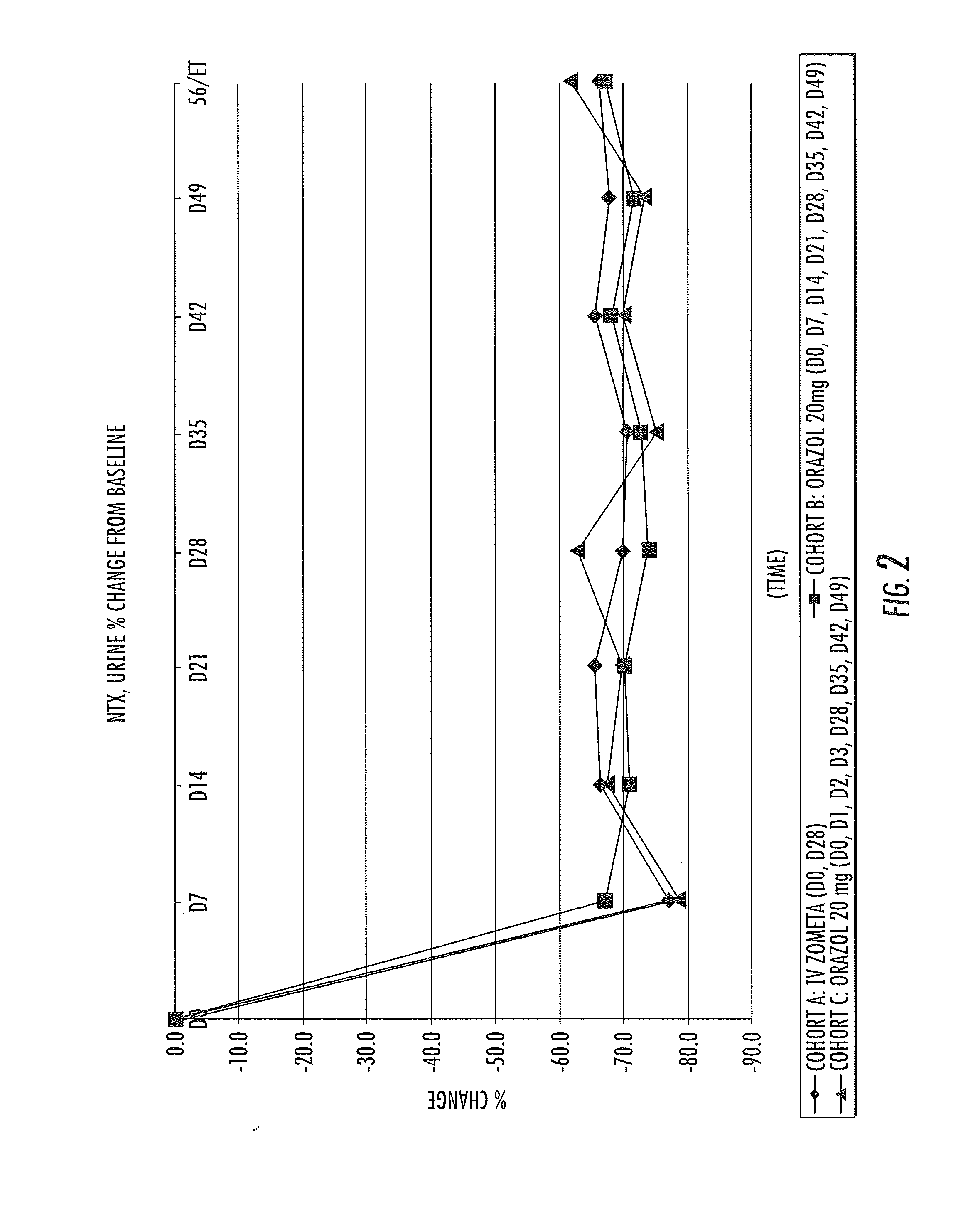
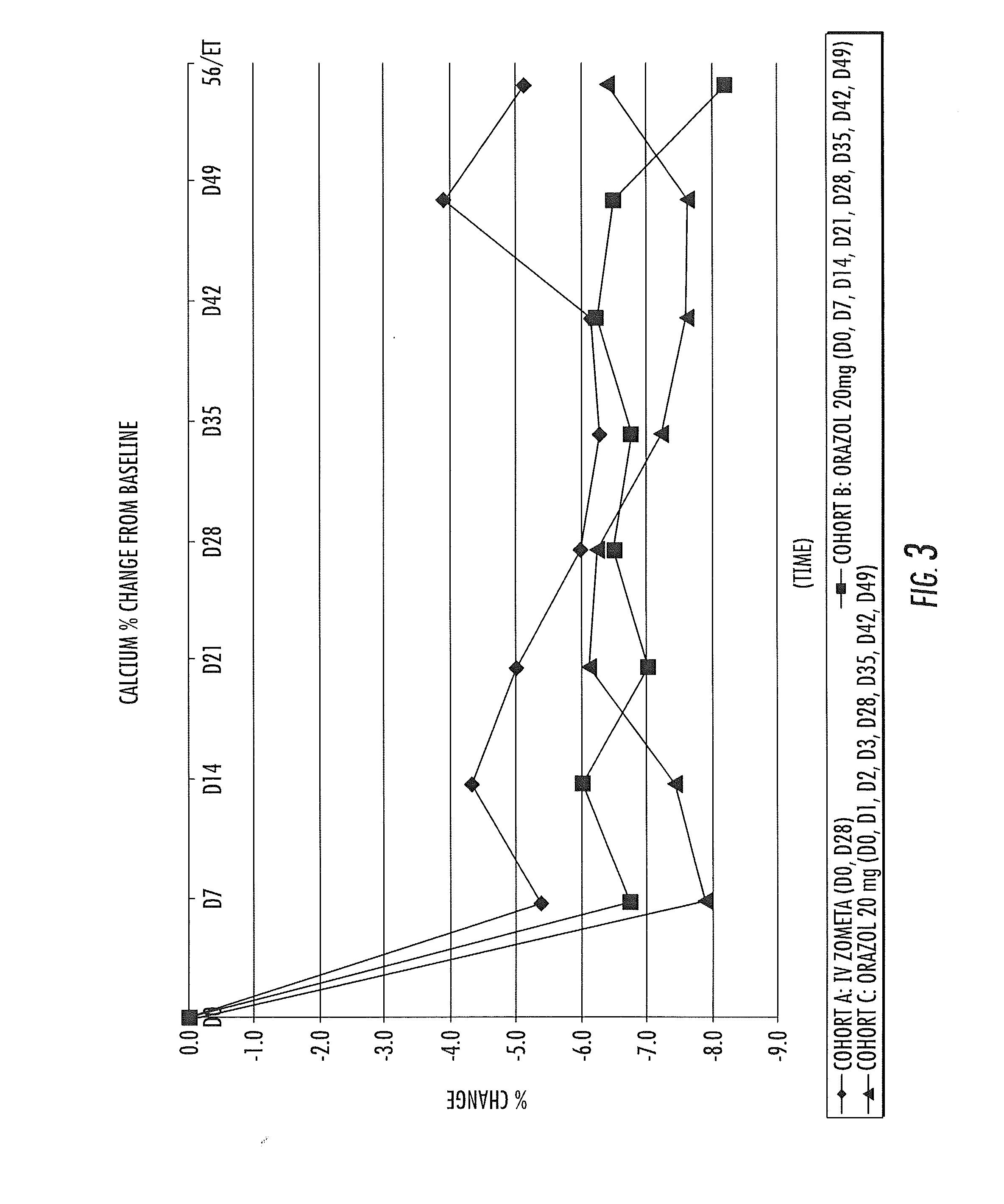
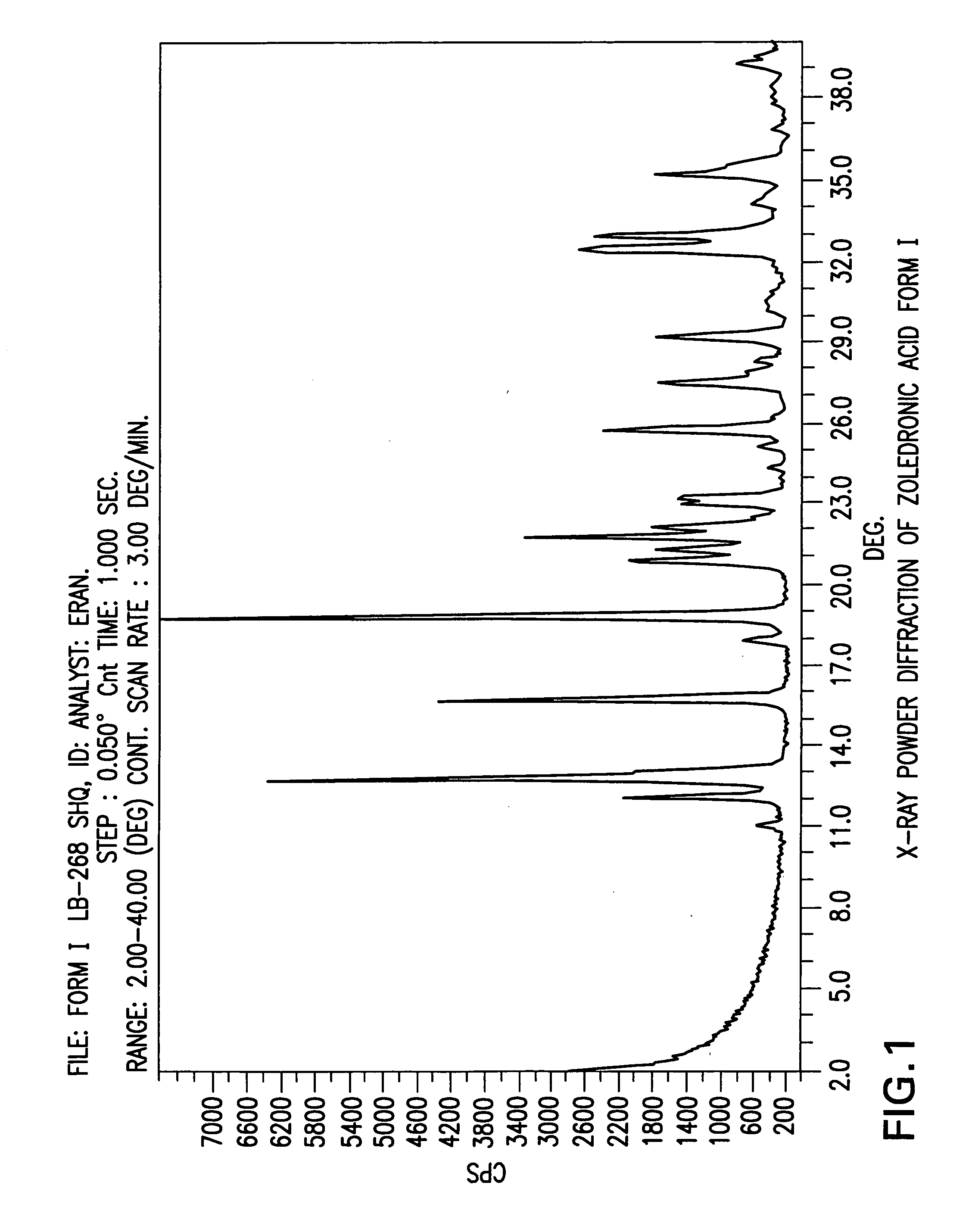
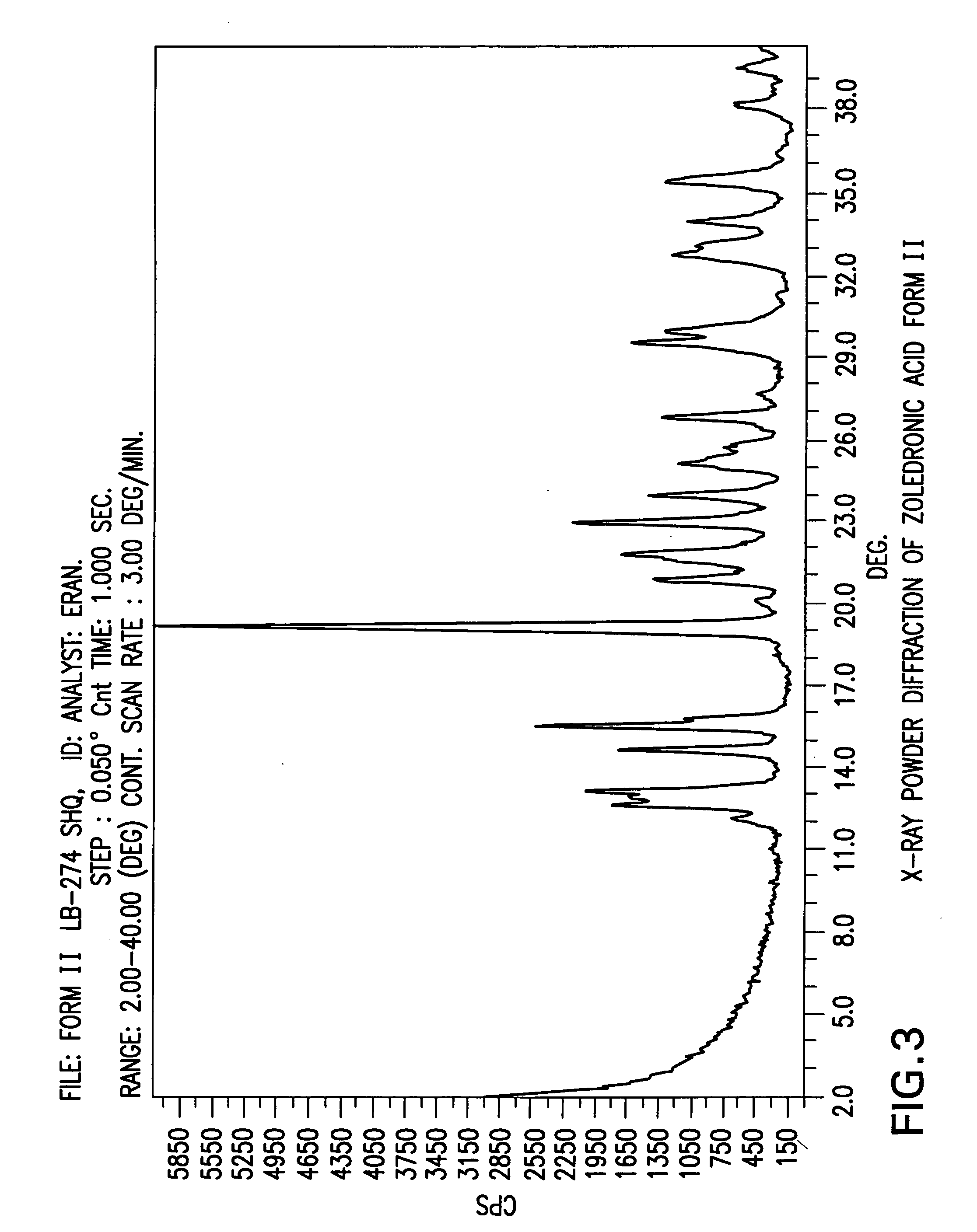
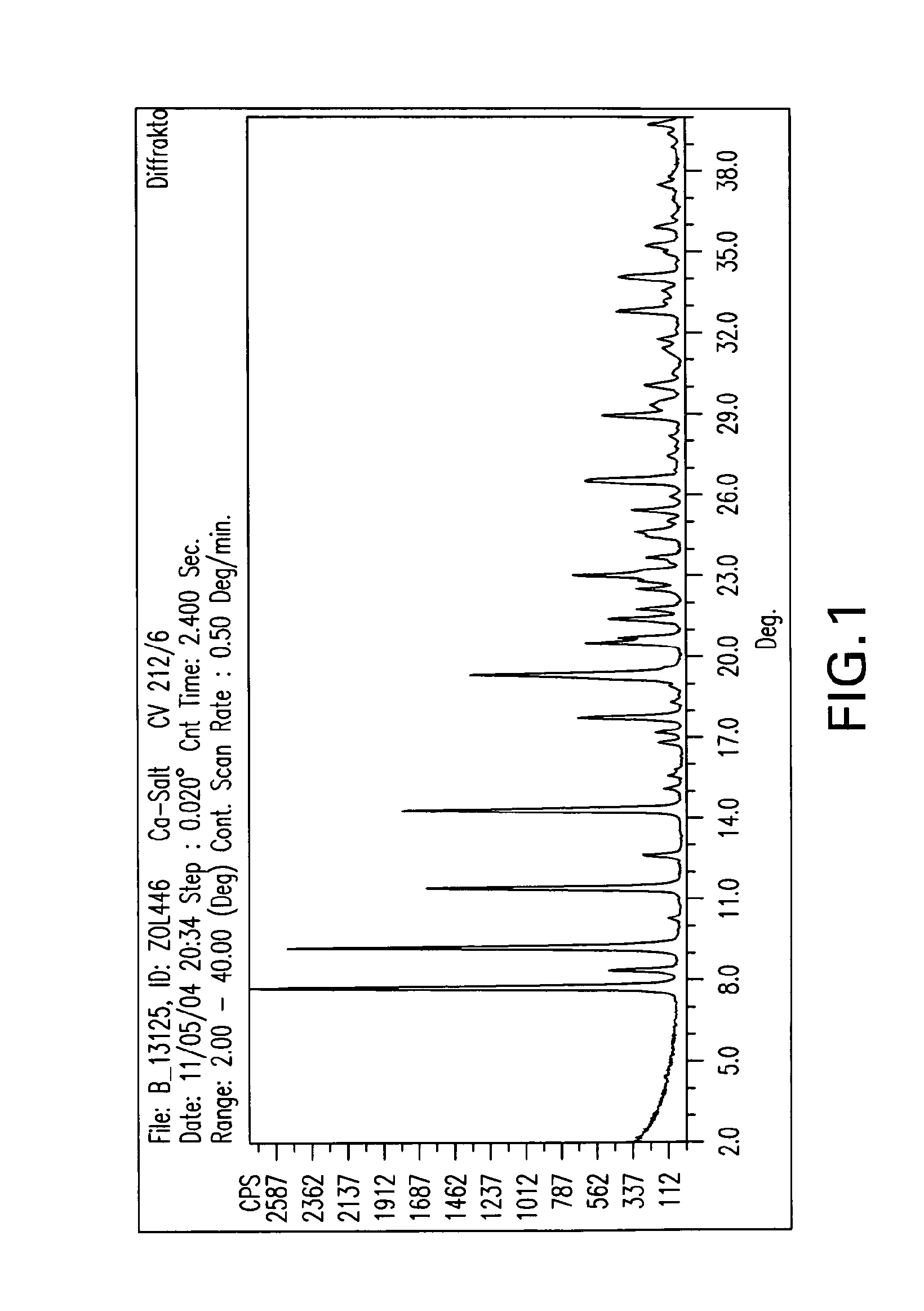
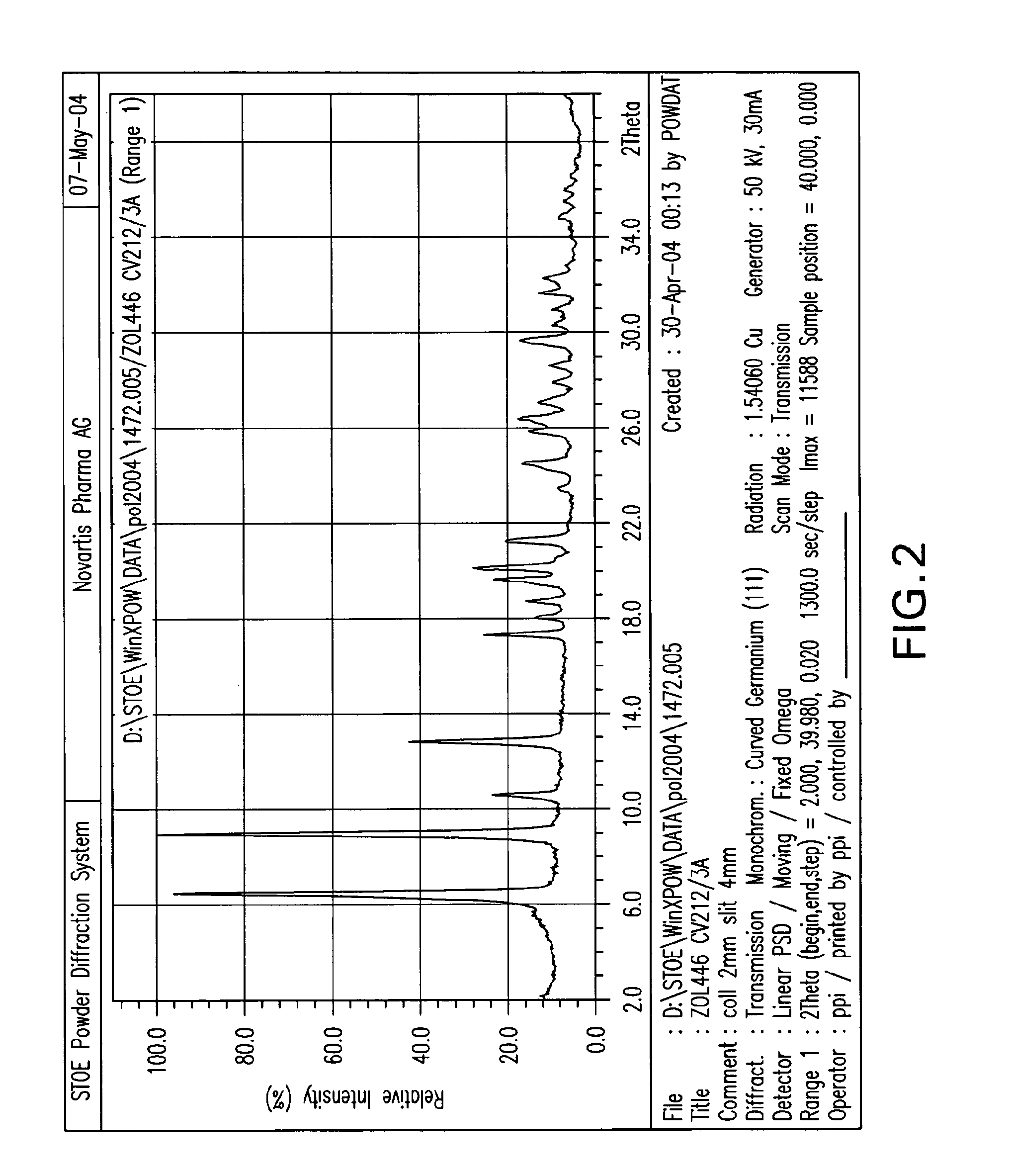
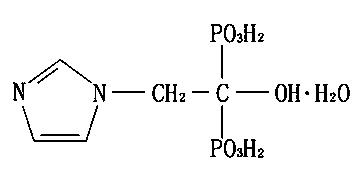
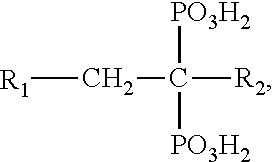Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
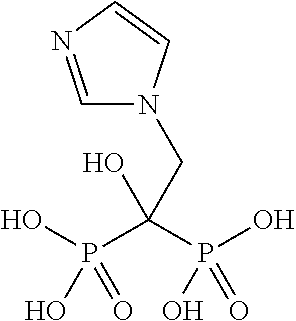
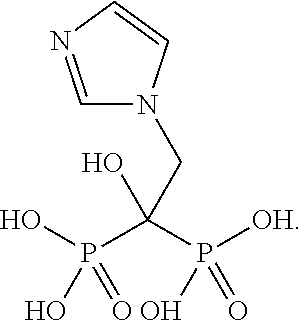
127 results about "Zoledronic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat high blood calcium levels (hypercalcemia) that may occur with cancer. Zoledronic acid is also used with cancer chemotherapy to treat bone problems that may occur with multiple myeloma and other types of cancer (such as breast, lung) that have spread to the bones.
Composition and drug delivery of bisphosphonates
InactiveUS20100215743A1Reduce adverse effectsBiocideOrganic active ingredientsMedicineDiphosphonates
The present invention provides methods of treating or preventing a medical condition that is responsive to a bisphosphonate compound in a subject. The methods comprise administering to the subject a pharmaceutical composition comprising a therapeutically effective amount of the bisphosphonate no less frequently than a bi-weekly dosage schedule. In some embodiment, the bisphosphonate compound is zoledronic acid.
Owner:NOVO NORDISK AS
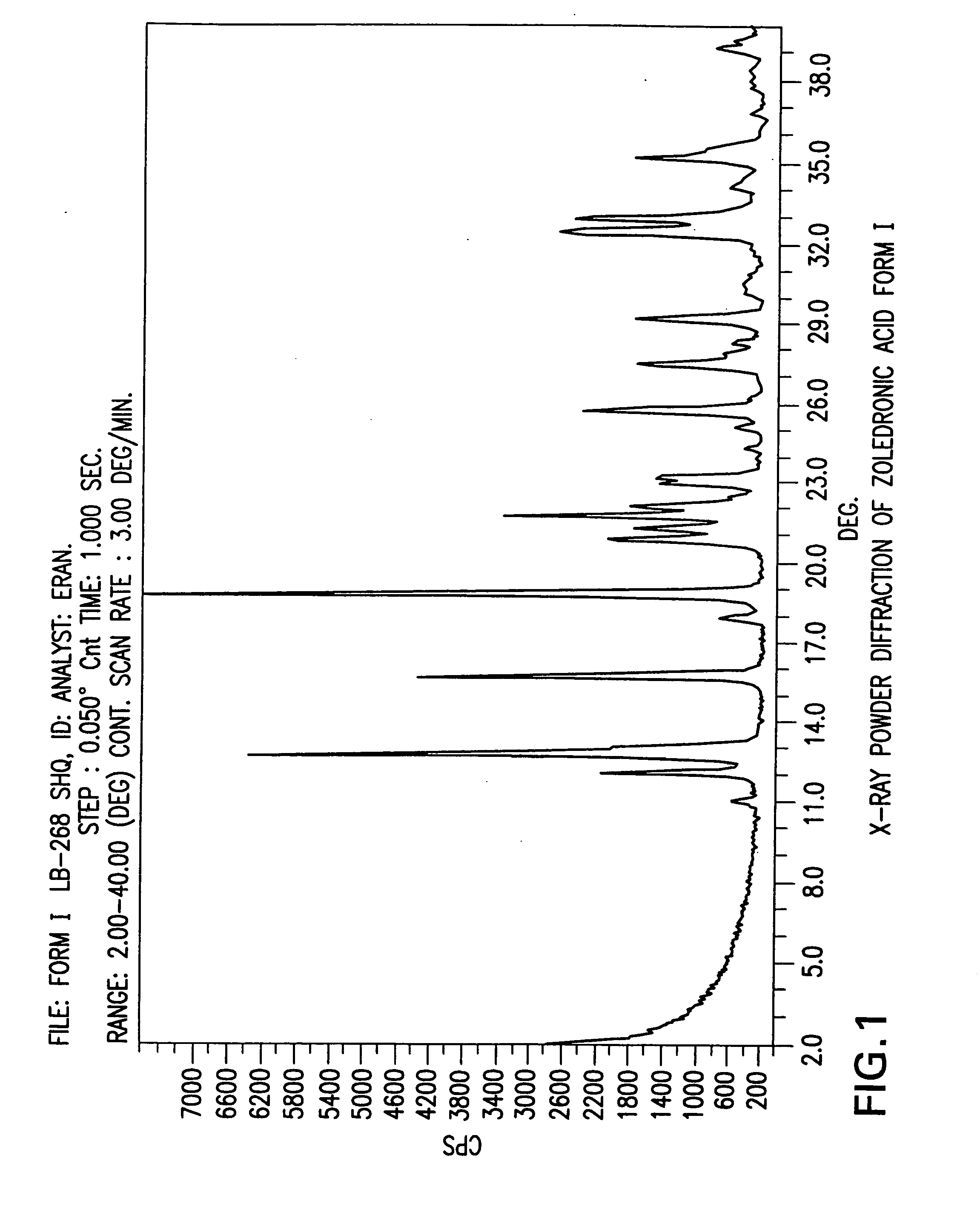
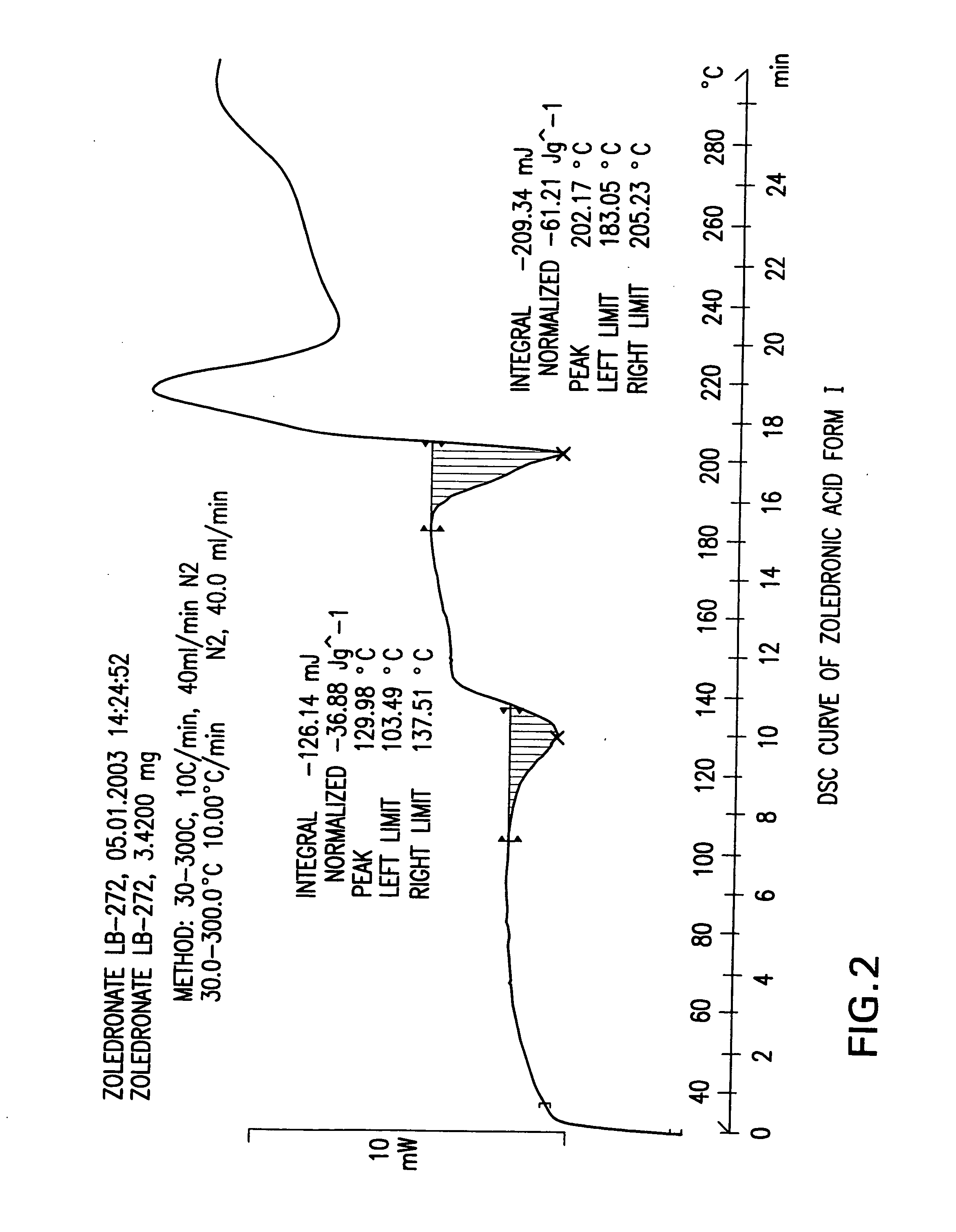
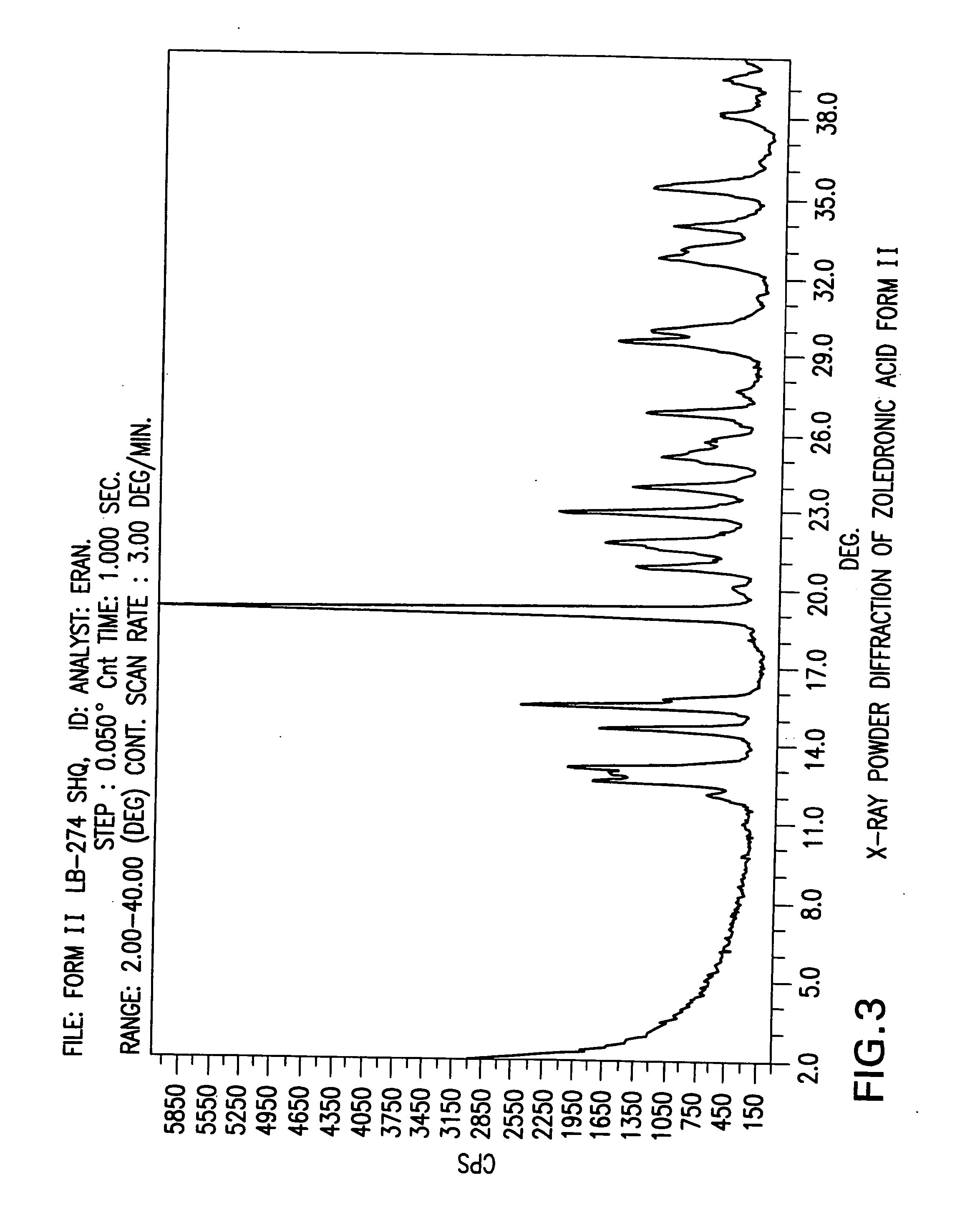
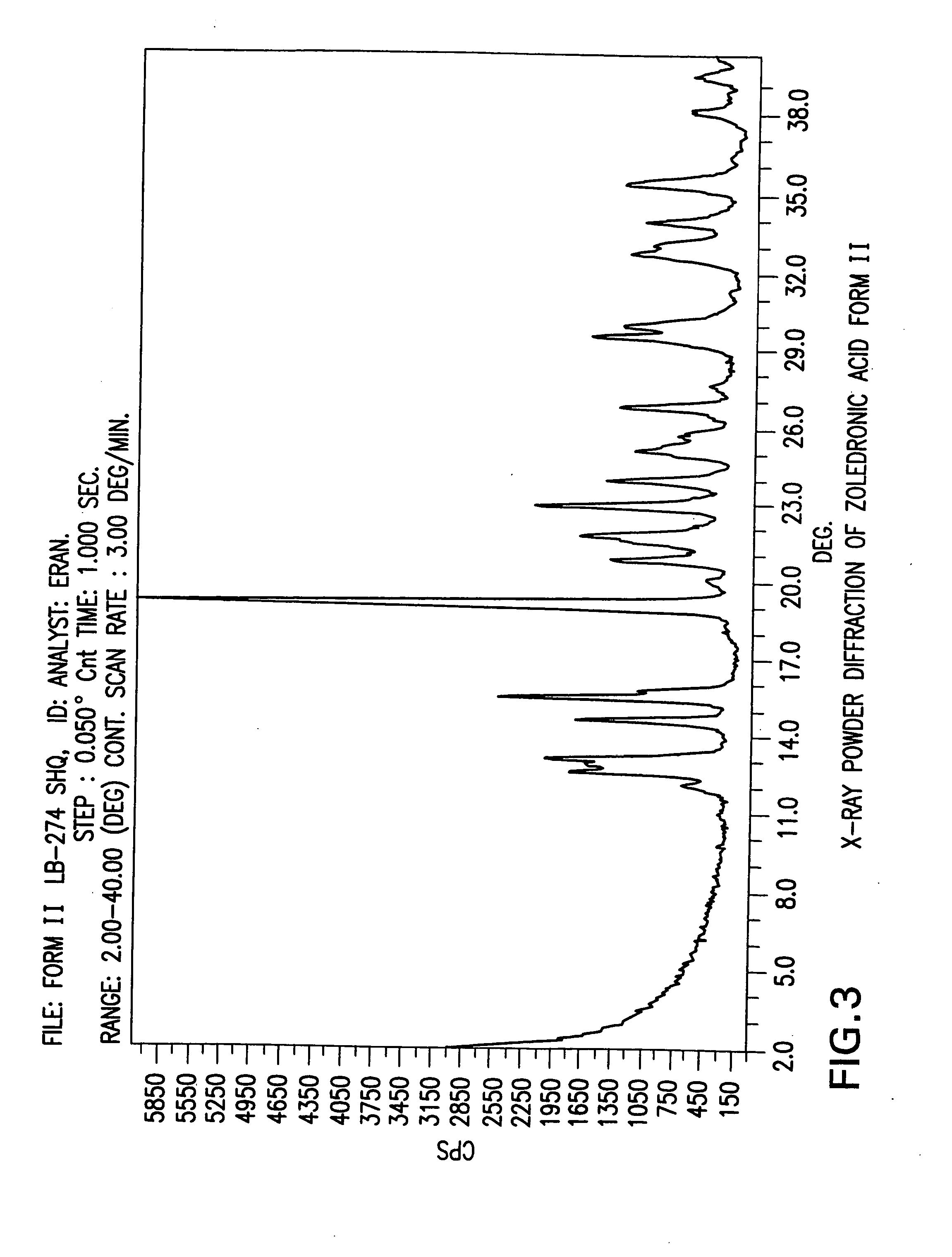
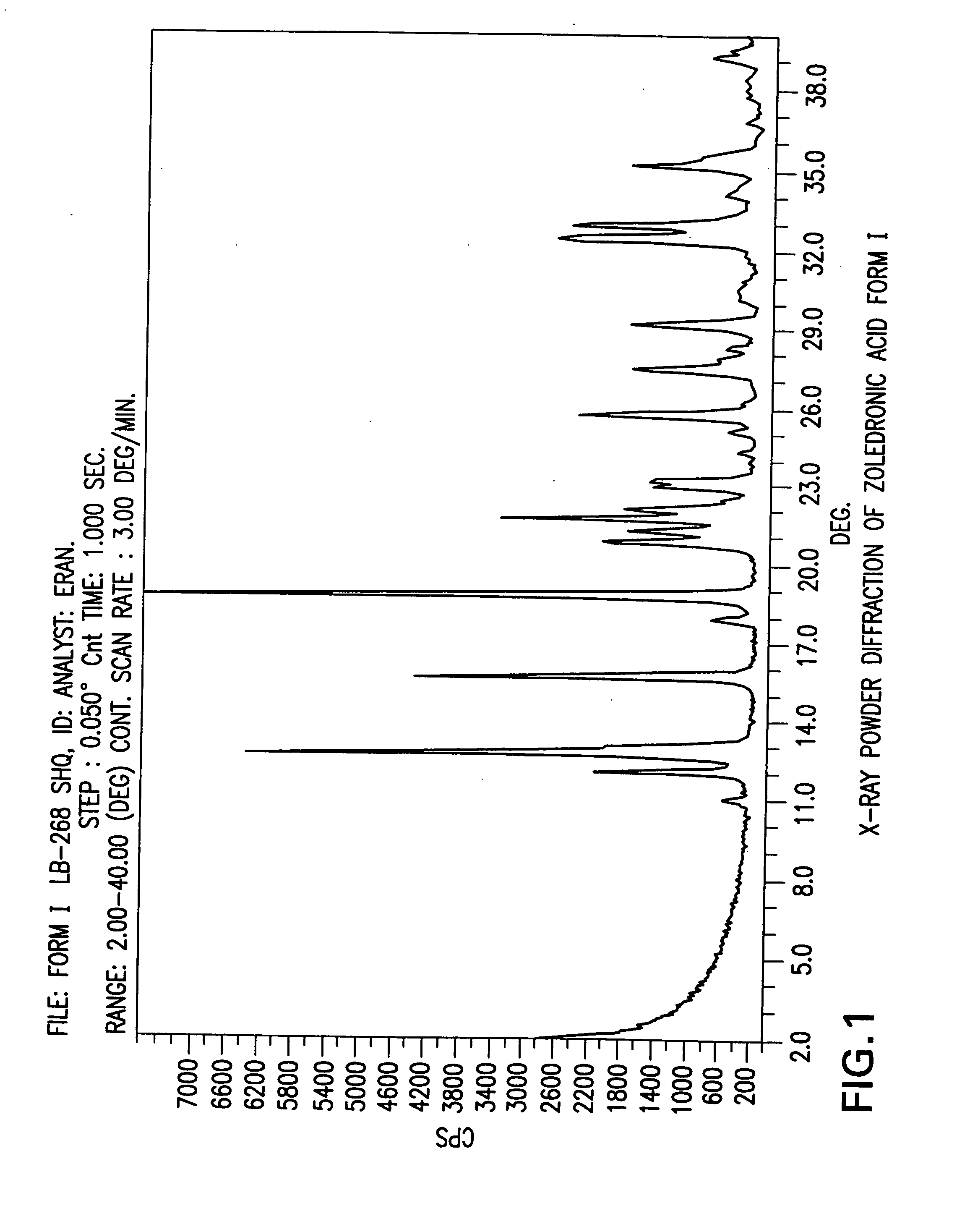
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
InactiveUS20050054616A1BiocideOrganic active ingredientsPharmaceutical SubstancesMedicinal chemistry
The invention relates to polymorphs of zoledronic acid and zoledronate sodium salts, amorphous zoledronate sodium salt, processes for making the polymorphs and amorphous zoledronate sodium salt and pharmaceutical compositions containing the polymorphs and amorphous zoledronate sodium salt.
Owner:TEVA PHARM USA INC
Novel oral forms of a phosphonic acid derivative
InactiveUS20120190647A1Improved aqueous solubilityIncrease ratingsBiocideOrganic active ingredientsEnprofyllineArginine
Novel solution complexes of zoledronic acid are described which give rise to improved properties of zoledronic acid. The invention includes aqueous solution and molecular complexes of zoledronic acid with and optical isomers of asparagine, histidine, arginine and proline as well as pharmaceutical complexes containing them and methods of treatment using them.
Owner:THAR PHARMA
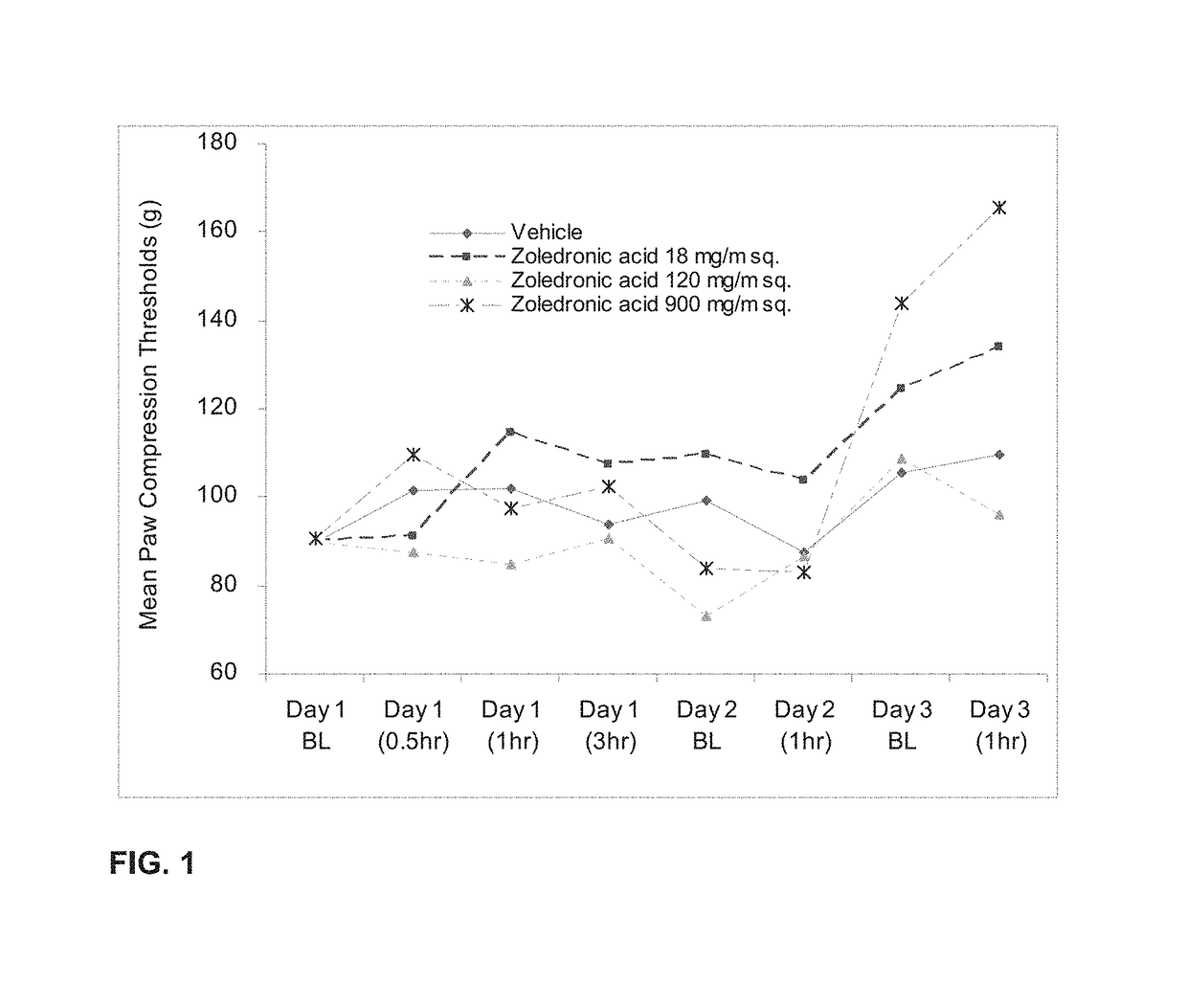
Co-administration of steroids and zoledronic acid to prevent and treat osteoarthritis
ActiveUS8859530B2Reduce productionAvoid seizuresBiocidePhosphorous compound active ingredientsCo administrationCombined Modality Therapy
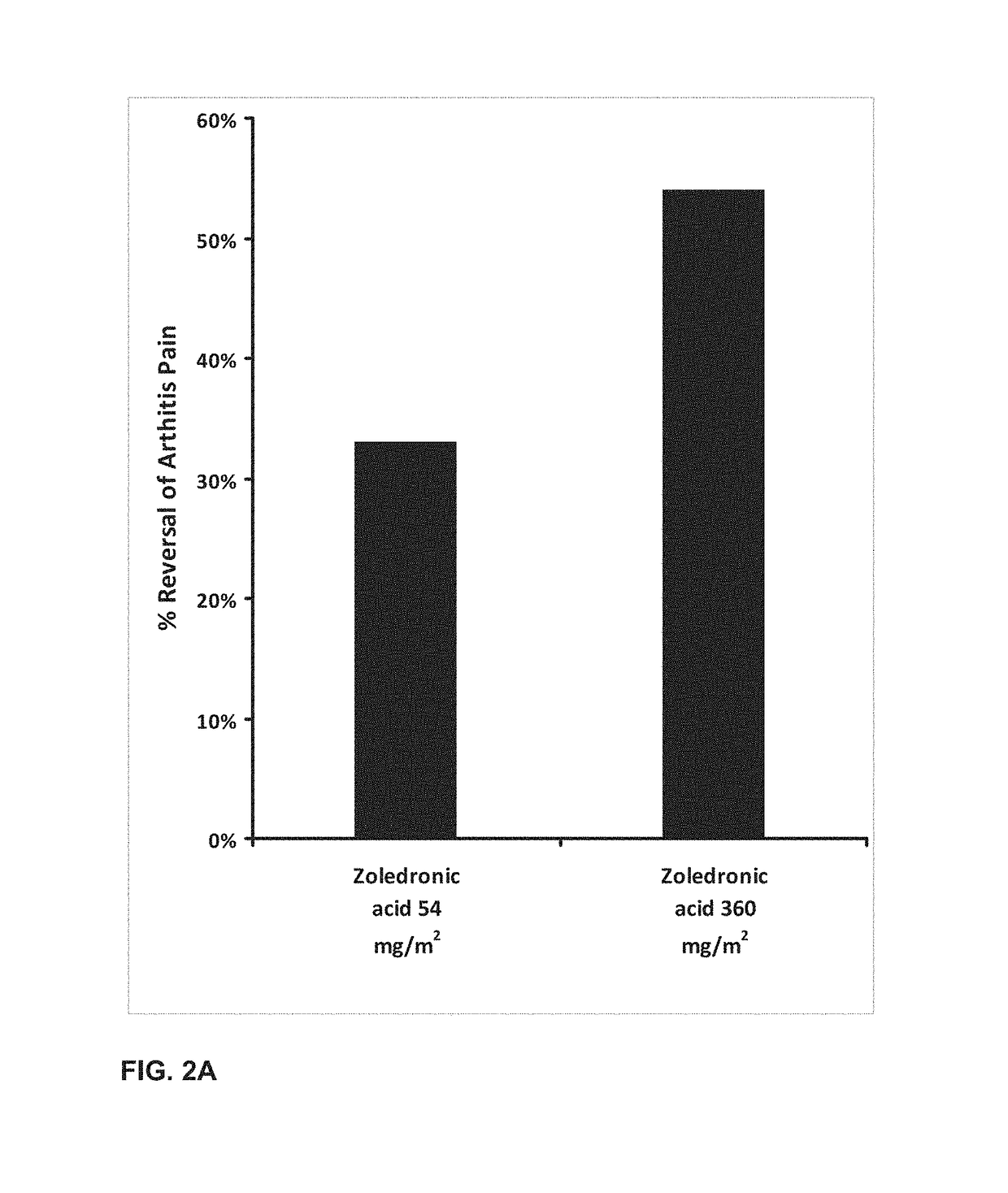
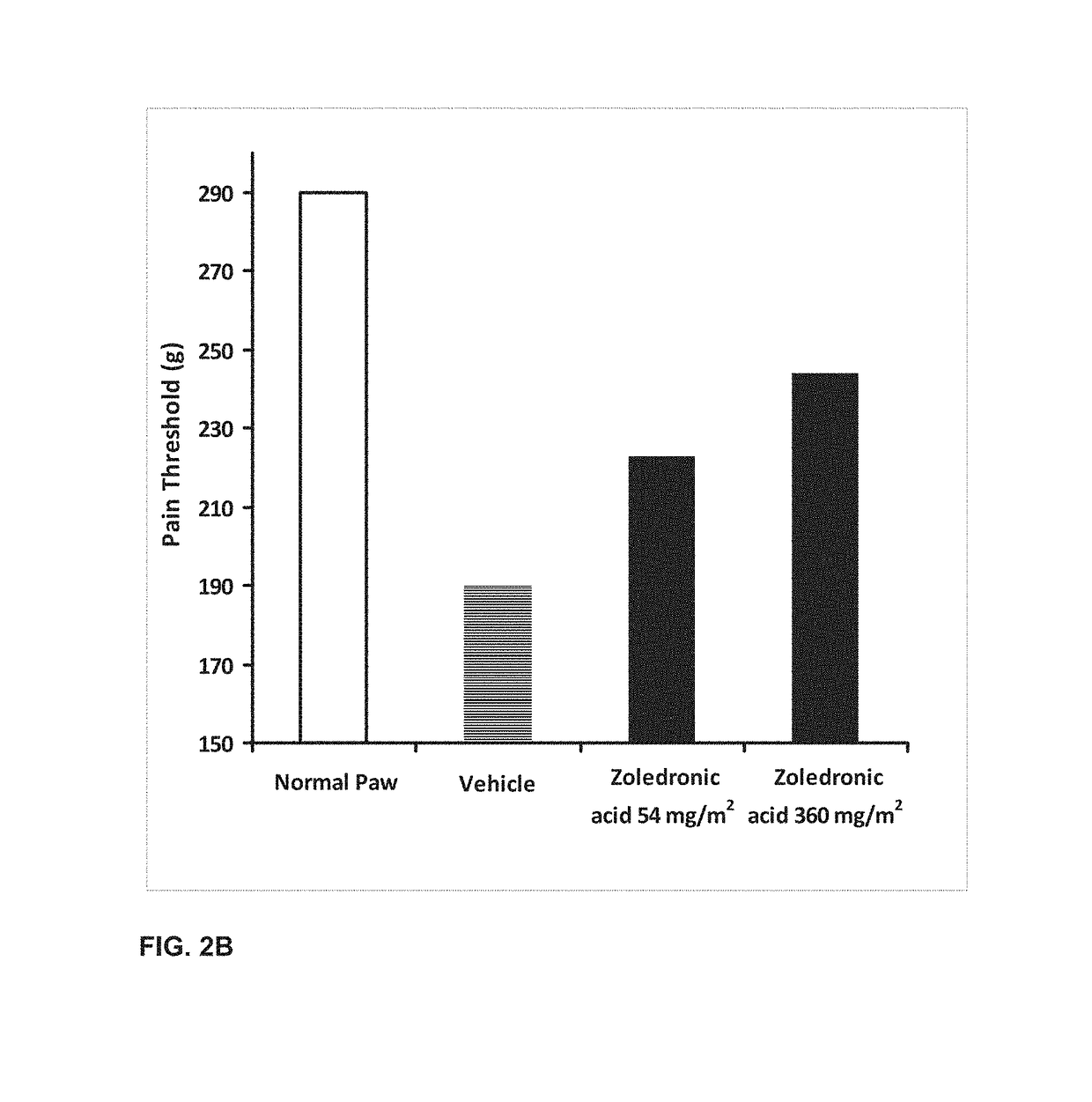
A combination therapy for treating osteoarthritis is disclosed. The combination therapy includes the co-administration of a steroid and Zoledronic Acid. The coadministration of a steroid decreases the production of cytokines, and, therefore, decreases the pro-inflammatory effects of Zoledronic Acid. The co-administration of Zoledronic Acid with steroids treats osteoarthritis, and helps to prevent the onset of osteoarthritis in patients at risk for osteoarthritis.
Owner:LEVOLTA PHARMA
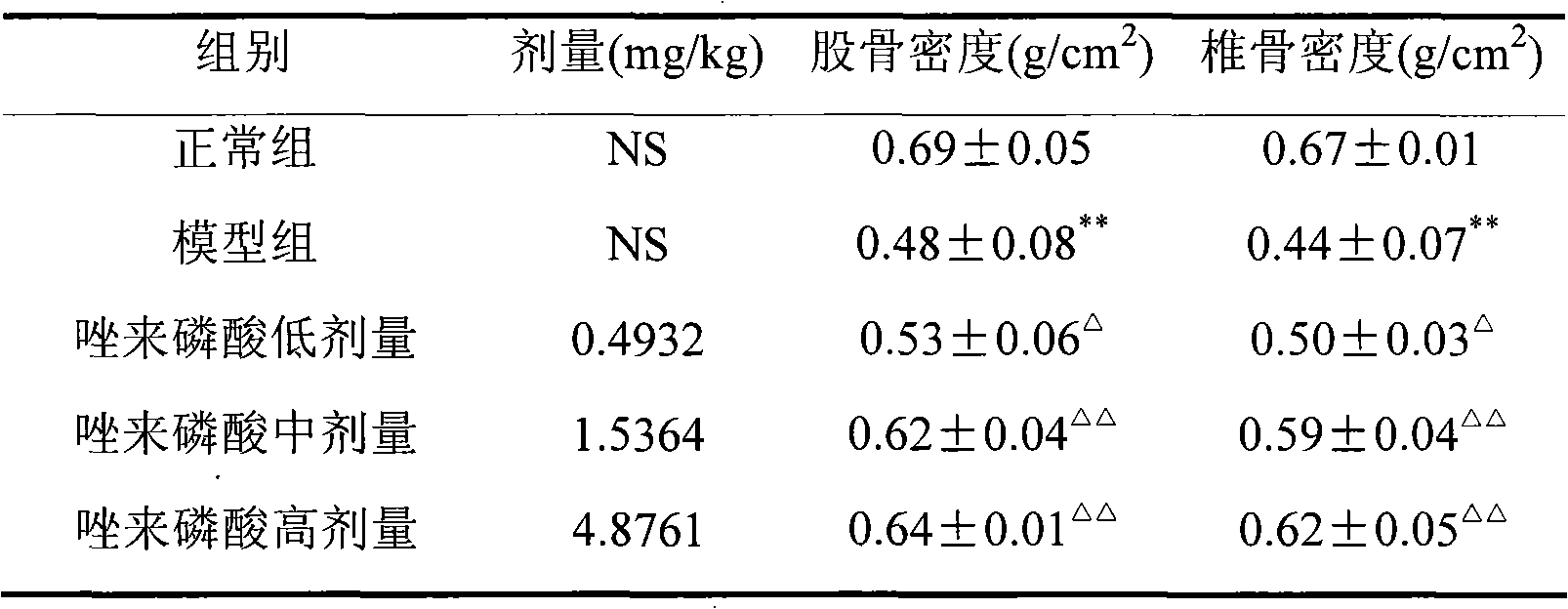
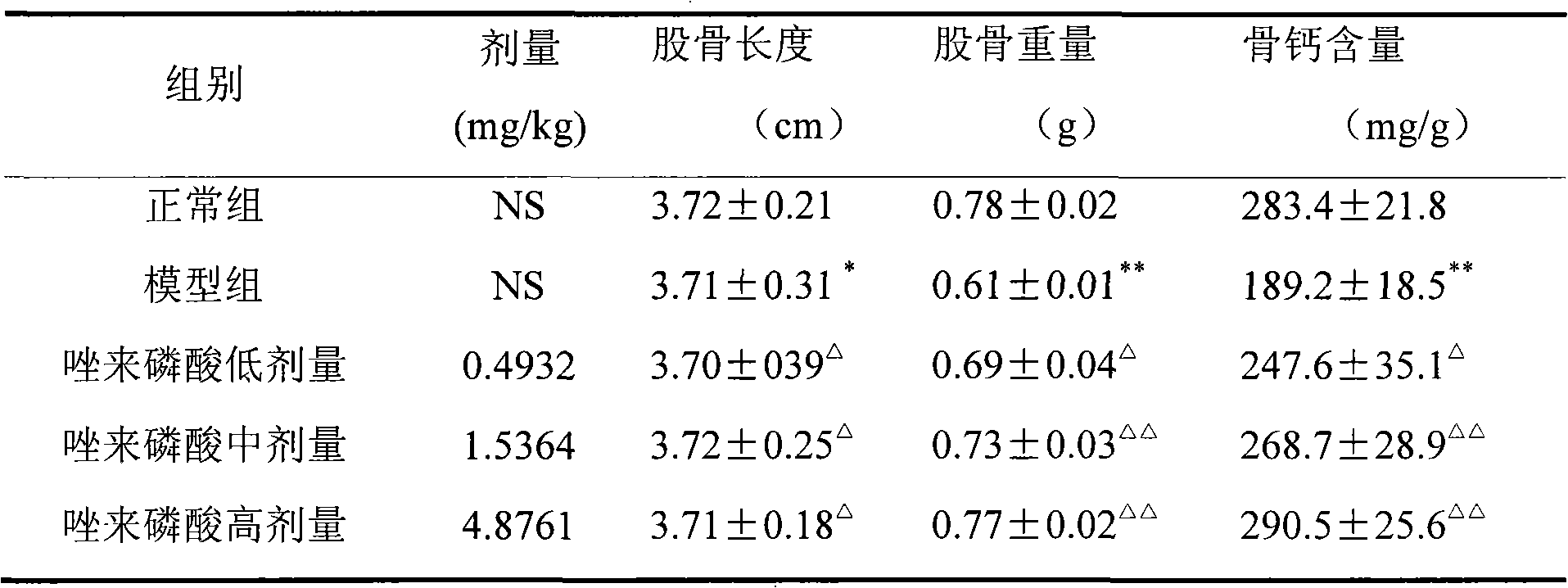
Zoledronic acid for preparing medicaments for preventing and treating osteoporosis
The invention discloses applications of zoledronic acid in preventing osteoporosis; the zoopery is providence that zoledronic acid has good preventing effect and therapeutical action to osteoporosis; zoledronic acid can be prepared into any clinically acceptable oral preparations such as tablets, capsules, granules, suspensions, dry suspensions and oral liquid, etc. so as to clinically prevent and treat osteoporosis.
Owner:王洪 +1
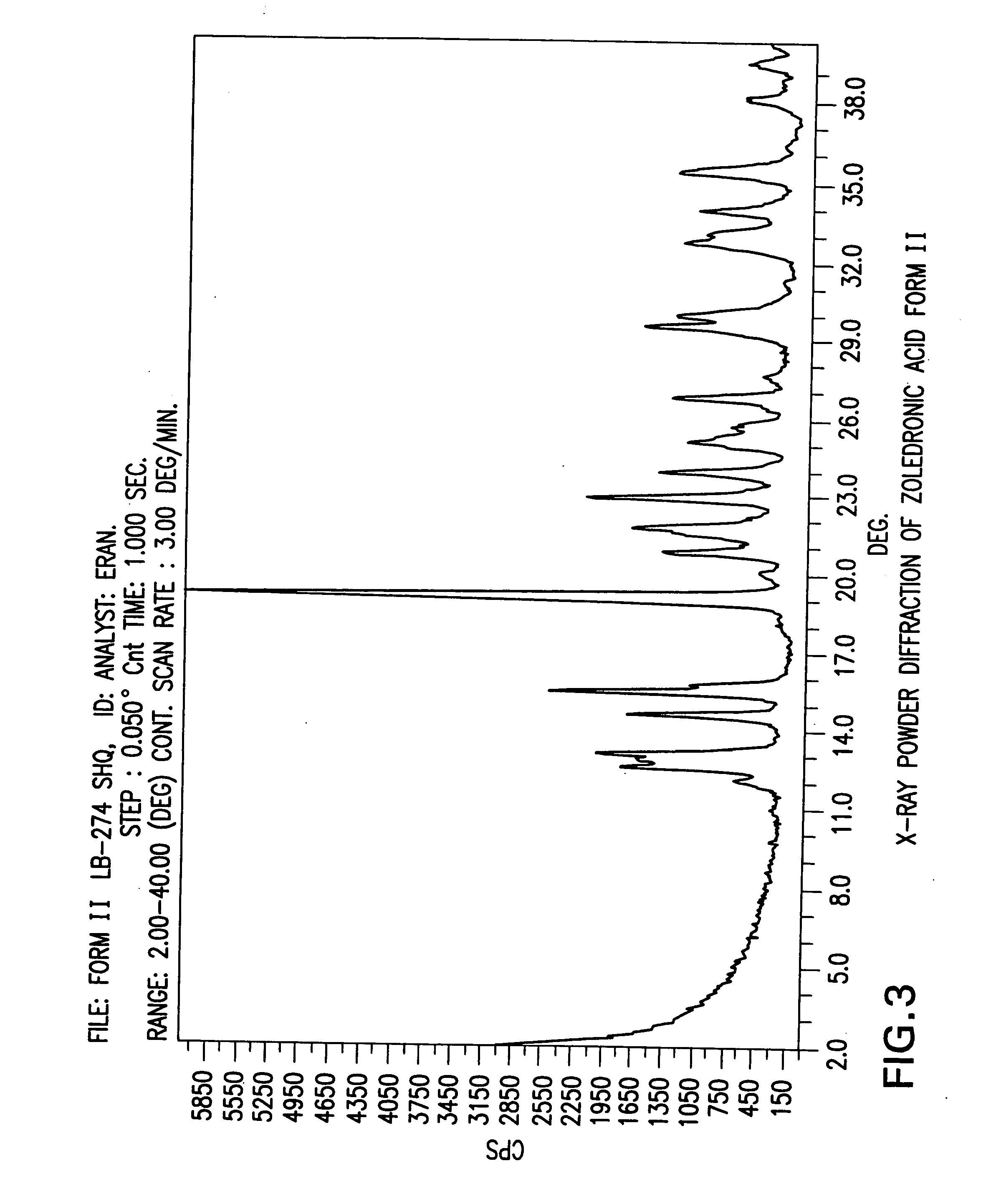
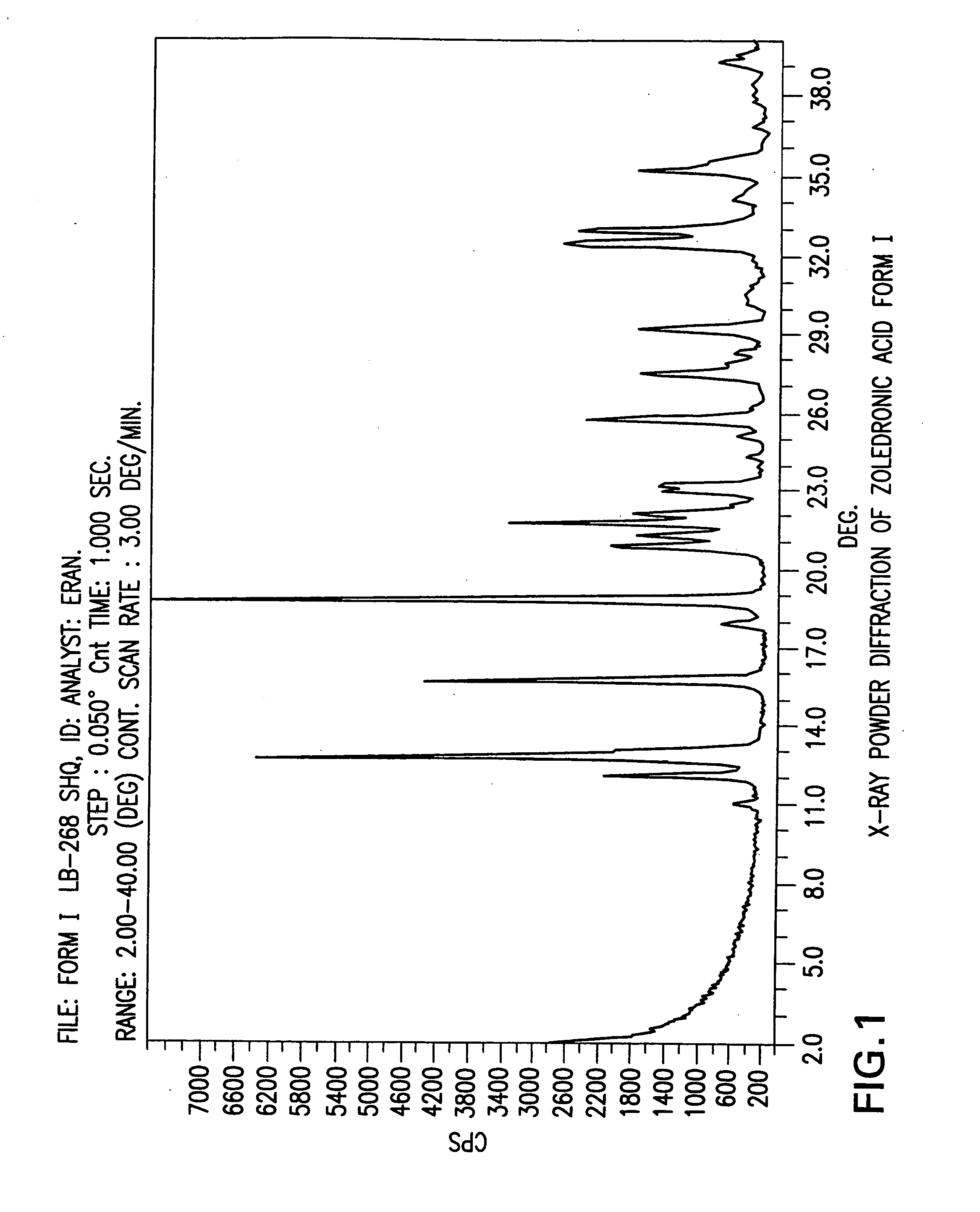
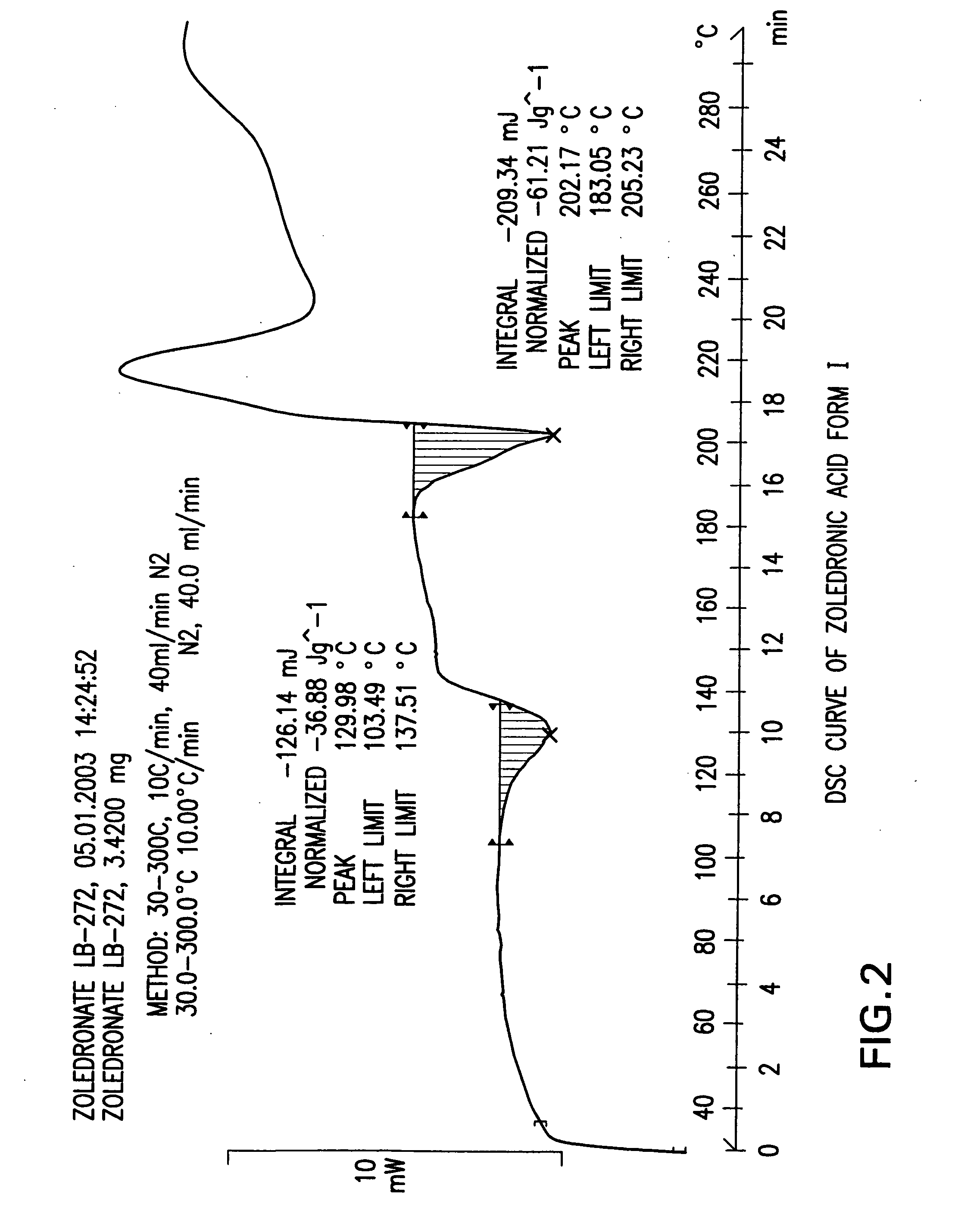
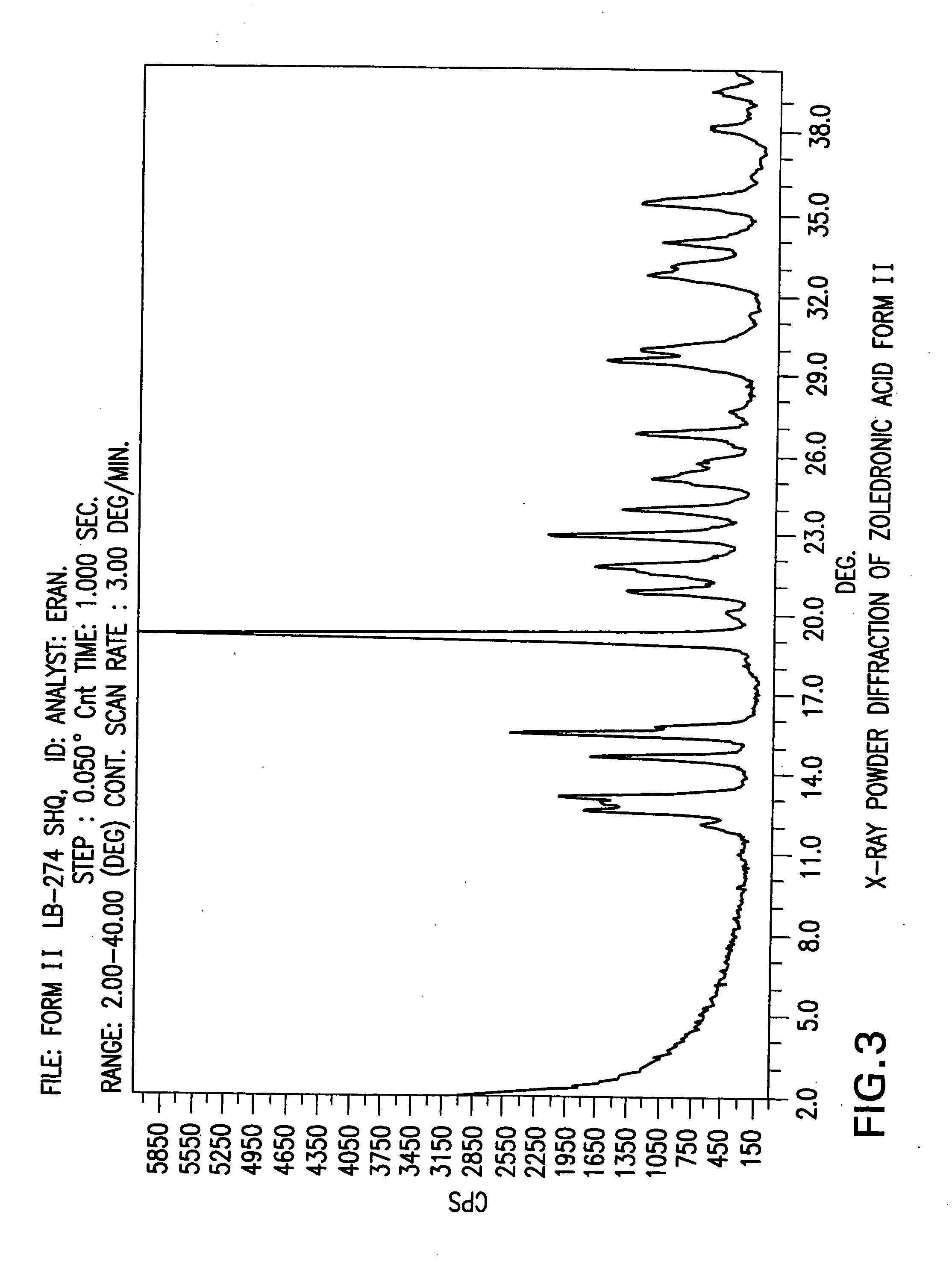
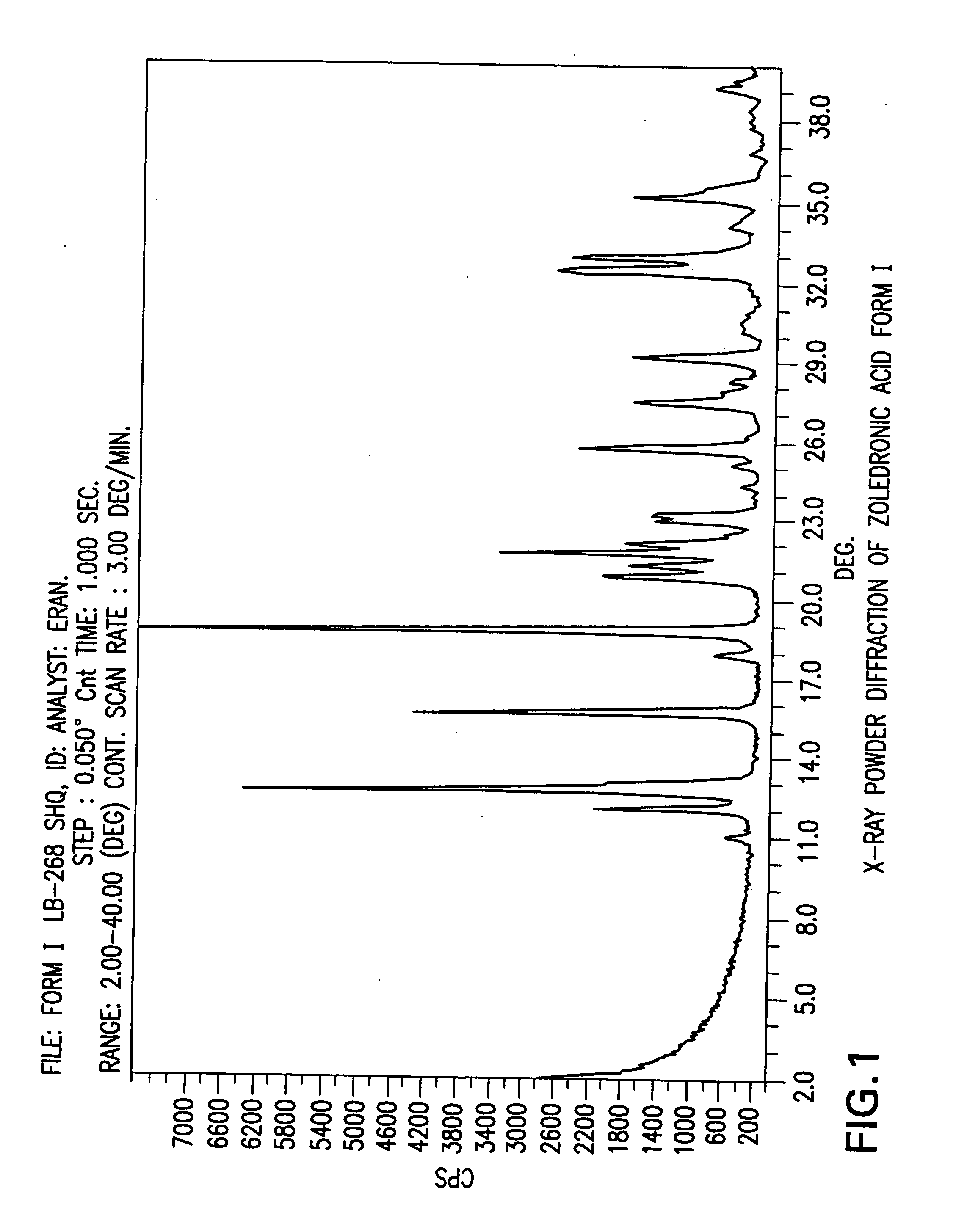
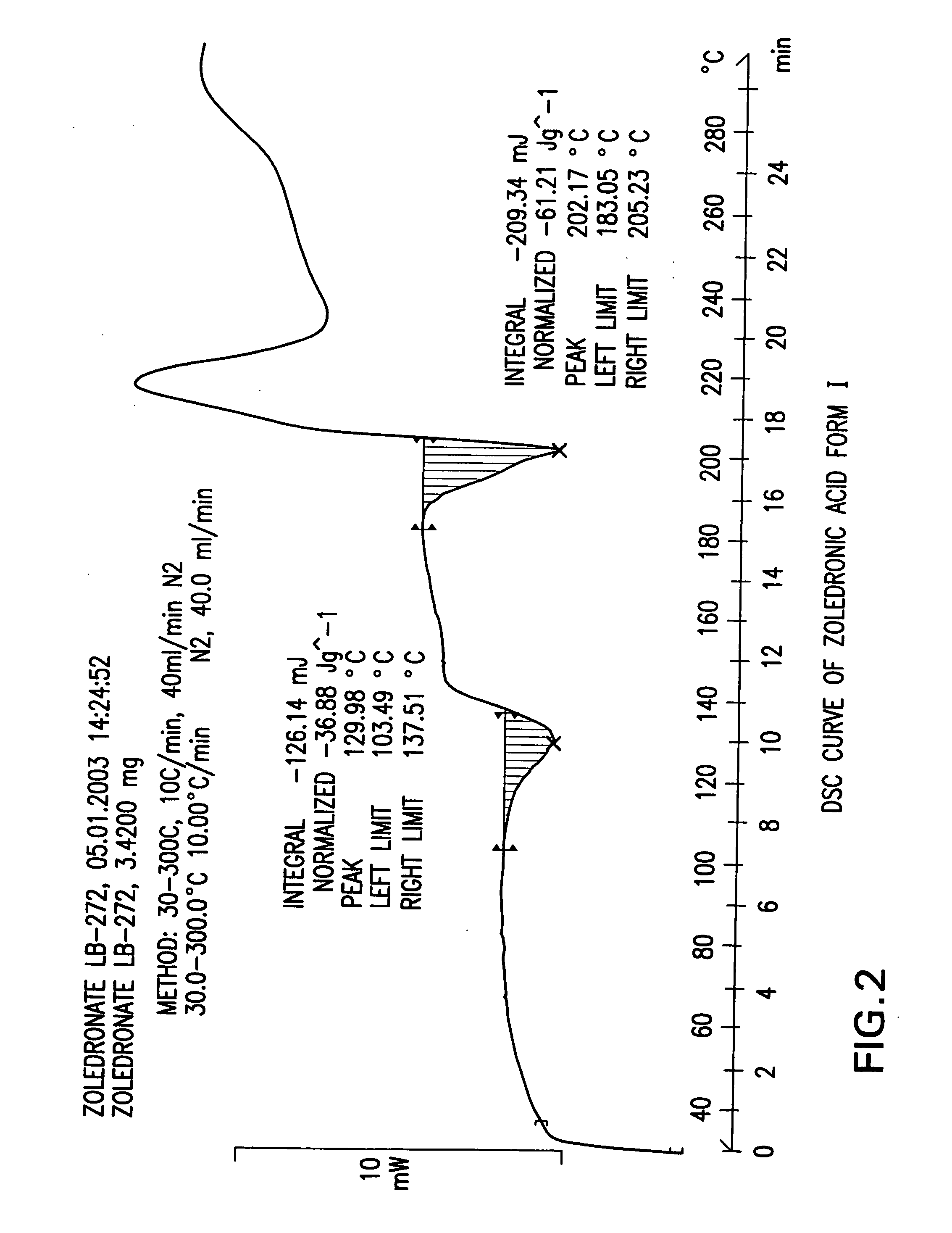
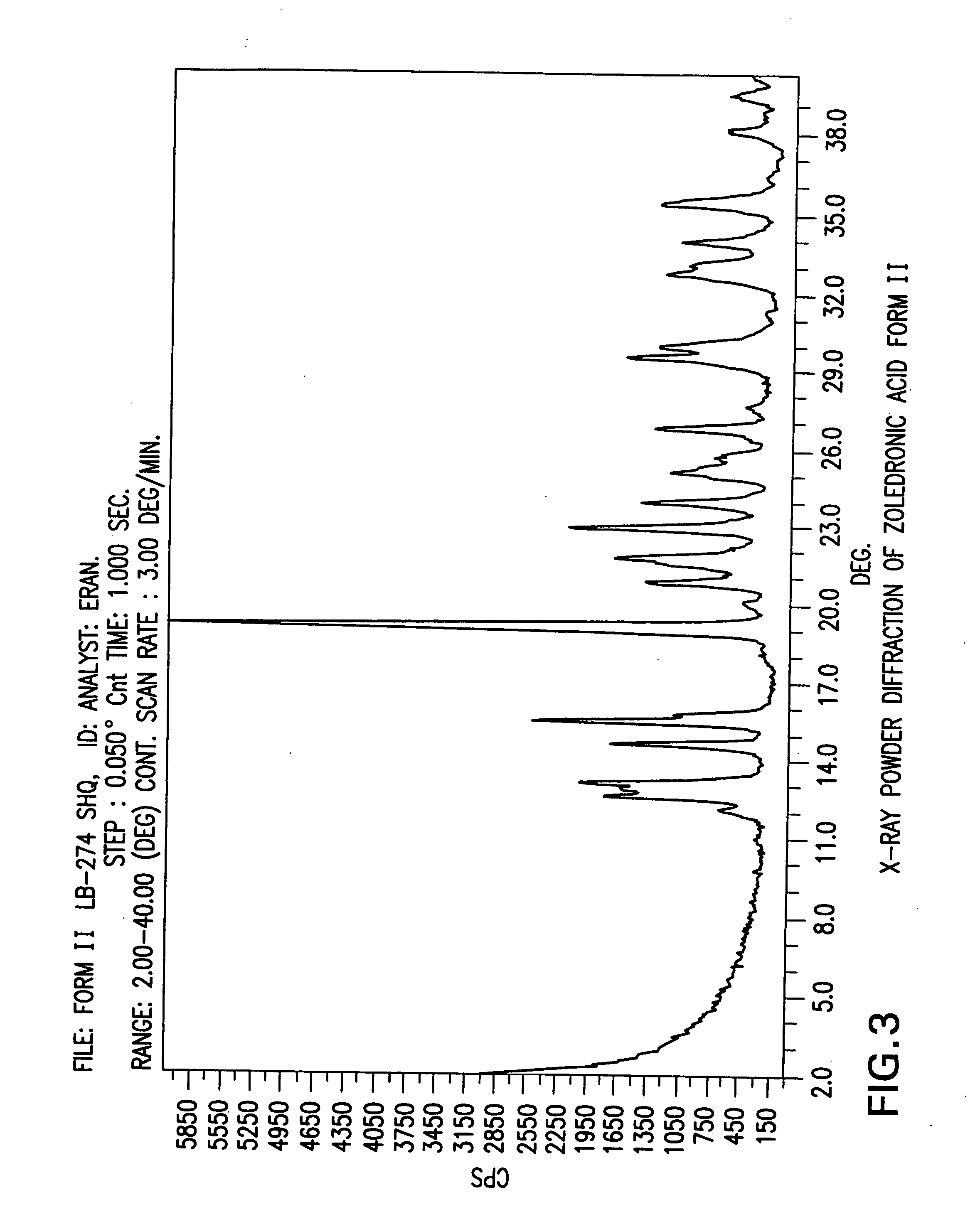
Crystalline form of zoledronic acid
Owner:DR REDDYS LAB LTD +1
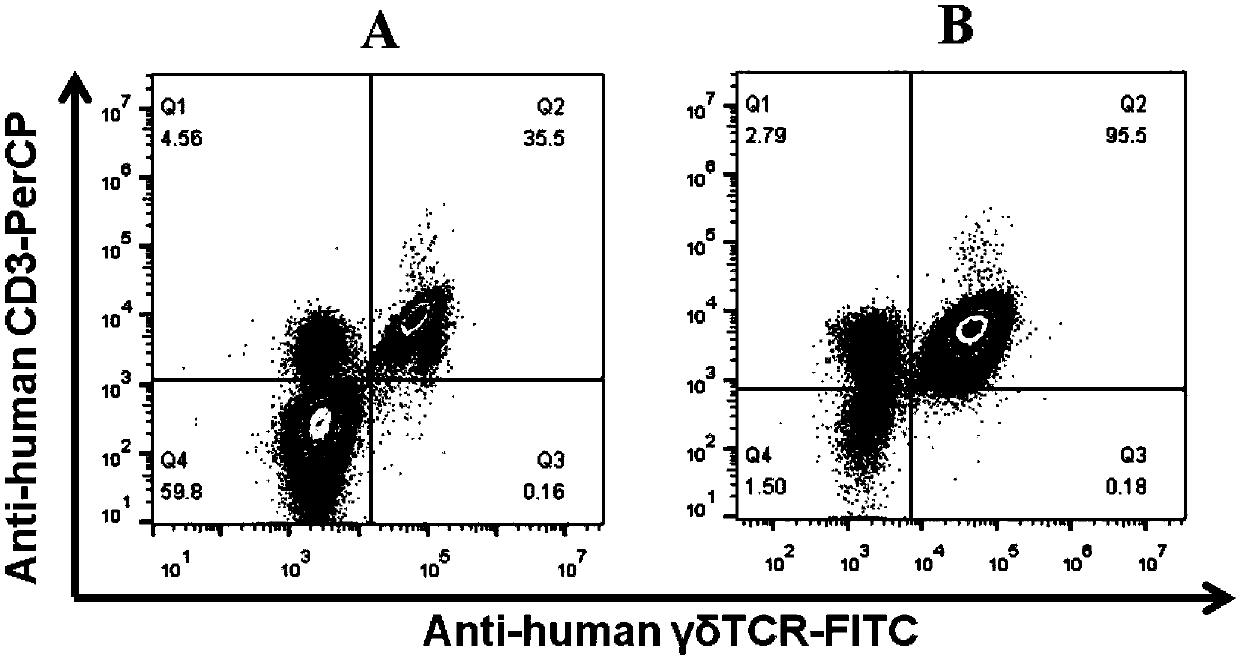
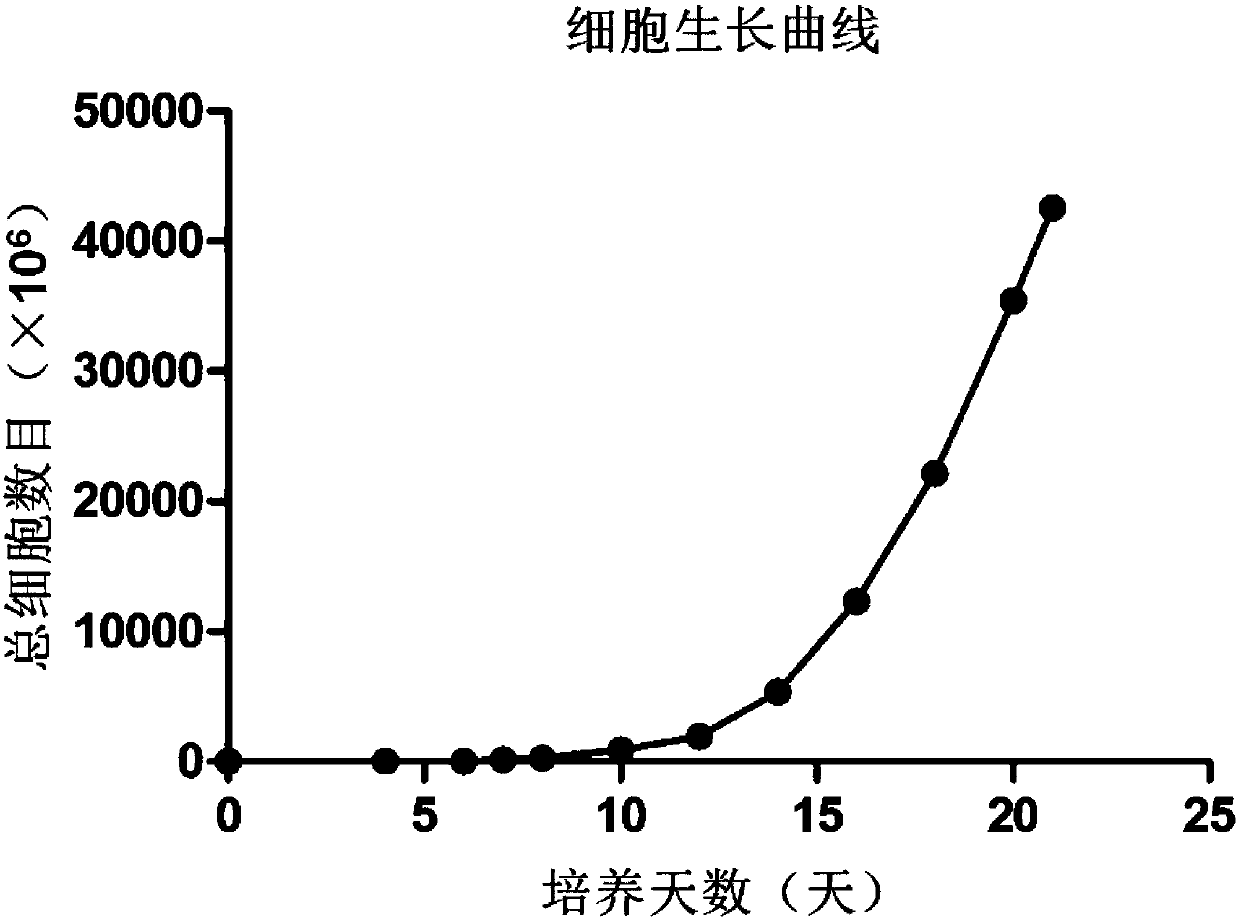
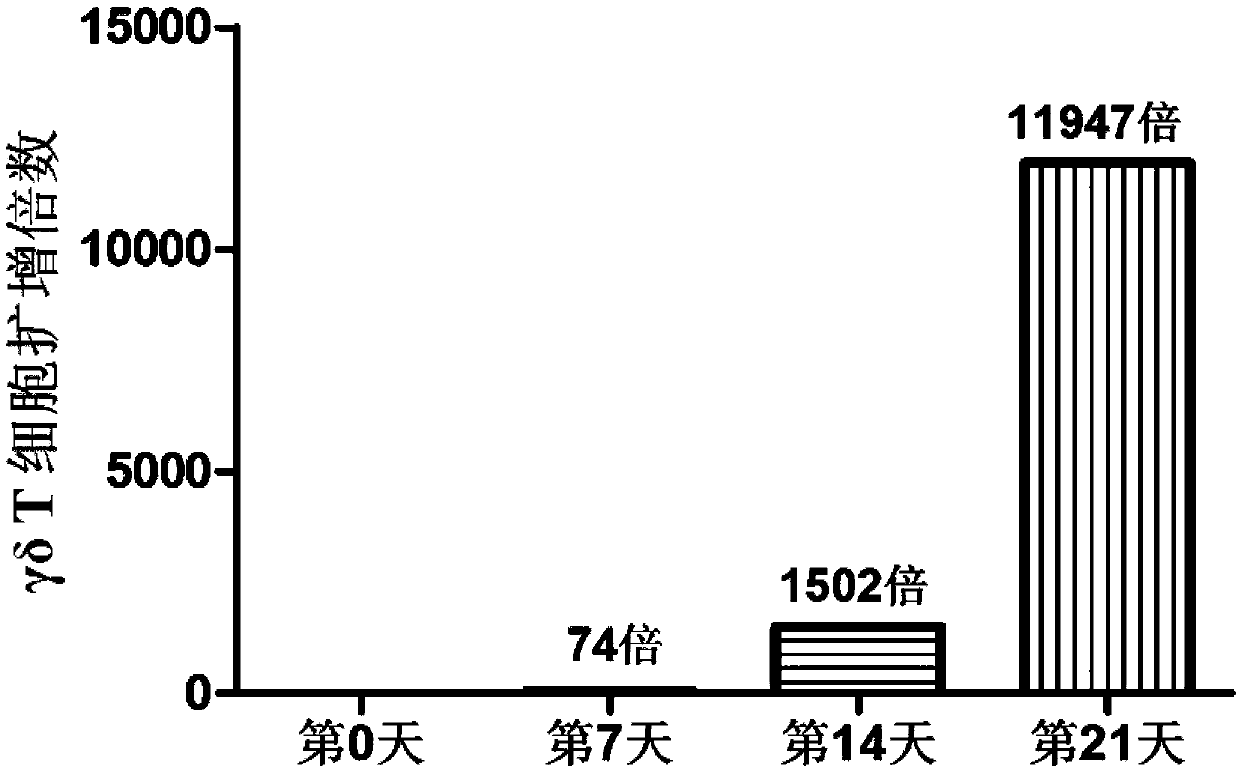
Method for efficiently multiplying gamma delta T cells by stimulating peripheral blood in vitro and application of method
ActiveCN105112370AIntact Antitumor CytotoxicityMammal material medical ingredientsBlood/immune system cellsWhite blood cellAntigen receptors
The invention belongs to the field of medical biology engineering, and particularly relates to a method for effectively multiplying gamma delta T cells by stimulating peripheral blood in vitro and application of the method. The method comprises the step of using feeder cells, an OKT3 (ornithine ketoacid transaminase) antibody, interleukin-2 and zoledronic acid. The feeder cells are formed by specifically inserting CD64, CD86 and CD137L genes in a target site of a genome of the feeder cells. After the zoledronic acid and the nterleukin-2 are used for increasing the proportion of the gamma delta T cells of the peripheral blood, protein products of genes, the OKT3 antibody and the interleukin-2 act in a combined manner, and the gamma delta T cells can be stimulated so that a large amount of gamma delta T cells can be multiplied. The multiplied gamma delta T cells can be used for killing tumor cells which are pretreated by the zoledronic acid, or the tumor cells can be directly killed by modifying and expressing chimeric antigen receptors (CAR) via a genetic engineering means. The gamma delta T cells which are obtained by the method have complete anti-tumor cytotoxicity, and can kill solid tumor cells and non-solid tumor cells.
Owner:杭州朔溪生物医药有限公司
Co-administration of steroids and zoledronic acid to prevent and treat side effects from zoledronic acid infusion
ActiveUS20110263537A1Prevent and treat side effectBiocideOrganic active ingredientsPaget DiseaseSide effect
Zoledronic Acid is used for treatment of hypercalcemia of malignancy, for the treatment of bone metastasis associated with malignancies such as prostate and breast cancer, for the prevention of and treatment of osteoporosis and for the treatment of Paget's disease. Administration of Zoledronic Acid is complicated by what is described as “post-dosing syndrome” (PDS) and osteonecrosis of the jaw (ONJ). Inflammation may be the cause of these side effects, which could be decreased by the co-administration of steroids. This application is a method of use patent for the co-administration of steroids (oral, IV, IM, rectal, or by inhalation) with Zoledronic Acid and a composition of matter patent for mixing Methyl Prednisolone with Zoledronic Acid for infusion.
Owner:DESAI KETAN
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
InactiveUS20070021618A1Organic active ingredientsPhosphorus organic compoundsSodium saltZoledronic acid
The invention relates to polymorphs of zoledronic acid and zoledronate sodium salts, amorphous zoledronate sodium salt, processes for making the polymorphs and amorphous zoledronate sodium salt, and pharmaceutical compositions containing the polymorphs and amorphous zoledronate sodium salt.
Owner:TEVA PHARMA IND LTD
Osteoclast inhibitors such as zoledronic acid for low back pain treatment
ActiveUS9943531B2Improve bioavailabilityAvoid developmentPhosphorous compound active ingredientsPill deliveryAnesthesiaModic changes
Oral dosage forms of osteoclast inhibitors, such as zoledronic acid, in an acid or a salt form can be used to treat or alleviate pain or related conditions, such as Modic changes type I.
Owner:ANTECIP BIOVENTURES II
Expansion method of various lymphocyte subpopulations and application of expansion method
ActiveCN105624107ASuppress deathImprove developmentGenetic material ingredientsMammal material medical ingredientsWhite blood cellNatural Killer Cell Inhibitory Receptors
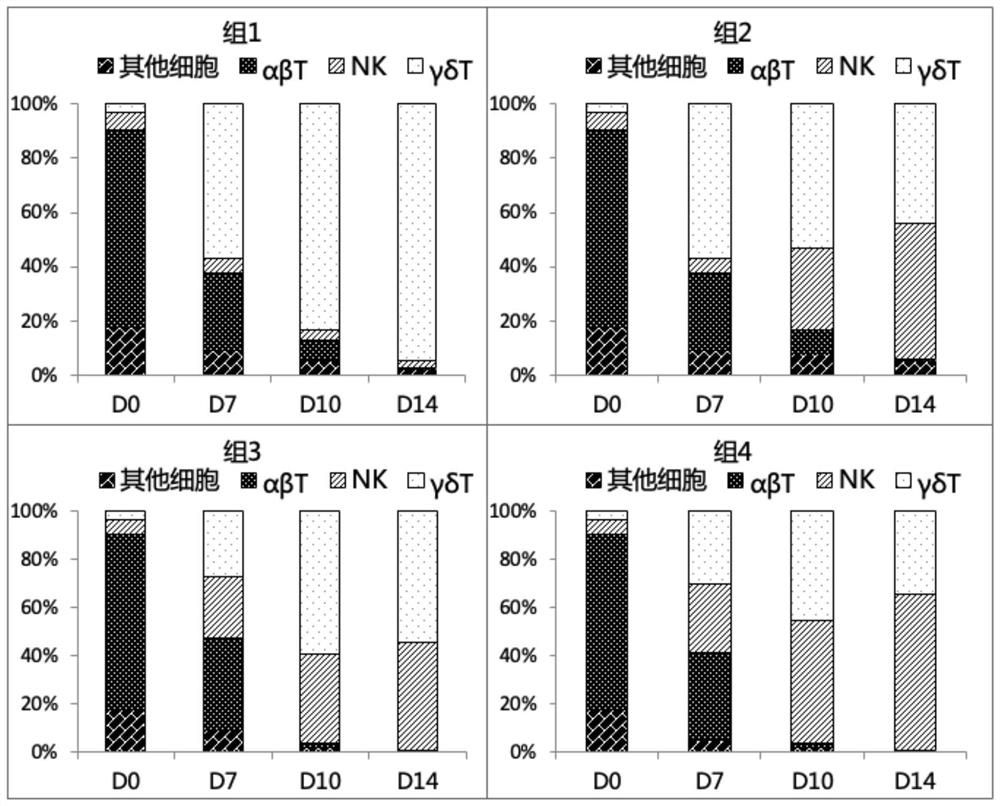
The invention discloses an expansion method of various lymphocyte subpopulations and application of the expansion method to preparation of an adoptive immunotherapy medicine for cancers. The expansion method comprises the following steps: S1, adding IFN gamma and zoledronic acid into a culture medium of PBMC collected in the blood of a tumor patient, and re-adding an OKT3 antibody and recombinant human interleukin-2 after culture to stimulate PBMC so as to finish preliminary expansion; S2, after finish of preliminary expansion, mixing immune lymphocytes with feeder layer cells, and after adding zoledronic acid, an OKT3 antibody and recombinant human interleukin-2, continually performing expansion. By adoption of the expansion method, after twice expansion, the sum of the immune cells can be 10,000 to 80,000 times by expansion, the expanded immune cells include alpha beta T cells, gamma delta T cells, NKT cells and NK cells, and tumor cells can be directly killed, or a cellular immunotherapy drug is prepared to kill the tumor cells.
Owner:杭州朔溪生物医药有限公司
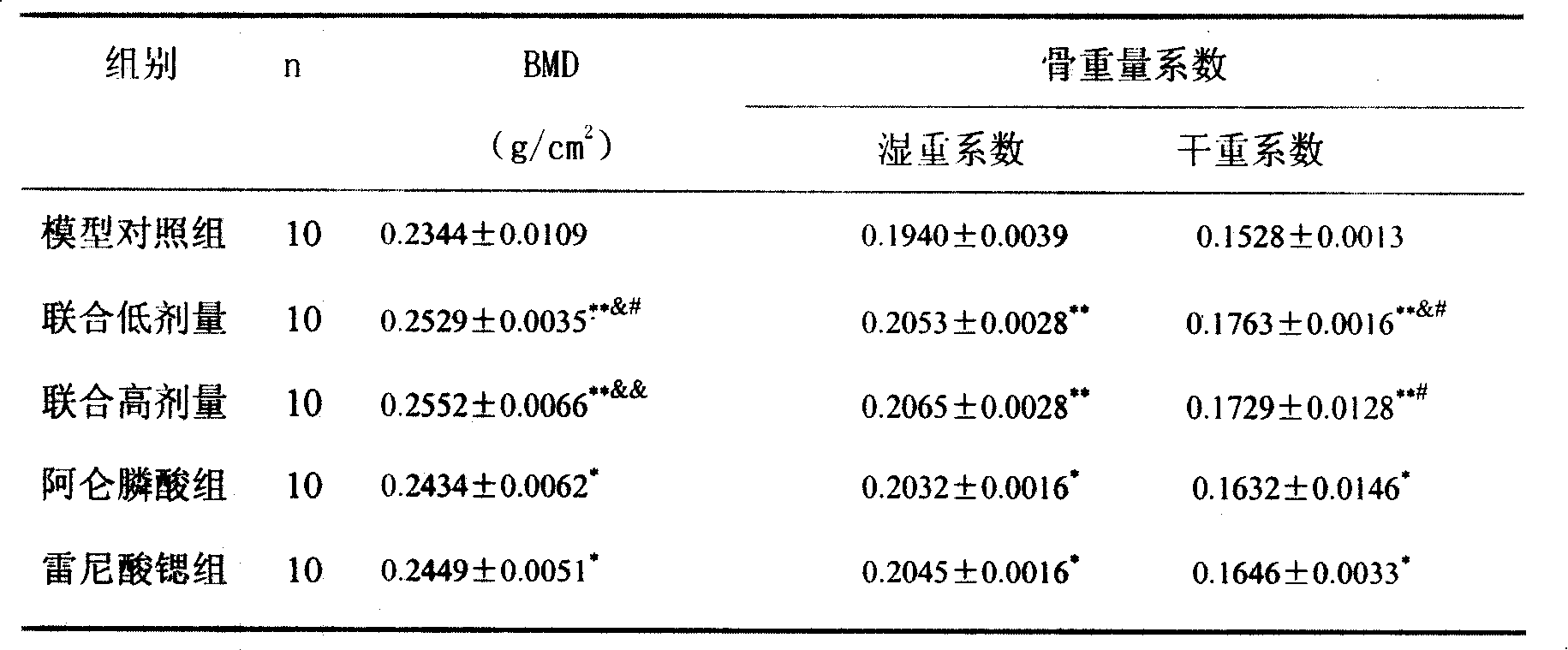
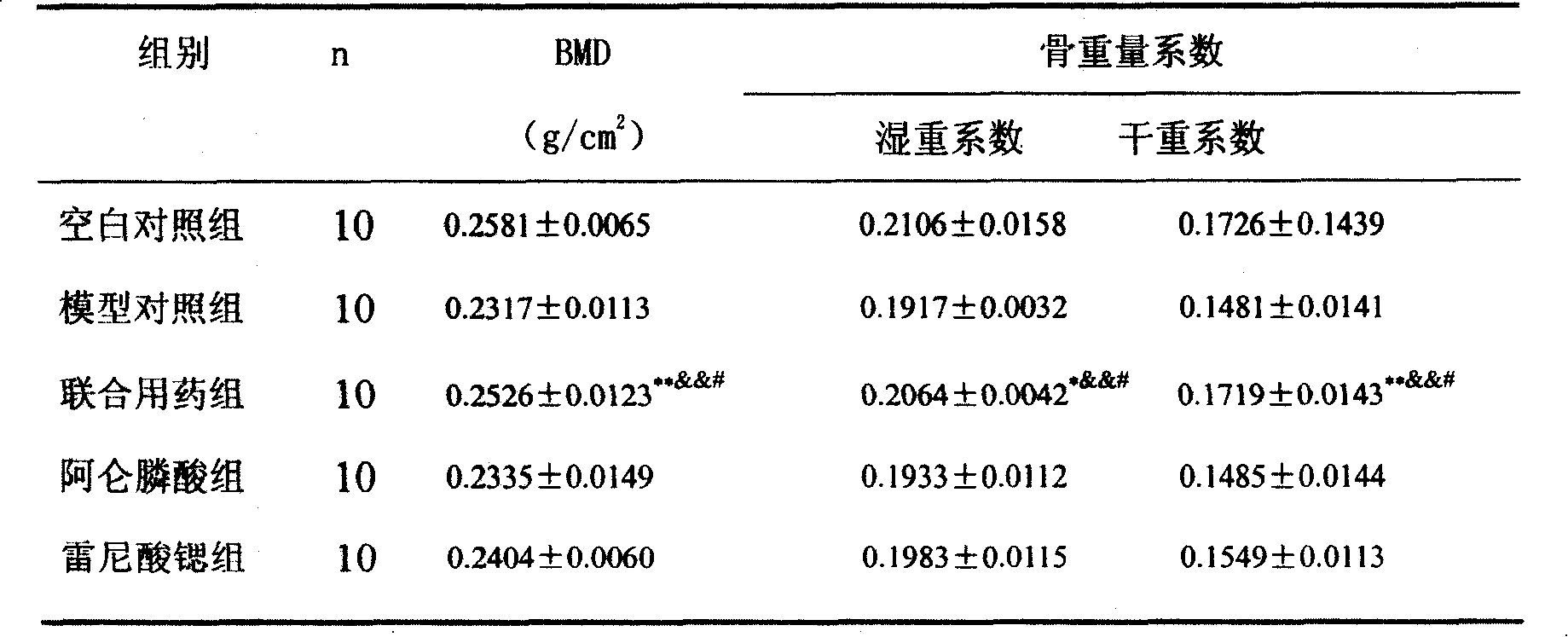
Medicine compounds for treating osteoporosis
ActiveCN101229177AReduce dosageSignificant effectOrganic active ingredientsSkeletal disorderMicro structureSide effect
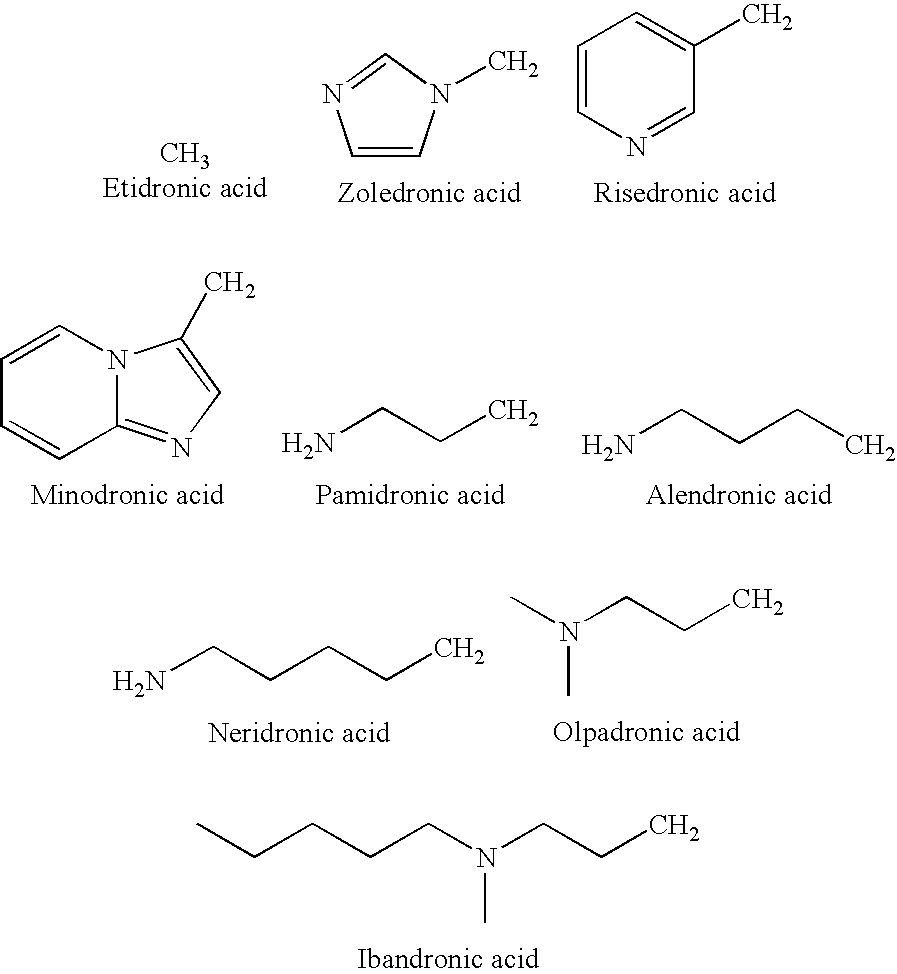
The invention provides a medical compound for treating osteoporosis which is characterized in that the invention contains strontium ranelate and bisphosphonate. Animal experiments indicate that the invention achieves the unexpected effect for treating the osteoporosis. The osteoporosis is a bone disease of the whole body characterized by the low bone mass and the degeneration of the micro structure of the bone organization, companying with the enhancement of the bone fragility and easy happened bone broken for which no ideal treatment medicine exists in the clinic. The bisphosphonate of the invention comprises alendronate, risedronate sodium, ibandronate, pamidronate, Etidronate, disodium clodronate and zoledronic acid, etc. In the invention, the dosage of the bisphosphonate is greatly reduced, which can effectively reduce the happening of side effects and is convenient to use the medicine.
Owner:LUNAN PHARMA GROUP CORPORATION
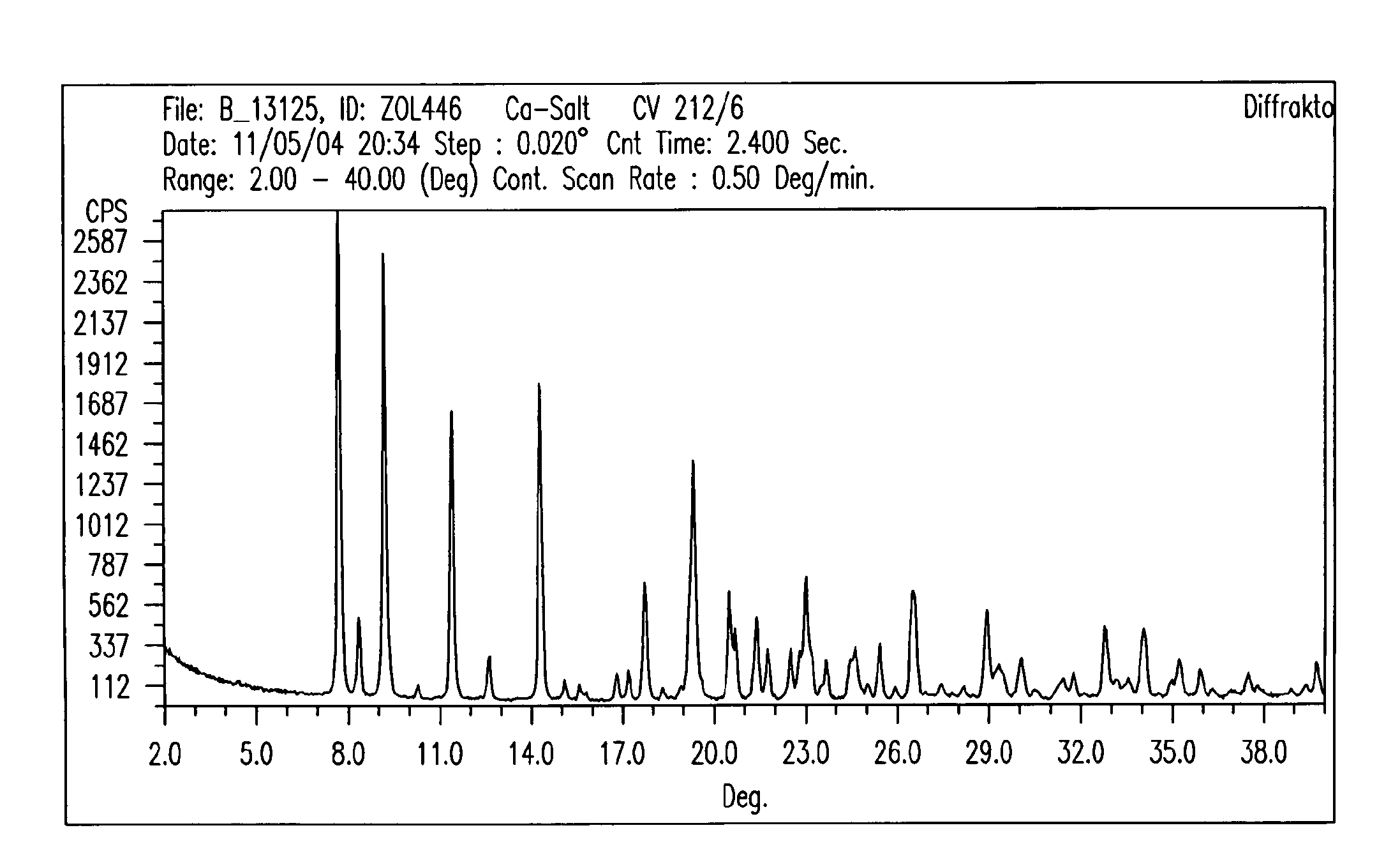
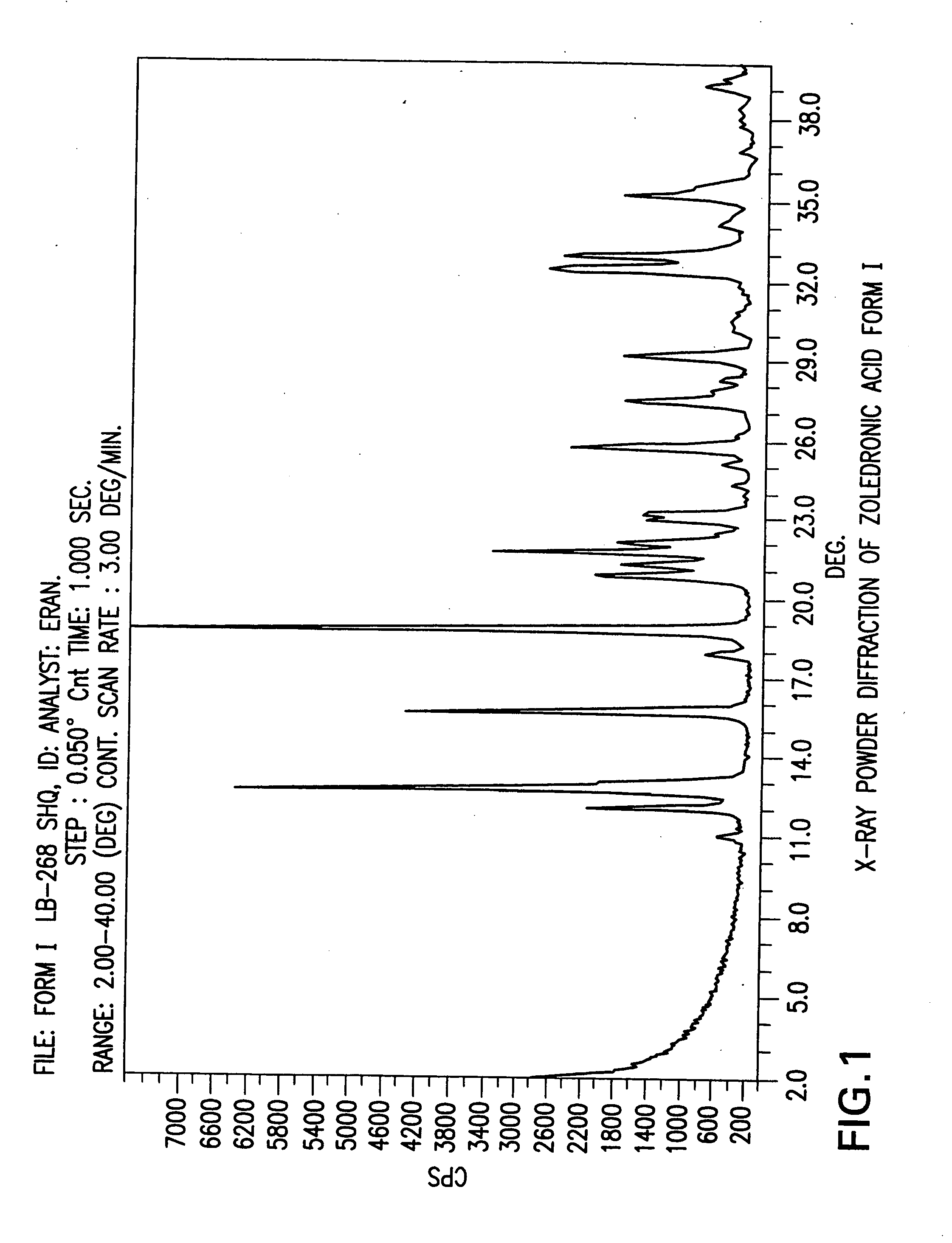
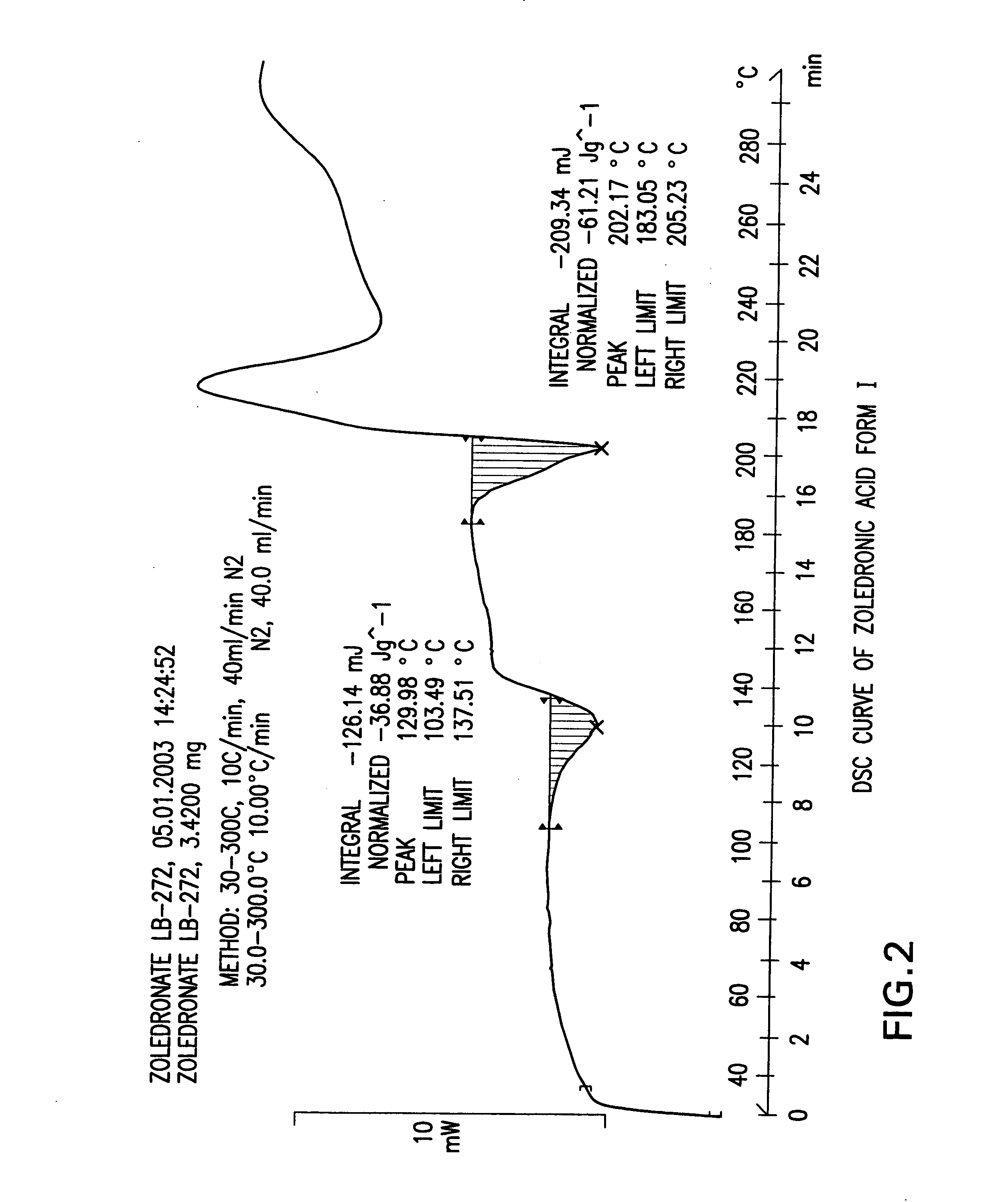
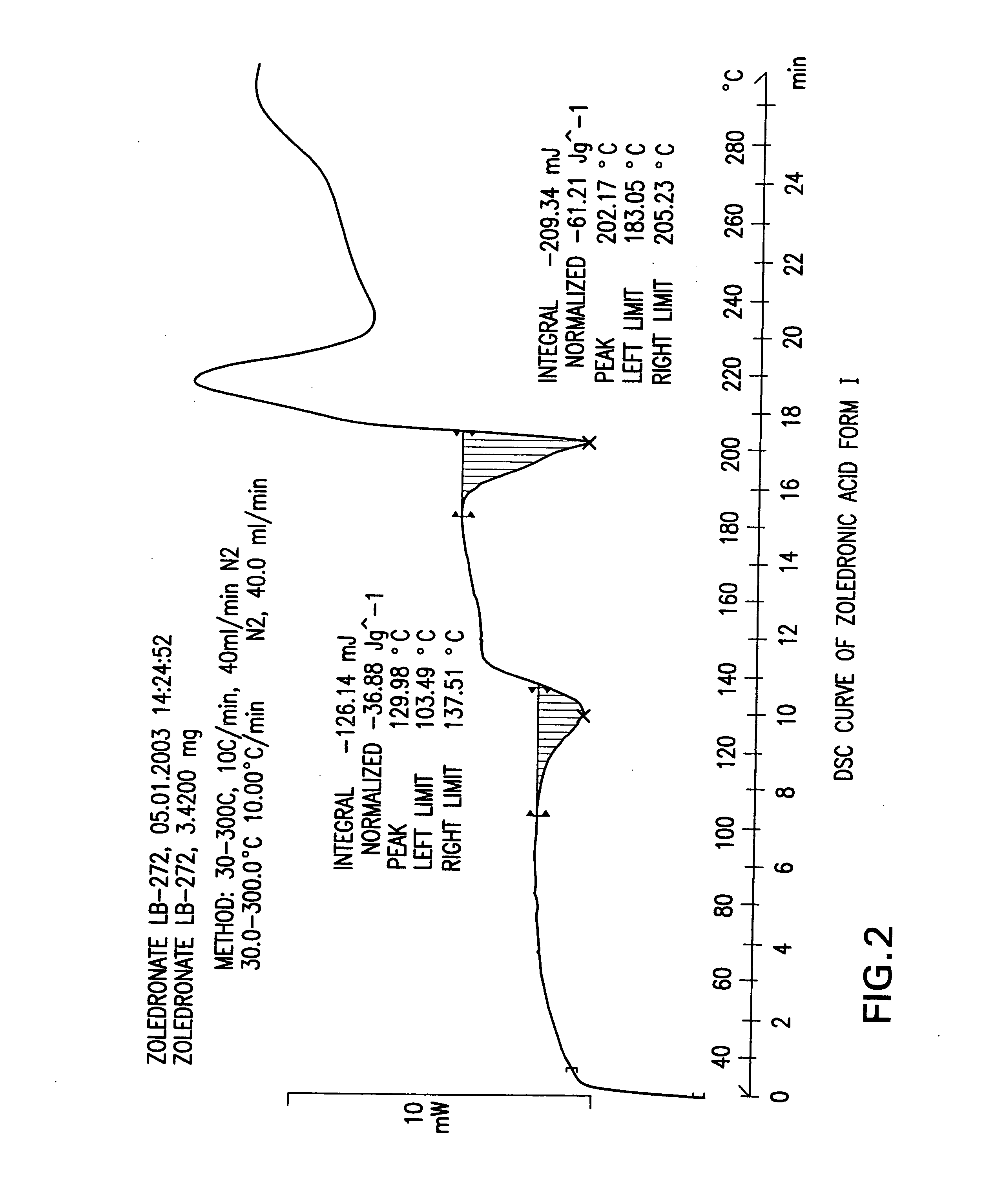
Crystalline forms of zoledronic acid
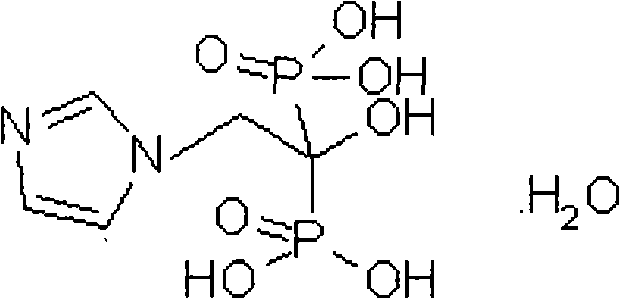
The invention relates to new crystalline forms of low water soluble salts of zoledronic acid, the process for preparation of these crystalline forms, compositions containing these crystalline forms, and the use of these crystalline forms in diagnostic methods or therapeutic treatment of warm-blooded animals, especially humans. The invention relates to the crystalline form of the free acid monohydrate of zoledronic acid, the process for preparation of the crystalline form of the free acid monohydrate of zoledronic acid, compositions containing the crystalline form of the free acid monohydrate of zoledronic acid, and the use of crystalline form of the free acid monohydrate of zoledronic acid in diagnostic methods or therapeutic treatment of warm-blooded animals, especially humans.
Owner:NOVARTIS AG
Zoledronic acid adjuvant and vaccine containing same
ActiveCN103768595AToxic, little side effectRaw materials are easy to getImmunological disordersAntibody medical ingredientsAntigenSide effect
The invention provides a zoledronic acid adjuvant and a vaccine containing the same. Each dose of the vaccine comprises 10-50mu g of zoledronic acid adjuvant. The zoledronic acid adjuvant provided by the invention is small in toxic and side effect, is safe and reliable in use in an immunizing does range, can be used for inducing specific humoral immune response of antigen, close to that of an aluminum adjuvant, is easily available in raw materials and stable in performance, and can be used as an adjuvant of multiple vaccines.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Composition for stimulating and inducing single karyocyte to be amplified to gamma deltaT cell and application of composition
ActiveCN108220239AImprove proliferative abilityHigh purityBlood/immune system cellsCell culture active agentsHigh cellPeripheral blood mononuclear cell
The invention relates to a composition for stimulating and inducing a single aryocyte to be amplified to a gamma delta T cell. The composition is prepared from zoledronic acid, anti-human CD3Ab, anti-human CD28Ab, IL-15, IL-21 and IL-2. The invention also provides a method for the in-vitro mass amplification of gamma deltaT cells by utilizing the composition, and particularly relates to a method for in-vitro inducing the mass amplification of gamma deltaT cells by utilizing PBMCs. The zoledronic acid is used for induction and activation, the anti-human CD3Ab, the anti-human CD28Ab, IL-15, IL-21 and IL-2 are used for inducing and activating the proliferation, and various amplification factor combinations to jointly stimulate and induce the amplification of gamma deltaT cells, and the obtained gamma deltaT cell has the characteristics of large quantity, high purity, high cell toxicity and the like and has good clinical application value.
Owner:安徽瑞达健康产业有限公司
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
InactiveUS20070021616A1Organic active ingredientsPhosphorus organic compoundsSodium saltZoledronic acid
The invention relates to polymorphs of zoledronic acid and zoledronate sodium salts, amorphous zoledronate sodium salt, processes for making the polymorphs and amorphous zoledronate sodium salt, and pharmaceutical compositions containing the polymorphs and amorphous zoledronate sodium salt.
Owner:ARONHIME JUDITH +1
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
InactiveUS20070021617A1Organic active ingredientsPhosphorus organic compoundsSodium saltZoledronic acid
Owner:TEVA PHARMA IND LTD
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
The invention relates to polymorphs of zoledronic acid and zoledronate sodium salts, amorphous zoledronate sodium salt, processes for making the polymorphs and amorphous zoledronate sodium salt, and pharmaceutical compositions containing the polymorphs and amorphous zoledronate sodium salt.
Owner:TEVA PHARMA IND LTD
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
InactiveUS20070021619A1Organic active ingredientsPhosphorus organic compoundsSodium saltZoledronic acid
Owner:ARONHIME JUDITH +1
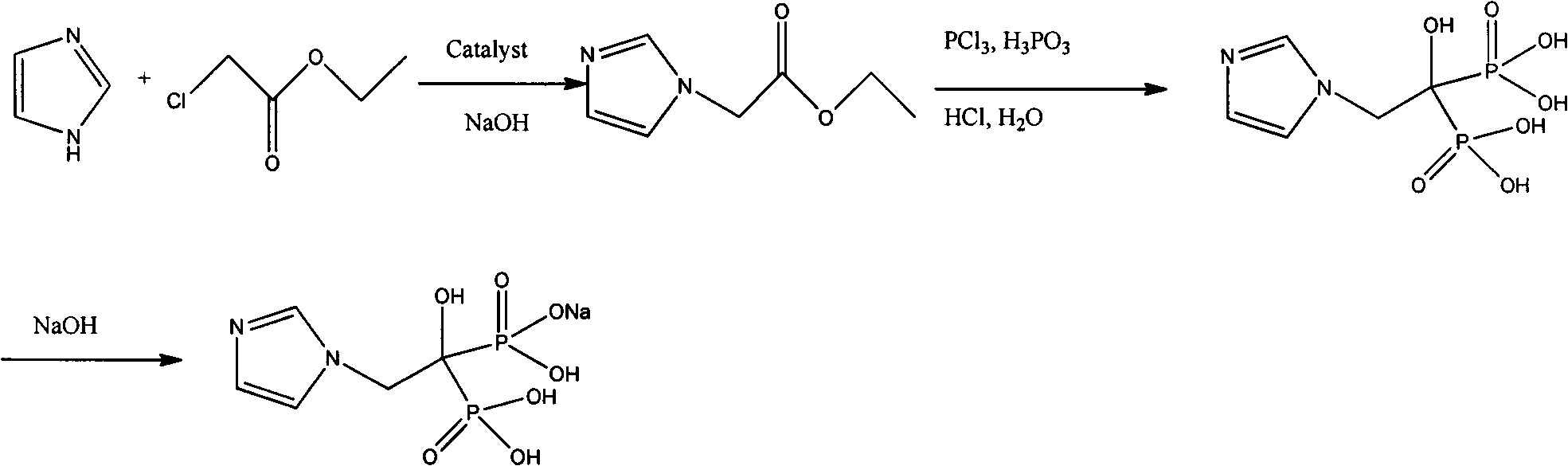
Process for preparing dazoline phospho acid
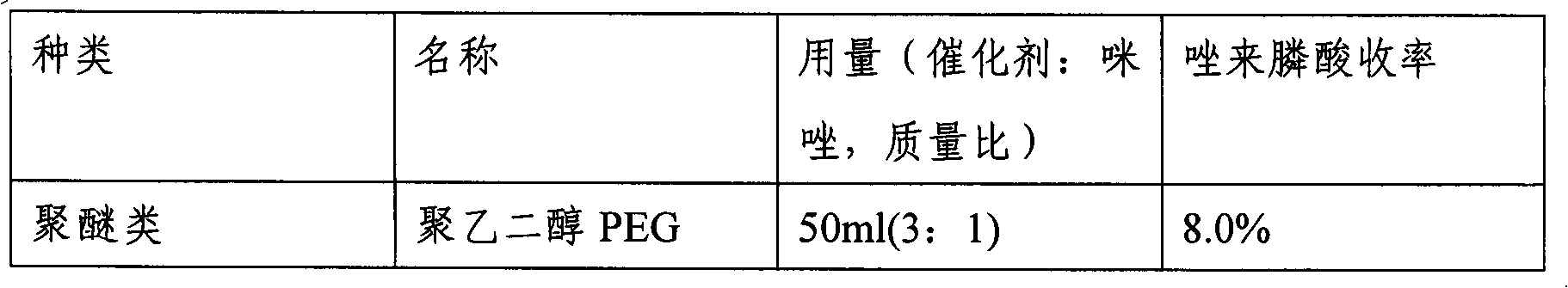
InactiveCN1693308AReduce usageEasy to operateGroup 5/15 element organic compoundsHydrogenPhosphoric acid
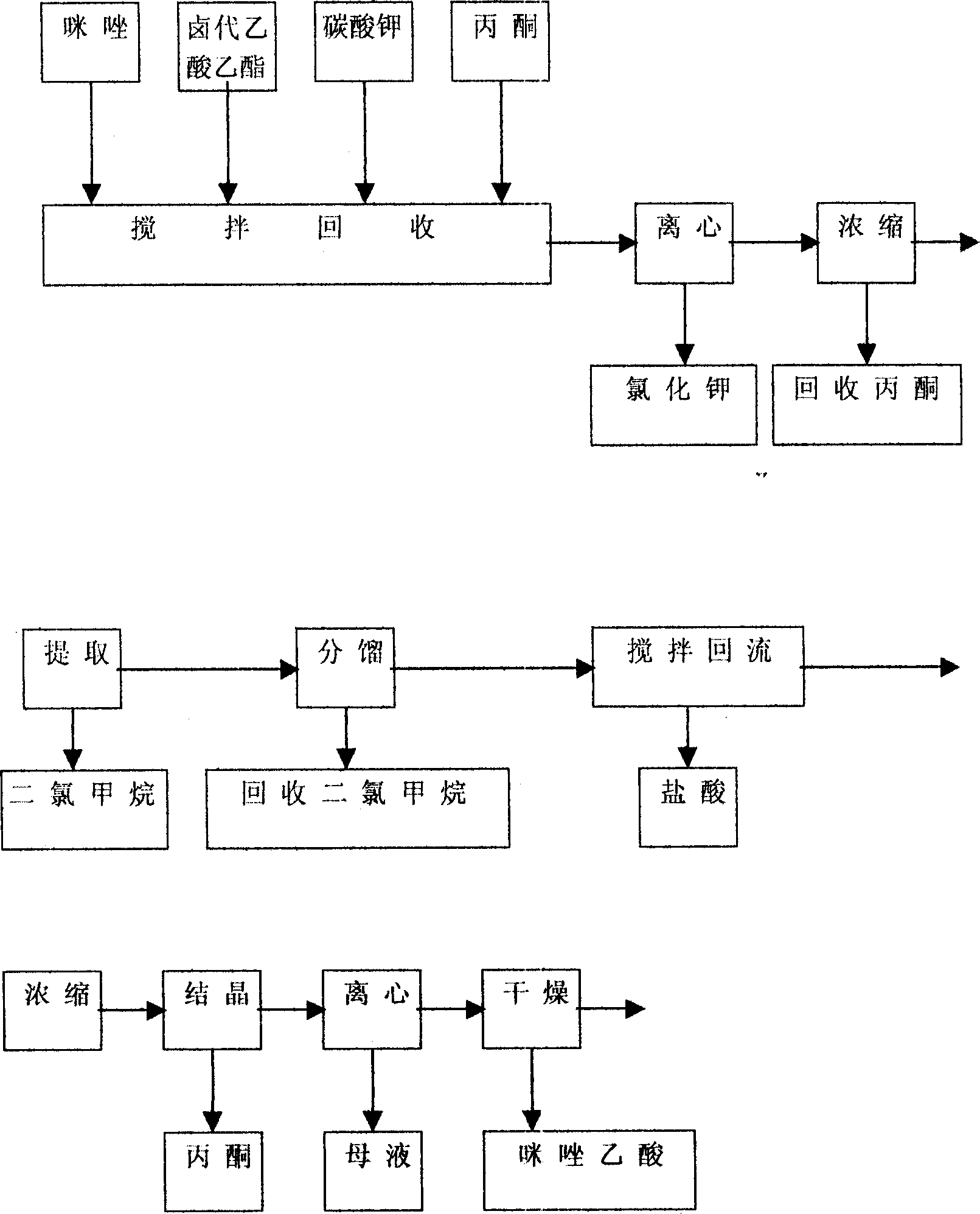
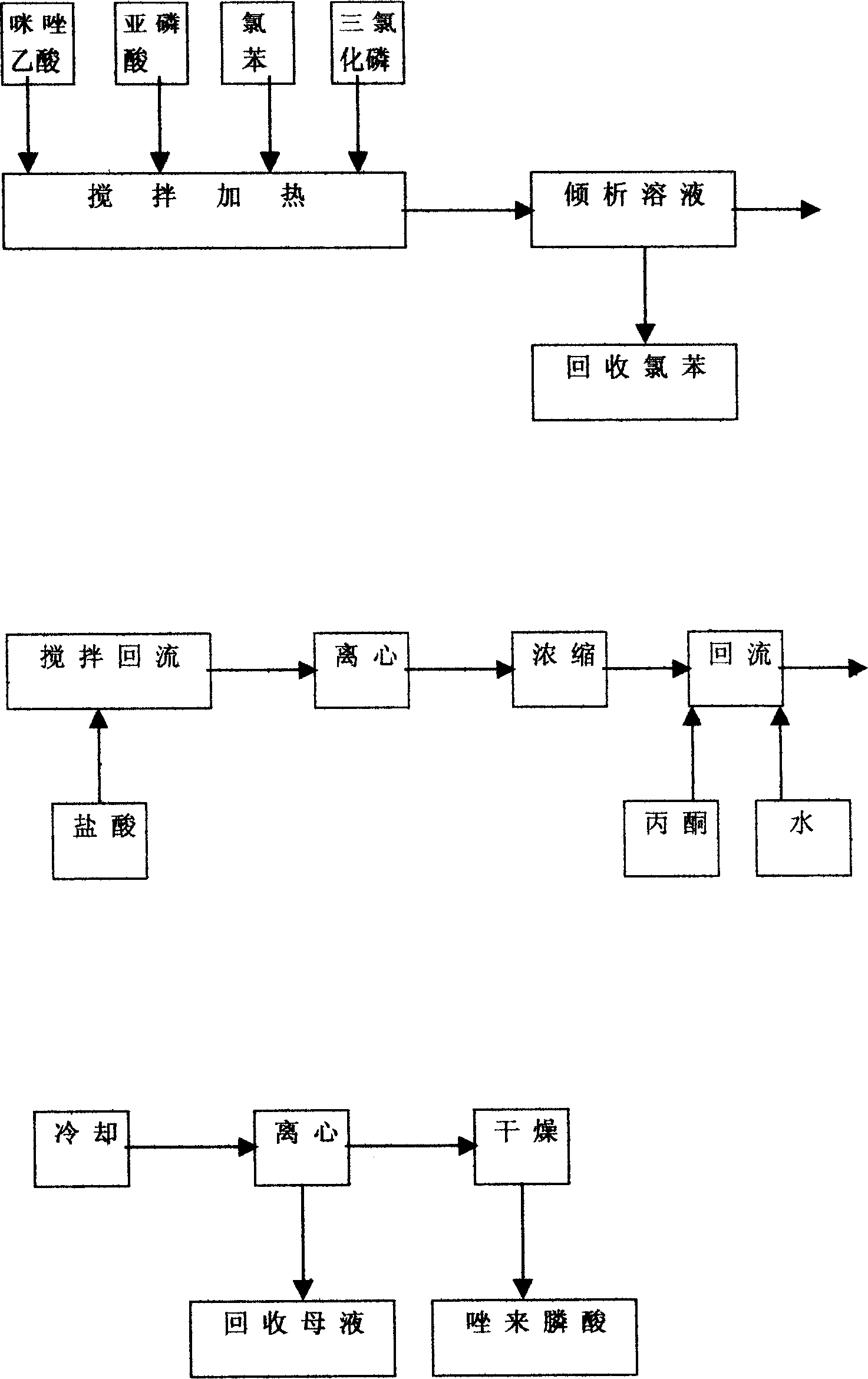
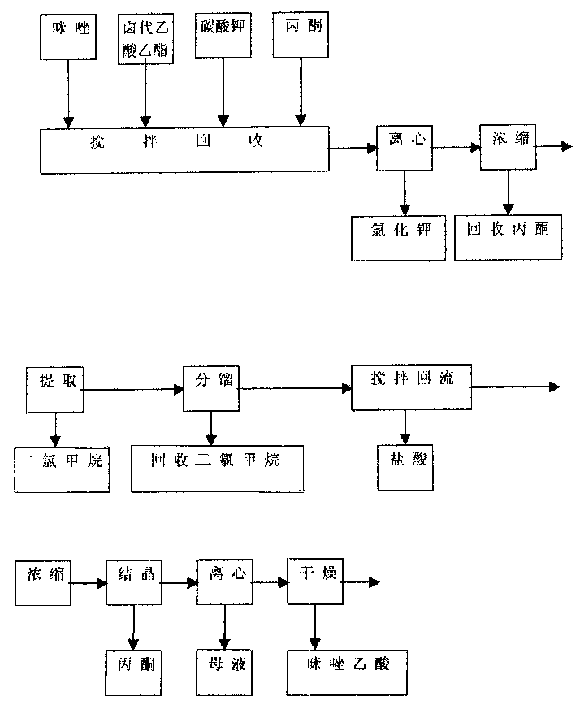
A process for preparing Zuolailingsuan from imidazole includes such steps as reaction between imidazole and alpha-haloacetate or alpha-haloacetonitrile in inertial solvent under catalysis of alkali, hydrolyzing in phosphoric acid solution, reacting on phosphorus trichloride (or trioxychloride), acidic hydrolyzing, and recrystallizing in distilled water.
Owner:YANGTZE RIVER PHARM GRP CO LTD
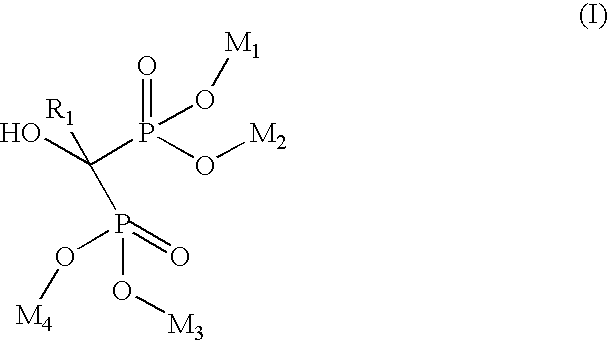
Process for manufacturing bisphosphonic acids
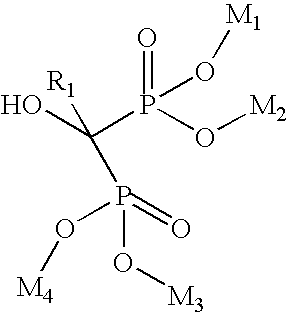
A manufacturing process for the preparation of bisphosphonic acids and in particular zoledronic acid is provided wherein diglyme, monoglyme, or a mixture thereof, is utilized to produce a homogenous, water soluble, solid reaction mass that upon cooling, dissolving in water and stripping results in a high purity product and comparatively good yield. wherein Ri is selected from the group consisting of
Owner:ALBEMARLE CORP
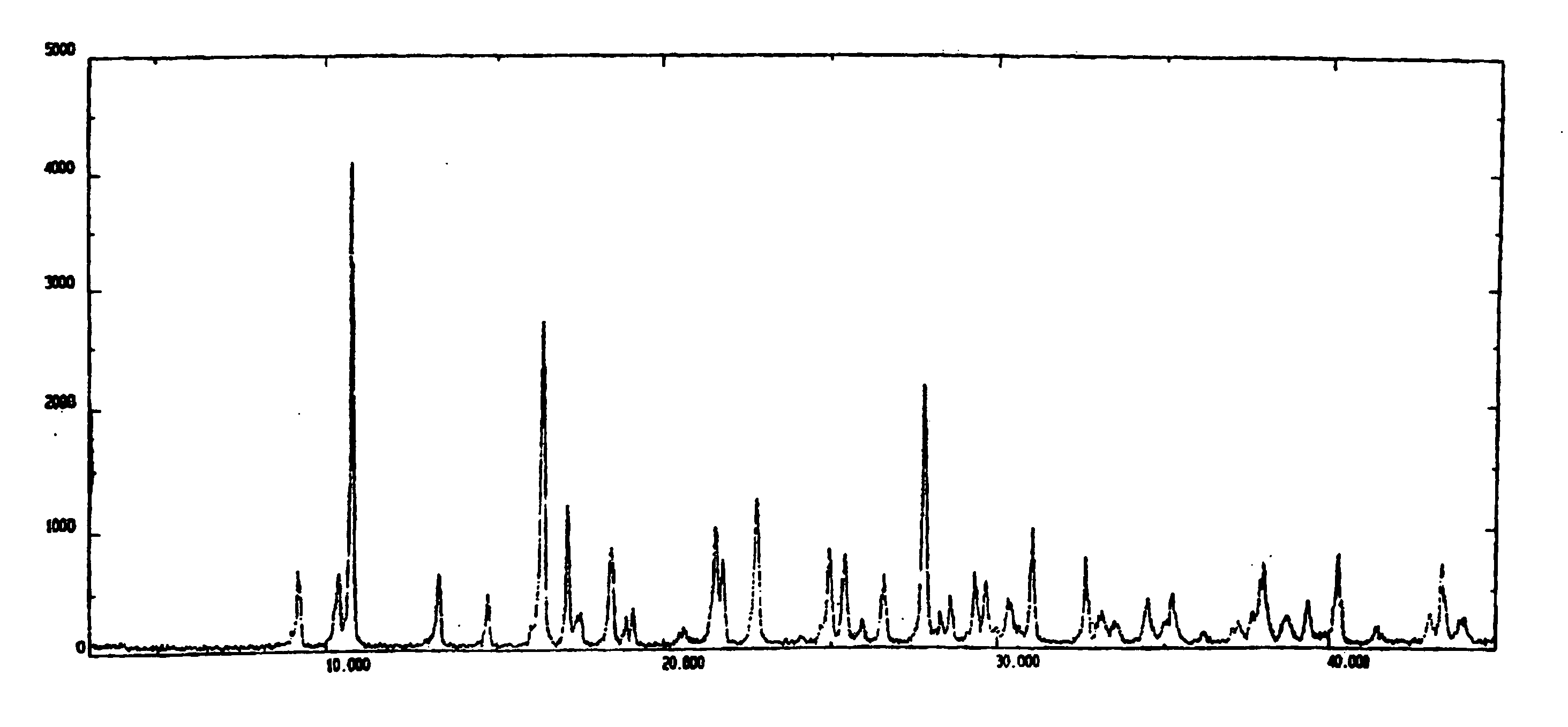
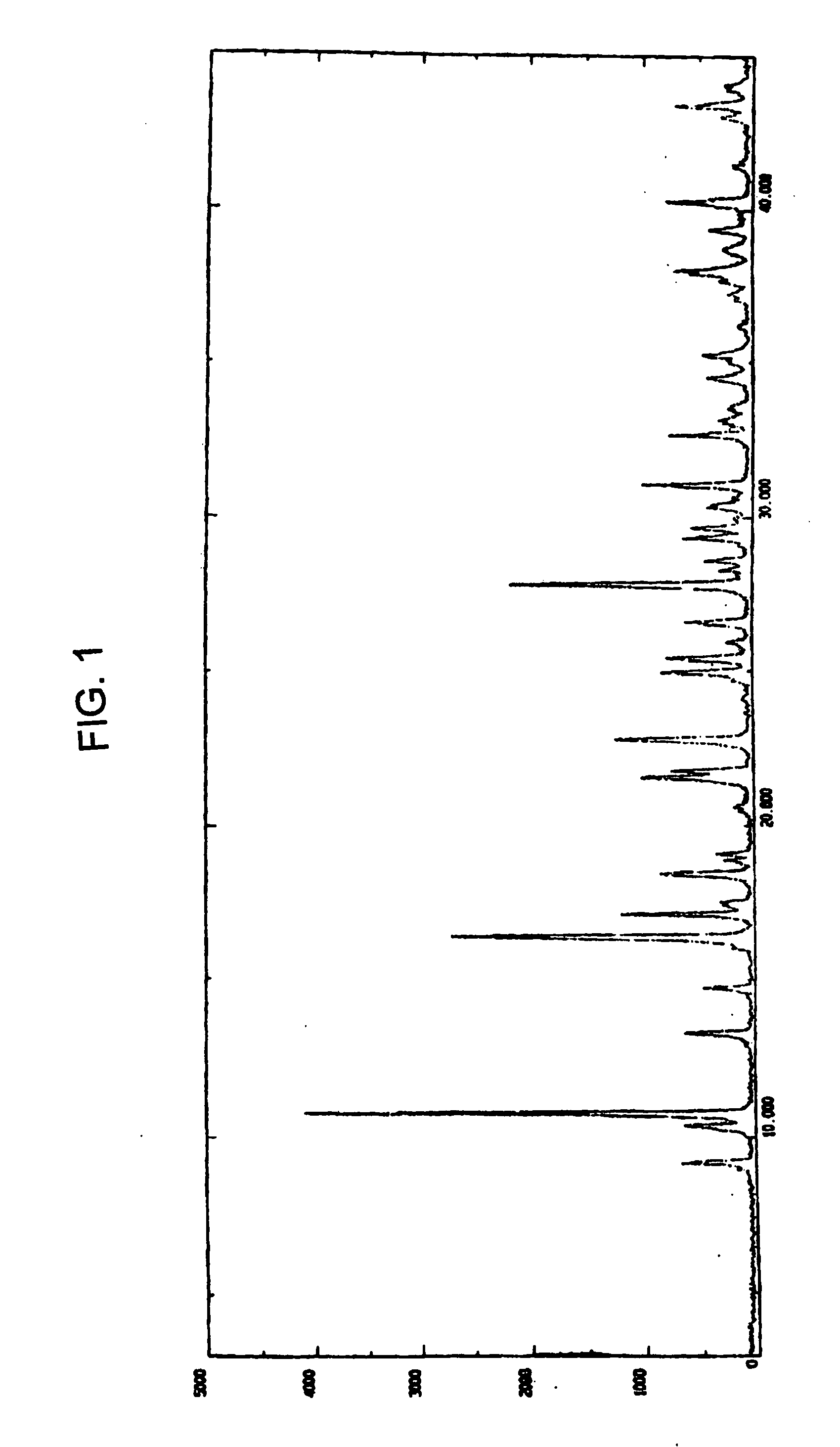
Investigating method for azolephosphate, freeze-dried and relevant mass and content of its injecta
The invention discloses a method for measuring the substance and content of azolephosphate material, freeze-dried, its injection.It takes the 18 alkyl connected silica gel as the filling reagent and 0.03% hydrogen-oxygen four butyl ammonium aqueous solution- B (100:20) as the flowage thing.The examining wave-length is 218 nms and the academic number of planks calculated by apex of can not be lower than 2500.The flowage thing can be prepared as follows: mix 10% hydrogen-oxygen four butyl ammonium aqueous solution(0.3 mls) and 100 ml water,then regulate the pH with the phosphoric acid to 2.55; Or take B- four hydrogen furan-0.05mols / L phosphoric acid 2hydrogen ammonium(4:1:100) as the flowage thing. The method is simple, fast and stable, thus it can control the quality of production effectively.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Therapeutic treatment of breast cancer based on c-maf status
ActiveUS20190269707A1Short overall survivalPreventing or inhibiting bone remodellingMicrobiological testing/measurementPharmaceutical delivery mechanismTherapeutic treatmentZoledronic acid
The present invention relates to the design of a customized therapy for a subject with breast cancer based on the c-MAF expression level and the menopausal status of the subject. In some embodiments, the customized therapy comprises an agent for avoiding or preventing bone degradation. In some embodiments, the agent for avoiding or preventing bone degradation is zoledronic acid.
Owner:INBIOMOTION
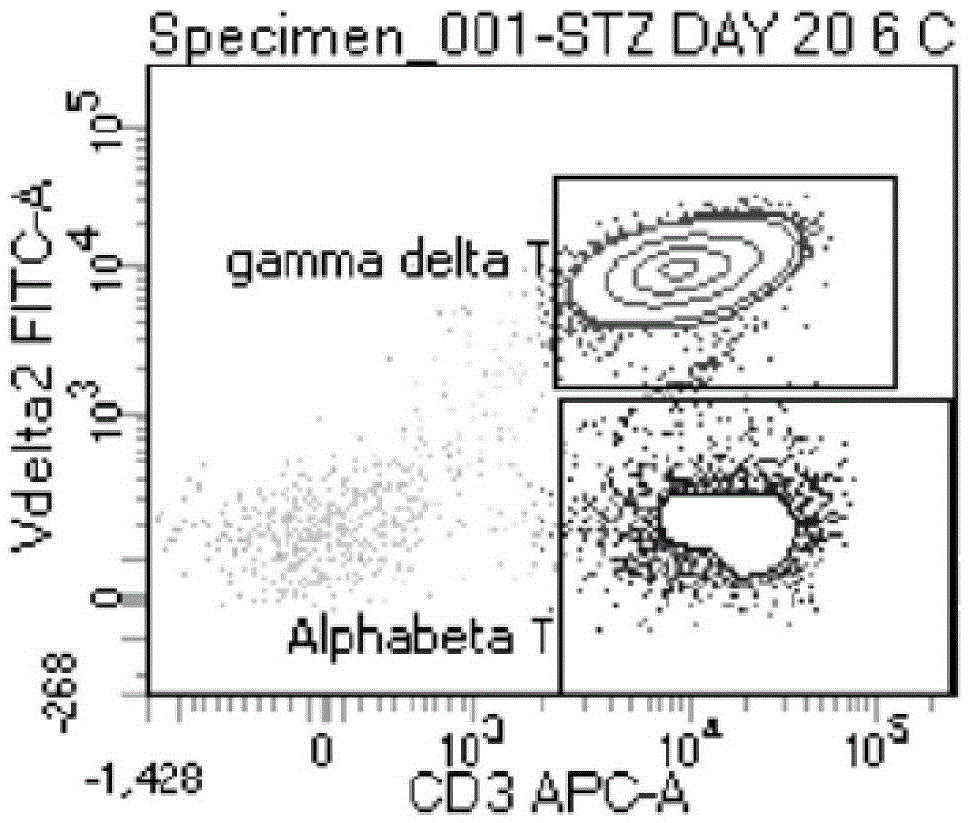
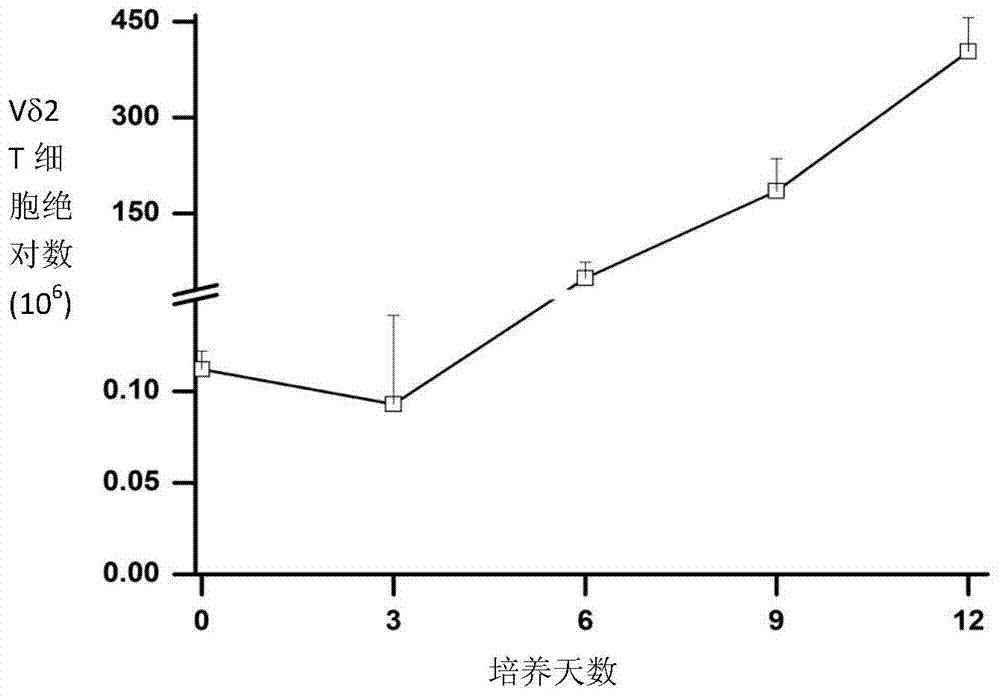
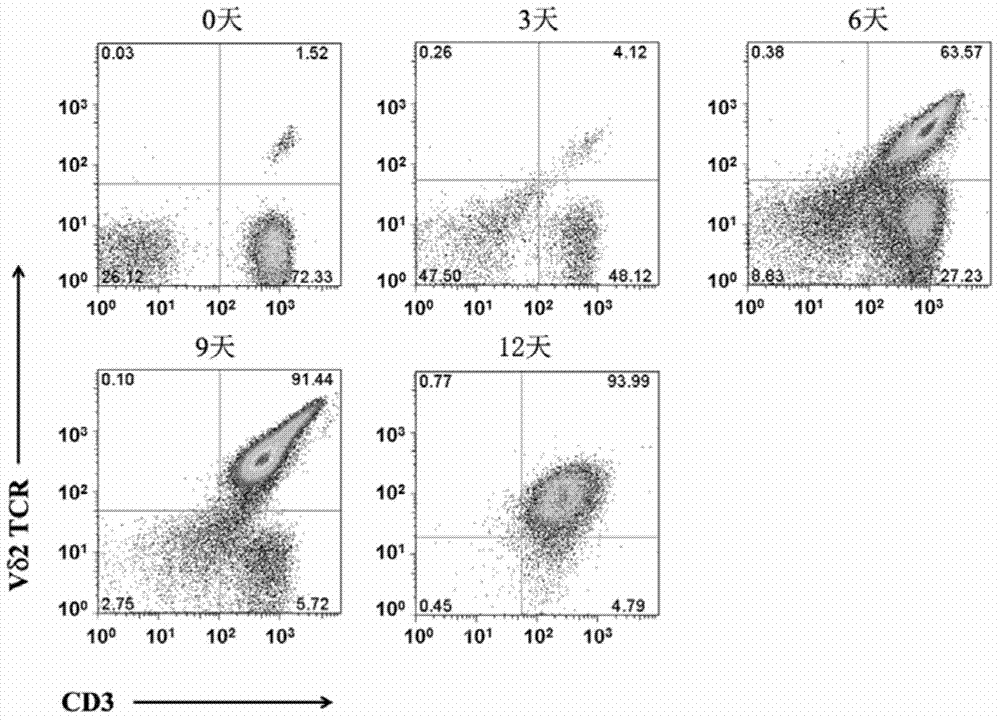
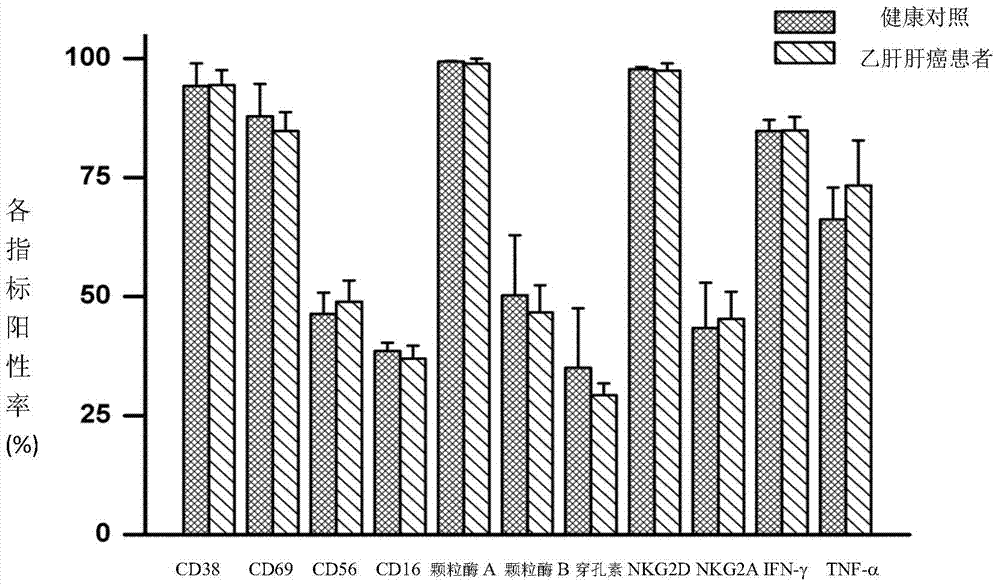
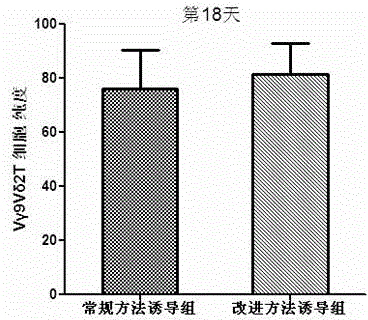
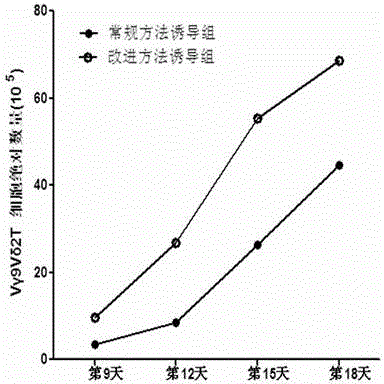
In-vitro culture method for increasing human Vdelta2 T cell amplification efficiency and application thereof
InactiveCN104711224AImprove induction efficiencyHigh activityBlood/immune system cellsAntineoplastic agentsBiotechnologyPeripheral blood mononuclear cell
The invention relates to an in-vitro culture method for increasing human Vdelta2 T cell amplification efficiency and an application thereof. The method is characterized in that peripheral blood mononuclear cells are separated, a medium (AIMU+10%FBS)is used to prepare a cell suspension, zoledronate is added for in-vitro stimulation; after one-day stimulation of zoledronate, cytokine rhIL-2 is added every day; the human peripheral blood mononuclear cells are cultured for 72-96 hours, half amount or 2 / 3 mount of nutrient solution can be replaced when the nutrient solution turns yellow; half amount of nutrient solution can be replaced when the nutrient solution turns yellow after 96 hours; when culture is carried out in 11th-14th day, when the amplified Vdelta2 T cell frequency and absolute amount reach the requirements, the cells can be acquired. The Vdelta2 T cell purity can reach more than 90%. The method has the advantages of simple culture method, the required peripheral blood amount is little, induction cell quantity is more, and the amplification efficiency is high, Vdelta2 T cell is expected to increase curative effect of for liver cancer immunity treatment, and has good application potential.
Owner:TIANJIN UNIV
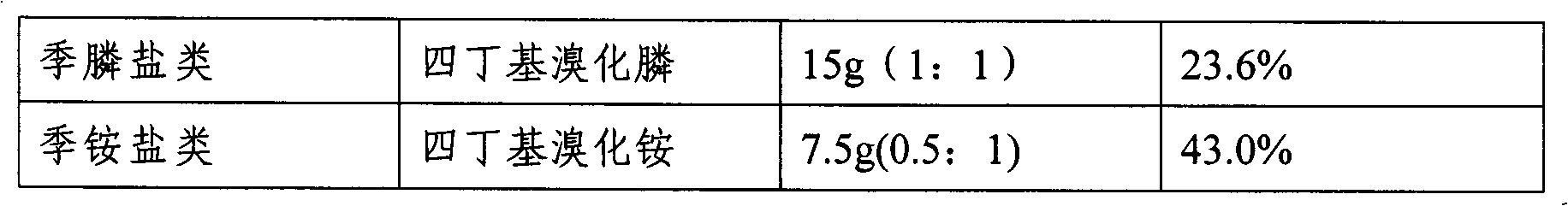
Method for preparing zoledronic acid and sodium salt thereof by utilizing phase transfer catalyst
ActiveCN102070668AHigh reaction yieldHigh yieldGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsSodium salt
The invention relates to a phase transfer catalyst used for preparing zoledronic acid and sodium salt thereof, and a method for preparing the zoledronic acid and sodium salt thereof by utilizing the phase transfer catalyst. Compared with the prior art, due to the adoption of the phase transfer catalyst, the method has the advantages that: the extent of reaction is accelerated, the reaction yield is high, and the total synthesis yield is improved by about 60 percent; and compared with the conventional method, the method at least doubles the yield at one time and has significance for productioninnovation of the zoledronic acid. Moreover, in the synthesis method, the zoledronic acid is synthesized by a one-pot method, and the method ensures high product quality and yield and has good industrial application prospect.
Owner:BENGBU BBCA MEDICINE SCI DEV
Culture method for increasing amplification efficiency and activity of Vgamma9Vdelta2T cells
InactiveCN103555666AImprove induction efficiencyHigh activityBlood/immune system cellsCell activityTyrosine-kinase inhibitor
The present invention provides a culture method for increasing amplification efficiency and activity of Vgamma9Vdelta2T cells, wherein IL-2 and zoledronic acid are combined with a tyrosine kinase inhibitor dasatinib to carry out in vitro Vgamma9Vdelta2T cell induction production to obtain the Vgamma9Vdelta2T cells with high induction efficiency and high activity. According to the present invention, the method has characteristics of simple and easy performing induction culture process, short period, low cost, high induction efficiency and good repeatability, the cells obtained through induction has enhanced activity, the tumor immunotherapy effect of the current Vgamma9Vdelta2T cells is expected to be increased with the number and the function of the induction-cultured cells, and good application potential is provided.
Owner:ZHEJIANG UNIV
Synthesis of product prepared from imidazole acted with halogenated acetate ethyl ester
InactiveCN1472215AReduce pollutionRaw materials are easy to getGroup 5/15 element organic compoundsAcetic acidCyanide
A process for snthesizing azolyphosphonic acid includes such steps as reacting of imidazo on ethyl thioacetate, hydrolyzing with acid to obtain imidazoleacetic acid, and preparing azolyphosphonic acid. Its advantages are easily available raw materials, low cost, short process, high productivity and no environmental pollution.
Owner:MAILIN HIGH TECH DEVING WUHAN CITY
Self-Assembling Nanoparticles for the Release of Bisphosphonates in the Treatment of Human Cancers
ActiveUS20140086979A1Easy to prepareHigh drug loadingPowder deliveryPeptide/protein ingredientsLymphatic SpreadProstate cancer
The present invention describe nanocomplexes also called auto-assembling nanoparticles comprising biphosphonates, lipid nanovectors and inorganic nanovectors.In particular the invention describes zoledronic acid complexed with calcium phosphate base nanoparticles; said particles in their turn mixed with lipidic particles e.g. liposomes.Said nanocomplexes are useful, and showed to be efficient in vivo, as pharmaceutical formulations of biphosphonates for the treatment or prevention of tumor growth and / or metastasis. Tumors can be solids and / or haematological such as prostate, lung, head / neck, colon, liver, breast, pancreas, kidneys, bladder, male and female urogenital tract, bones, multiple myeloma, primitive and secondary tumours of the central nervous system and lymphomas.
Owner:IST FISIOTERAPICI OSPITALERI +4
Culture method for simultaneously amplifying gamma delta T and NK
PendingCN113430167AQuality assuranceGuaranteed quantityGenetically modified cellsBlood/immune system cellsAntigenNatural Killer Cell Inhibitory Receptors
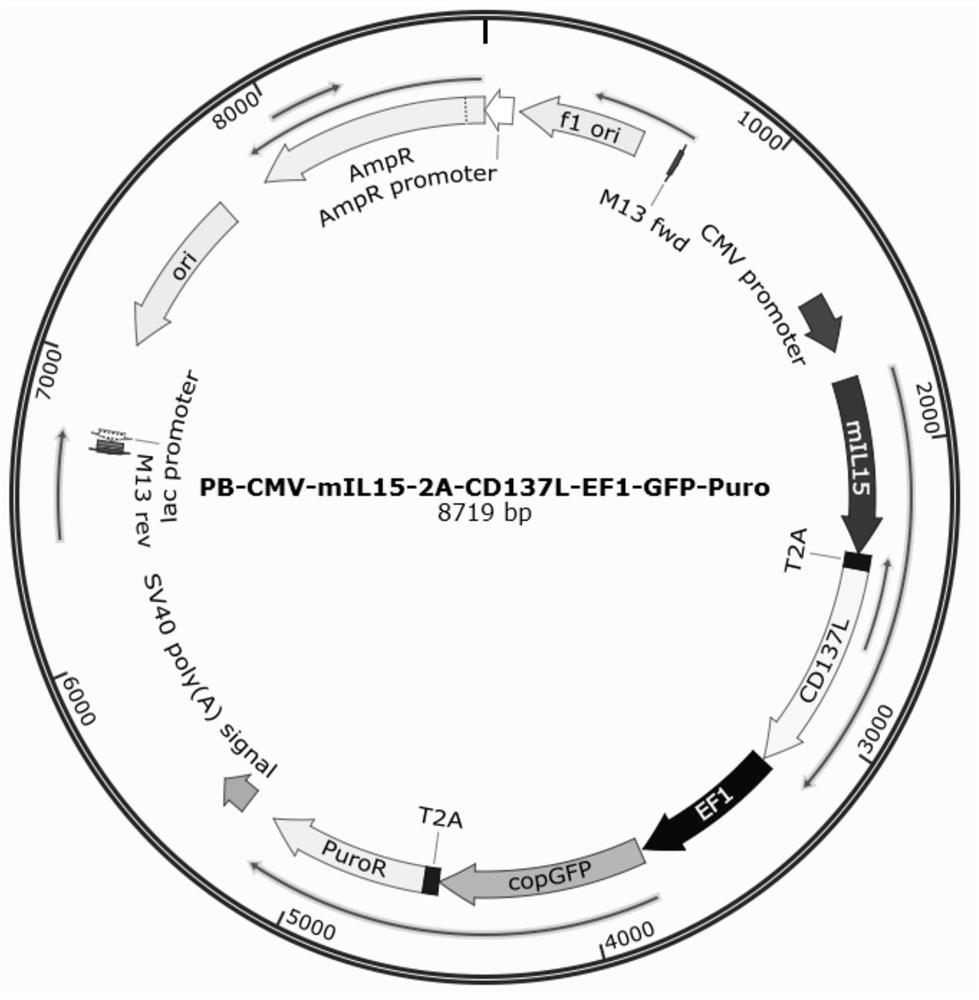
The invention relates to the field of immune medicine, in particular to a cell culture method which comprises the following steps: co-culturing an inactivated artificial antigen presenting cell with high expression of mIL15 and CD137L and PBMC in a culture medium, wherein the culture medium comprises a basic culture medium and additional components, the additional components comprise IL2 with the concentration of 100 IU / mL to 1200 IU / mL and zoledronic acid with the concentration of 3 mu M to 7 mu M. The ratio of NK cells to gamma delta T cells cultured by the method is high, and the cells have a more excellent anti-tumor effect.
Owner:GUANGZHOU BIO GENE TECH CO LTD
Kit for forecasting NSCLC (non-small-cell lung cancer) patient prognosis and application of osteopontin acting as specific marker
InactiveCN103926408AImprove long-term survival rateImproved prognosisDisease diagnosisBiological testingCell cancerImmunofluorescence
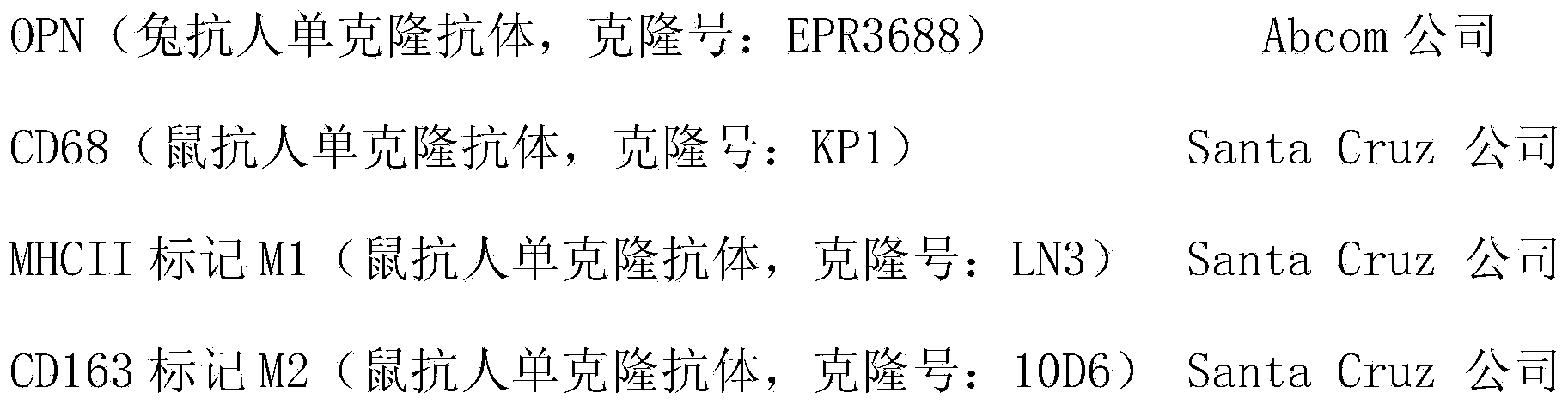
The invention discloses a kit for constructing forecasting NSCLC (non-small-cell lung cancer) patient prognosis and guiding clinic application of zoledronic acid and application of osteopontin (OPN) acting as a specific marker of M2 type macrophage; the application of the kit provided by the invention is evaluation of the NSCLC patient prognosis by using the expression of OPN in an immunofluorescence double standard technology detection tissue chip in TAMs and tumor cells, and comprehensive measures are taken in real time to interfere aiming at the detection result of the kit, so that the long-term survival rate of patients is increased, and the prognosis is improved; the method is simple and convenient and can be widely applied to clinic.
Owner:TIANJIN MEDICAL UNIV CANCER INST & HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com