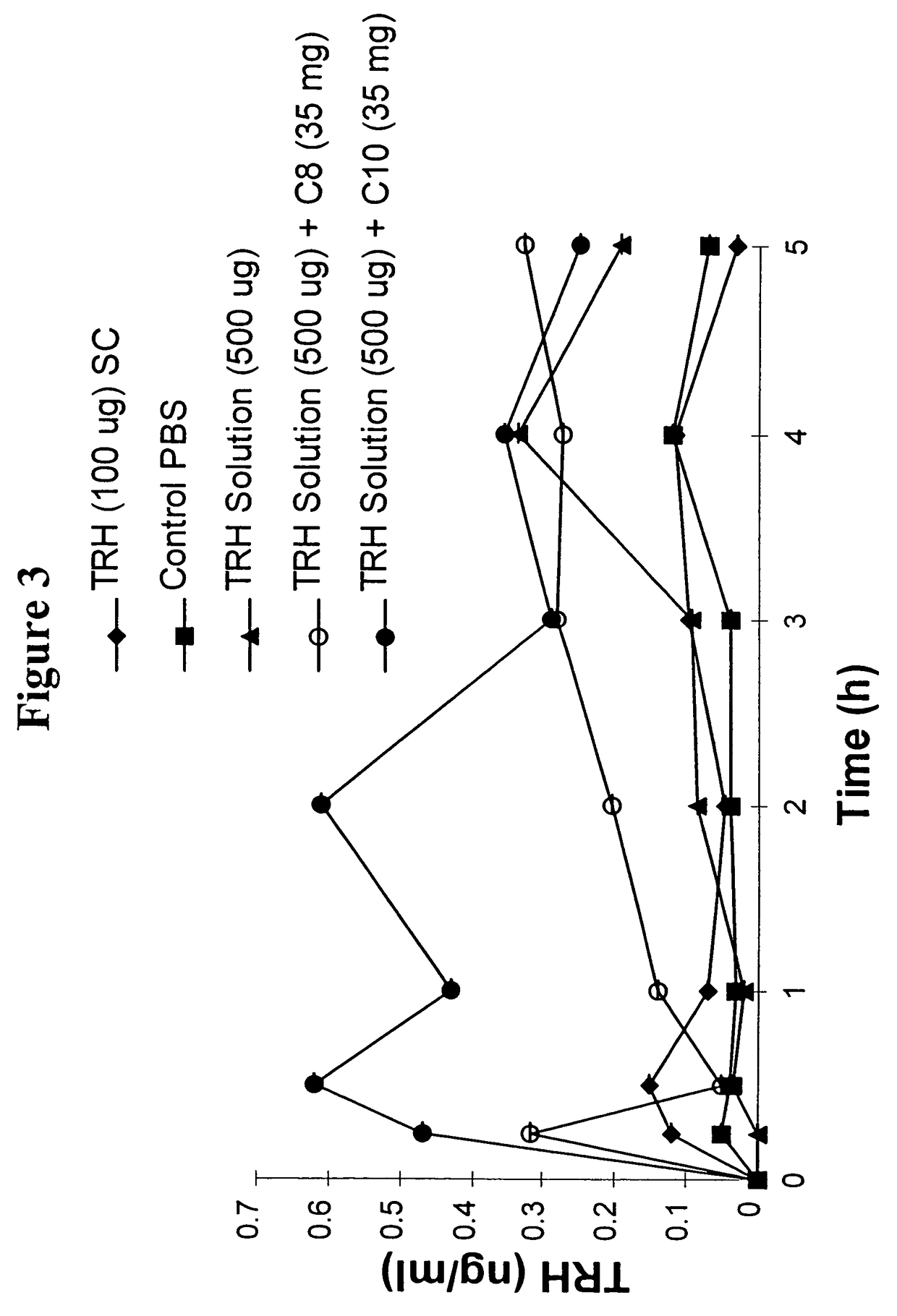
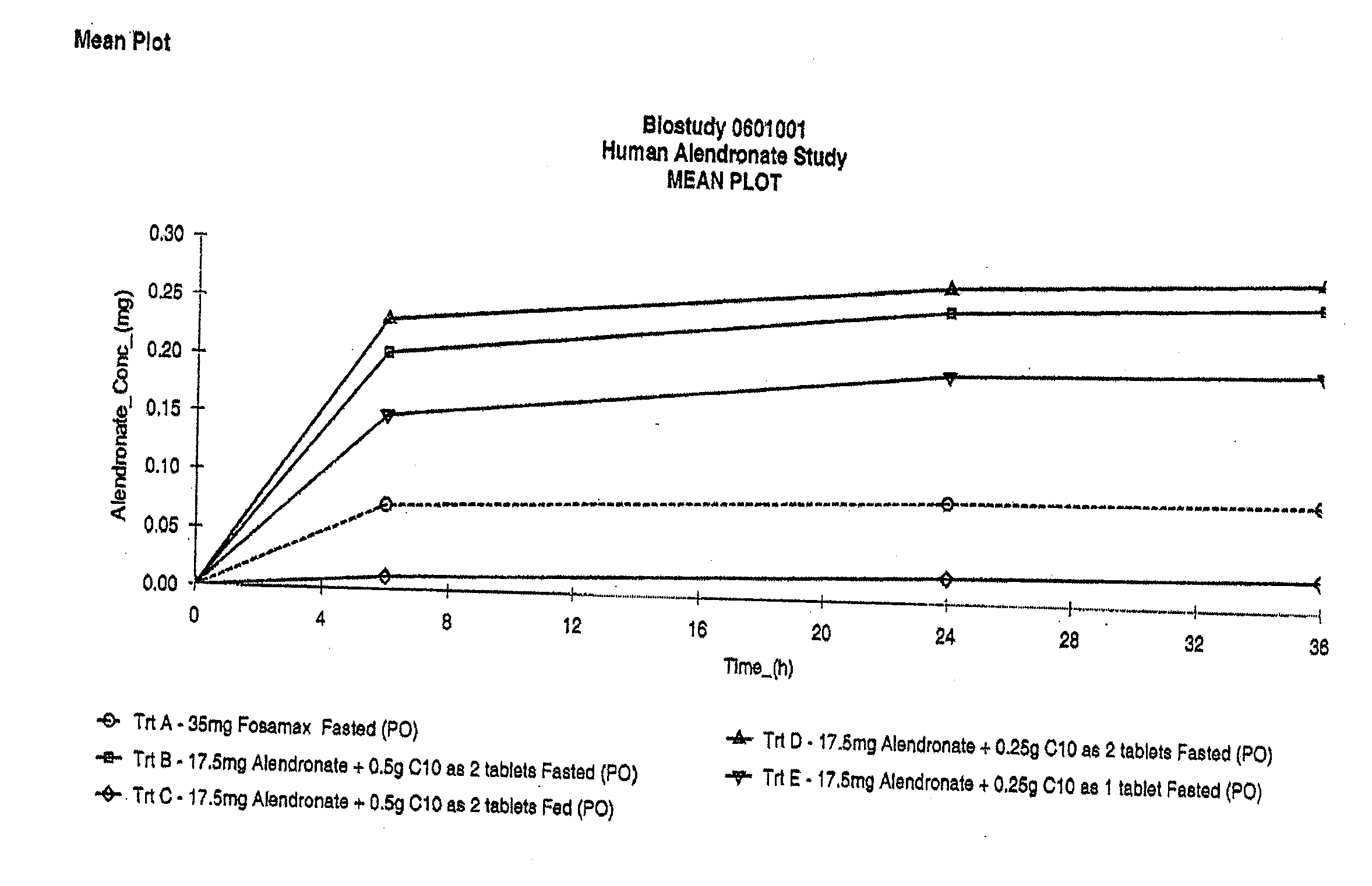
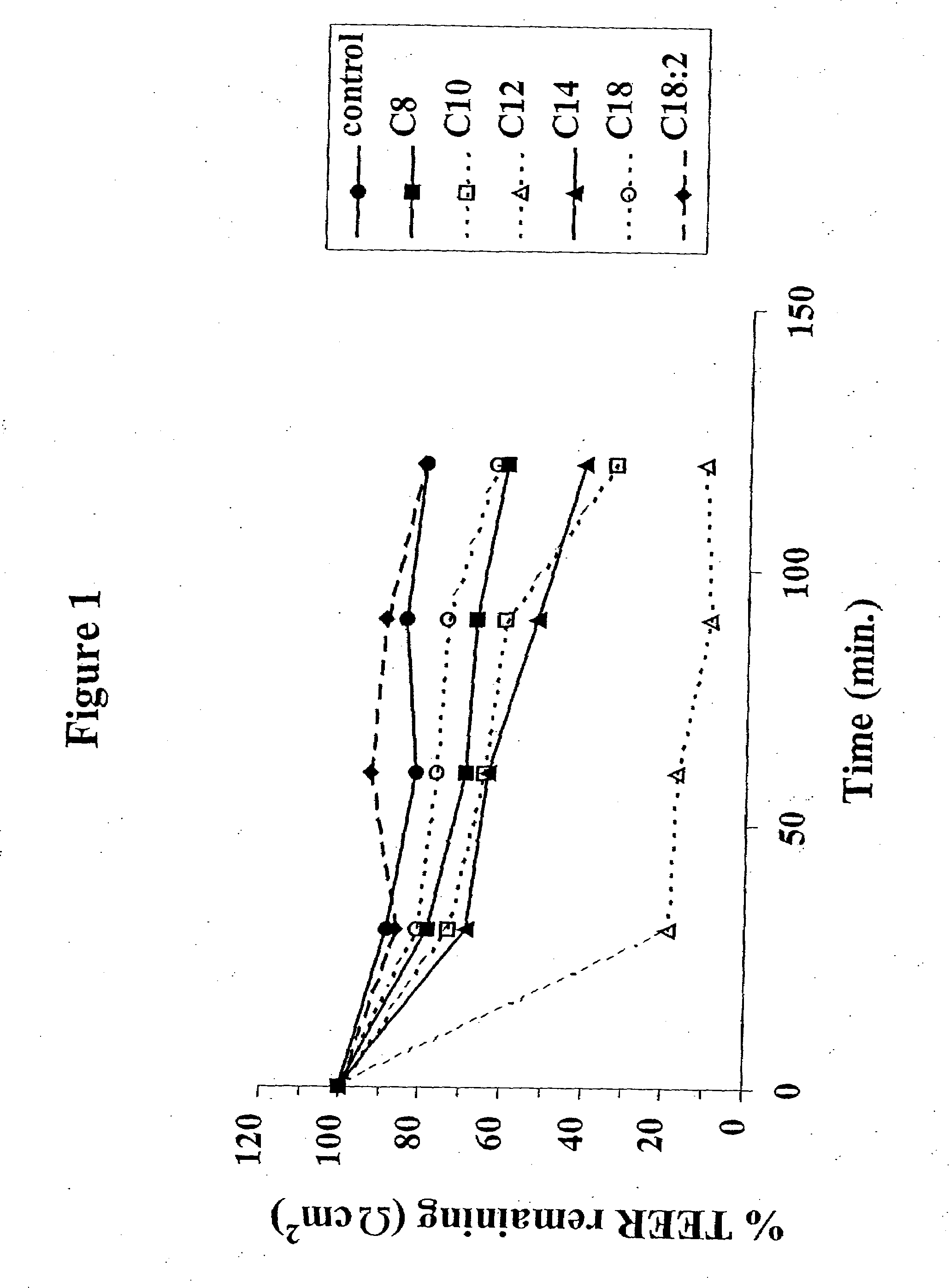
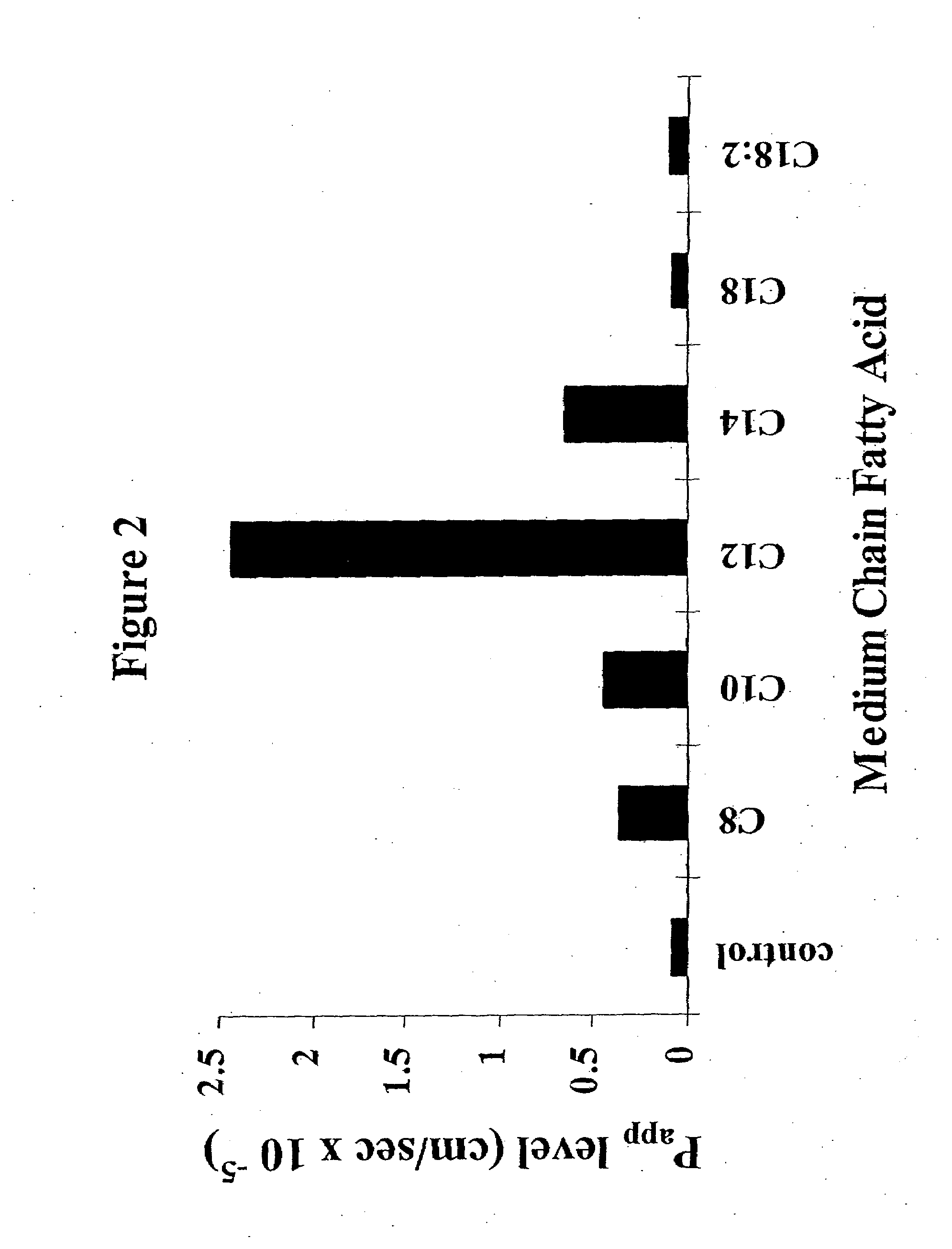
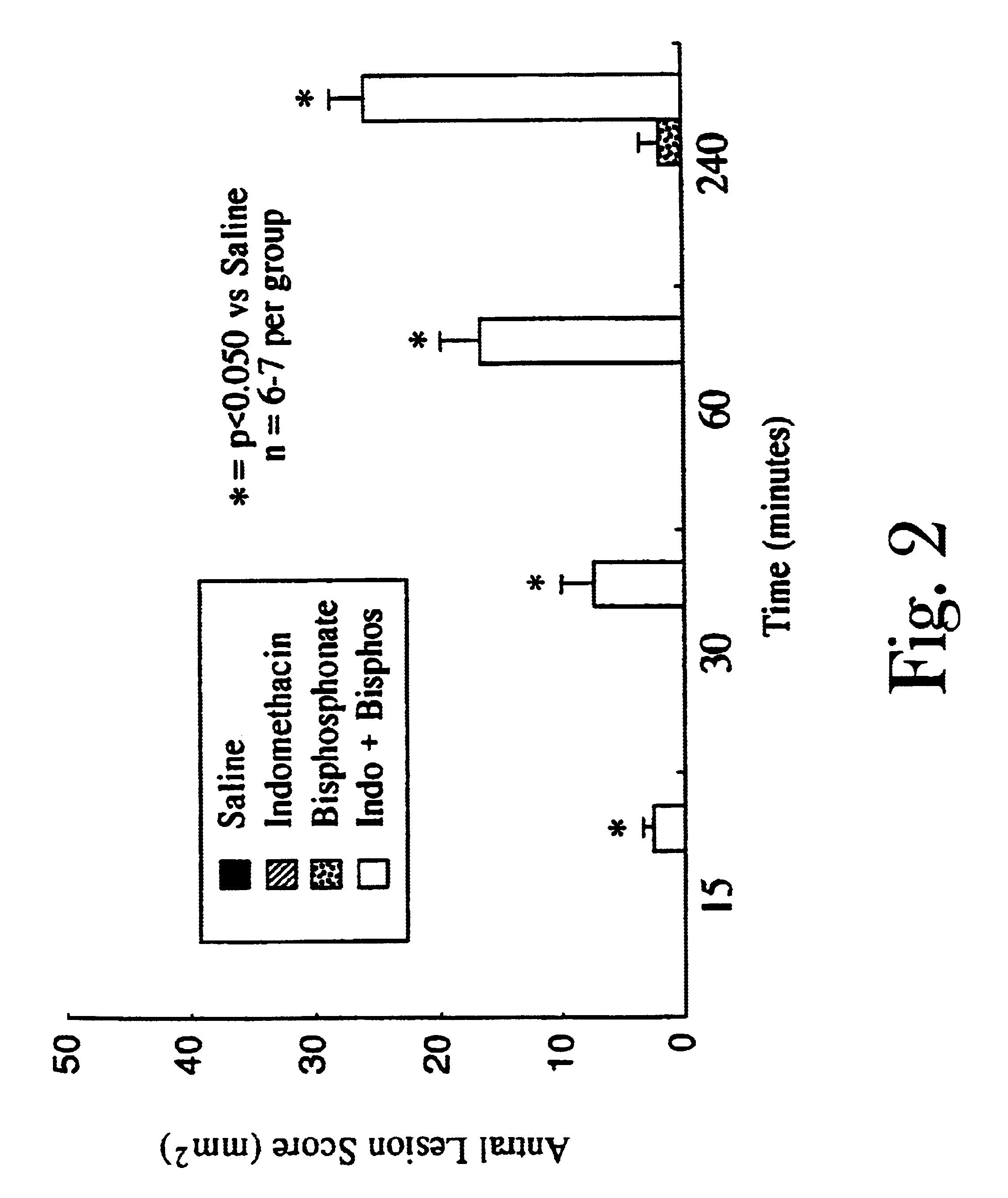
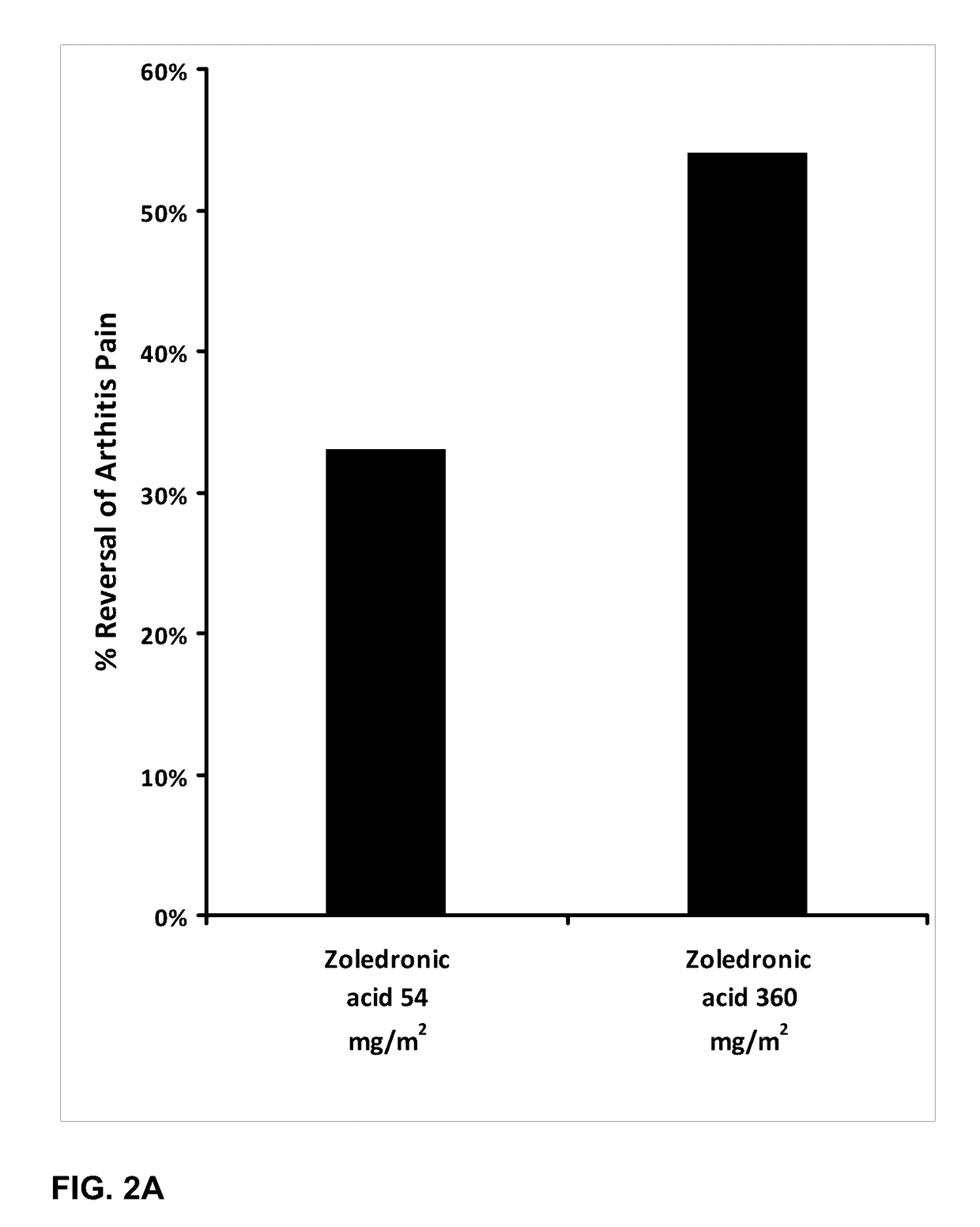
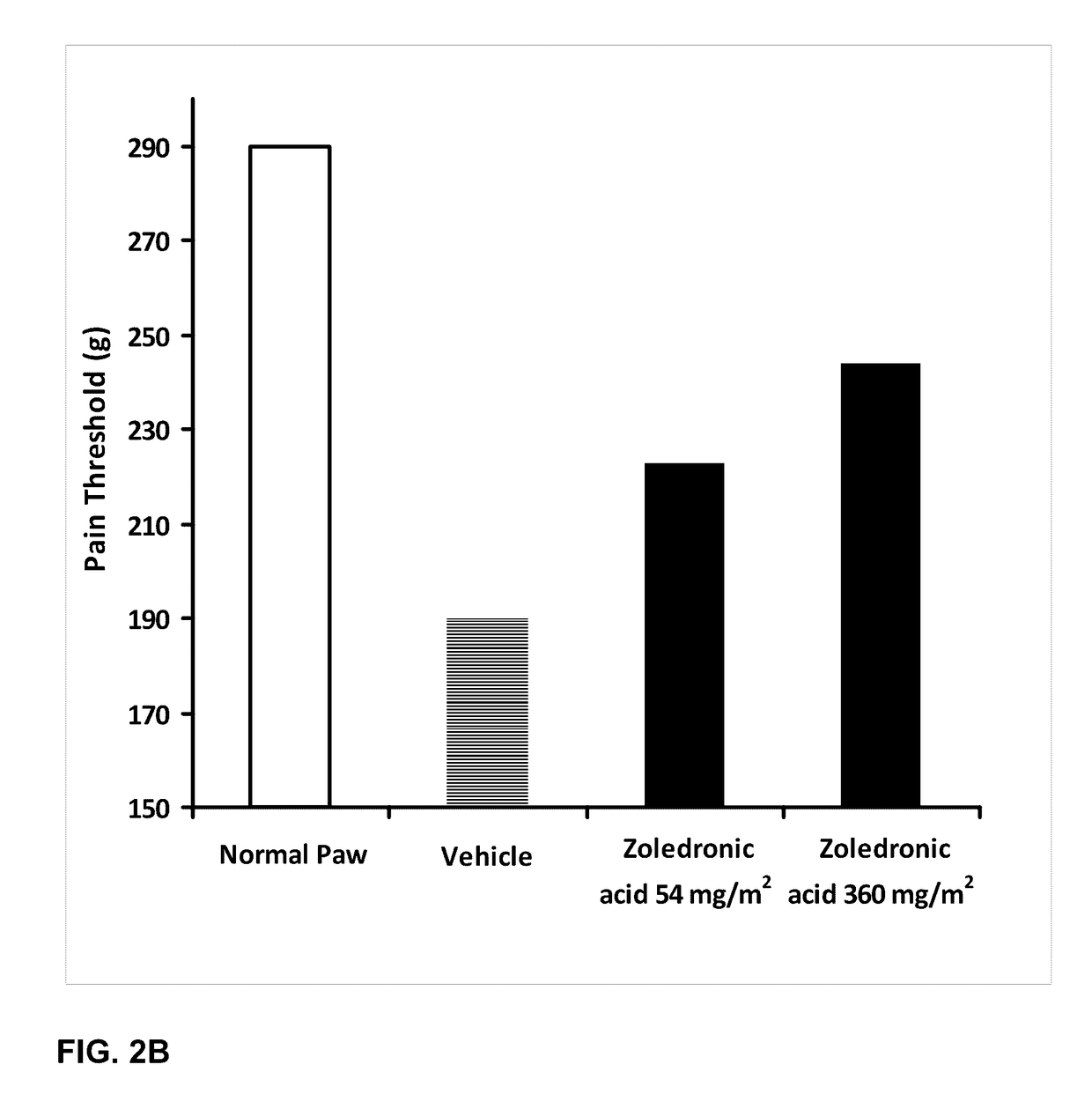
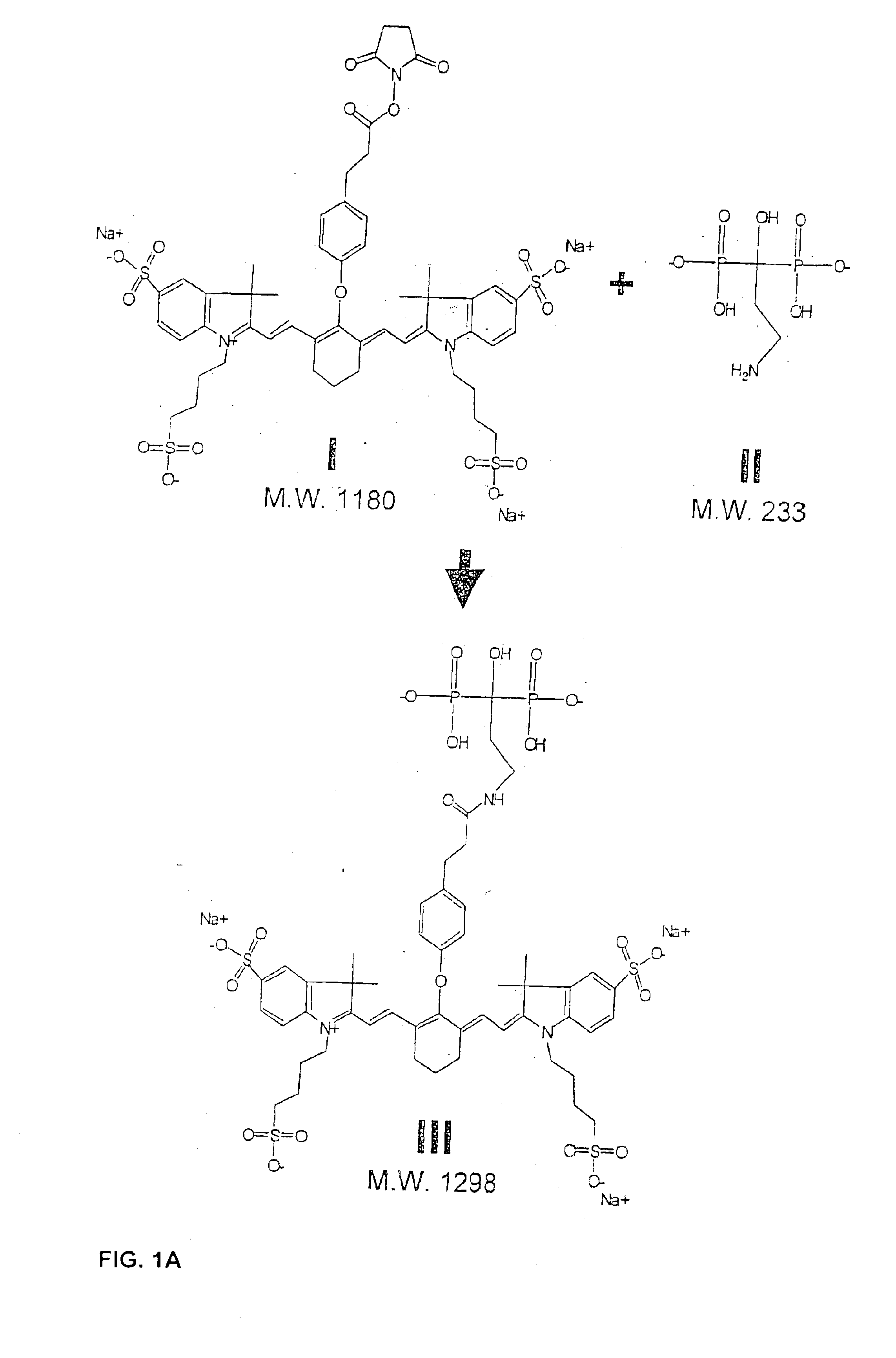
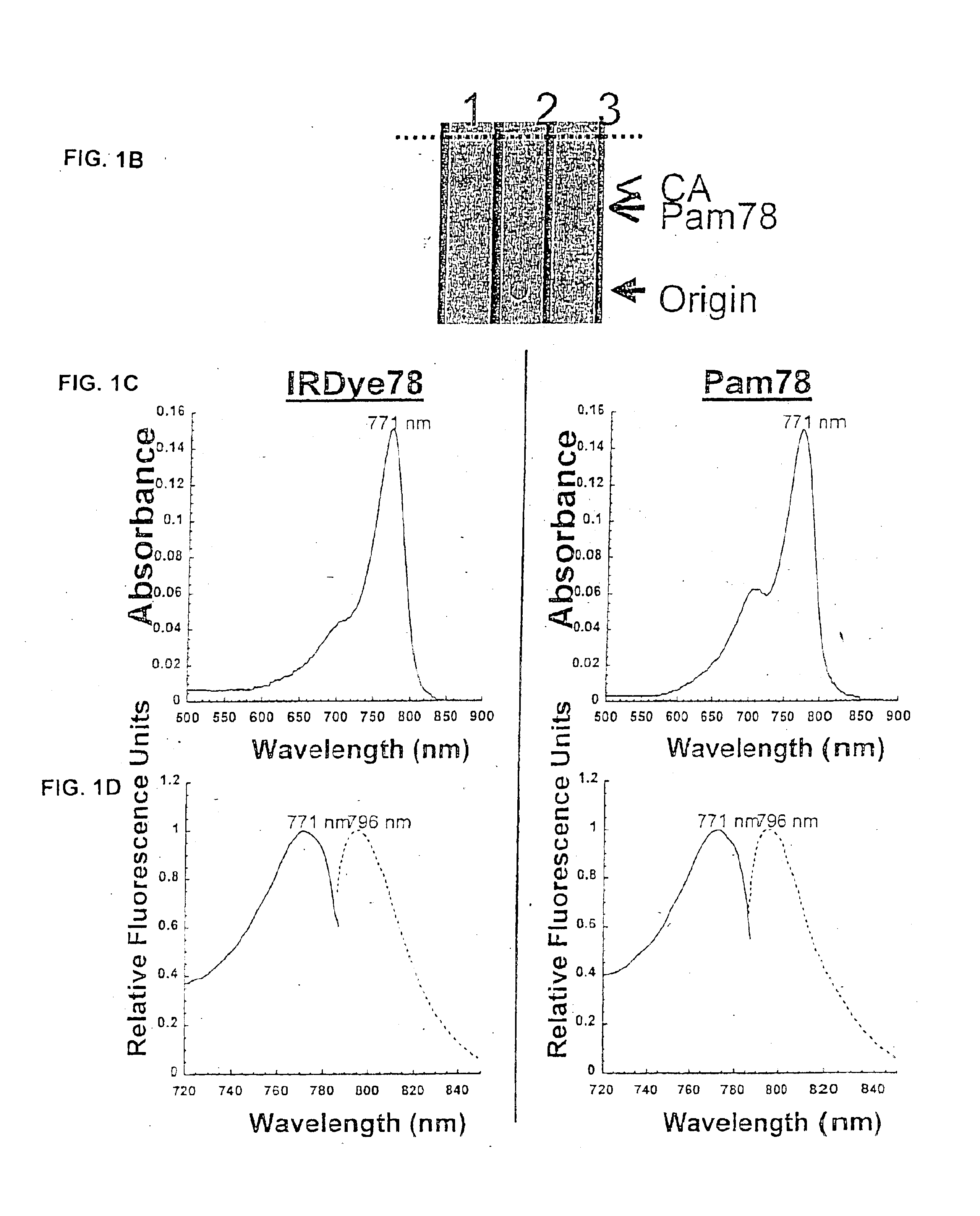
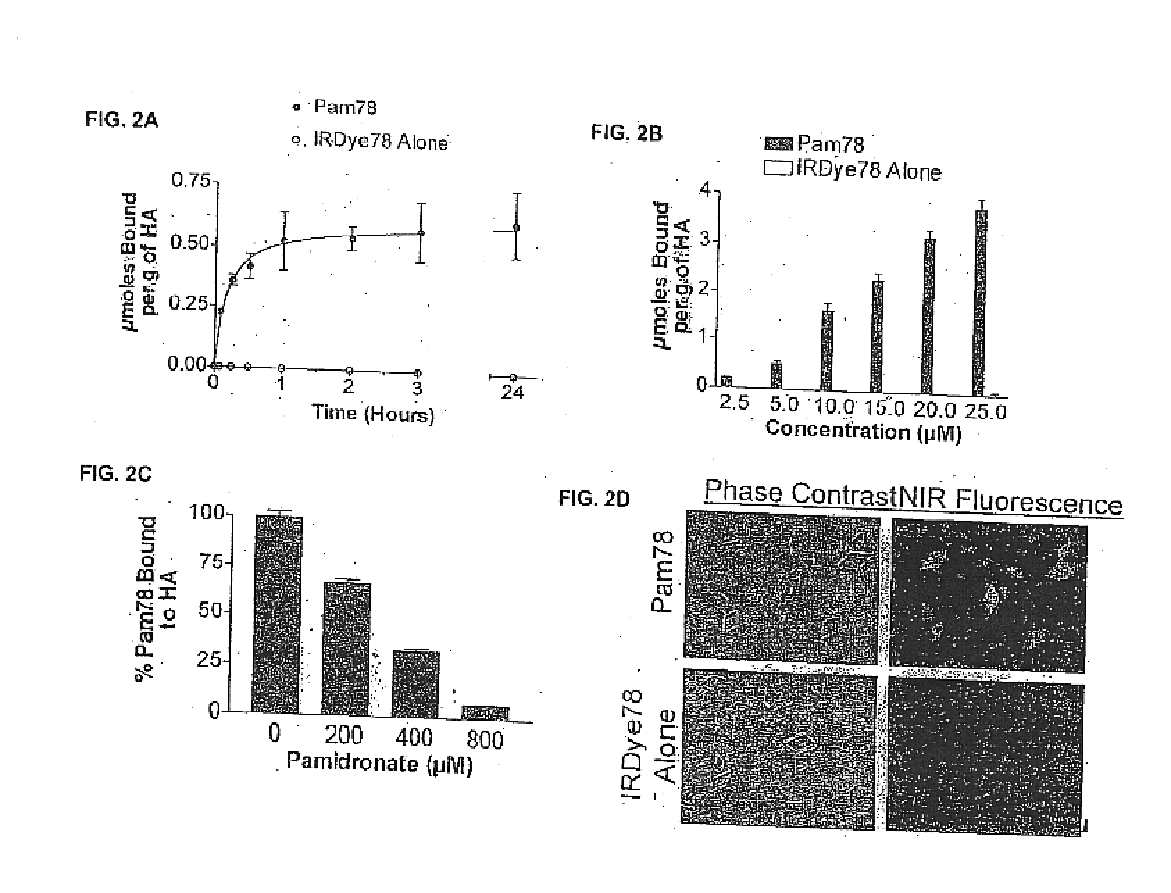
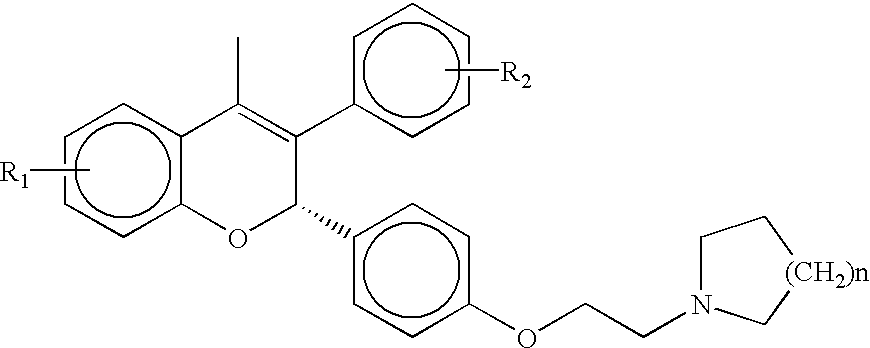
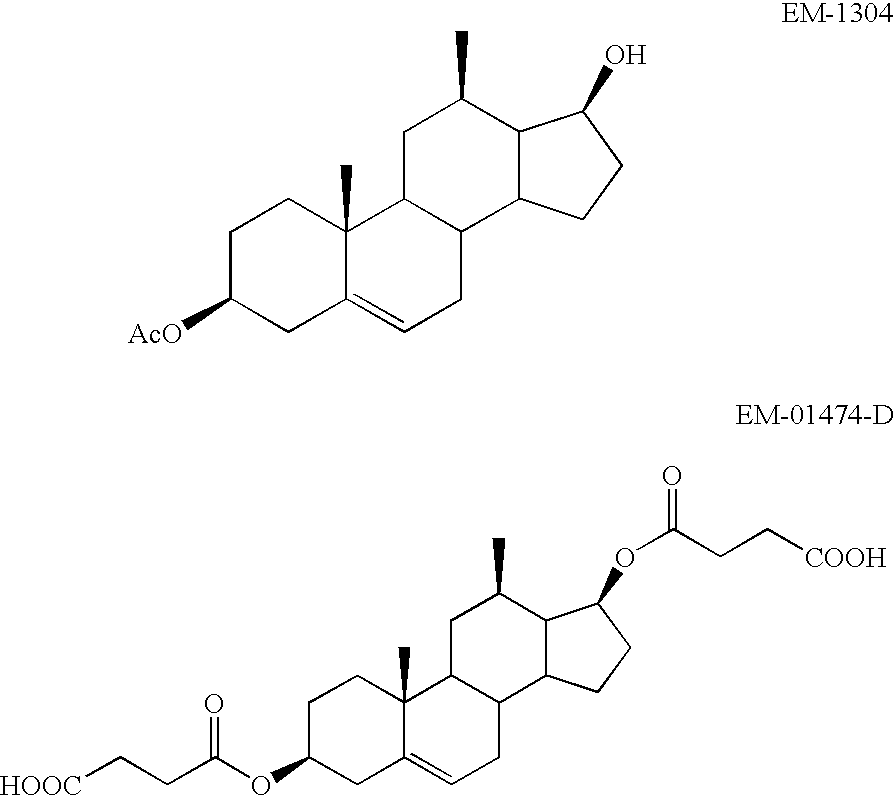
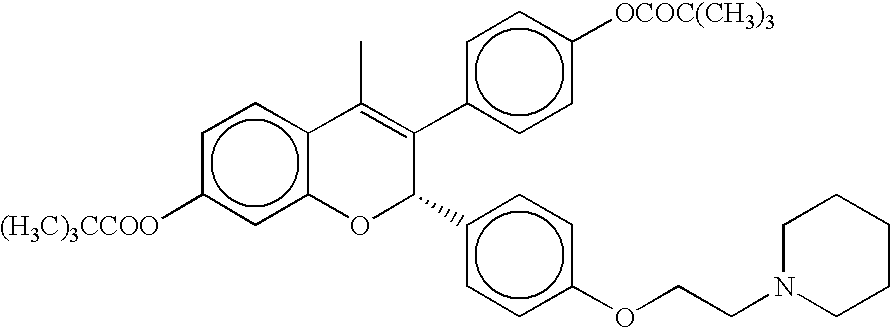
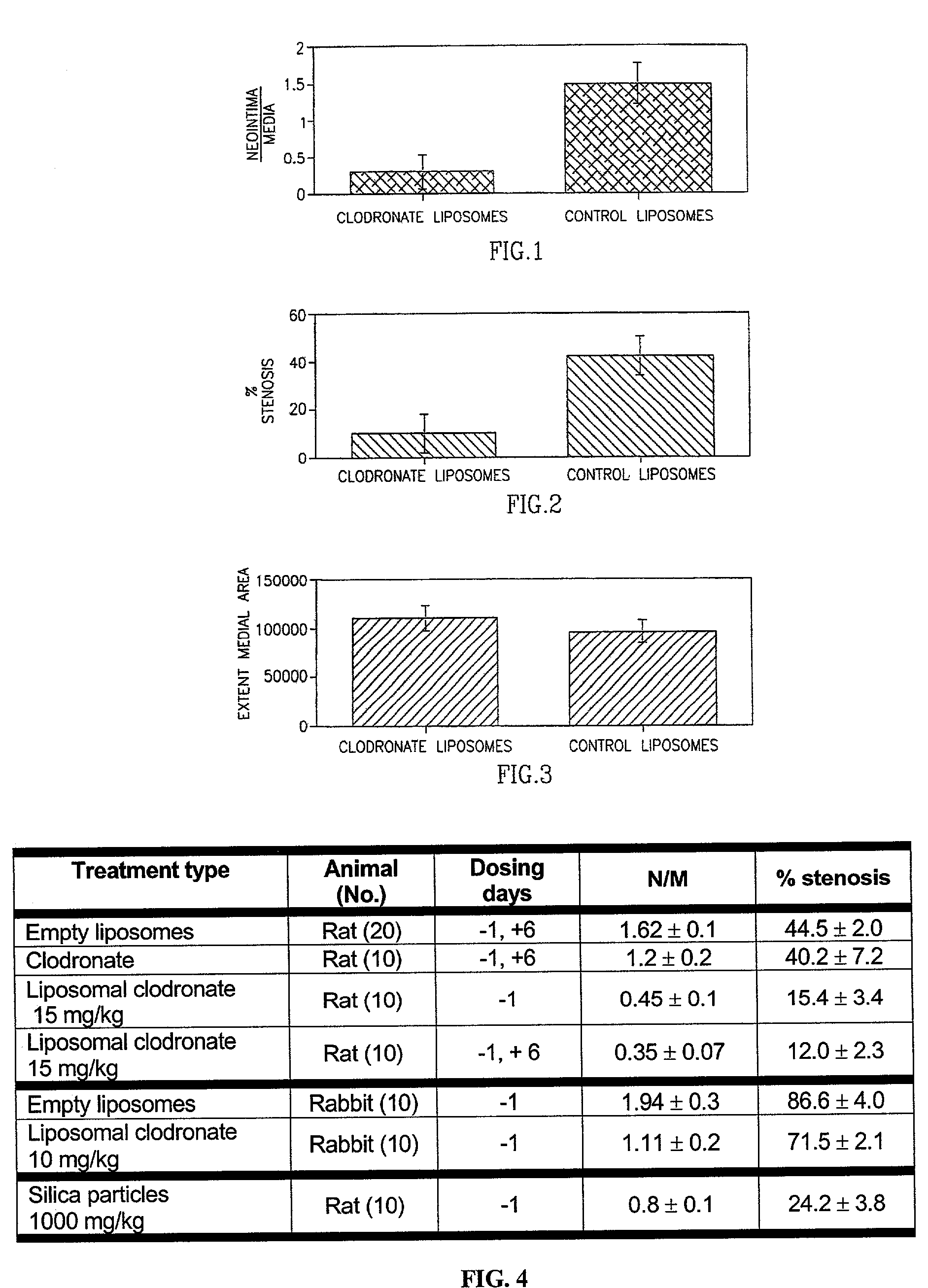
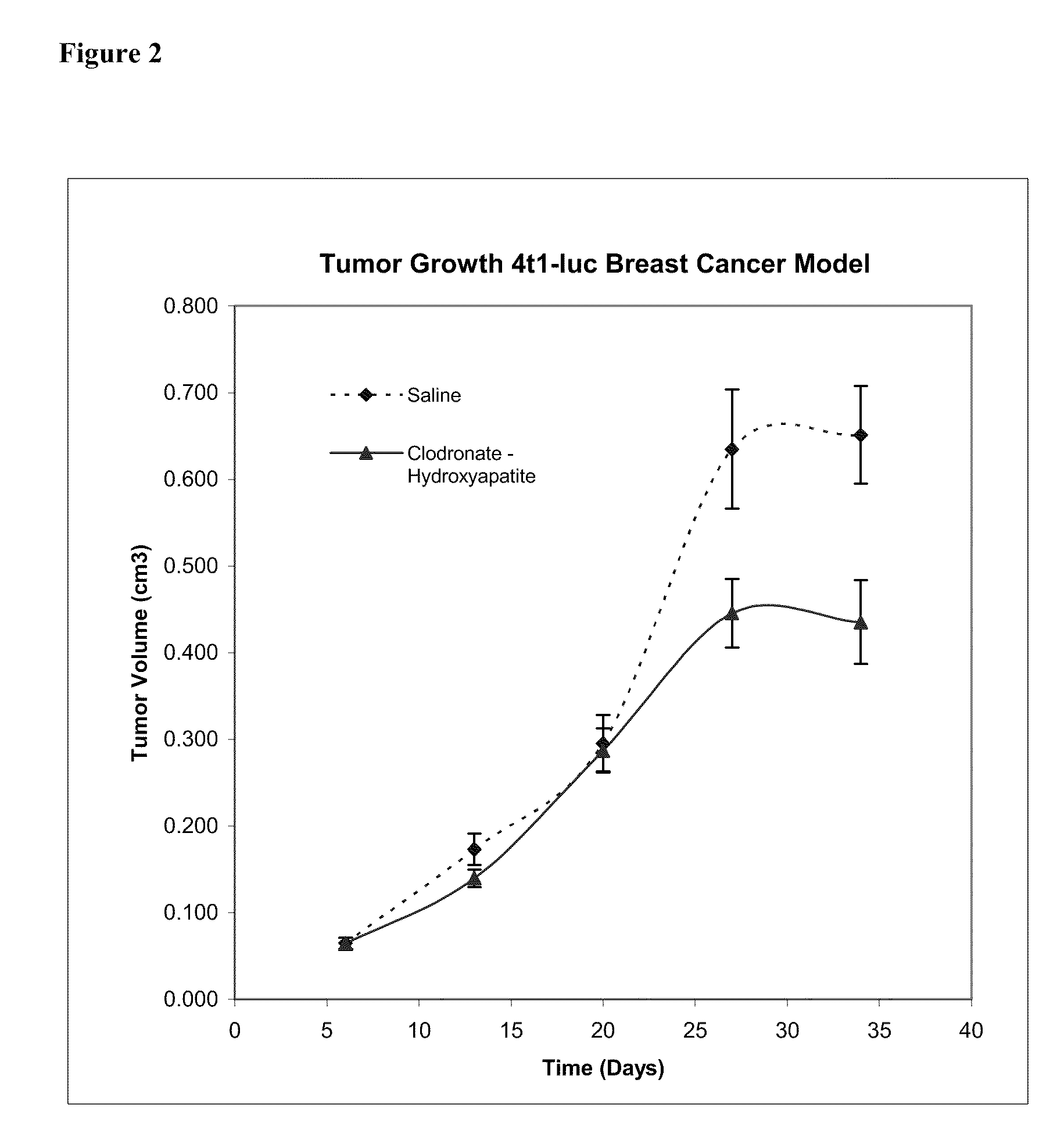
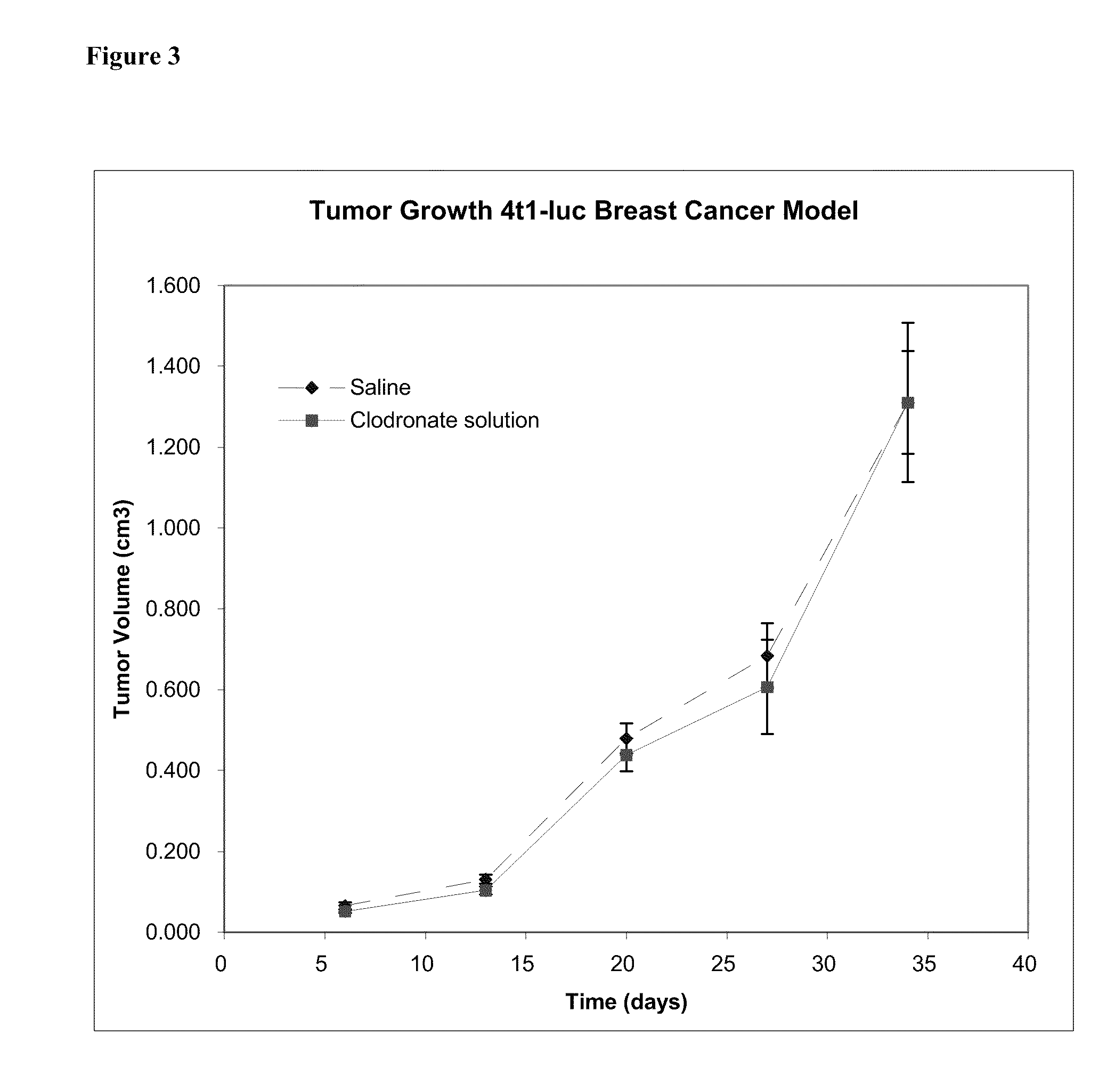
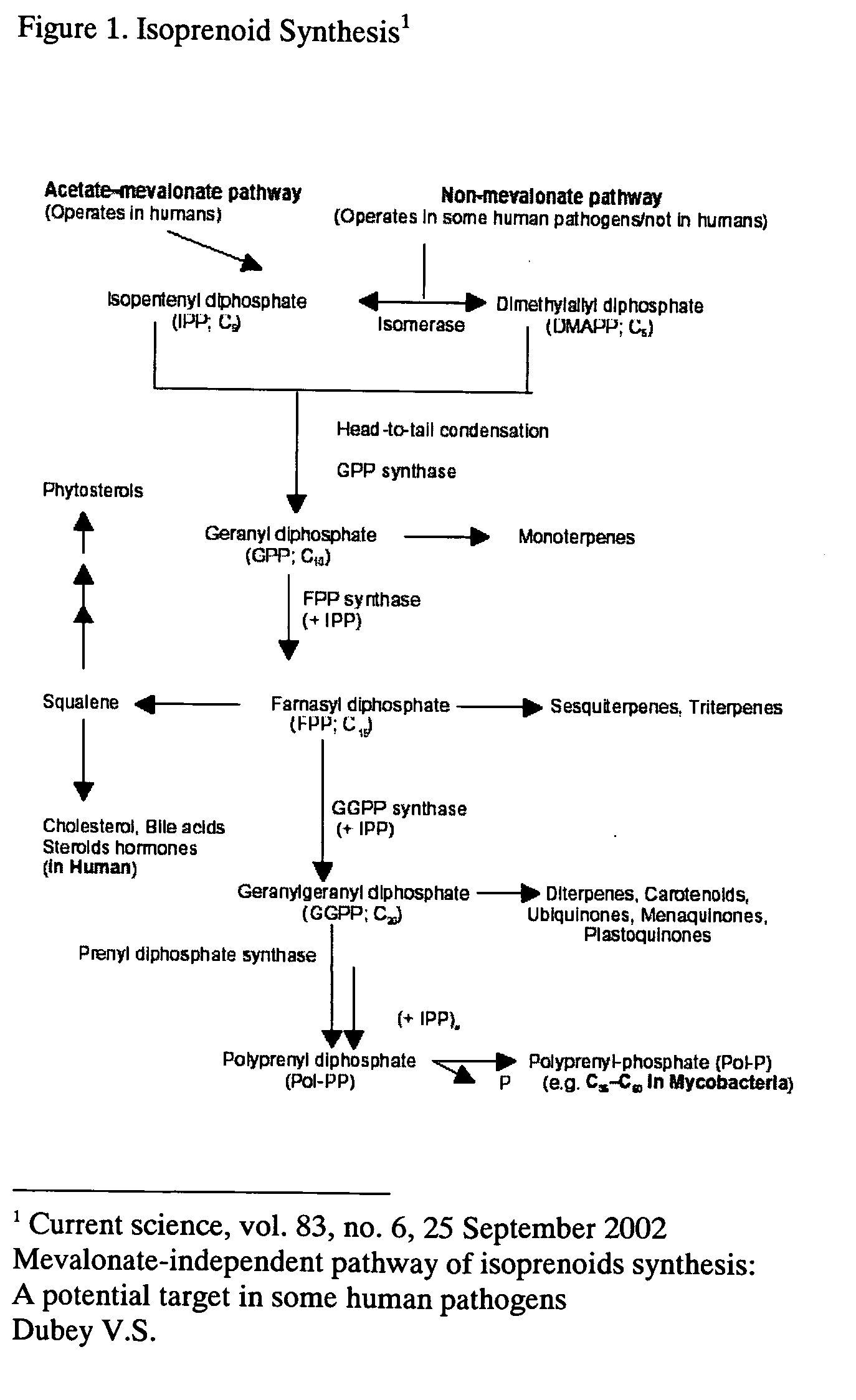
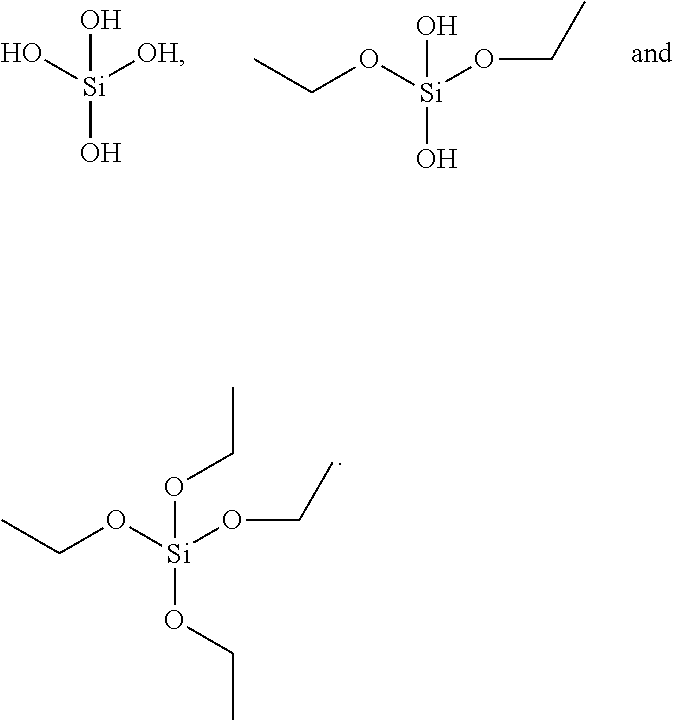
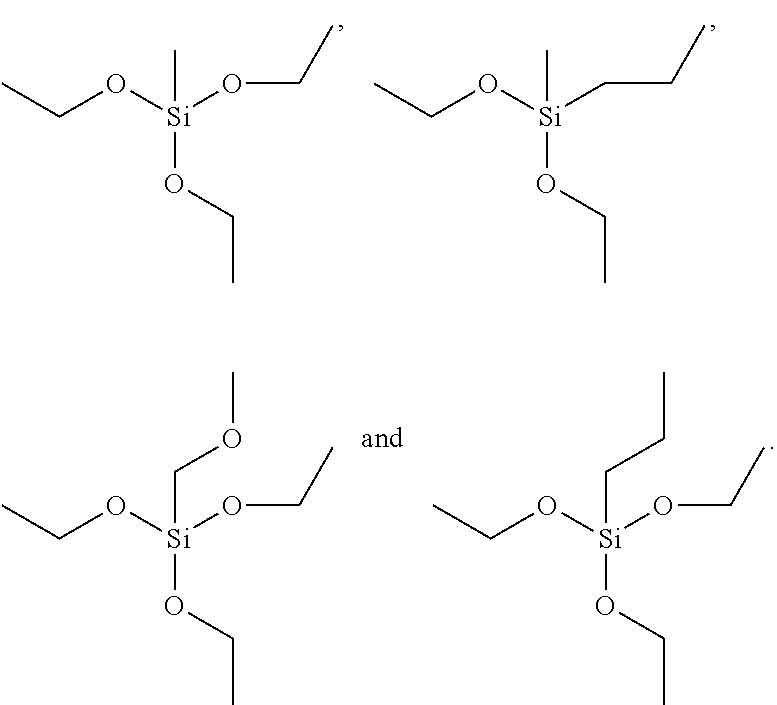
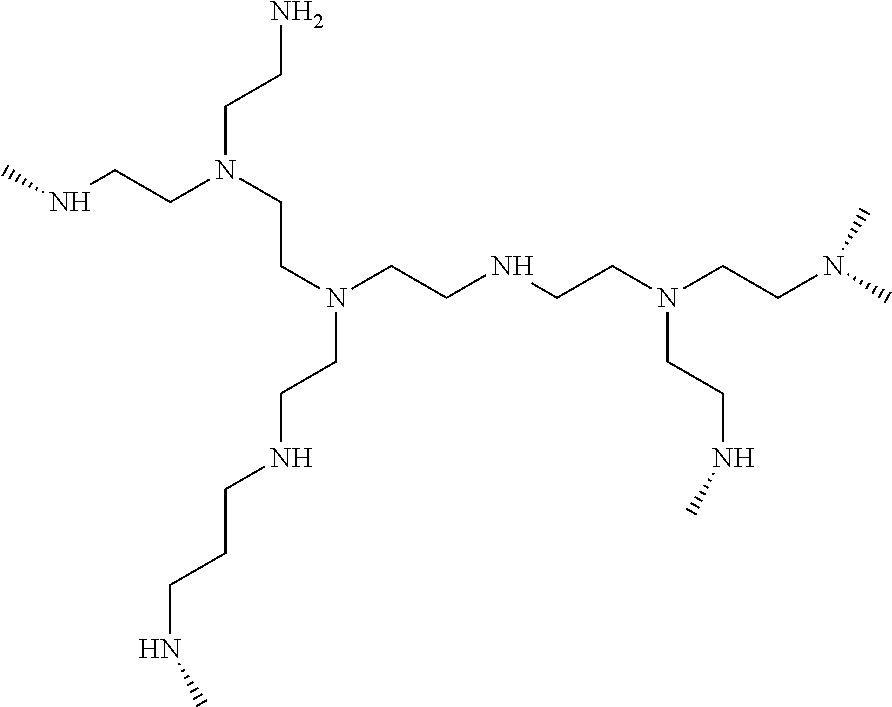
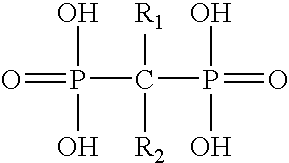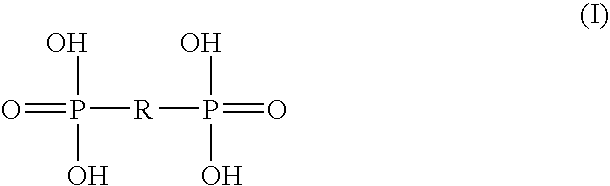Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
265 results about "Oral bisphosphonates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
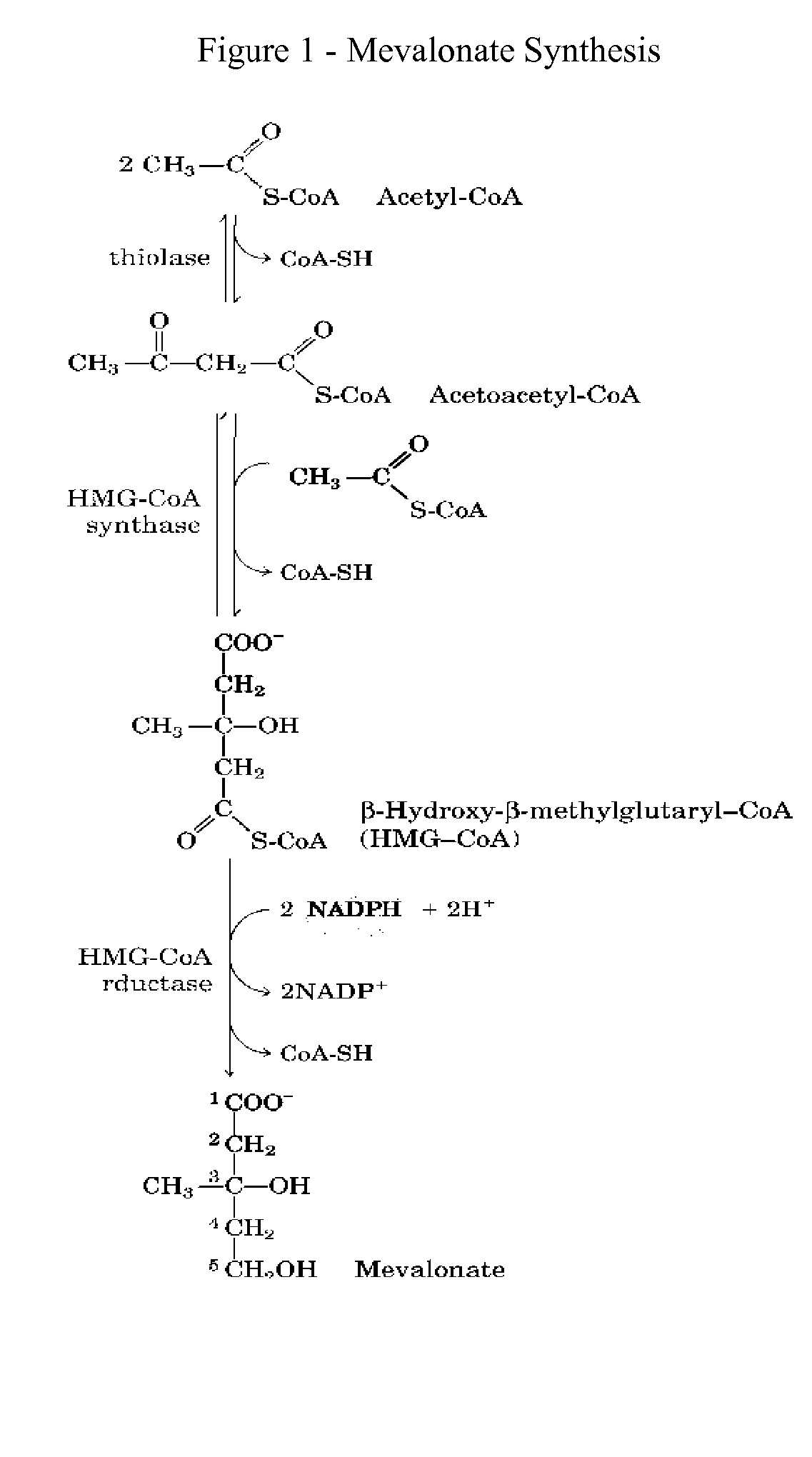
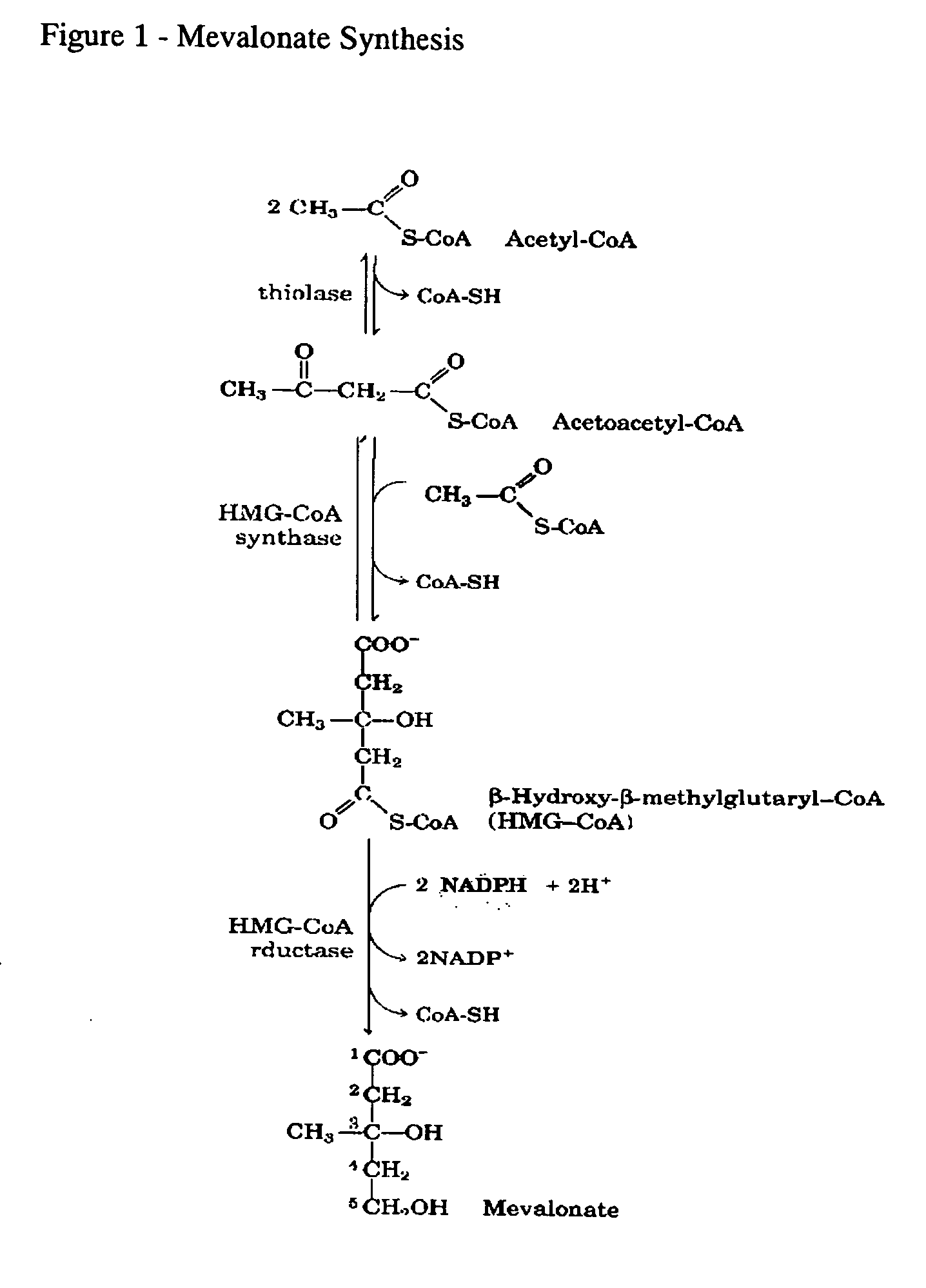
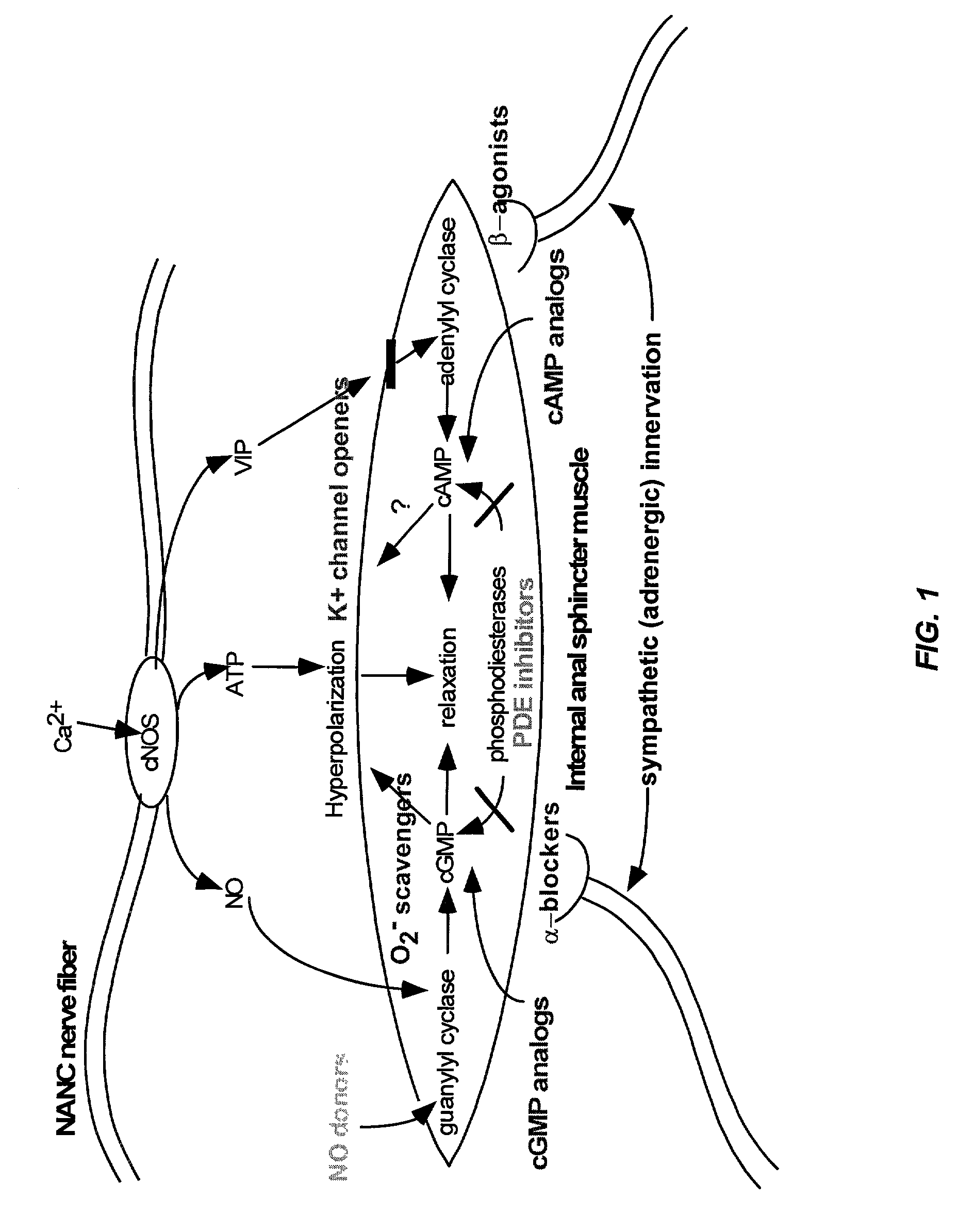
Of the bisphosphonate that is resorbed (from oral preparation) or infused (for intravenous drugs), about 50% is excreted unchanged by the kidney. The remainder has a very high affinity for bone tissue, and is rapidly adsorbed onto the bone surface.
Method of prevention and treatment of aging, age-related disorders and/or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimers disease and cancer
InactiveUS20060275294A1Halogenated hydrocarbon active ingredientsBiocideAbnormal tissue growthSTAT Transcription Factors
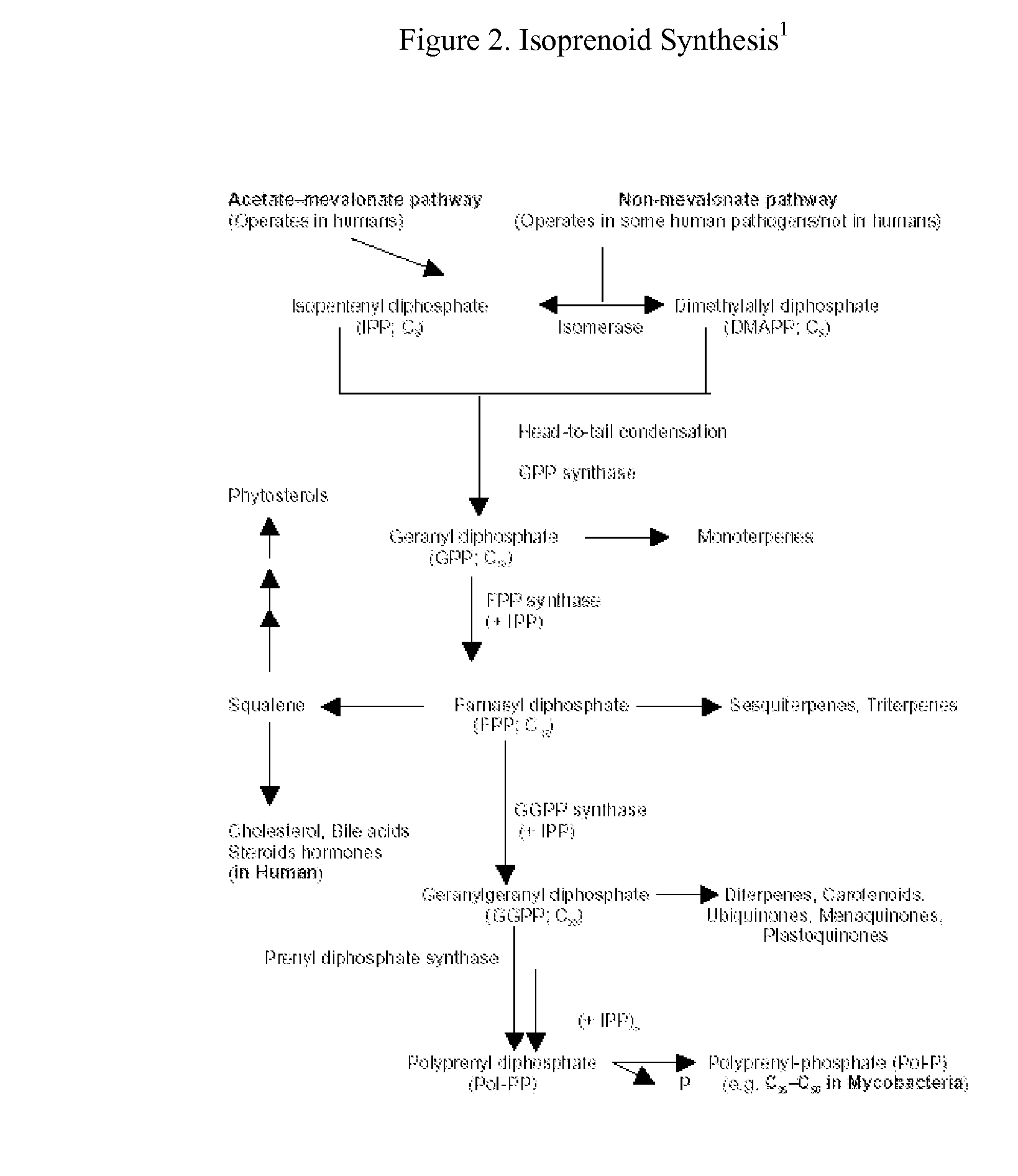
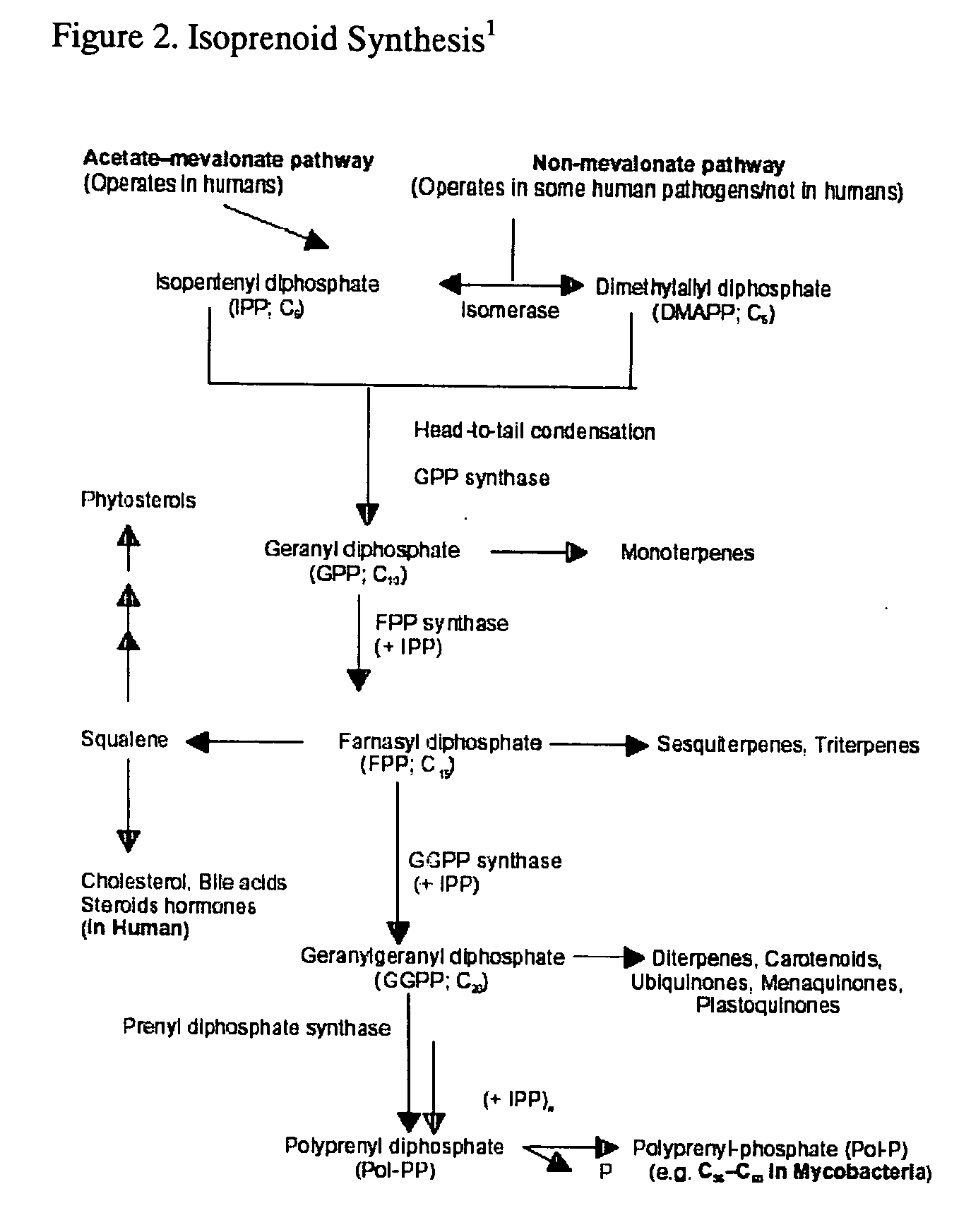
This invention relates to a method for prevention and treatment of aging, age-related disorders and / or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging, age-related disorders and / or age-related manifestations including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway
Owner:OMOIGUI OSEMWOTA SOTA
Composition and drug delivery of bisphosphonates
InactiveUS20100215743A1Reduce adverse effectsBiocideOrganic active ingredientsMedicineDiphosphonates
The present invention provides methods of treating or preventing a medical condition that is responsive to a bisphosphonate compound in a subject. The methods comprise administering to the subject a pharmaceutical composition comprising a therapeutically effective amount of the bisphosphonate no less frequently than a bi-weekly dosage schedule. In some embodiment, the bisphosphonate compound is zoledronic acid.
Owner:NOVO NORDISK AS
Method of prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimer's disease and cancer
This invention relates to a method for prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging and age-related disorders including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway.
Owner:OMOIGUI OSEMWOTA SOTA
Dosage forms of bisphosphonates
ActiveUS20050260262A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyUpper gastrointestinal
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Solid oral dosage form containing an enhancer
InactiveUS8119159B2Minimizes risk of local irritationImprove oral bioavailabilityBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
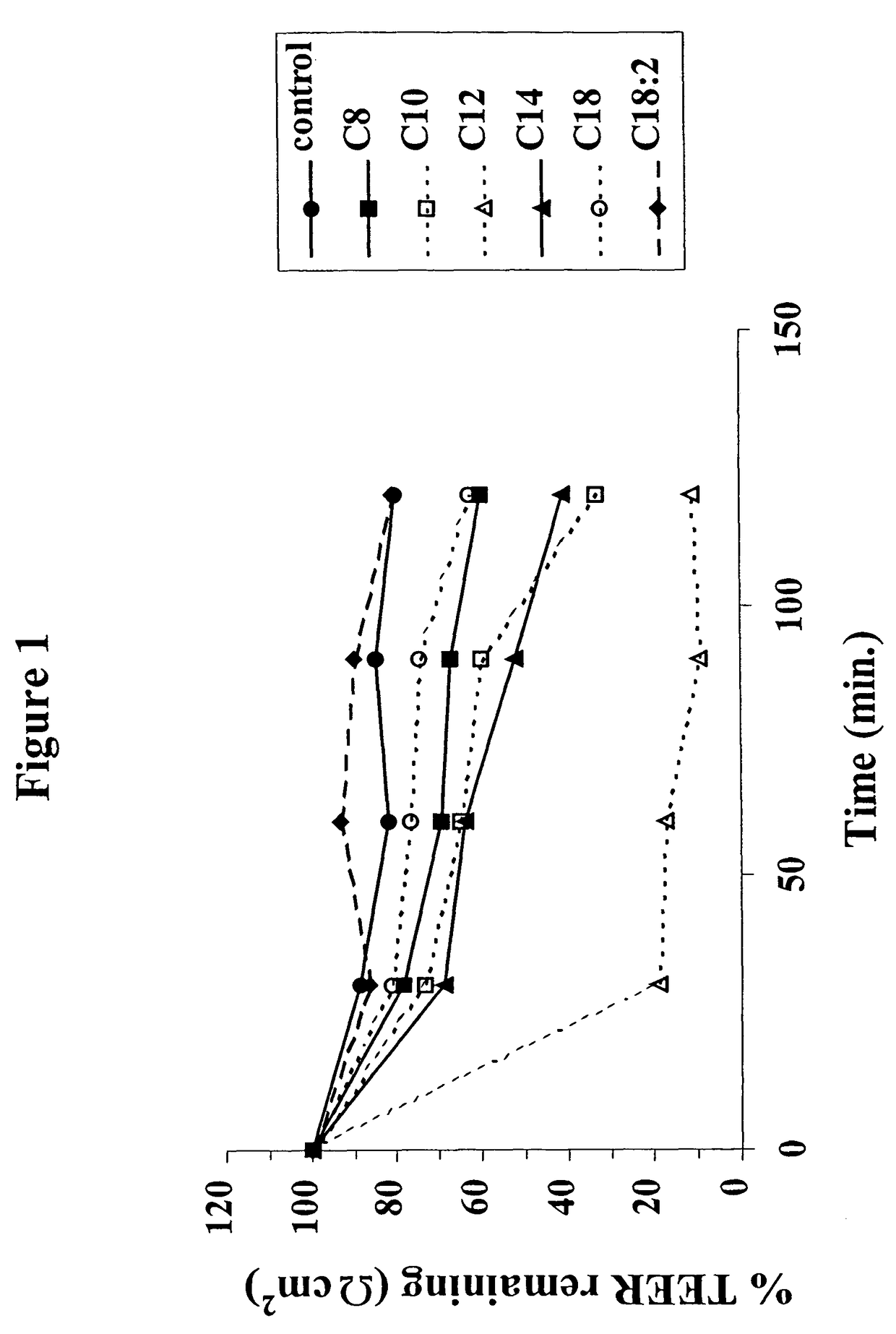
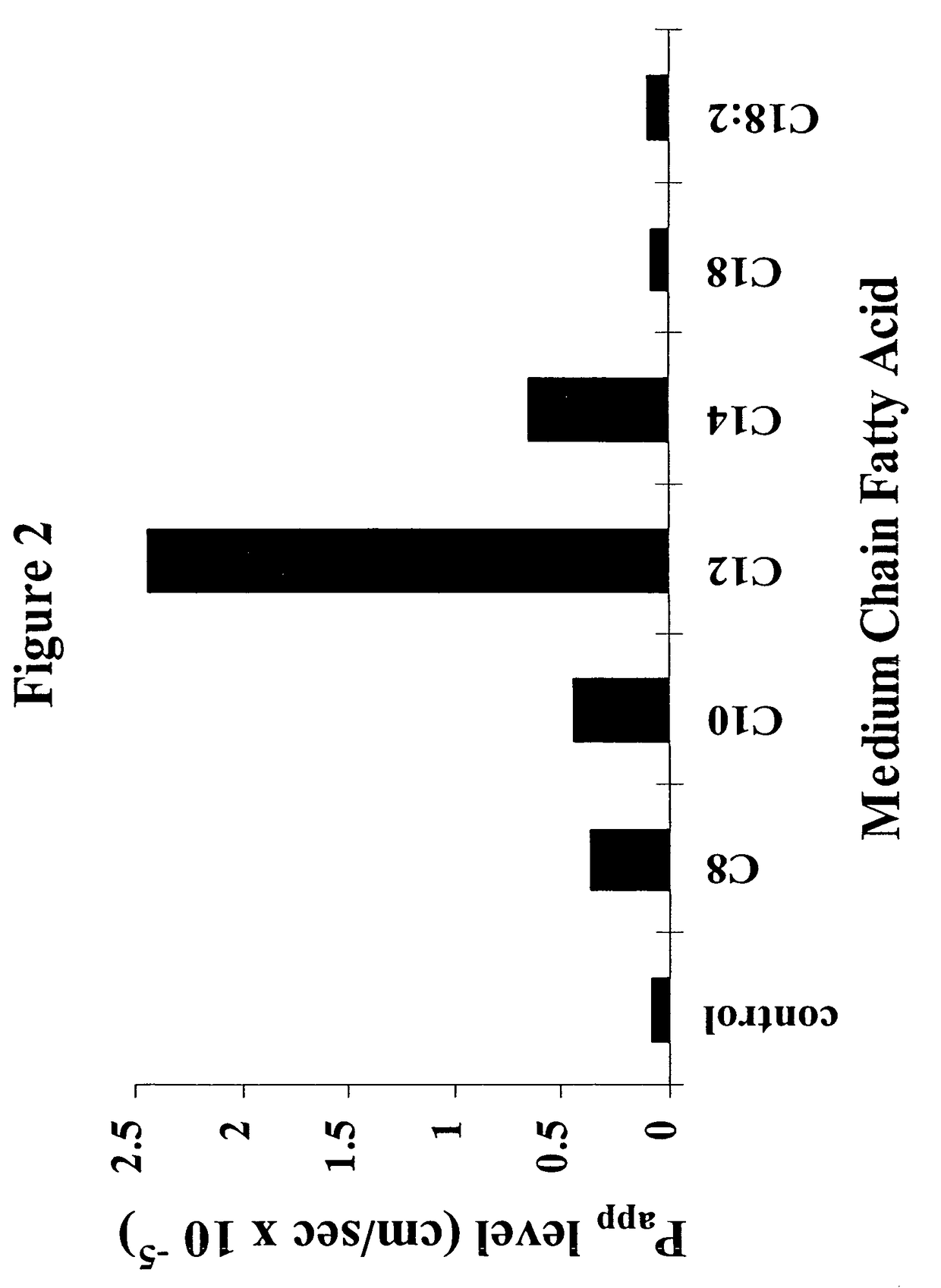
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Treatment of Spinal Mechanical Pain
The invention is directed to a method of treating chronic spinal mechanical pain by intravenous administration to a subject in need of chronic spinal mechanical pain relief of an effective amount of bisphosphonate.
Owner:NEW YORK UNIV
Medical devices and compositions for delivering biophosphonates to anatomical sites at risk for vascular disease
Methods, compositions and devices for inhibiting restenosis are provided. Specifically, bisphosphonate compositions and medical devices useful for the site specific delivery of bisphosphonates are disclosed. In one embodiment the medical device is a vascular stent coated with a bisphosphonate selected from the group consisting of zolendronate and pamedronate and derivatives and analogues thereof. In another embodiment an injection catheter for delivery an anti-restenotic effective amount of bisphosphonate to the adventitia is provided.
Owner:MEDTRONIC VASCULAR INC
Nanoparticulate bisphosphonate compositions
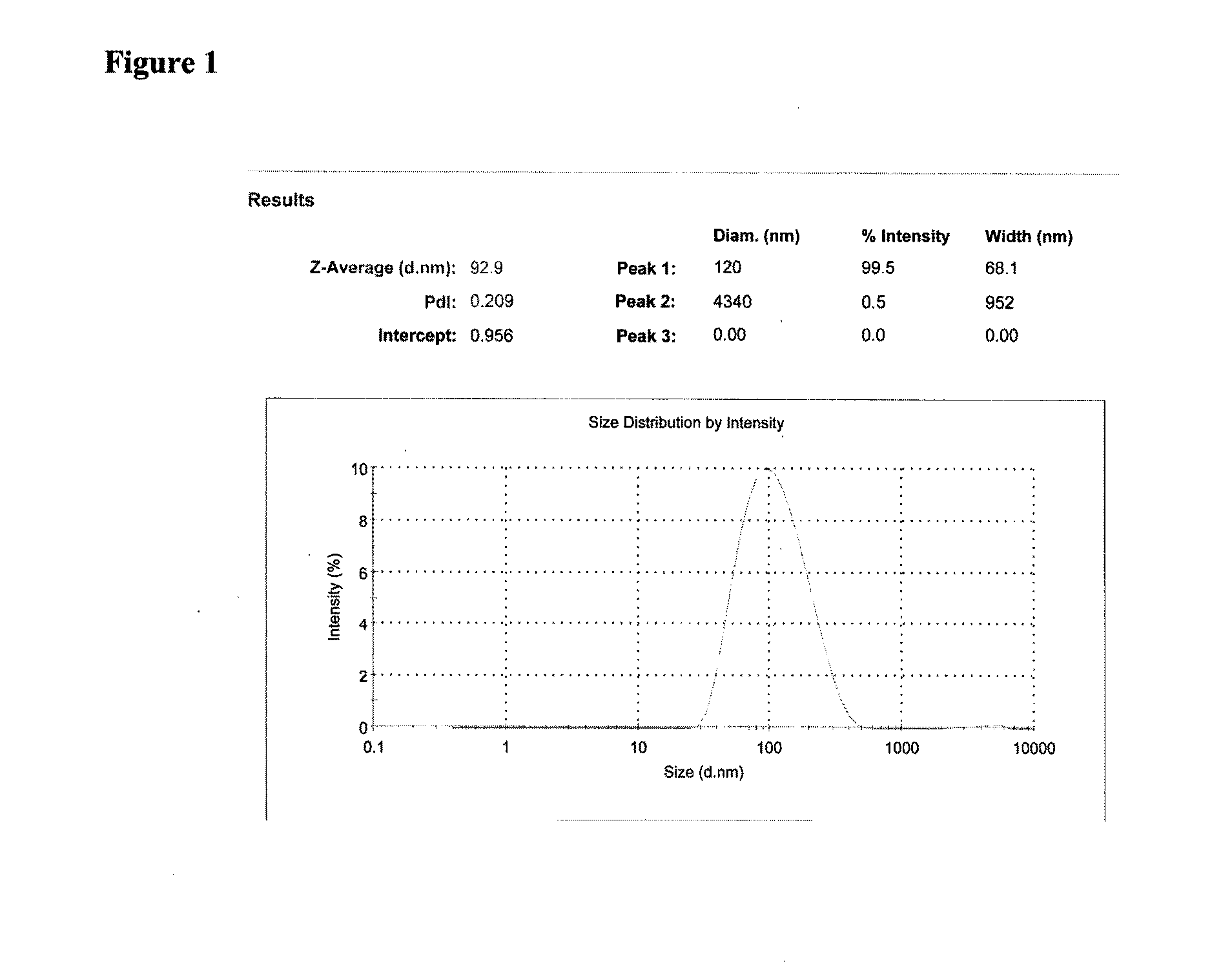
InactiveUS20060210639A1Avoid gastrointestinal irritationLow water solubilityBiocidePowder deliveryNanoparticleMedicine
Nanoparticulate bisphosphonate compositions, having an effective average particle size of less than 2000 nm, are described. The compositions are useful in treating bone resorption in a mammal.
Owner:ALKERMES PHARMA IRELAND LTD
Solid pharmaceutical dosage forms comprising bisphosphonates and modified amino acid carriers
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
Unique compositions of zwitterionic phospholipids and bisphosphonates and use of the compositions as bisphosphate delivery systems with reduced GI toxicity
InactiveUS6943155B2Reduce GI toxicityImprove bioavailabilityBiocideInorganic active ingredientsDiphosphonatesMedicine
Compositions and methods for treating osteoporosis using the compositions are disclosed where the compositions have reduced GI toxicity and improved bio-availability and include a bisphosphonate and zwitterionic phospholipid.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Treatment of spinal mechanical pain
The invention is directed to a method of treating chronic spinal mechanical pain by intravenous administration to a subject in need of chronic spinal mechanical pain relief of an effective amount of bisphosphonate.
Owner:NEW YORK UNIV
Method for inhibiting bone resorption
ActiveUS20090074763A1Inhibiting bone resorptionLower Level RequirementsAntibacterial agentsNervous disorderBone densityIncreased bone mineral density
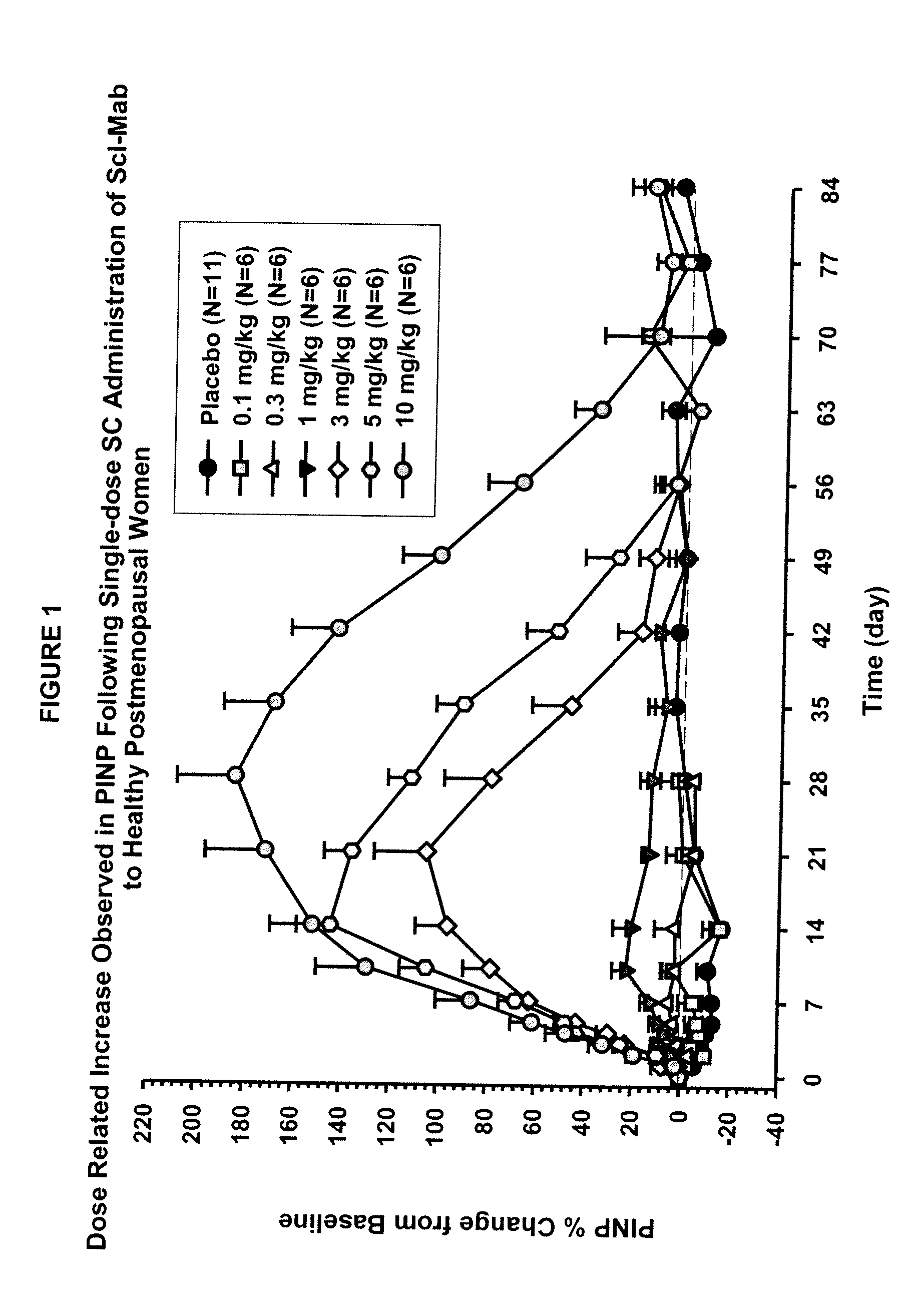
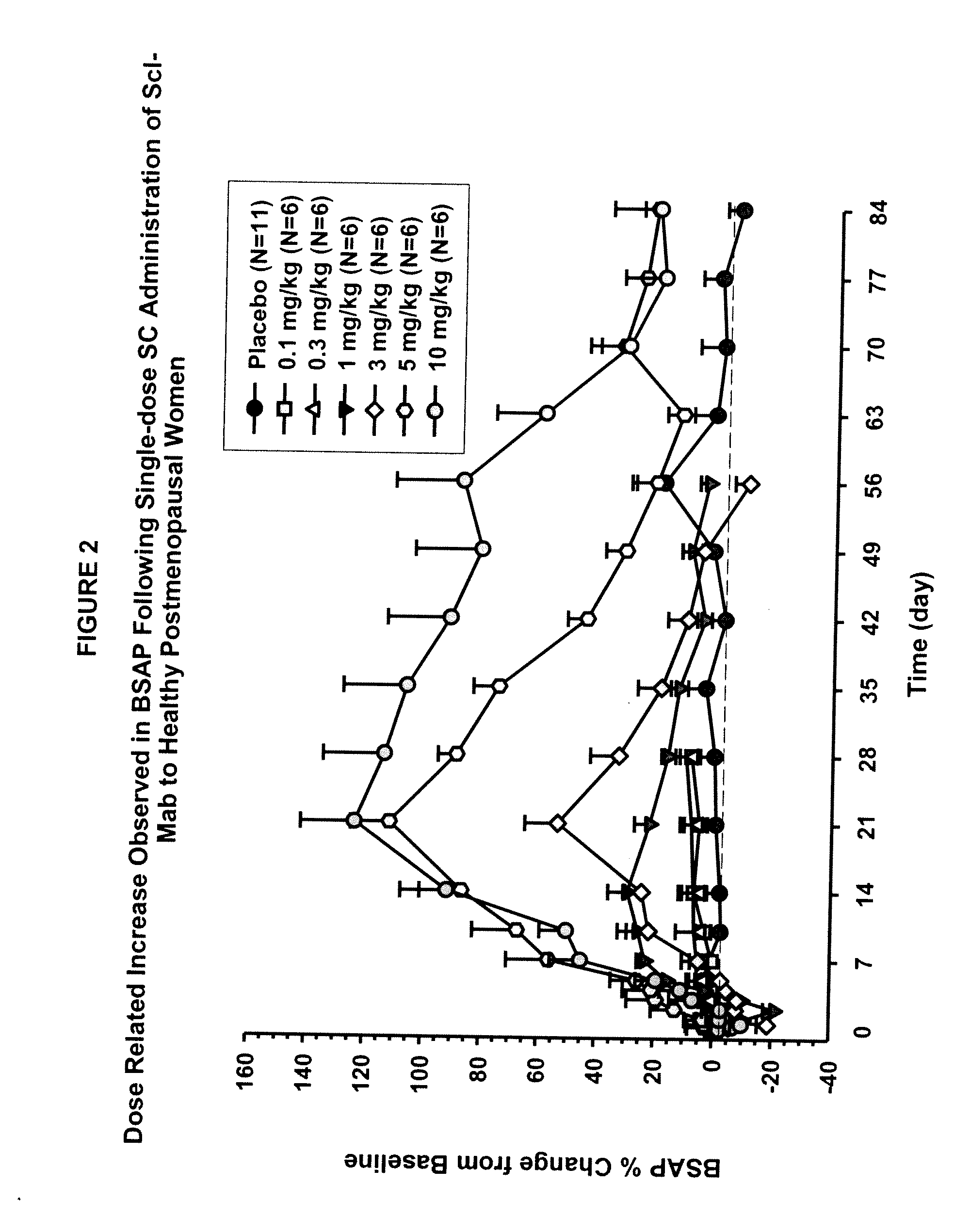
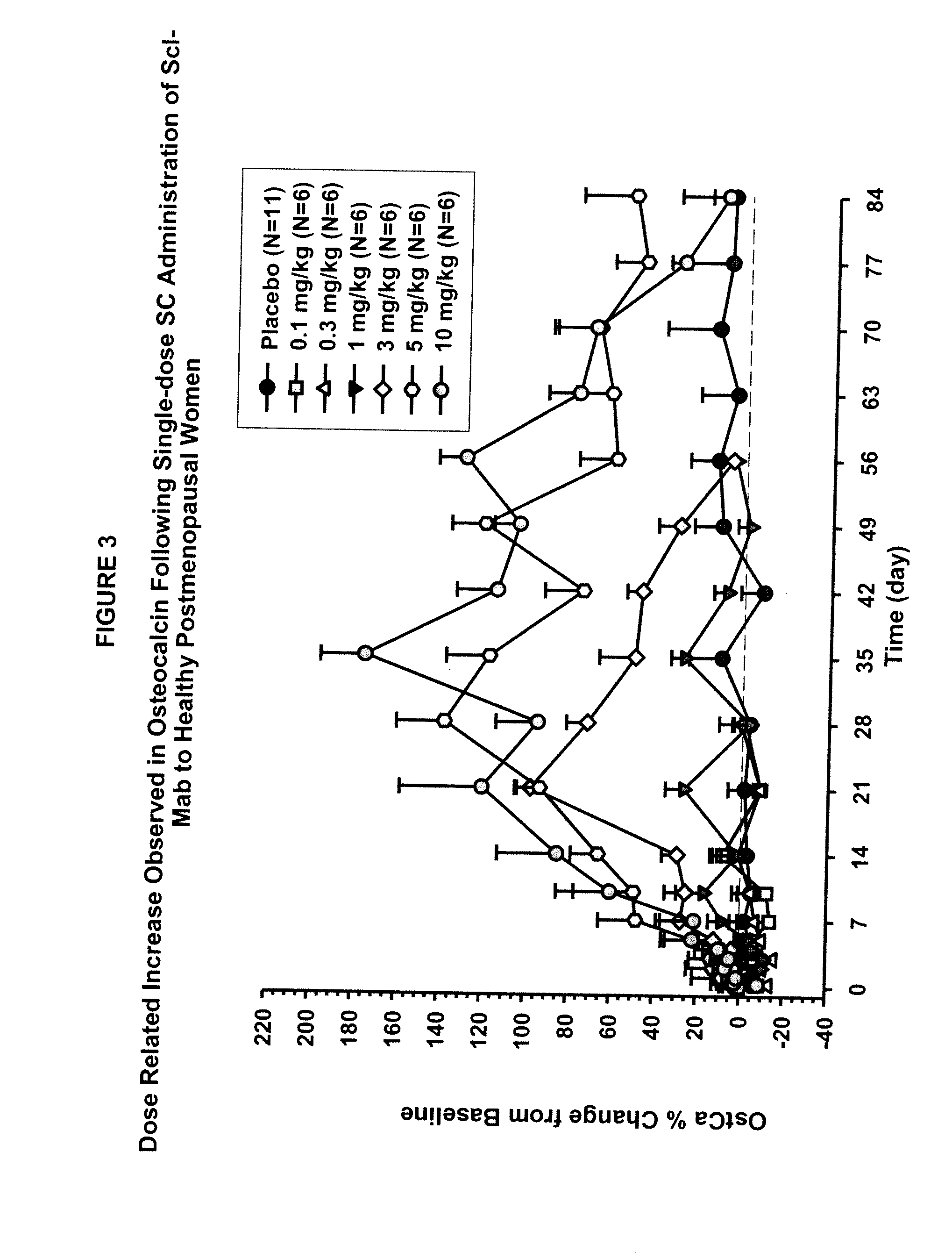
The invention is directed to a method of inhibiting bone resorption. The method comprises administering to a human an amount of sclerostin inhibitor that reduces a bone resorption marker level for at least 2 weeks. The invention also provides a method of monitoring anti-sclerostin therapy comprising measuring one or more bone resorption marker levels, administering a sclerostin binding agent, then measuring the bone resorption marker levels. Also provided is a method of increasing bone mineral density; a method of ameliorating the effects of an osteoclast-related disorder; a method of treating a bone-related disorder by maintaining bone density; and a method of treating a bone-related disorder in a human suffering from or at risk of hypocalcemia or hypercalcemia, a human in which treatment with a parathyroid hormone or analog thereof is contraindicated, or a human in which treatment with a bisphosphonate is contraindicated.
Owner:AMGEN INC
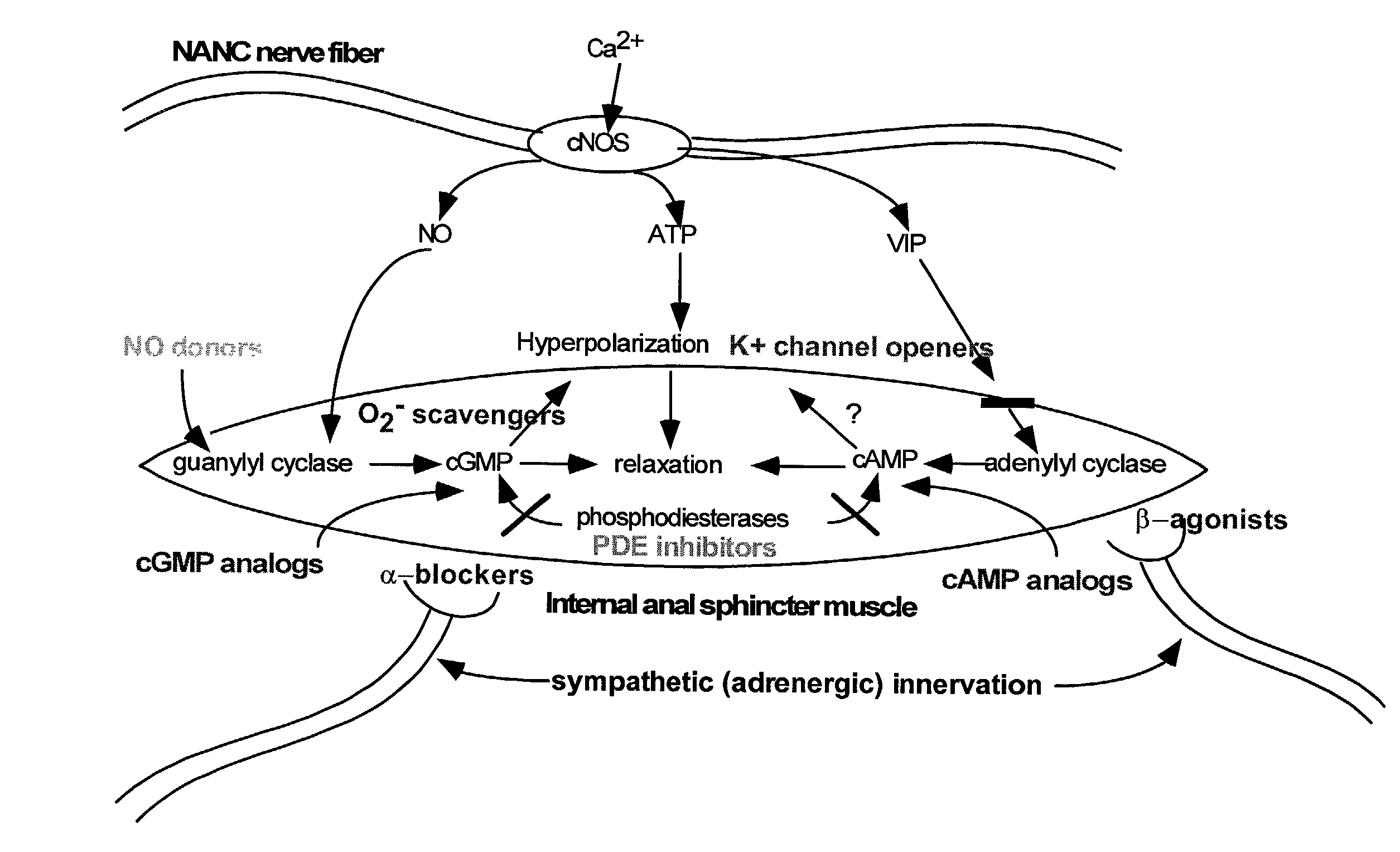
Compounds and methods for the treatment of urogenital disorders
InactiveUS6987129B2Reduce painLess discomfortBiocidePeptide/protein ingredientsDiseaseFemale Sexual Arousal Disorder
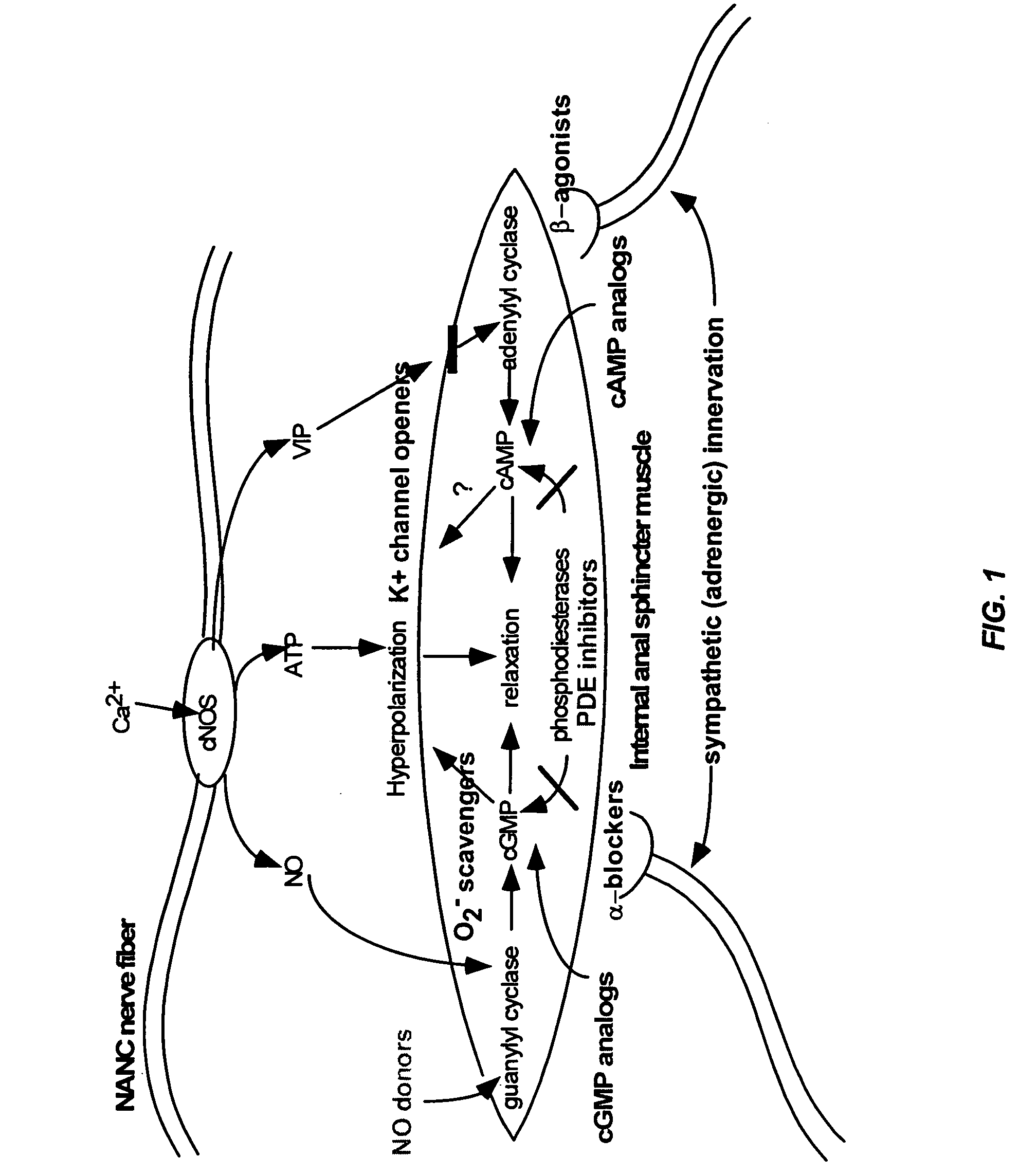
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
Implant improving local bone formation
InactiveUS20060188542A1Improve effectivenessBiocideDental implantsChemical LinkageCalcium biphosphate
A bone implant comprises an active agent on at least a portion thereof. The active agent is locally deliverable to bone proximate the implant in at least a two-phased release scheme. A first phase rapidly releases a first quantity of the active agent, and at least a second phase gradually releases a second quantity of the active agent, whereby bone formation stimulated by the active agent is modulated. In one embodiment, a porous implant comprises a porous portion coated with a calcium phosphate compound and which is contacted with a bisphosphonate compound to form a bisphosphonate layer chemically bound to the calcium phosphate at the surface of the porous portion and to form bisphosphonate molecules being non-chemically attached inside the pores of the porous portion. The non-chemically attached bisphosphonate molecules are released in the subject at a rate greater than that of the chemically bound bisphosphonate layer.
Owner:BOBYN JOHN DENNIS +1
Osteoclast inhibitors for joint conditions
ActiveUS9610300B2Improve bioavailabilityPhosphorous compound active ingredientsPill deliveryNitrogenAnesthesia
Oral dosage forms of osteoclast inhibitors, such as nitrogen-containing bisphosphonates, can be used to treat or alleviate pain or related conditions.
Owner:ANTECIP BIOVENTURES II
Dosage forms of bisphosphonates
ActiveUS7645459B2Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyLower Gastrointestinal Tract
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
Non-isotopic detection of osteoblastic activity in vivo using modified bisphosphonates
InactiveUS6869593B2Accurately cardiovascular riskUltrasonic/sonic/infrasonic diagnosticsLuminescence/biological staining preparationIn vivoOsteocyte
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
Medical uses of a selective estrogen receptor modulator in combination with sex steroid precursors
InactiveUS6465445B1Reduce riskGood effectBiocideOrganic chemistrySelective progesterone receptor modulatorOsteopetrosis
Novel methods for the medical treatment and / or inhibition of the development of osteoporosis, breast cancer, hypercholesterolemia, hyperlipidemia or atherosclerosis in susceptible warm-blooded animals including humans involving administration of selective estrogen receptor modulator particularly compounds having the general structure:and an amount of a sex steroid precursor selected from the group consisting of dehydroepiandrosterone, dehydroepiandrosterone sulfate, androst-5-ene-3beta,17beta-diol and compounds converted in vivo to one of the foregoing presursor. Further administration of bisphosphonates in combination with selective estrogen receptor modulators and / or sex steroid precursor is disclosed for the medical treatment and / or inhibition of the development of osteoporosis. Pharmaceutical compositions for delivery of active ingredient(s) and kit(s) useful to the invention are also disclosed.
Owner:ENDORES & DEV
Method of electrolytically depositing a pharmaceutical coating onto a conductive osteal implant
InactiveUS20080011613A1Electrolytic inorganic material coatingPretreated surfacesCalcium biphosphateConductive materials
A method of electrolytically depositing a pharmaceutical coating onto a conductive osteal implant. The implant is submerged into an electrolytic cell containing an electrolysis solution of the pharmaceutical and acts as a cathode. When current is applied to the electrolytic cell, the pharmaceutical coating forms on the implant. The pharmaceutical can comprise bisphosphonates, including calcium salts. The implants can comprise any conductive material suitable for use as an osteal implant. The implants can also be electrolytically coated with calcium phosphate before coating with a pharmaceutical.
Owner:THE UNIV OF BRITISH COLUMBIA
Multifunctional implant device
InactiveUS20070191851A1Mechanical strengthAchieve mechanical strengthInternal osteosythesisBone implantSelf reinforcedBioactive glass
Bone fixation or augmentation in a mammalian body to enhance the mechanical strength of a fracture is provided by reinforcement fixing bone ends together using the implant device. A resorbable device can be rendered anti-osteolytic by incorporating materials such as bisphosphonates. It can also be rendered osteoconductive by the incorporation of an osteoconductive material such as bioactive glass or TCP. The implant device has a matrix as one phase, where the matrix is made of a bioresorbable polymer. One phase of the implant is made from self-reinforcing elements and the matrix contains an antiosteolytic agent component. The implant contains further osteoconductive and / or osteoconductive material.
Owner:ASHAMMAKHI NUREDDIN
Method of inhibiting restenosis using bisphosphonates
InactiveUS7008645B2Affect activityEfficient transportPowder deliveryBiocidePlatelet-Derived Growth Factor BetaParticulates
A method of inhibiting the activity or production of cytokines or growth factors associated with vascular restenosis, by administering to an individual an effective amount of an active ingredient comprising a bisphosphonate particle or a bisphosphonate particulate. The bisphosphonate may be encapsulated, embedded or adsorbed within the particle, dispersed uniformly in the polymer matrix, adsorbed on the particle surface, or in combination of any of these forms. The particles include liposomes or inert polymeric particles, such as microcapsules, nanocapsules, nanoparticles, nanospheres, or microparticles. The particulates include any suspended or dispersed form of the bisphosphonate which is not encapsulated, entrapped, or adsorbed within a polymeric particle. The particulates include suspended or dispersed colloids, aggregates, flocculates, insoluble salts and insoluble complexes of the active ingredient. The cytokines and growth factors include, but are not limited to interleukin 1-β, matrix metalloproteinase-2, and platelet-derived growth factor β (PDGFβ).
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD
Method of Improving Treatments in Rheumatic and Arthritic Diseases
Improved treatments of joint diseases, such as, e.g. osteoarthritis and rheumatoid arthritis, and pain, wherein a strontium-containing compound is administered alone or in combination with one or more second therapeutically and / or prophylactically active substances, selected from the group consisting of bisphosphonates, glucosamine, pallitative agents, analgesic agents, disease modifying anti-rheumatic compounds (DMARDs), selective estrogen receptor modulators (SERMs), aromatase inhibitors, non-steroidal anti-inflammatory agents (NSAIDs), COX-2 inhibitors, COX-3 inhibitors, opioids, inhibitors / antagonists of IL-1, inhibitors / antagonists of TNF-alpha, inhibitors of matrix metallo-proteinases (MMPs), cathepsin K inhibitors, inhibitors / antagonists of RANK-ligand, statins, glucocorticoids, chondroitin sulphate, NMDA receptor antagonists, inhibitors of interleukin-I converting enzyme, Calcitonin gene related peptide antagonists, glycine antagonists, vanilloid receptor antagonists, inhibitors of inducible nitric oxide synthetase (iNOS), N-acetylcholine receptor agonists, neurokinin antagonists, neuroleptic agents, PAR2 receptor antagonists and anabolic growth factors acting on joint tissue components. Pharmaceutical compositions comprising a strontium-containing compound and a second therapeutically and / or prophylactically active substance as defined above.
Owner:OSTEOLOGIX AS
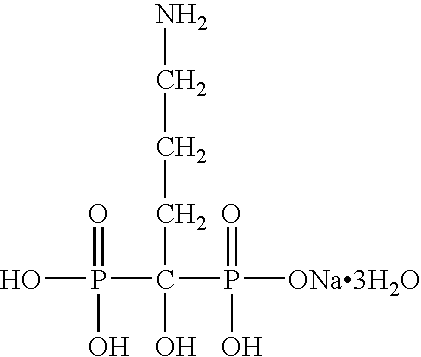
Delivery of a bioactive material
A solid pharmaceutical composition comprising a water-soluble bioactive material and an encapsulating material which is present in the composition in the form of continuous solid phase, and in which solid particles of the bioactive material are dispersed and encapsulated in the continuous solid phase of the encapsulating material, wherein each of the bioactive material and the encapsulating material is normally a solid at room temperature and the melting point of the encapsulating material is lower than the melting point of the bioactive material, the bioactive material being preferably a bisphosphonate, most preferably alendronate, and the encapsulating material includes an enhancer, preferably a mono- or di-glyceride, or an encapsulating surfactant, preferably, a polyoxyethylene / polyoxypropylene block copolymer having surface active properties, and a process for preparing the composition in which solid particles of the bioactive material are mixed with and dispersed in the encapsulating material which is in molten (liquid) form; and cooling the molten form of the encapsulating material to form a solid pharmaceutical composition having the solid particles of the bioactive material dispersed and encapsulated in a continuous solid phase of the encapsulating material.
Owner:NOVO NORDISK AS
Drug for the treatment of osteonecrosis and for the management of patients at risk of developing osteonecrosis
InactiveUS7425549B2Permit new bone formationMinimising collapseBiocidePeptide/protein ingredientsDiseaseOral bisphosphonates
A bisphosphonate for the treatment of osteonecrosis and / or osteonecrosis dissecans. The drug may further be used to prevent the onset of osteonecrosis and / or osteonecrosis dissecans and any complications associated with both diseases.
Owner:THE SYDNEY CHILDRENS HOSPITALS NETWORK RANDWICK & WESTMEAD
Treatment of restenosis
Bisphosphonate (BP), pyrophosphate (PP) a complex of BP or PP, a polymer of BP or PP or pharmaceutically acceptable salt or ester thereof, are used for the prevention or treatment of vascular restenosis.
Owner:YISSUM RES DEV CO OF THE HEBREW UNIV OF JERUSALEM LTD +1
Compounds and methods for the treatment of urogenital disorders
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
Microparticle compositions to modify cancer promoting cells
This invention provides pharmaceutical compositions and methods related to the prevention and treatment of primary tumors and metastatic, malignant or spreading cancers by selectively targeting cancer associated myeloid derived cells by the targeted delivery of a bisphosphonate formulated with a non-liposomal particle carrier. In some aspects, the bisphosphonate particles have one or more properties suitable for phagocytosis by cancer associated myeloid derived cells and release of the bisphosphonate within the macrophages. Advantageously, administering the particles to a subject reduces the level and / or activity of cancer associated myeloid derived cells in the subject.
Owner:JOVESIS
Method of prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, and age-related disorders including osteoporosis, arthritis, type 2 diabetes, dementia and Alzheimer's disease
InactiveUS20060078531A1Avoid problemsBiocidePhosphorous compound active ingredientsInterleukin 6Age related disease
This invention relates to a method for prevention and treatment of Atherosclerosis, Peripheral Vascular Disease, Coronary Artery Disease, and age-related disorders including Osteoporosis, Arthritis, Type II Diabetes, Dementia and Alzheimer's disease in a subject comprising administering to said subject a therapeutically effective dosage of each component or combination of statins, bisphosphonates and / or cholesterol lowering agents or techniques, administered separately, in sequence or simultaneously. Cholesterol Metabolites (isoprenoids) are an integral component of the signaling pathway for Interleukin 6 mediated inflammation. Interleukin 6 inflammation is the common causative origin for Atherosclerosis, Peripheral Vascular Disease, Coronary Artery Disease, and age-related disorders including Osteoporosis, Arthritis, Type II Diabetes, Dementia and Alzheimer's disease. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism and isoprenoid depletion.
Owner:SOTA OSEMWOTA
Novel manganese comprising nanostructures
ActiveUS20140350193A1Cost advantageGood biotolerabilityGroup 5/15 element organic compoundsNanomedicineArylMRI contrast agent
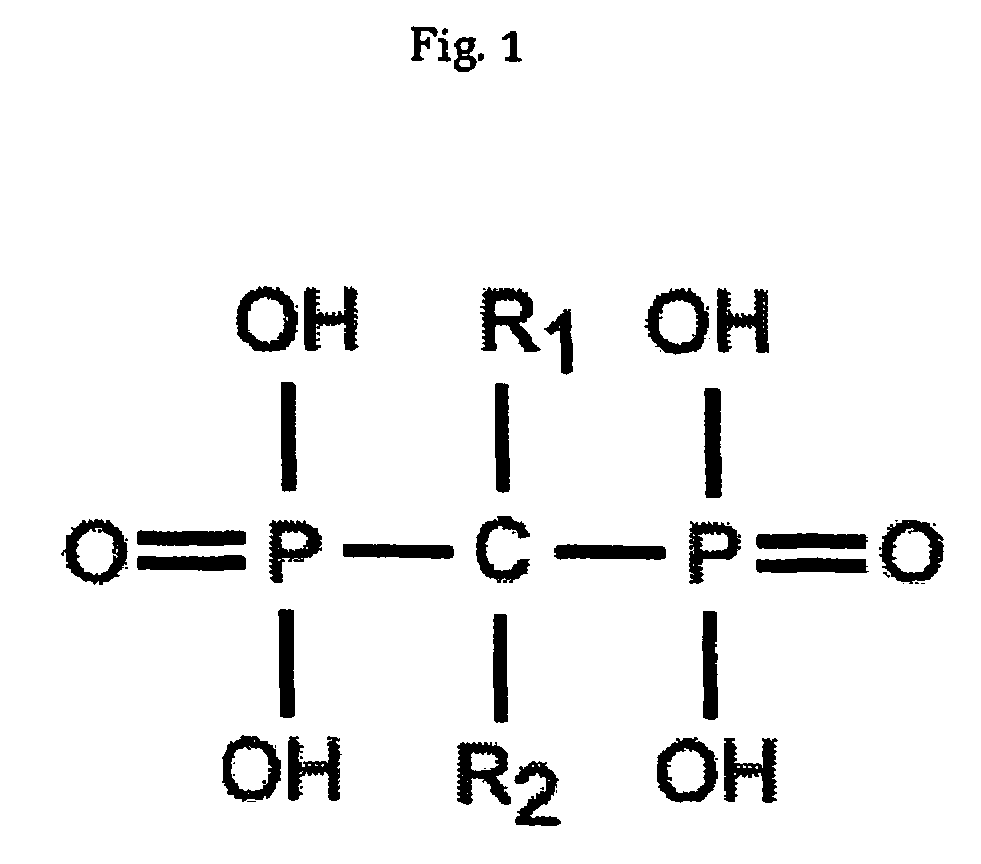
Disclosed herein are nanostructures comprising a polymeric framework comprising at least five geminal bisphosphonate groups, wherein the geminal bisphosphonate groups independently of each other are incorporated as —R3R4C(P═O(OR1)(OR2))2, wherein R1 and R2 are independently selected from the group consisting of a negative charge, H, alkyl and aryl, and wherein at least one of R3 and R4 is a group connected to the polymeric framework with the proviso that when only one of R3 and R4 is such a connected group, the other of R3 and R4 is either a group being able to connect to the polymeric framework, or the residue of such a group, or selected from the group consisting of H, OH, OR5 and R5, wherein R5 is a lower alkyl. The polymeric framework may comprise manganese ions. Disclosed are also methods for producing such manganese containing nanostructures, compositions comprising such manganese containing nanostructures and use of such manganese containing nanostructures, i.a. as MRI contrasting agents.
Owner:SPAGO NANOMEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com