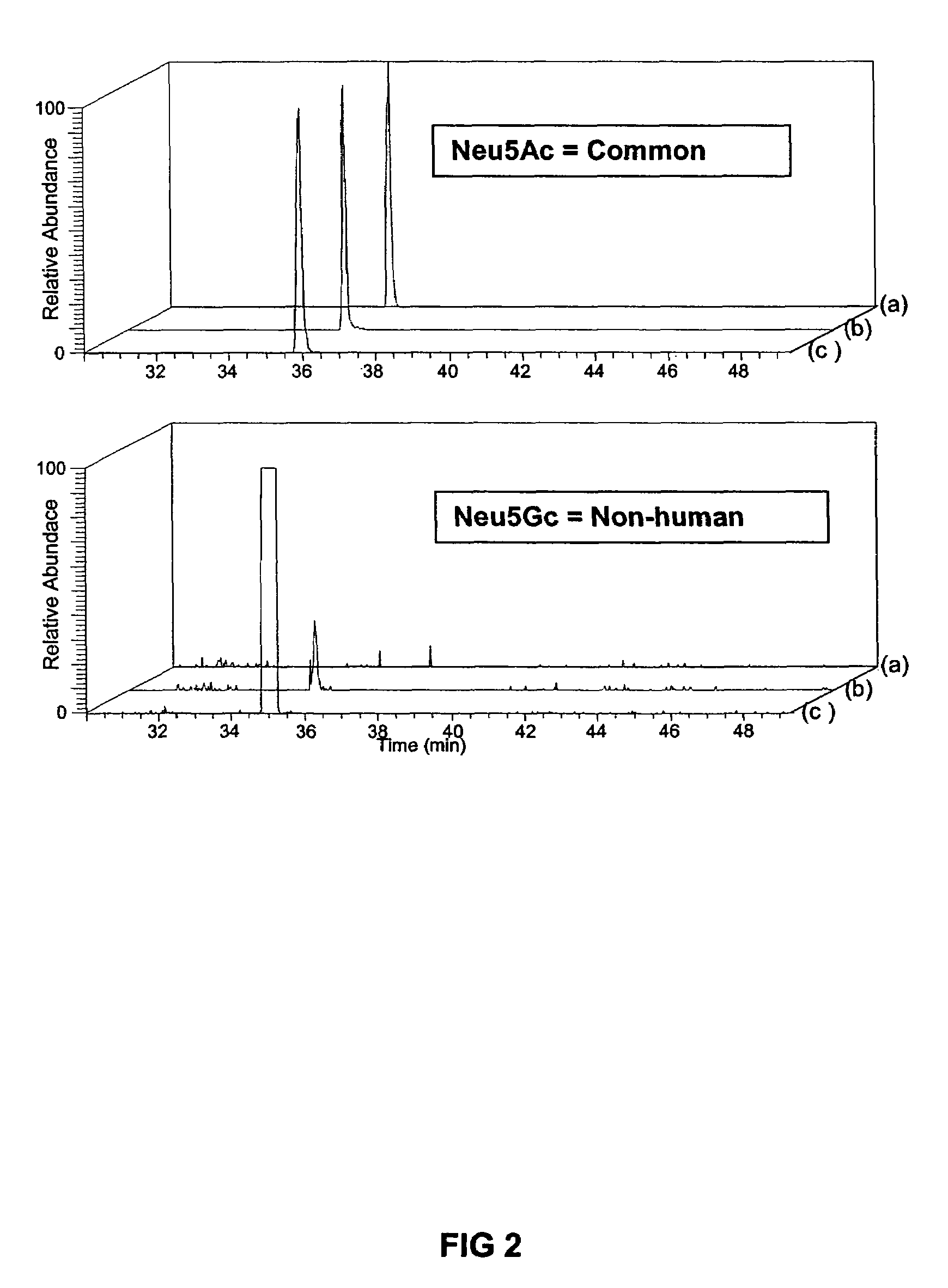
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor are used in a medium with gamma amino butyric acid, pipecholic acid, lithium and lipids, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium. A humanized matrix of human proteins can be used as a basement matrix to culture the cells. New lines of human embryonic stem cells made using these culture conditions, the medium and the matrix, will never have been exposed to animal cells, animal products, feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Medium containing pipecholic acid and gamma amino butyric acid and culture of embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor, gamma amino butyric acid, pipecholic acid, lithium and transforming growth factor beta are added to the medium in which the stem cells are cultured, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Biodegradable polymer coils for intraluminal implants
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
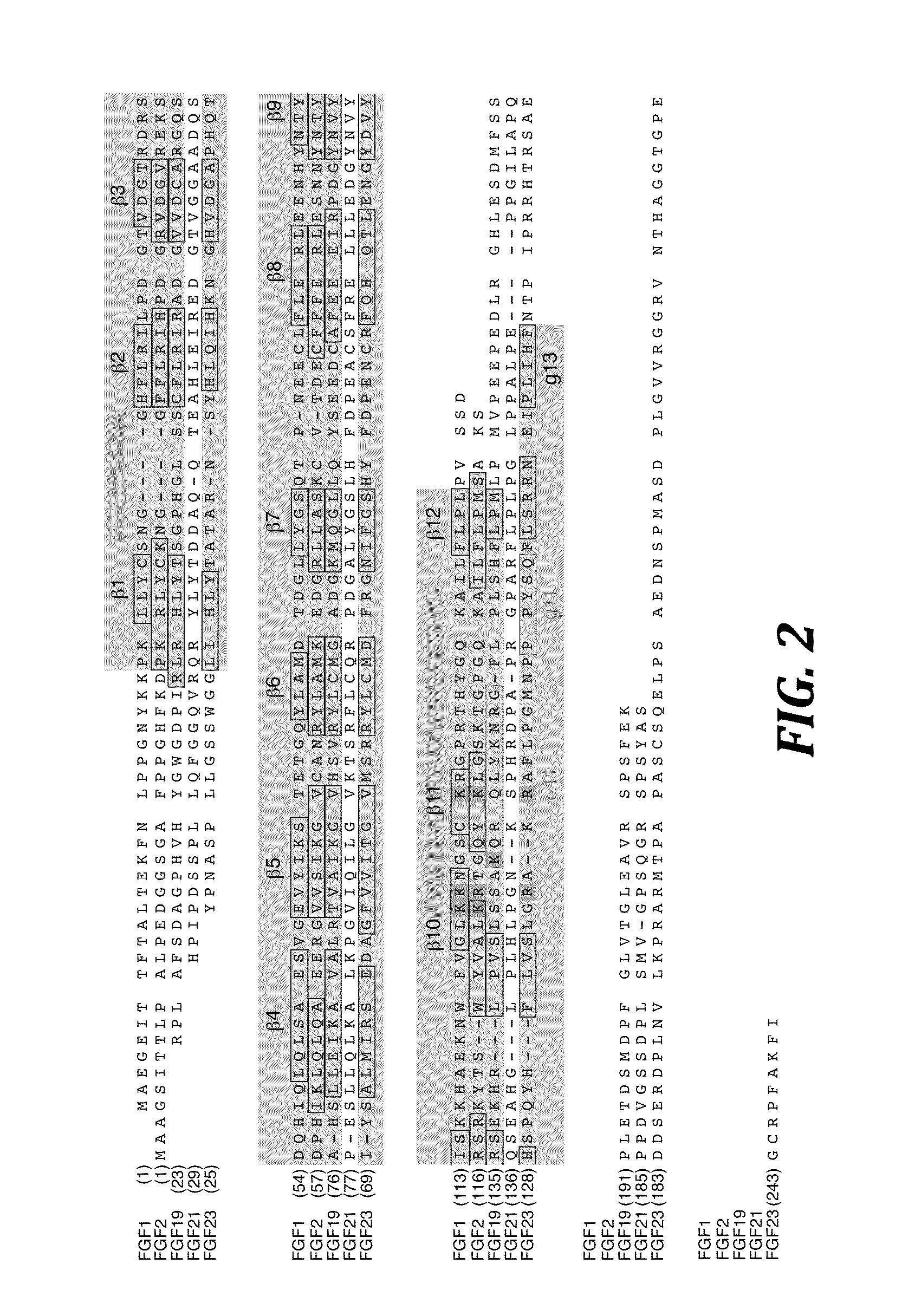
Active variants of FGF with improved specificity
InactiveUS7288406B2Enhanced receptor specificityImprove in vivo activitySugar derivativesPeptide/protein ingredientsDiseaseReceptor subtype
The present invention provides active fibroblast growth factor variants demonstrating enhanced receptor subtype specificity. The preferred novel variants retain binding to FGF Receptor Type 3 (FGFR3) triggering intracellular downstream mechanisms leading to activation of a biological response. Methods of utilizing preferred FGF mutants in preparation of medicaments for the treatment of malignancies and skeletal disorders including osteoporosis and enhancing fracture healing and wound healing processes are provided.
Owner:PROCHON BIOTECH
Muteins of Fibroblast Growth Factor 21
InactiveUS20070293430A1Reduced deamidationReduce capacityPeptide/protein ingredientsMetabolism disorderWild typeNucleic acid sequencing
The present invention relates to novel muteins of human fibroblast growth factor 21 with reduced deamidation compared to wild-type human FGF-21. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome.
Owner:ELI LILLY & CO
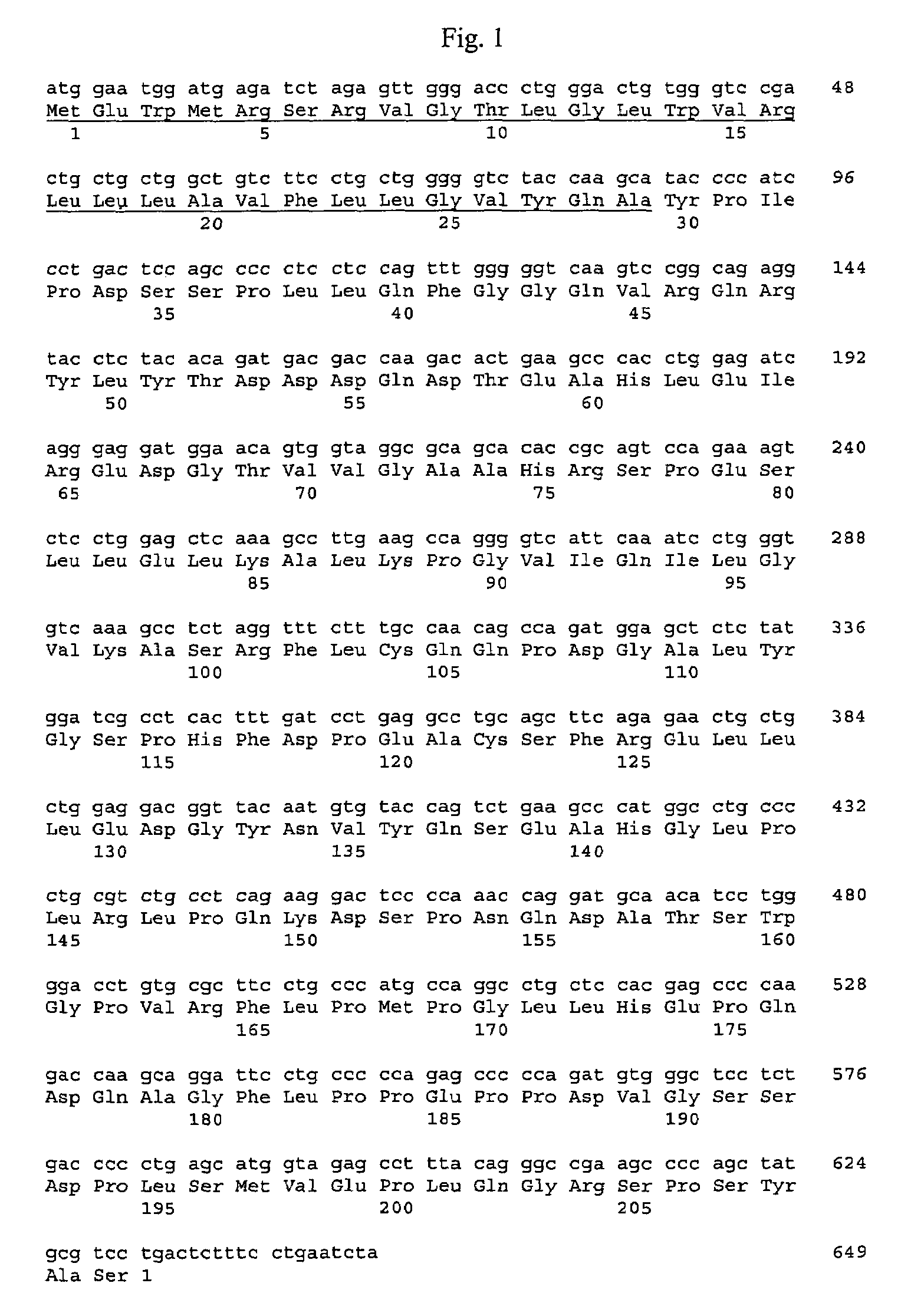
Chimeric fibroblast growth factors with altered receptor specificity
ActiveUS20110104152A1Peptide/protein ingredientsAntibody mimetics/scaffoldsDna encodingTherapeutic treatment
The present invention is directed to novel chimeric fibroblast growth factor (FGF) polypeptides, novel DNA encoding chimeric FGF polypeptides, and to the recombinant production of chimeric FGF polypeptides, and to methods, compositions and assays utilizing chimeric FGF polypeptides for the therapeutic treatment of metabolic-related disorders and other conditions, and for producing pharmaceutically active compositions including chimeric FGF polypeptides, the compositions having therapeutic and pharmacologic properties including those associated with the treatment of metabolic-related disorders and other conditions.
Owner:F HOFFMANN LA ROCHE & CO AG
Fibroblast growth factor-like polypeptides
Owner:AMGEN INC
Remodeling and Glycopegylation of Fibroblast Growth Factor (Fgf)
InactiveUS20080176790A1Improve pharmacokineticsCost effectiveOrganic active ingredientsFungiMutantPolynucleotide
The present invention relates to mutants of Fibroblast Growth Factor (FGF), particularly FGF-20 and FGF-21, which contain newly introduced N-linked or O-linked glycosylation site(s). The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:89BIO LTD +1
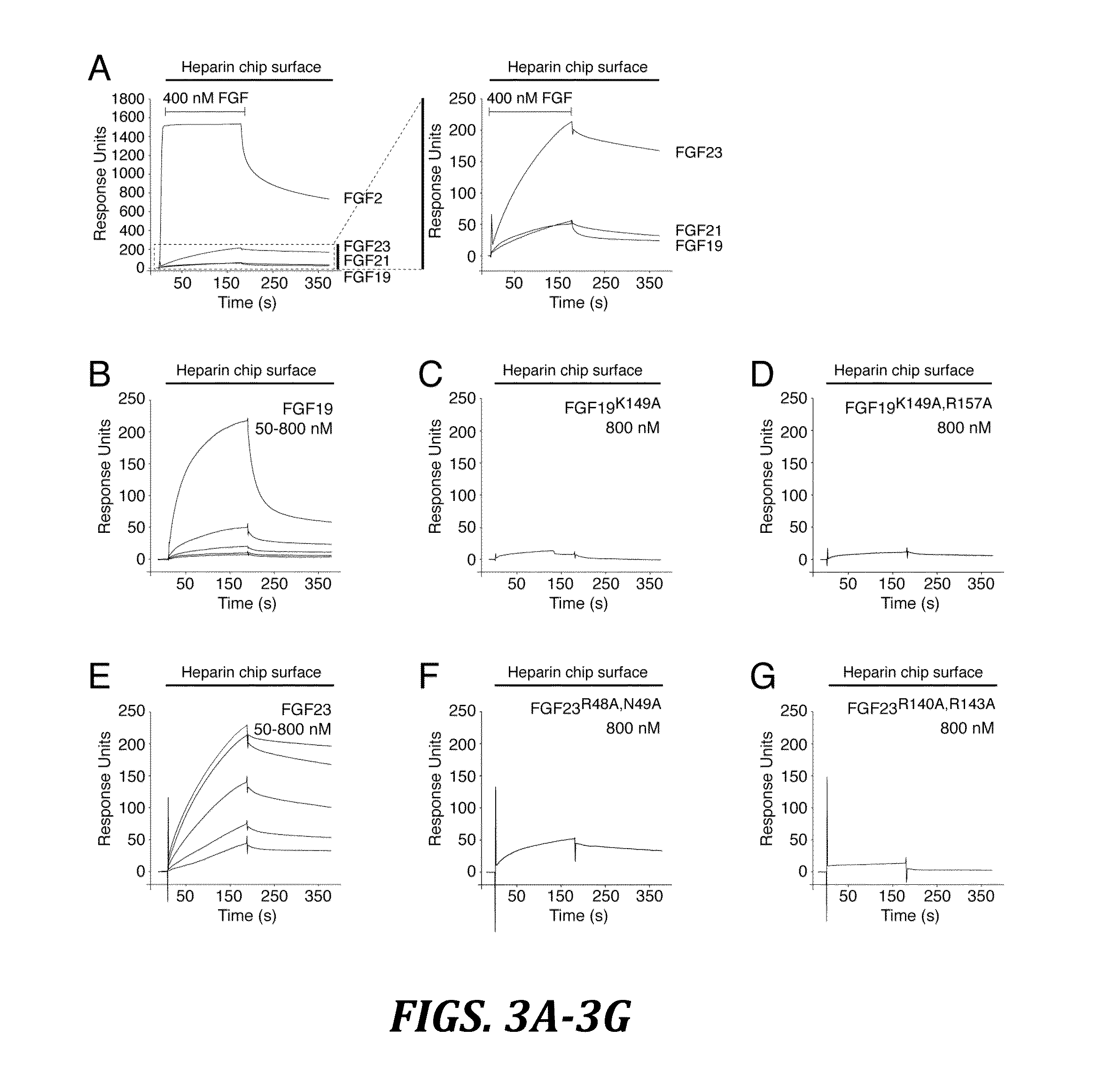
Fibroblast Growth Factor-23 molecules and uses thereof
InactiveUS20060160181A1Sugar derivativesPeptide/protein ingredientsFibroblast growth factor 23Biology
The present invention provides Fibroblast Growth Factor-23 (FGF-23) polypeptides and nucleic acid molecules encoding the same. The invention also provides selective binding agents, vectors, host cells, and methods for producing FGF-23 polypeptides. The invention further provides pharmaceutical compositions and methods for the diagnosis, treatment, amelioration, and / or prevention of diseases, disorders, and conditions associated with FGF-23 polypeptides.
Owner:AMGEN INC
Culturing human embryonic stem cells
Previous methods for culturing human embryonic stem cells have required either fibroblast feeder cells or a medium which has been exposed to fibroblast feeder cells in order to maintain the stem cells in an undifferentiated state. It has now been found that if high levels of fibroblast growth factor are used in a medium with gamma amino butyric acid, pipecholic acid, lithium and lipids, the stem cells will remain undifferentiated indefinitely through multiple passages, even without feeder cells or conditioned medium. A humanized matrix of human proteins can be used as a basement matrix to culture the cells. New lines of human embryonic stem cells made using these culture conditions, the medium and the matrix, will never have been exposed to animal cells, animal products, feeder cells or conditioned medium.
Owner:WISCONSIN ALUMNI RES FOUND
Method for in vitro amplifying, and in 3D solid culturing nerve stem
InactiveCN101092606AUniform penetrationIncrease the cultivation areaNervous system cellsCuticleCell growth
This invention relates to a method for amplifying neural stem cells in vitro by 3-dimensional culture. The method comprises: selecting microcarrier with 3-dimensional environment, pre-treating, coating the microcarrier with 40-60 ng / mL alkaline fibroblast growth factor, 40-60 ng / mL epidermal growth factor, and B27 DMEM / F12 neural stem cell serum-free culture medium, adding 1X105-1X106 neural stem cells into the culture bottle, taking out the microcarrier grown with neural stem cells, removing the microcarrier, and rinsing cells to obtain neural stem cells. The porous microcarrier can enlarge the culture area. The alkaline fibroblast growth factor and epidermal growth factor can promote cell multiple fission and improve cell microenvironment, which is advantageous for multiple fission of neural stem cells.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Muteins OF Fibroblast Growth Factor 21
ActiveUS20070299007A1Reduced O-glycosylationImprove drug stabilityPeptide/protein ingredientsMetabolism disorderYeastNucleic acid sequencing
The present invention relates to novel muteins of human fibroblast growth factor-21 with reduced susceptibility for proteolytic degradation when expressed in yeast. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, or metabolic syndrome. X-16816
Owner:ELI LILLY & CO
Muteins of fibroblast growth factor 21
The present invention relates to novel muteins of human fibroblast growth factor 21 with improved pharmaceutical properties. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, metabolic syndrome, and in reducing the mortality and morbidity of critically ill patients.
Owner:ELI LILLY & CO
Method and composition for restoration of age related tissue loss in the face or selected areas of the body
InactiveUS20060073178A1Restoring age related tissue lossEasy to produceCosmetic preparationsBiocideInsulin-like growth factorThyroid hormones
A treatment method for restoring of age related tissue loss in the face or selected areas of the body is disclosed which includes injecting an injectable composition containing a growth factor and hyaluronic acid as a carrier into the dermis, the hypodermis, or both, in various areas of the face, or selected areas of the body of a person to stimulate collagen, elastin, or fat cell production, thereby restoring age related tissue loss in the face and selected areas of the body. Further disclosed is an injectable composition for restoring of age related tissue loss in the face and selected areas of the body, which contains a growth factor and hyaluronic acid as a carrier for providing time release of the growth factor into tissues. The growth factor can be insulin, insulin-like growth factor, thyroid hormone, fibroblast growth factor, estrogen, retinoic acid, or their combinations.
Owner:CELLHEALTH TECH
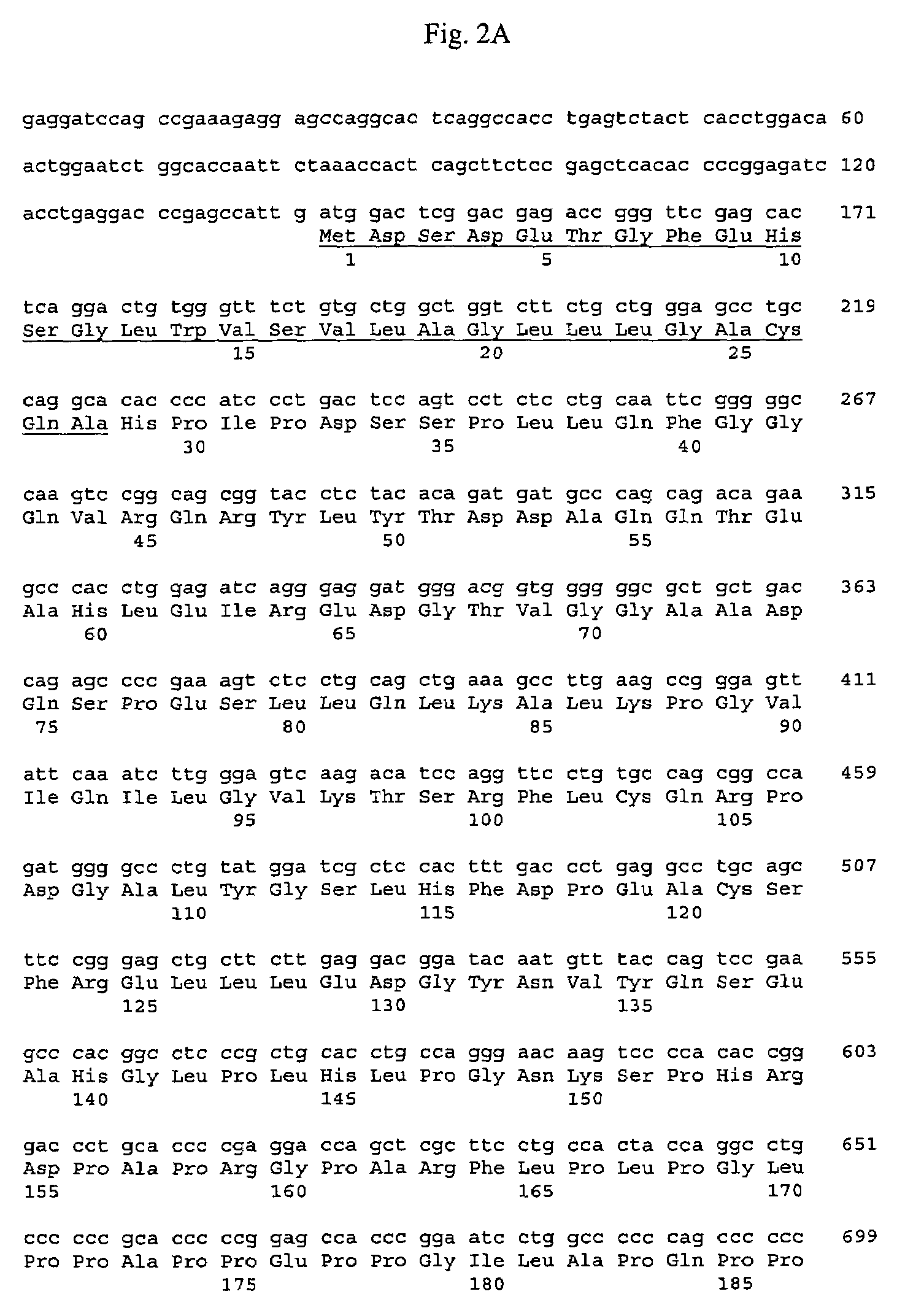
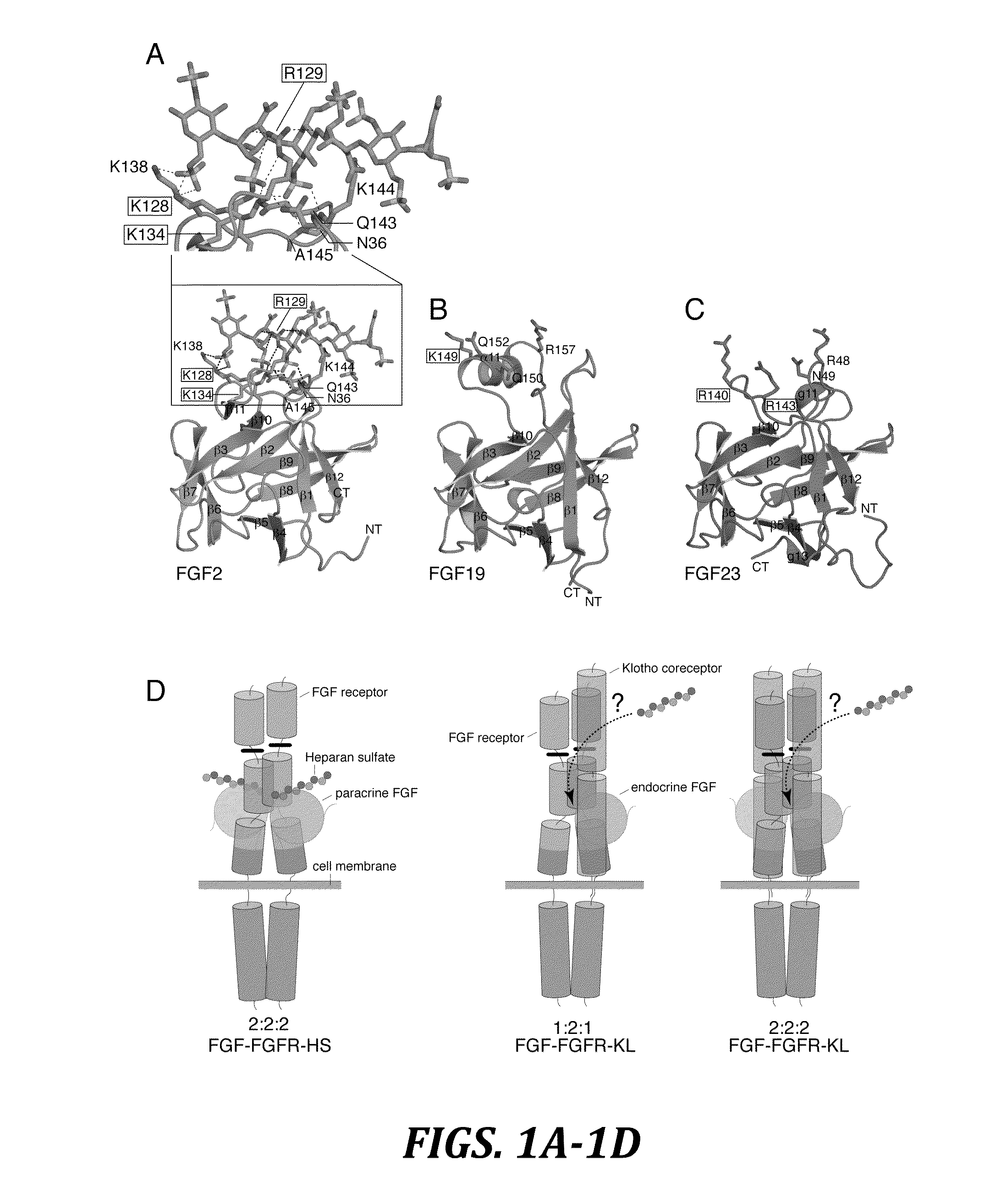
Chimeric fibroblast growth factor 23 proteins and methods of use
ActiveUS20140243260A1Peptide/protein ingredientsAntibody mimetics/scaffoldsChimera ProteinFibroblast growth factor 23
The present invention relates to an isolated chimeric protein. The isolated chimeric protein includes an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion from a fibroblast growth factor (“FGF”) 23 molecule and the C-terminus includes a C-terminal portion from an FGF19 molecule. The present invention also relates to a pharmaceutical composition including an isolated chimeric protein and a pharmaceutically acceptable carrier. The isolated chimeric protein includes an N-terminus coupled to a C-terminus, where the N-terminus includes an N-terminal portion from a fibroblast growth factor (“FGF”) 23 molecule and the C-terminus includes a C-terminal portion from an FGF19 molecule, and a pharmaceutically-acceptable carrier. Yet another aspect of the present invention relates to a method for treating a subject suffering from a disorder. This method includes selecting a subject suffering from the disorder and administering to the subject a therapeutically effective amount of a chimeric protein according to the present invention.
Owner:NEW YORK UNIV
Muteins of fibroblast growth factor 21
InactiveUS20090118190A1Antibacterial agentsPeptide/protein ingredientsCritically illNucleic acid sequencing
The present invention relates to novel muteins of human fibroblast growth factor 21 with improved pharmaceutical properties. Both protein and the respective encoding nucleic acid species are disclosed. The invention also embodies vectors and host cells for the propagation of said nucleic acid sequences and the production of said muteins. Also disclosed are methods for treating type 2 diabetes, obesity, metabolic syndrome, and in reducing the mortality and morbidity of critically ill patients.
Owner:BEALS JOHN MICHAEL +7
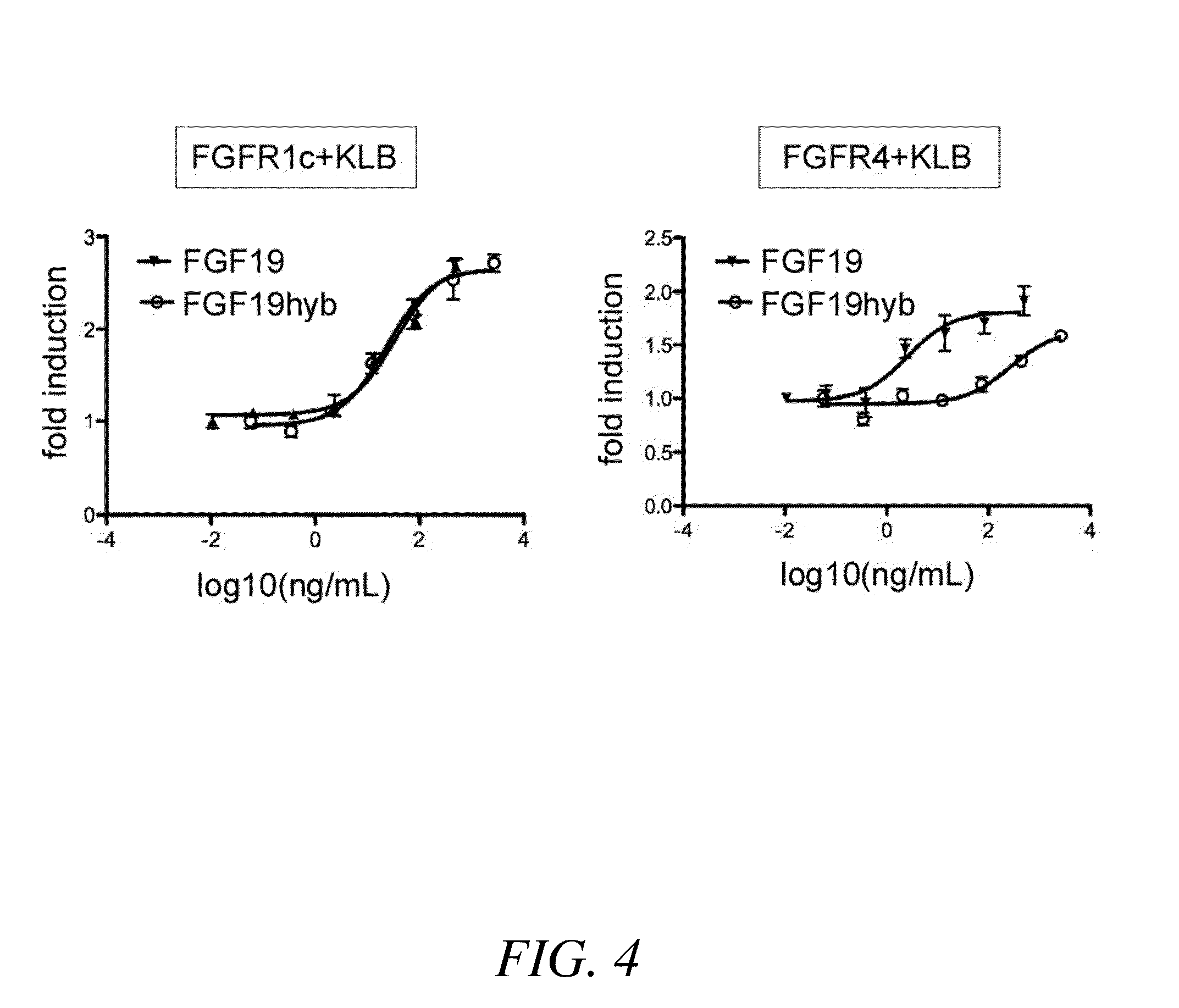
Chimeric fibroblast growth factor 19 proteins and methods of use
ActiveUS20130331317A1Enhanced endocrine activityLow affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsKlothoHeparin-DHE
The present invention relates to a chimeric protein that includes an N-terminus coupled to a C-terminus, where the N-terminus includes a portion of a paracrine fibroblast growth factor (“FGF”) and the C-terminus includes a C-terminal portion of an FGF19 molecule. The portion of the paracrine FGF is modified to decrease binding affinity for heparin and / or heparan sulfate compared to the portion without the modification. The present invention also relates to pharmaceutical compositions including chimeric proteins according to the present invention, methods for treating a subject suffering from diabetes, obesity, or metabolic syndrome, and methods of screening for compounds with enhanced binding affinity for the βKlotho-FGF receptor complex involving the use of chimeric proteins of the present invention.
Owner:SALK INST FOR BIOLOGICAL STUDIES +2
Differentiation modulating agents and uses therefor
InactiveUS20120059047A1Reduce expressionIncrease gene expressionOrganic active ingredientsPeptide/protein ingredientsSignalling pathwaysObesity
The present invention is directed to methods and agents for modulating the differentiation potential and / or proliferation of preadipocytes. More particularly, the present invention discloses methods and agents for modulating a fibroblast growth factor (FGF) signaling pathway, especially the FGF-1 or FGF-2 signaling pathway, for treating or preventing adiposity-related conditions including, but not limited to, obesity, lipoma, lipomatosis, cachexia or lipodystrophy or the loss of adipose tissue in trauma or atrophic conditions.
Owner:VERVA PHARMA
Graft materials containing ECM components, and methods for their manufacture
ActiveUS20080107750A1Peptide/protein ingredientsMammal material medical ingredientsCell-Extracellular MatrixECM Protein
Described are packaged, sterile medical graft products containing controlled levels of a growth factor such as Fibroblast Growth Factor-2 (FGF-2). Also described are methods of manufacturing medical graft products wherein processing, including sterilization, is controlled and monitored to provide medical graft products having modulated, known levels of a extracellular matrix factor, such as a growth factor, e.g. FGF-2. Preferred graft materials are extracellular matrix materials isolated from human or animal donors, particularly submucosa-containing extracellular matrix materials. Further described are ECM compositions that are or are useful for preparing gels, and related methods for preparation and use.
Owner:COOK BIOTECH +1
FGF variants and methods for use thereof
ActiveUS7563769B2High activityHigh affinityPeptide/protein ingredientsSkeletal disorderDiseaseReceptor subtype
The present invention provides fibroblast growth factor variants demonstrating enhanced receptor subtype specificity and / or affinity. Preferred embodiments include both variants having enhanced activity that act as improved agonists and variants having reduced activity that act as antagonists. Methods of utilizing preferred FGF variants in preparation of medicaments for the treatment of skeletal disorders including skeletal dysplasia, osteoporosis and enhancing bone fracture healing and cartilage healing processes are provided.
Owner:PROCHON BIOTECH
Cultivation of human embryonic stem cells in the absence of feeder cells or without conditioned medium
InactiveUS7439064B2Maintain normalMaintaining the karyotype of the stem cellsCulture processArtificial cell constructsFeeder LayerStem cell culture
The invention relates to methods for culturing human embryonic stem cells by culturing the stem cells in an environment essentially free of mammalian fetal serum and in a stem cell culture medium including amino acids, vitamins, salts, minerals, transferring, insulin, albumin, and a fibroblast growth factor that is supplied from a source other than just a feeder layer the medium. Also disclosed are compositions capable of supporting the culture and proliferation of human embryonic stem cells without the need for feeder cells or for exposure of the medium to feeder cells.
Owner:WICELL RES INST
Complete medium with low serum concentration for cultivating mesenchymal stem cells and method for cultivating mesenchymal stem cells using same
The invention discloses a complete medium with low serum concentration for cultivating mesenchymal stem cells and a method for cultivating the mesenchymal stem cells using same. The complete medium comprises a cell basic medium, fetal calf serum with final concentration of 1-100 mul / ml, an epidermal growth factor with final concentration of 1-100 ng / ml, and a basic fibroblast growth factor with final concentration of 1-100 ng / ml. The complete medium with low serum concentration successfully reaches equal or even better function of promoting cell proliferation than a culture reagent with high serum concentration. The cultured cells have the typical biological characteristics of mesenchymal stem cells, and can also express an omnipotent mark of the embryonic stem cell and high express the idiosyncratic mark of the neuron under the condition of in vitro inducement. And the difference between the cell batches is little, the cost is low and the security is good. Compared with the prior cultivating method, the method has advantages of simple operation, low probability of pollution and high success ratio of cultivating cells.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Chimeric fibroblast growth factors with altered receptor specificity
The present invention is directed to novel chimeric fibroblast growth factor (FGF) polypeptides, novel DNA encoding chimeric FGF polypeptides, and to the recombinant production of chimeric FGF polypeptides, and to methods, compositions and assays utilizing chimeric FGF polypeptides for the therapeutic treatment of metabolic-related disorders and other conditions, and for producing pharmaceutically active compositions including chimeric FGF polypeptides, the compositions having therapeutic and pharmacologic properties including those associated with the treatment of metabolic-related disorders and other conditions.
Owner:F HOFFMANN LA ROCHE & CO AG
Methods of treating fgf21-associated disorders
InactiveUS20120129766A1Good biological propertiesPeptide/protein ingredientsAntibody mimetics/scaffoldsFibroblast growth factorFGF21
The invention relates to the identification of new polypeptide and protein variants of fibroblast growth factor 21 (FGF21) that have improved pharmaceutical properties. Also disclosed are methods for treating FGF21-associated disorders, including metabolic conditions.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com