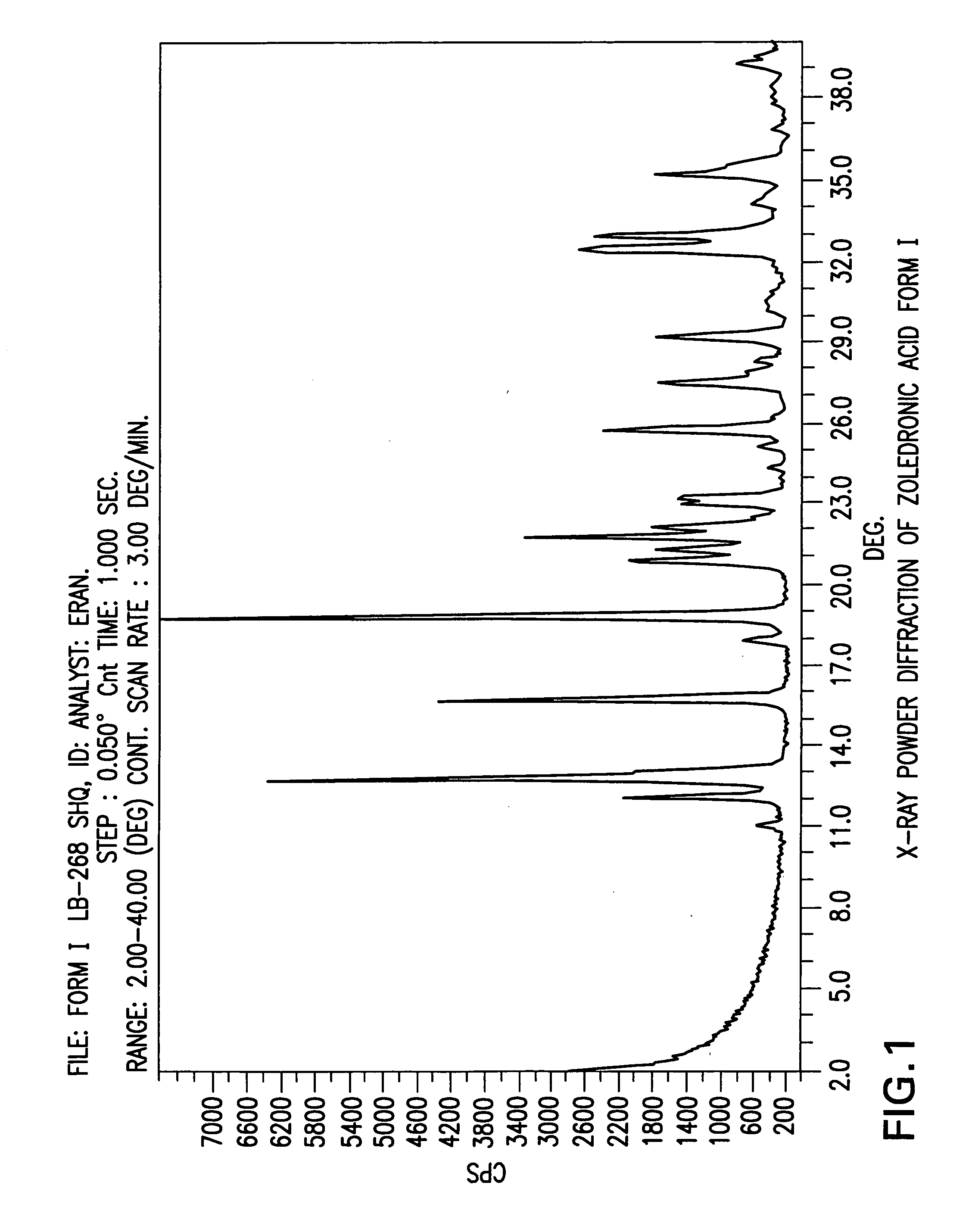
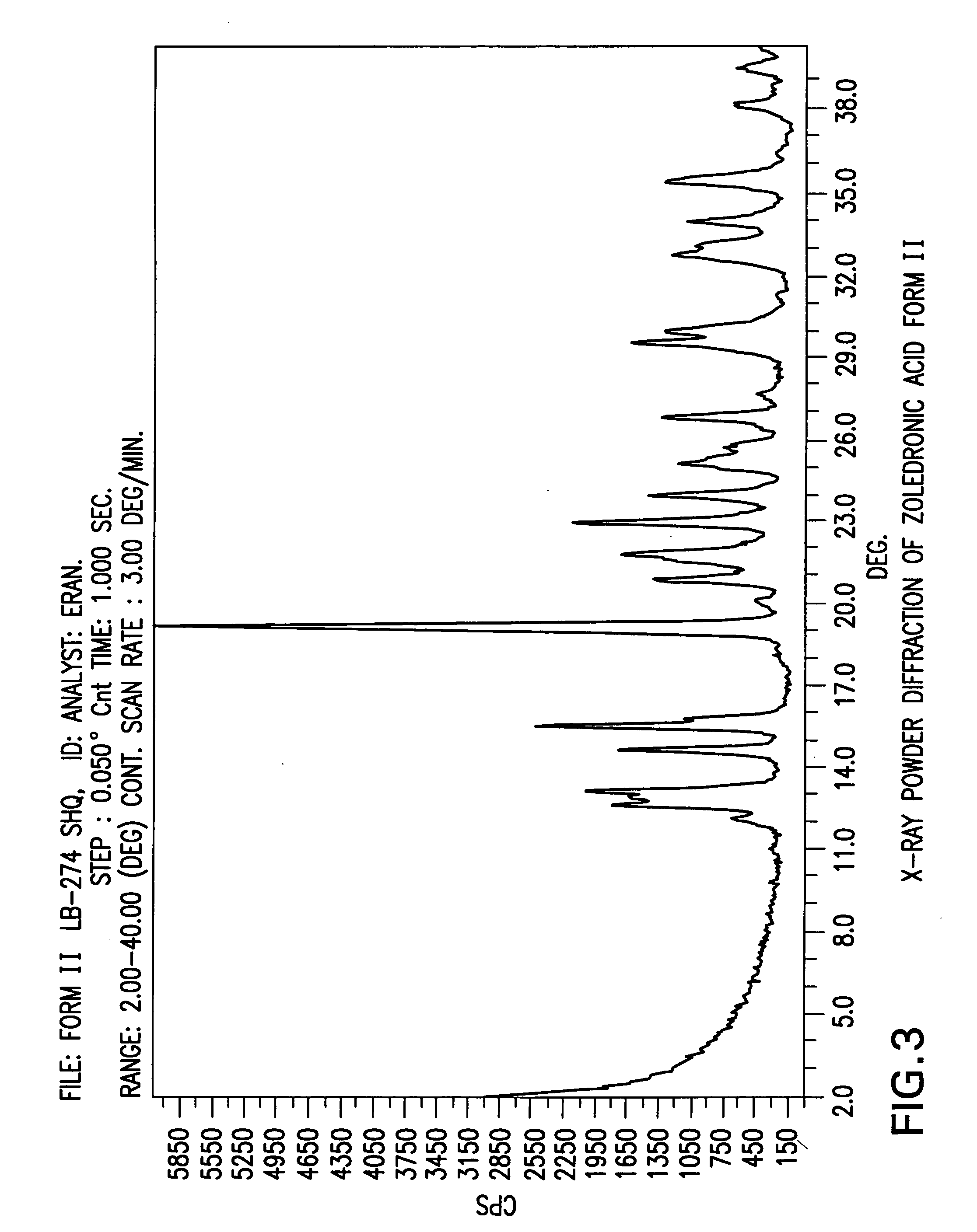
Zoledronic acid crystal forms, zoledronate sodium salt crystal forms, amorphous zoledronate sodium salt, and processes for their preparation
a technology of zoledronate sodium salt and crystal form, which is applied in the field of polymorphs of zoledronic acid and zoledronate sodium salt, amorphous zoledronate sodium salt, can solve the problems of not being able to repeat the last crystallization step exactly, and there is nothing in the literature that discloses polymorphs or different crystal forms of zoledronic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 10
Sodium hydroxide (pearls, 91.1 g) was added to a suspension of Zoledronic acid crystal form XII (200.0 g) in water (2000 ml) at room temperature (pH=14) to obtain a clear solution. Then the pH of the solution was adjusted to pH 1 by addition of 32% aqueous HCl (300 ml). The solution was then cooled gradually to 5° C. and the white precipitate was filtered, washed with cold water (2×150 ml) and dried in a vacuum oven at 50° C. for 24 hours to obtain 161.7 g (84%) of Zoledronic acid crystal form I (LOD by TGA=6.7%). Purity by HPLC 99.9%.
Preparation of ZLD-Ac Crystal Form II
example 11
A 250 ml three-necked flask equipped with a mechanical stirrer, a reflux condenser and a dropping funnel, was loaded with 1-Imidazoleacetic acid hydrochloride (4.9 g, 0.03 mole), phosphorous acid (4.9 g, 0.06 mole) and Silicon oil (Merck) (27 ml). The suspension was heated to 75° C. and phosphorous oxychloride (10.5 ml, 0.11 mole) was added drop-wise during 30 minutes. The reaction mixture was then heated to 80° C. for 27 hours. Then water (27 ml) and toluene (30 ml) were added at 80° C. The mixture was stirred vigorously for about 15 minutes. Then the toluene phase (containing the silicon oil) and the aqueous phase were separated. The aqueous phase was put in a clean flask and heated to 90° C. for 16 hours. Then it was cooled to room temperature and absolute Ethanol (27 ml) was added during the cooling process to obtain a white precipitate immediately. The mixture was stirred at 5° C. for 4 hours. The white product was then filtered, washed with absolute Ethanol (2×15 ml) and drie...
example 12
Zoledronic acid crystal form I (2.0 g) was stirred in Toluene (20 ml) at reflux temperature for 14 hours. Then the suspension was cooled to room temperature, filtered, washed with Toluene (1×15 ml) and dried in a vacuum oven at 50° C. for 24 hours to obtain 1.6 g of Zoledronic acid crystal form II.
Preparation of ZLD-Ac Crystal Form XII
PUM
| Property | Measurement | Unit |
|---|---|---|
| 2θ | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com