In vitro preparation method of NK cells
A NK cell and cell technology, applied in the biological field, can solve the problems of limited curative effect, difficult industrialized production, insufficient NK cell sources, etc., and achieve the effects of high NK cell activity, improved viability, and significant tumor killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
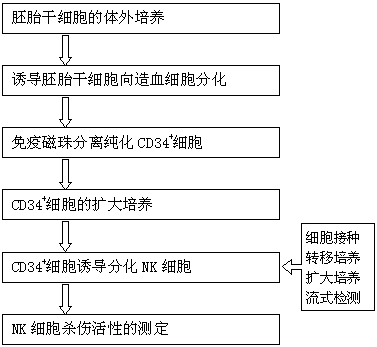
[0033] Example 1 In vitro culture of embryonic stem cells:
[0034] 1. Put the embryonic stem cells stored in liquid nitrogen in a 37°C water bath to dissolve quickly, and the cell suspension changes from solid to liquid.
[0035] 2. Inoculate the above-mentioned cell suspension into gelatin-pretreated culture dishes, and place them at 37°C, 5% CO 2 , Cultured in an incubator with 95% saturated humidity, cultivated for 2-3 days, and after the cell confluence reached 85%-90%, it was digested and passaged with digestive juice.
[0036] 3. Passage to a new culture dish according to 1:3-1:5, replenish the culture medium and continue the culture.
[0037] 4. Digest and passage with digestive juice every 2-3 days.
Embodiment 2
[0038] Example 2 Inducing embryonic stem cells to differentiate into hematopoietic stem cells
[0039] 1. Prepare frozen OP9 cells 1 day before use in a volume of 1.5×10 5 The cells / mL cell concentration was inoculated into 6-well culture plates pre-coated with gelatin.
[0040] 2. On the second day, centrifuge the embryonic stem cells, discard the supernatant, resuspend in the differentiation medium, inoculate in a 6-well plate with OP9 cells in advance, and place at 37°C, 5% CO 2 , 95% saturated humidity incubator culture, and add cytokines 5ng / mL FL, 20ng / mL IL-15, 20ng / mL IL-6, 10 ng / mL IL-7, 10ng / mL KL.
[0041] 3. After culture 1d, transfer the differentiating ES to a new ultra-low adsorption culture dish, and supplement the above cytokines.
[0042] 4. After culturing for 2-3 days, collect the cells by centrifugation, add PBS solution to wash the cells, remove the supernatant after centrifugation (1500r / min, 10min), add PBS solution to resuspend the cells, and centri...
Embodiment 3
[0043] Example 3 Separation and purification of CD34 by immunomagnetic beads + cell
[0044] 1. Divide the collected cells into 1×10 8 Mononuclear cells were suspended in 300 μl PBS buffer, 100 μl Fc receptor blocker was added, and 100 μl anti-CD34 immunomagnetic beads were added to it, mixed evenly, incubated at 4°C for 30 min, and then centrifuged and washed twice with PBS buffer. Centrifuge at a speed of 1000rpm for 10min each time, resuspend the washed cells in 500μl of PBS buffer at a density of 10 8 each / 500μl.
[0045] 2. Fix the separation column in the MACS magnetic field, slowly pass the labeled cells through the MiniMACS separation column, and elute to remove CD34 - cells, remove the separation column from the magnetic field, pressurize the elution column with 1mL buffer, and collect CD34 + cells and counted. Analysis of CD34 by flow cytometry + Cell Purity. The results showed that the CD34 + The purity of hematopoietic stem cells is above 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com