Recombinant lentivirus and application thereof
A technology of recombinant lentivirus and viral vector, applied in the field of cellular immunotherapy of tumors, can solve the problems of affecting the therapeutic effect and unsatisfactory chimeric antigen targeting effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of Chimeric Antigen Receptor Lentiviral Vector
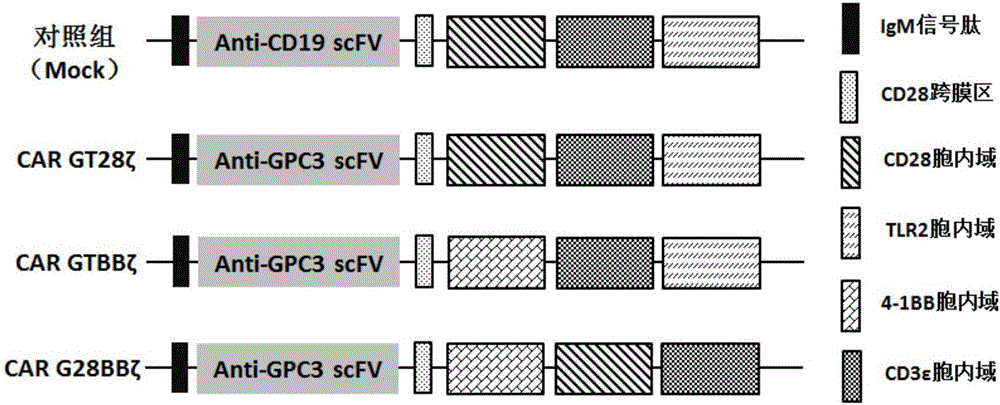
[0052] (1) Synthesize human IgM signal peptide, anti-GPC3 antibody extracellular region, human CD28 transmembrane region, human 41BB intracellular region, human TLR2 intracellular region and human CD3ζ signaling domain through whole gene synthesis, namely CAR-GPC3-41BB- CD3ξ-TLR2 (CAR-GTBBξ), the whole gene synthetic human IgM signal peptide, anti-GPC3 antibody extracellular domain, human CD28 transmembrane region and intracellular region, human TLR2 intracellular region and human CD3ζ signaling domain, namely CAR-GPC3 - CD28-CD3ξ-TLR2 (CAR-GT28ξ), its sequence is shown in SEQ ID NO.4, and then the whole gene synthesis CAR-CD19-CD28-CD3ξ-TLR2 (Mock) nucleic acid sequence is used as a negative control, CAR-GPC3-41BB -CD28-CD3ξ (CAR-G28BBξ) nucleic acid sequence is used as a positive control and its gene sequence map is as follows figure 1 As shown, the C-terminal of the synthesized gene contains a ...
Embodiment 2
[0055] Example 2: GT28ξCAR lentiviral packaging
[0056] (1) Cultivate 293T cells in a 10cm culture dish, the culture medium is: DMEM high glucose medium + 10% FBS (fetal bovine serum) + 1% double antibody (100 × penicillin-streptomycin mixed solution);
[0057] (2) When the 293T cell density in the 150mm culture dish reaches 80-90%, replace the medium: DMEM high glucose medium + 1% FBS + 1% double antibody;
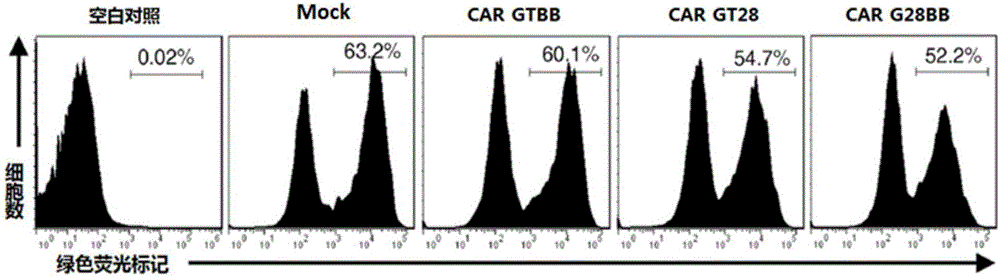
[0058] (3) After replacing the medium and culturing for 2-6 hours, the pWPXLd-CAR-EGFP plasmids (pWPXLd-CAR-GTBBξ-2A-EGFP, pWPXLd-CAR-GT28ξ-2A-EGFP, pWPXLd-CAR-G28BBξ- 2A-EGFP, pWPXLd-Mock-2A-EGFP) were co-transduced into 293T cells with lentiviral packaging helper plasmids pMD2.G and psPAX2 respectively, and the reagents and dosages were as follows:
[0059] Reagent dose pWPXLd-CAR-EGFP plasmid 9μg pMD2.G helper plasmid 3μg psPAX2 12μg PEI 72μg
[0060] (4) 24, 48 and 72 hours after transformation, collect the culture supern...
Embodiment 3
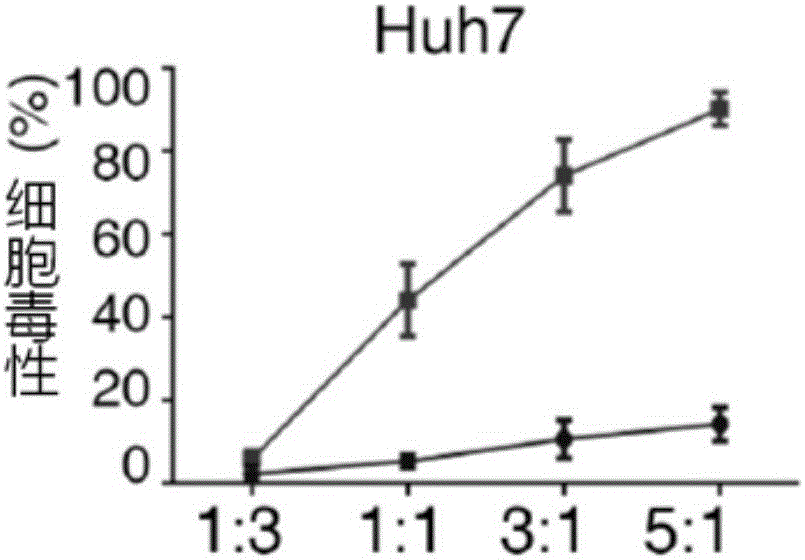
[0065] Example 3: Construction of CAR T cells
[0066] (1) Separation and purification of human T cells: the mononuclear cells in human blood are separated by the Ficoll density gradient method, and the red blood cells are removed by red blood cell lysate, and then the T cells are sorted by MACS Pan-T magnetic beads;
[0067] (2) Dilute the sorted T cells with medium: AIM-V medium+5% FBS+penicillin 100U / ml+streptomycin 0.1mg / ml to a cell concentration of 2.5×10 6 pcs / mL for use;
[0068] (3) Stimulate T cells by magnetic beads coated with CD2, CD3, and CD28 antibodies (Miltenyi, Germany), that is, the coated magnetic beads and T cells are mixed at a ratio of 1:2, and the final density of T cells should be 5×10 6 piece / mL / cm 2 . After mixing, place at 37°C, 5% CO 2 Incubator culture stimulation for 48 hours;
[0069] (4) Lentiviral transfection of T cells: remove the magnetic beads in the activated T cell-magnetic bead mixture by magnetic field, centrifuge at 300g for 5min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com