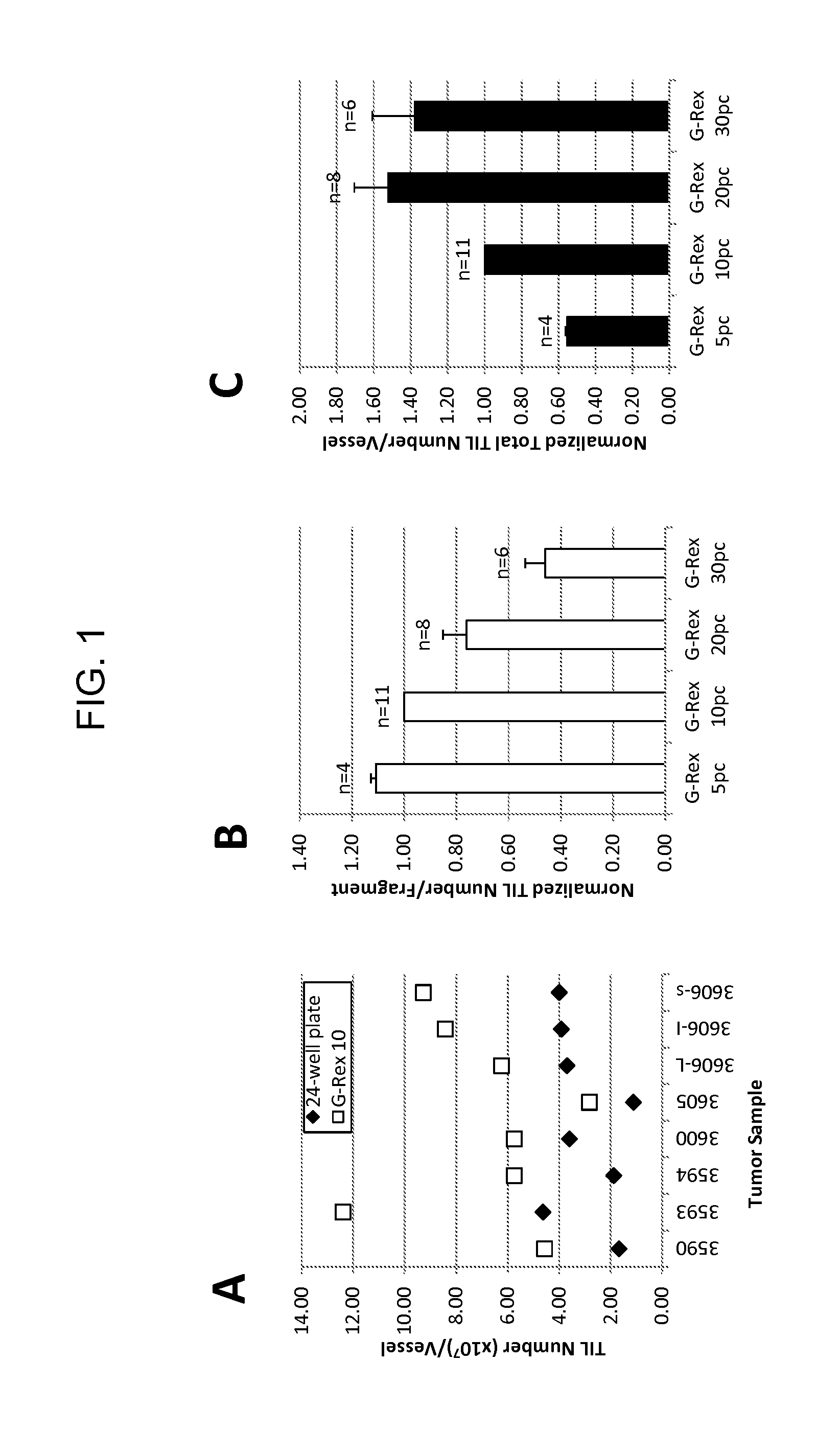
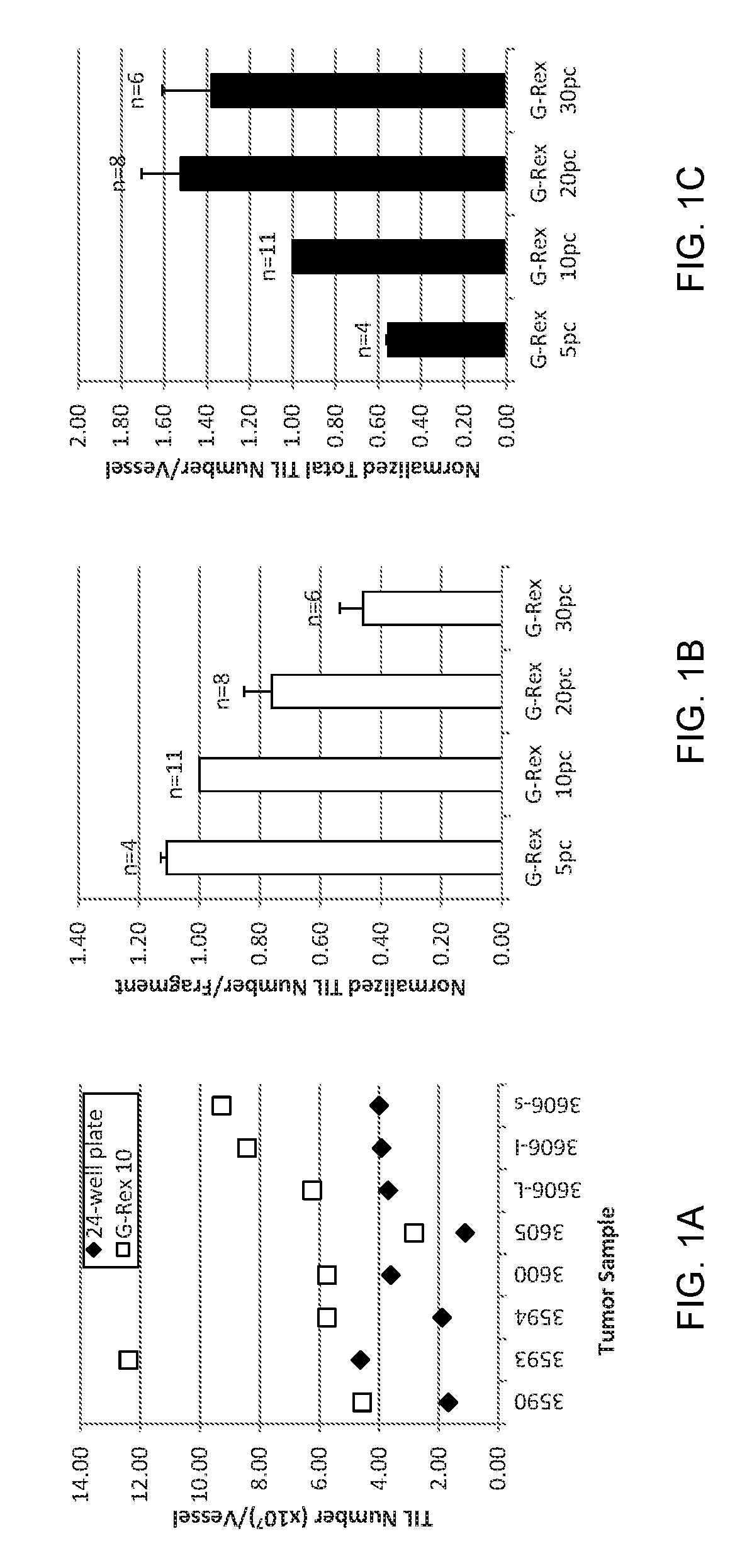
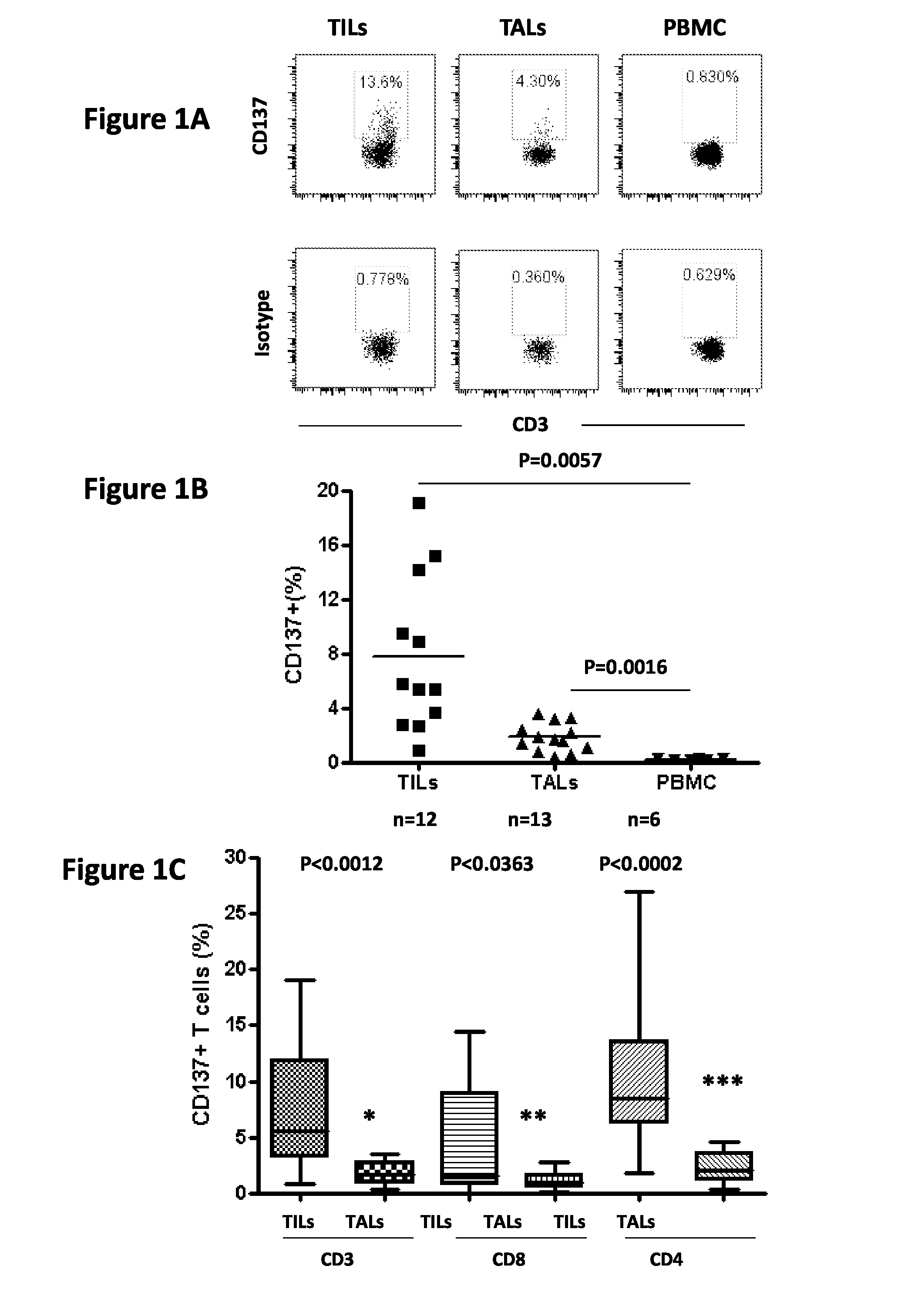
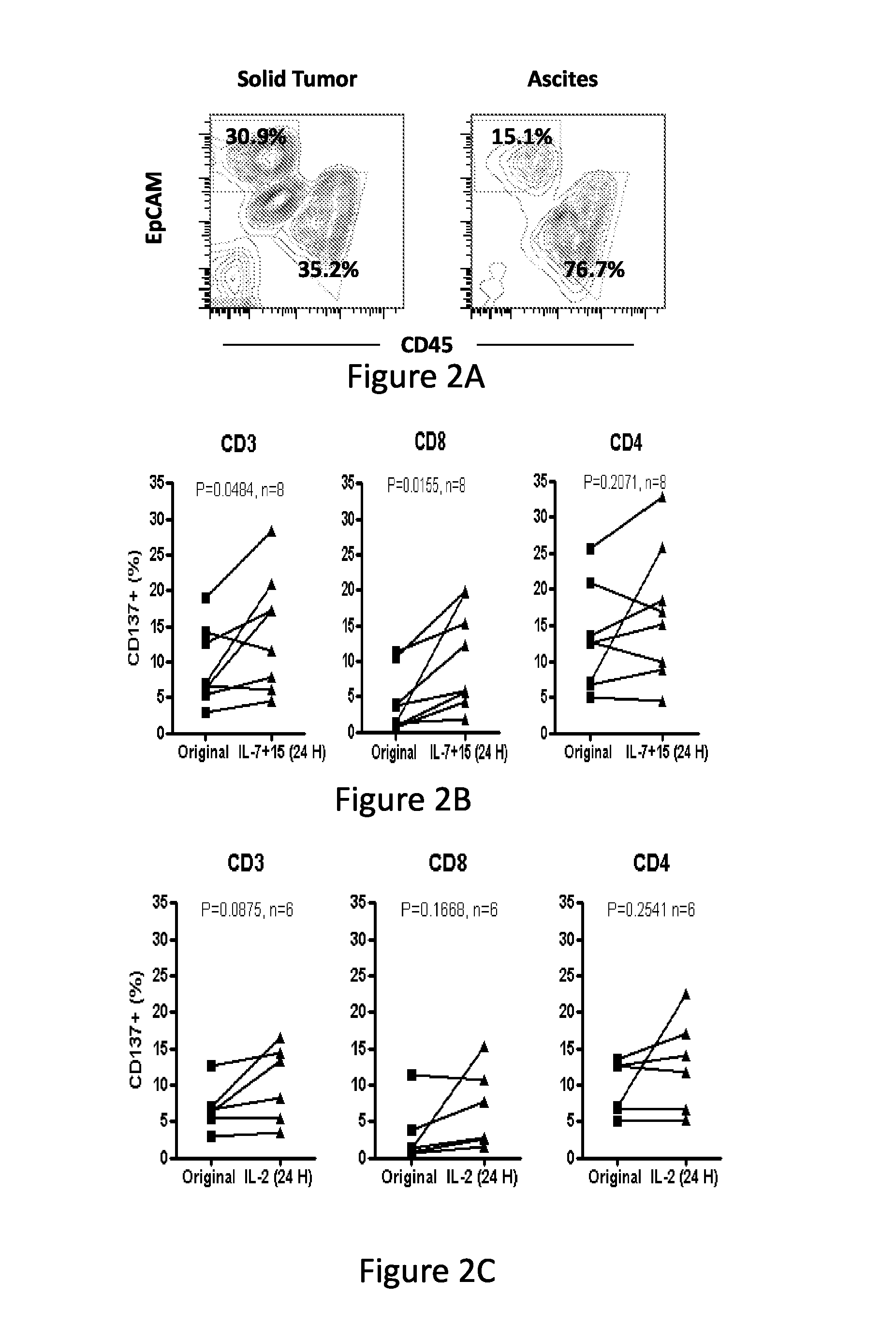
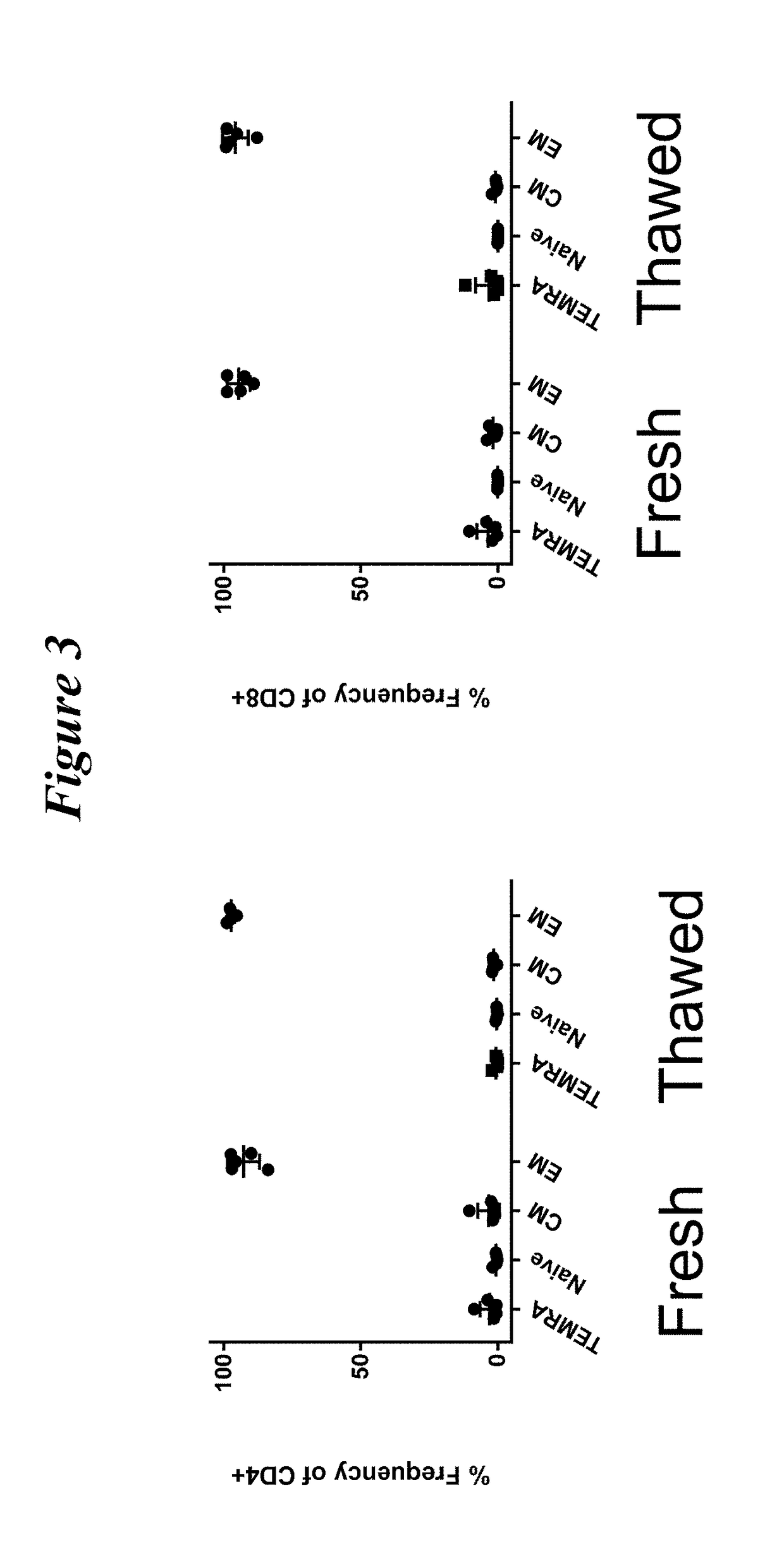
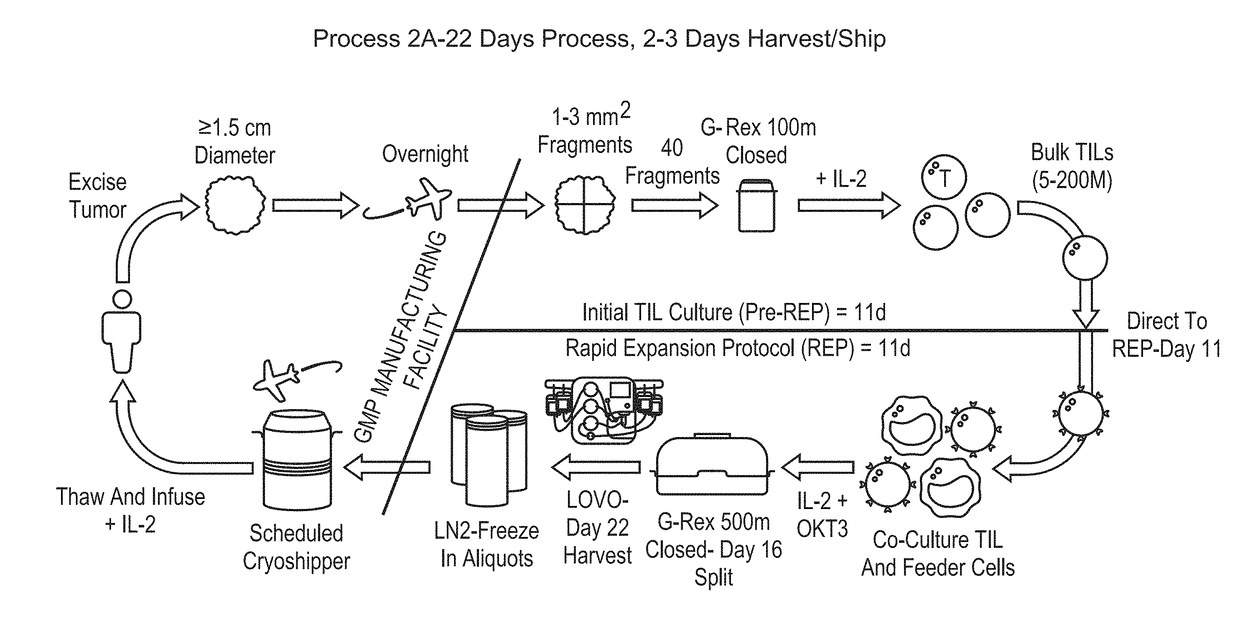
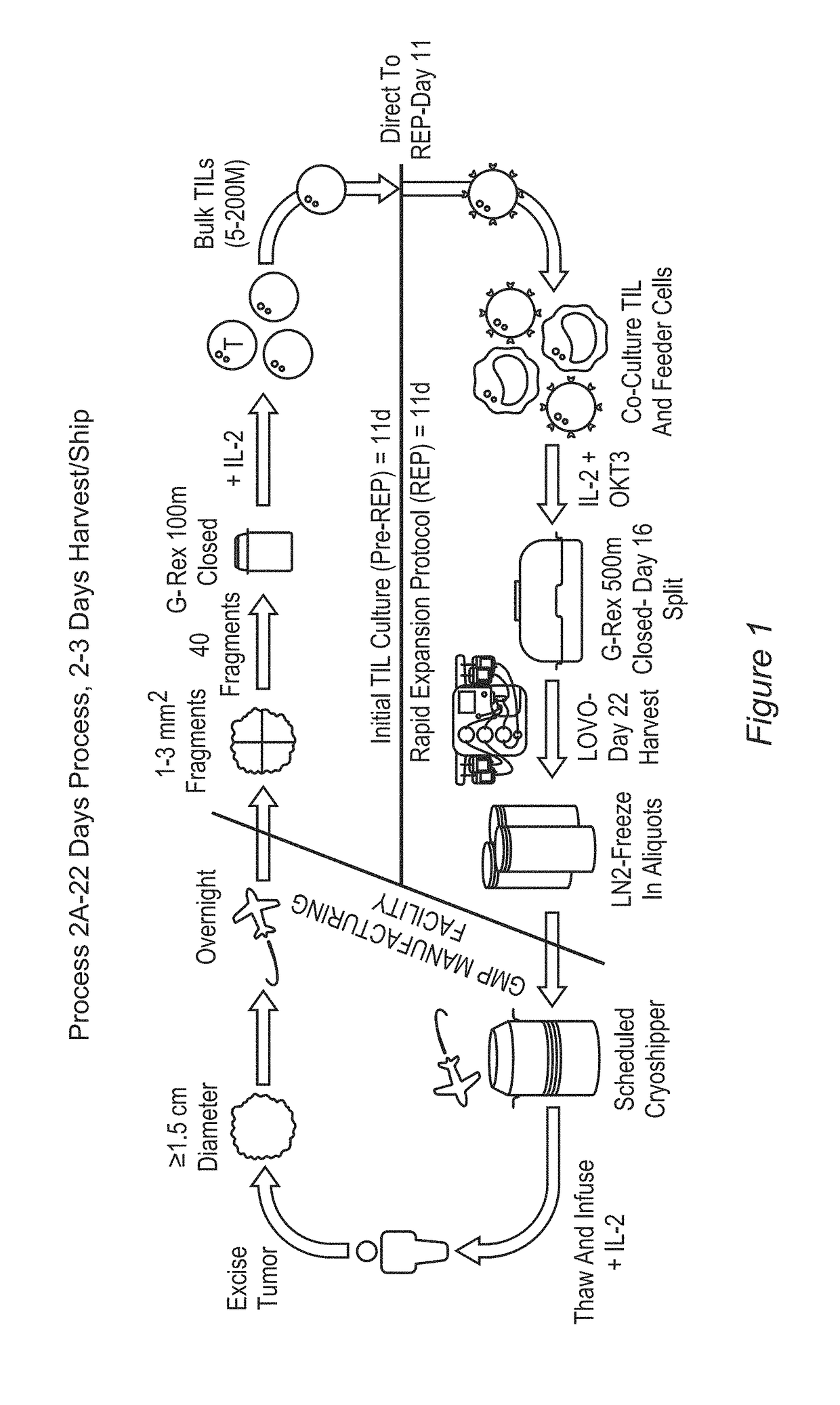
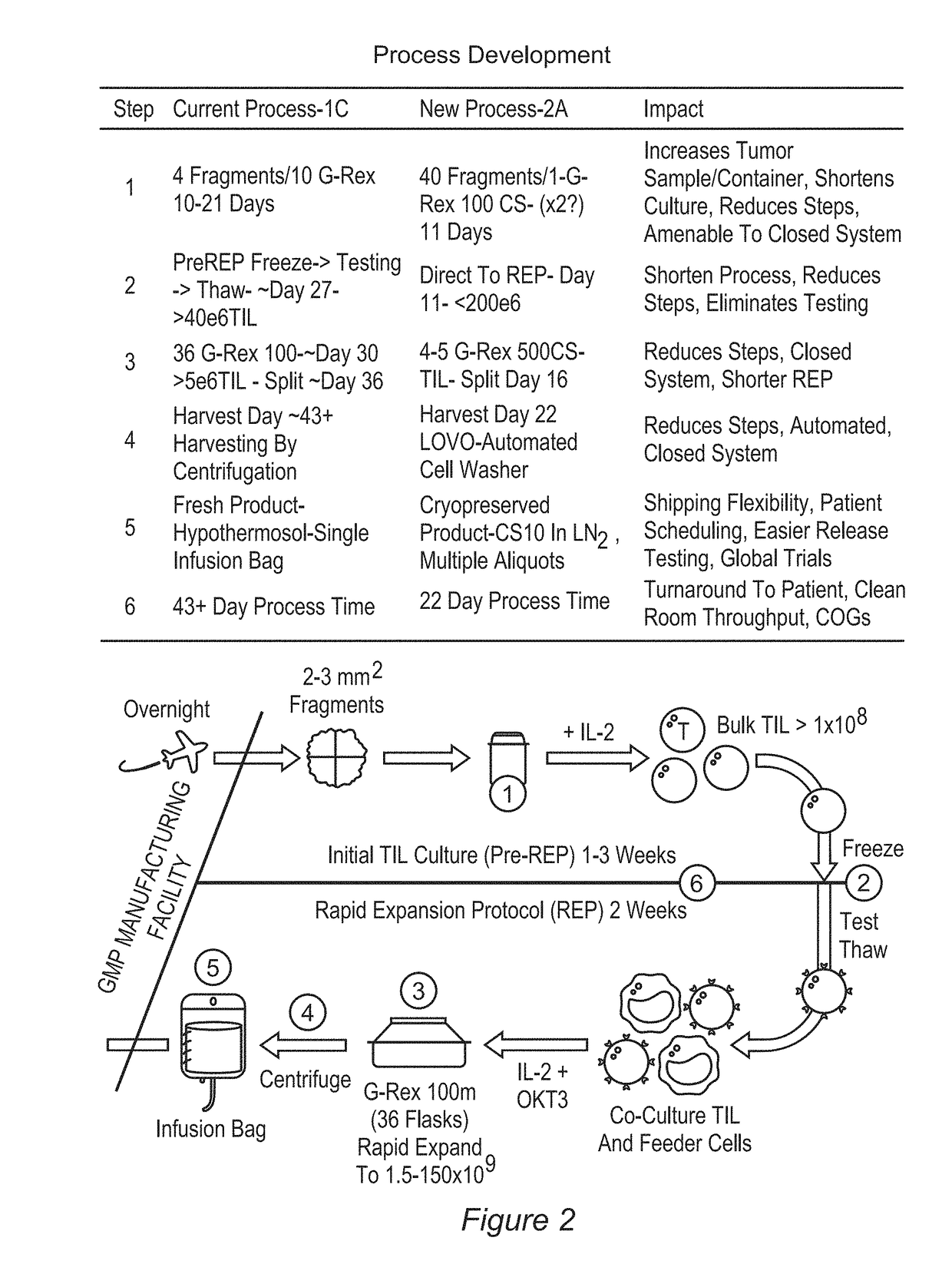
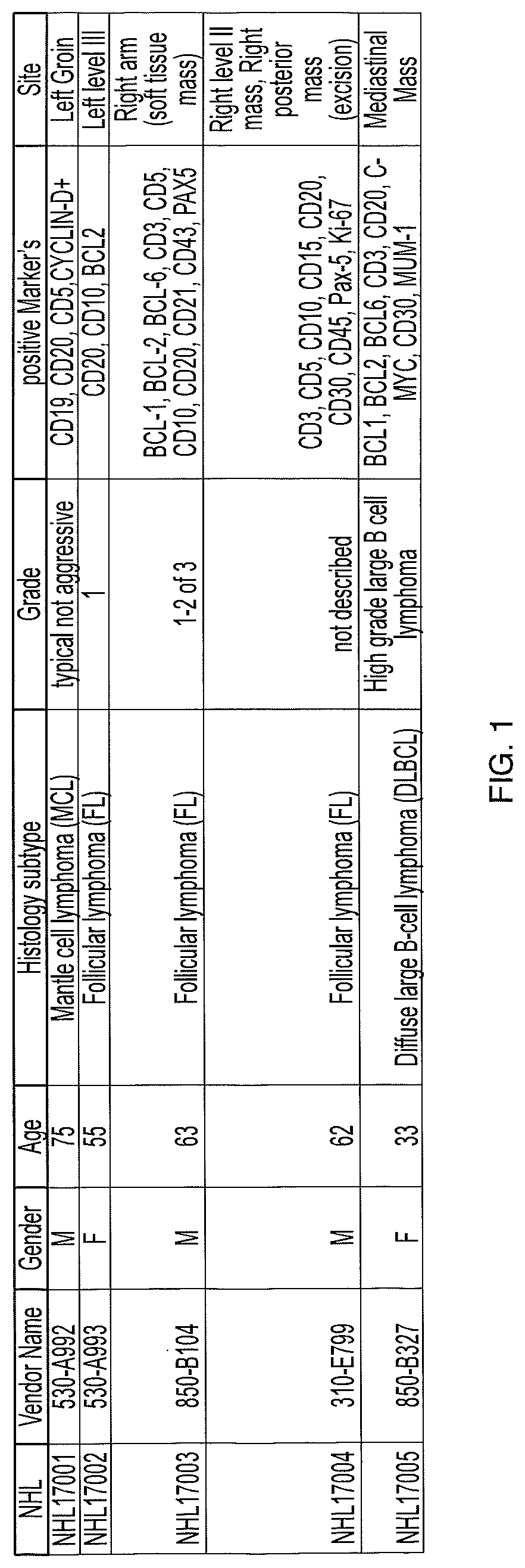
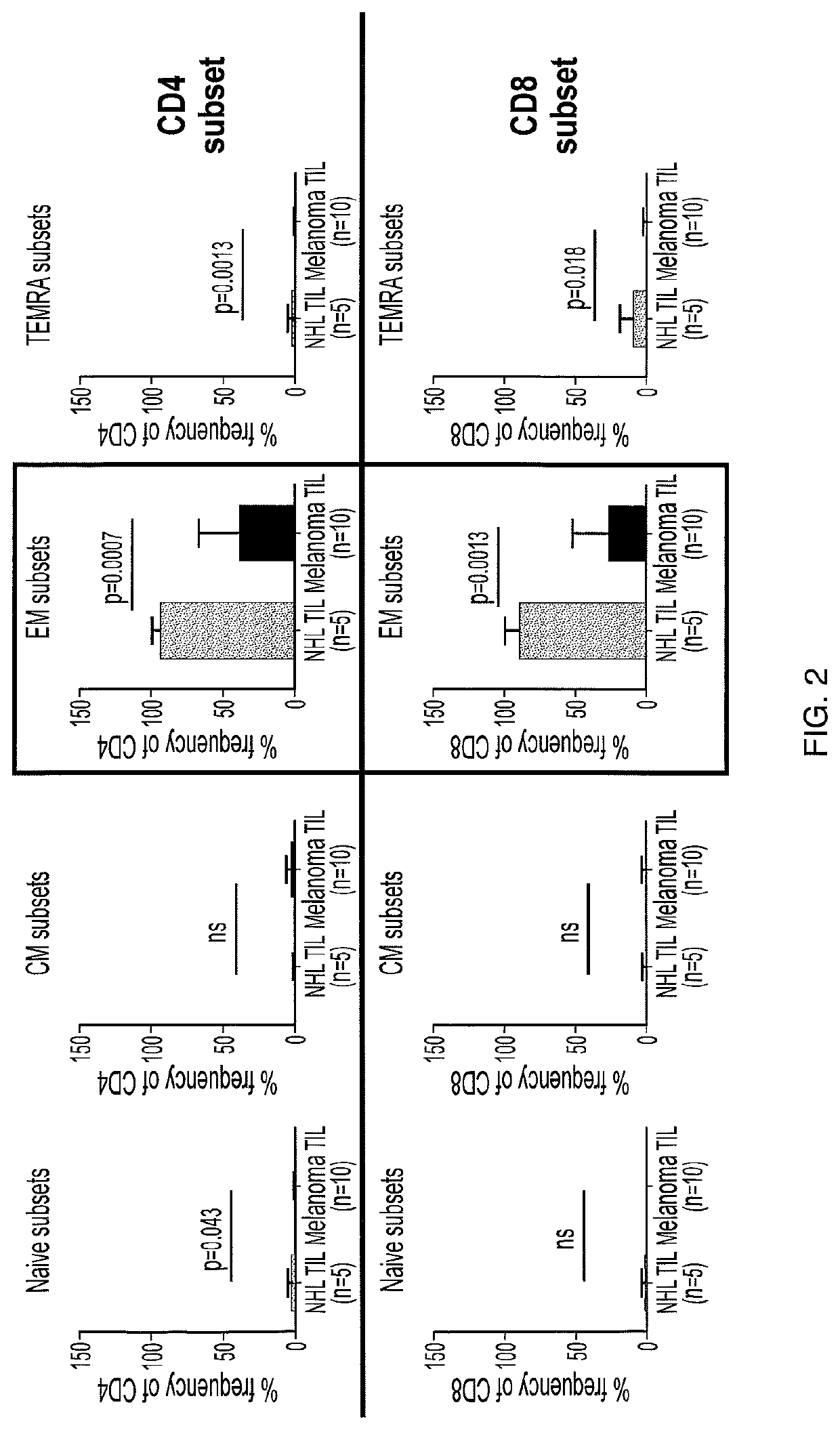
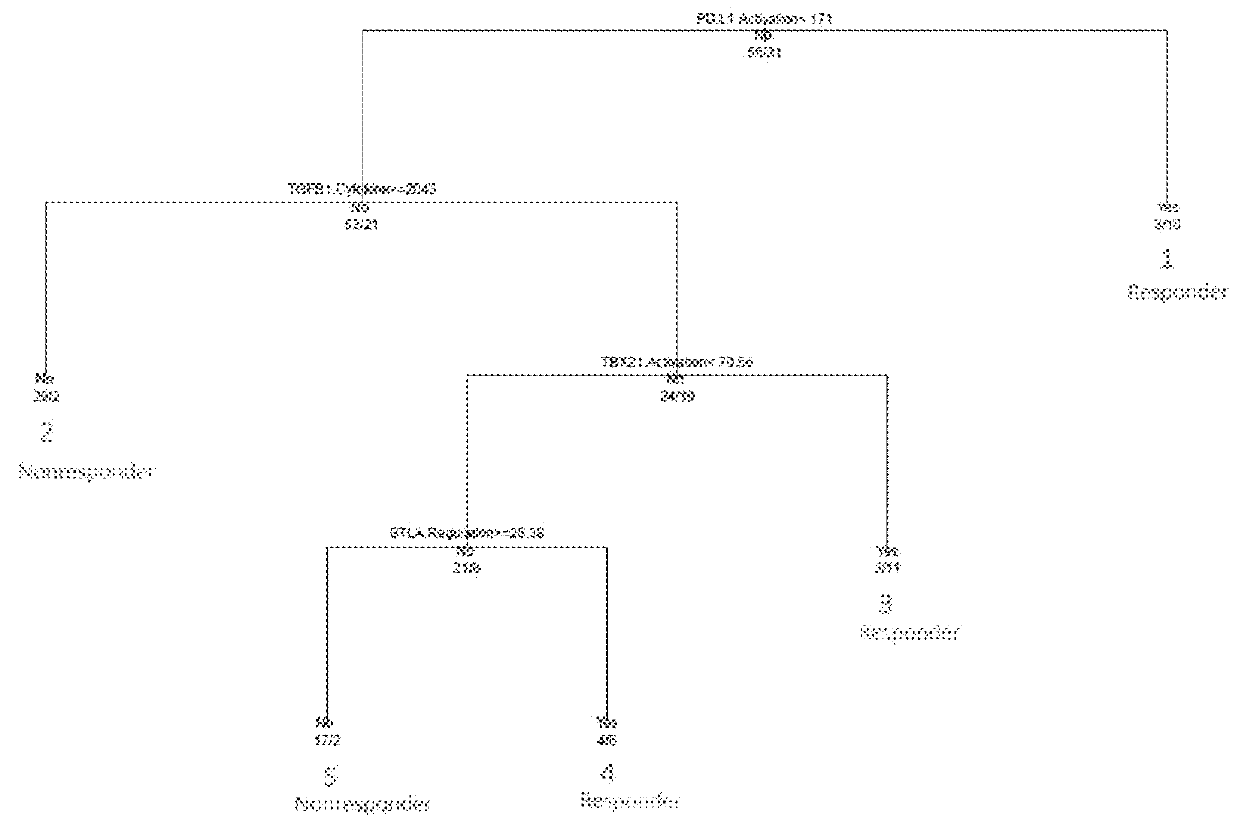
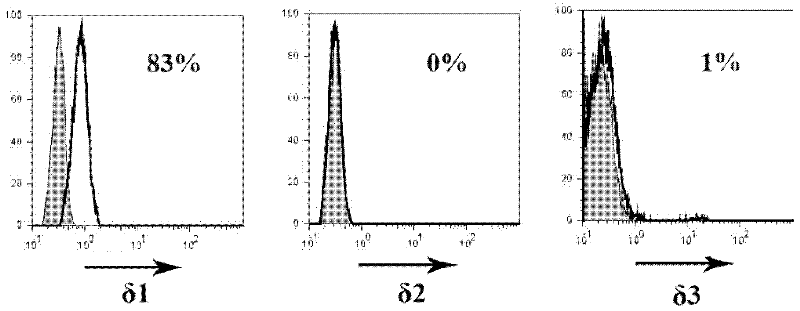
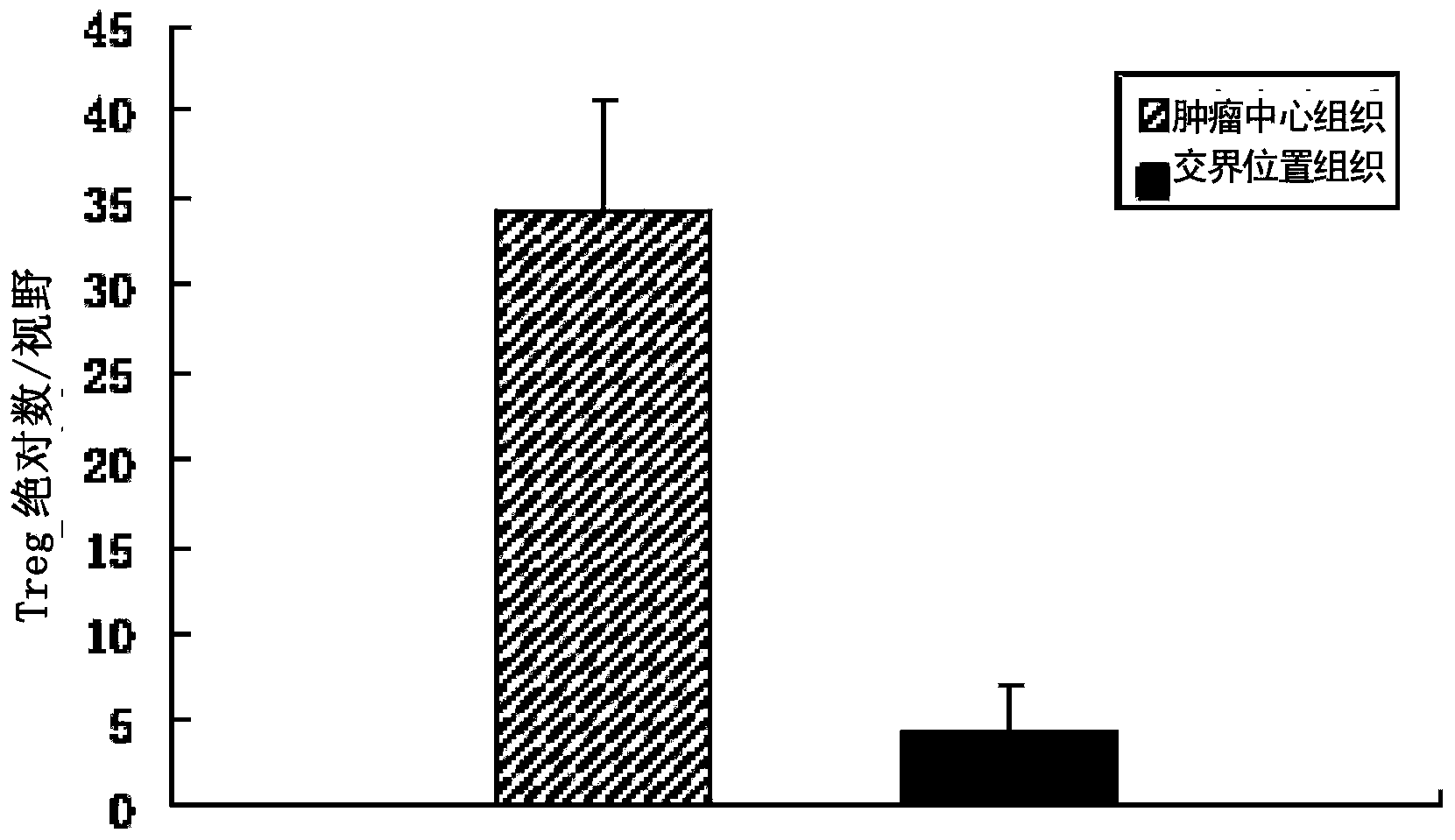
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
128 results about "Tumor-infiltrating lymphocytes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tumor-infiltrating lymphocytes are white blood cells that have left the bloodstream and migrated towards a tumor. They include T cells and B cells and are part of the larger category of ‘tumor-infiltrating immune cells’ which consist of both mononuclear and polymorphonuclear immune cells, (i.e., T cells, B cells, natural killer cells, macrophages, neutrophils, dendritic cells, mast cells, eosinophils, basophils, etc.) in variable proportions. Their abundance varies with tumor type and stage and in some cases relates to disease prognosis...
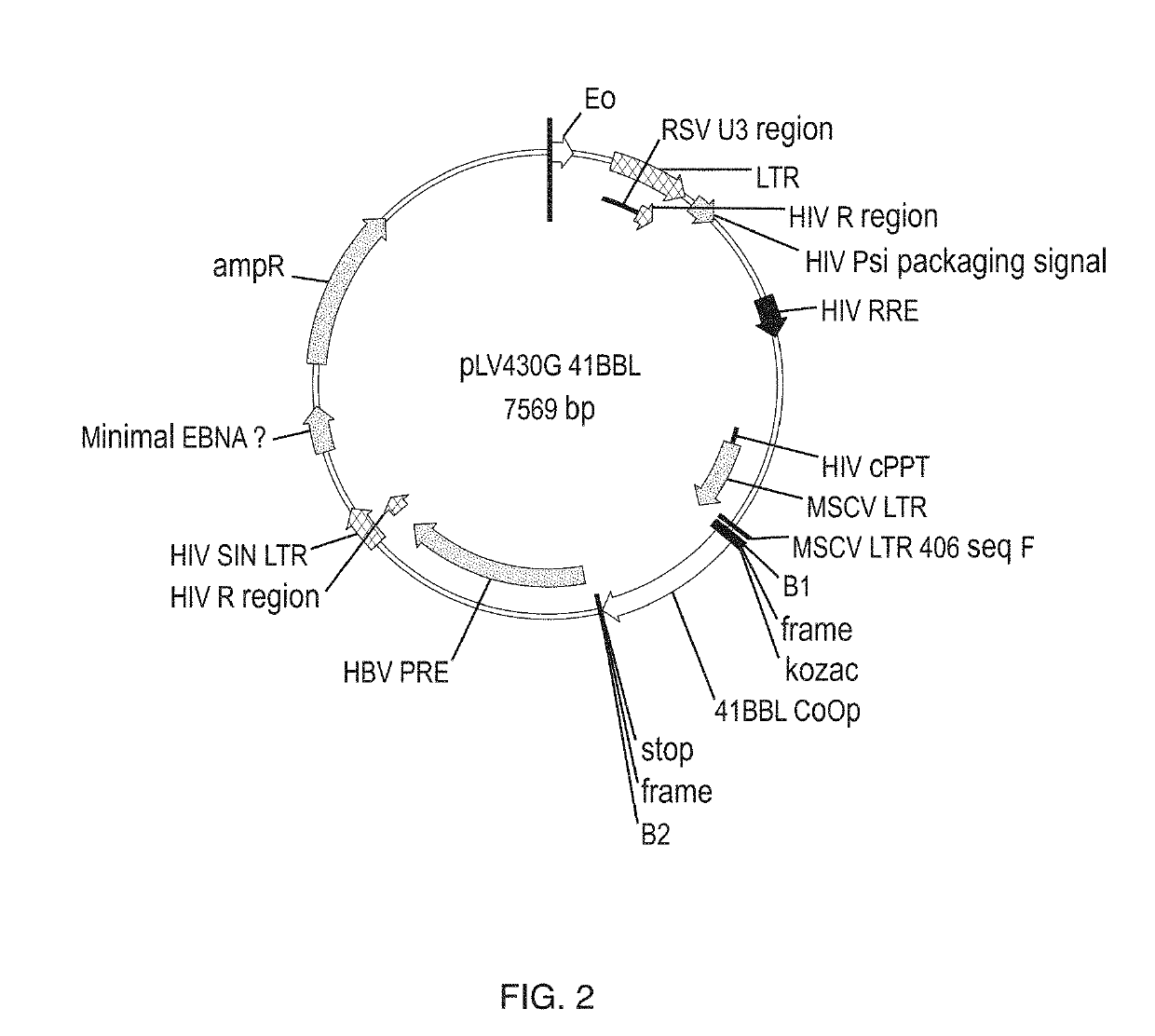
Methods of growing tumor infiltrating lymphocytes in gas-permeable containers
InactiveUS20120244133A1Increase the number ofBiocideArtificial cell constructsTumour tissueCell culture media
An embodiment of the invention provides a method of promoting regression of cancer in a mammal comprising obtaining a tumor tissue sample from the mammal; culturing the tumor tissue sample in a first gas permeable container containing cell medium therein; obtaining tumor infiltrating lymphocytes (TIL) from the tumor tissue sample; expanding the number of TIL in a second gas permeable container containing cell medium therein using irradiated allogeneic feeder cells and / or irradiated autologous feeder cells; and administering the expanded number of TIL to the mammal. Methods of obtaining an expanded number of TIL from a mammal for adoptive cell immunotherapy are also provided.
Owner:UNITED STATES OF AMERICA +1
Methods of growing tumor infiltrating lymphocytes in gas-permeable containers
InactiveUS20170152478A1Mammal material medical ingredientsCancer antigen ingredientsAbnormal tissue growthTumour tissue
An embodiment of the invention provides a method of promoting regression of cancer in a mammal comprising obtaining a tumor tissue sample from the mammal; culturing the tumor tissue sample in a first gas permeable container containing cell medium therein; obtaining tumor infiltrating lymphocytes (TIL) from the tumor tissue sample; expanding the number of TIL in a second gas permeable container containing cell medium therein using irradiated allogeneic feeder cells and / or irradiated autologous feeder cells; and administering the expanded number of TIL to the mammal. Methods of obtaining an expanded number of TIL from a mammal for adoptive cell immunotherapy are also provided.
Owner:UNITED STATES OF AMERICA +1
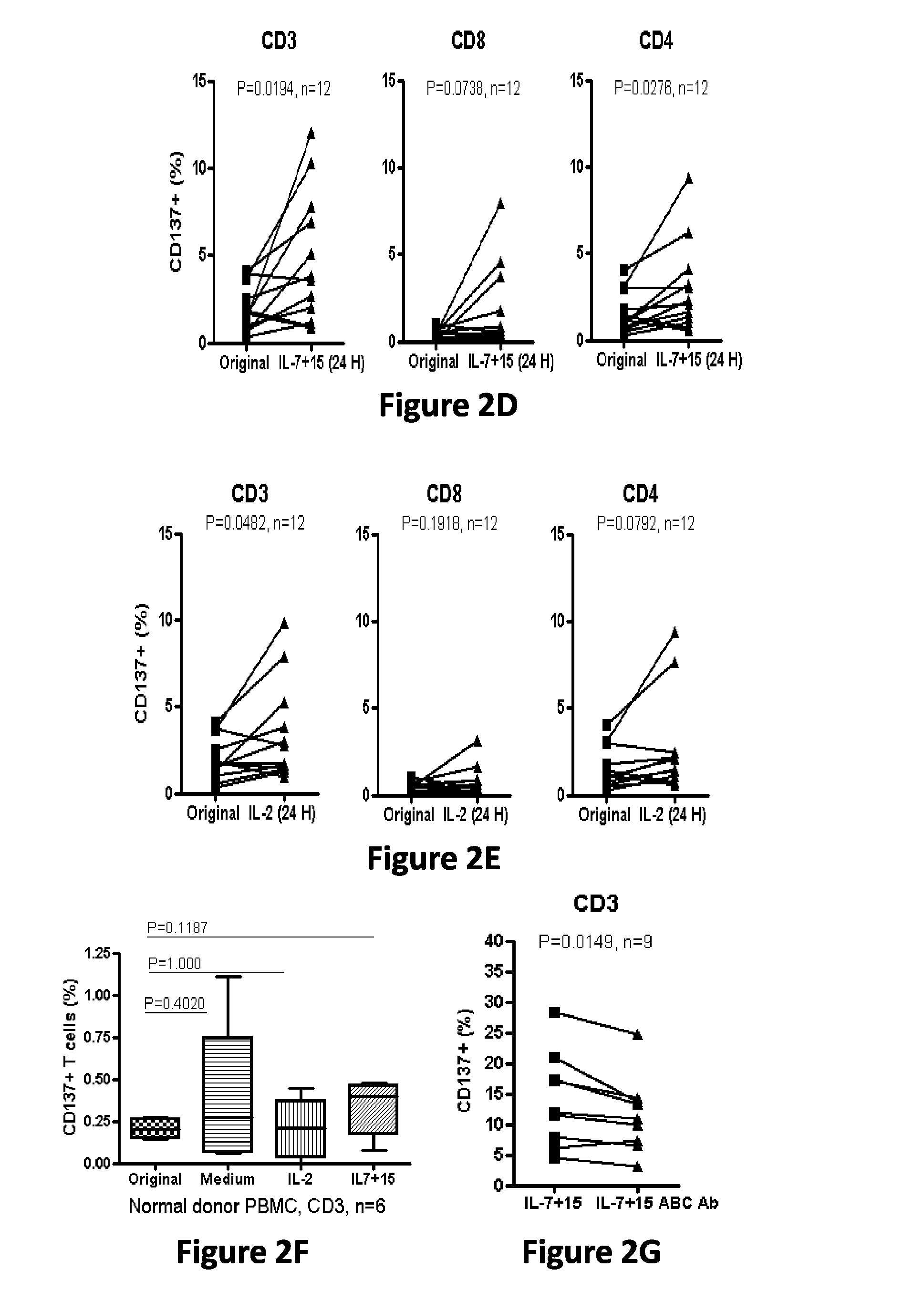
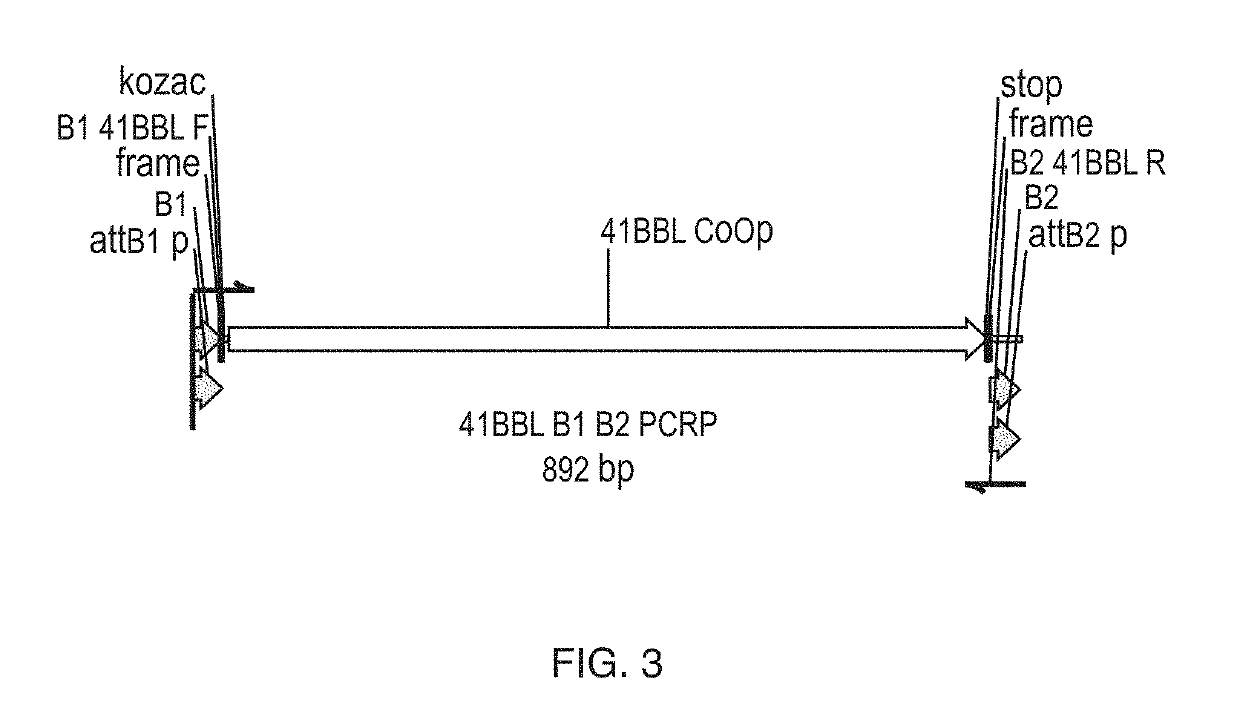
Cd137 enrichment for efficient tumor infiltrating lymphocyte selection
ActiveUS20160215262A1Effective anti-cancer T cell productMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthCD137
The invention includes compositions and methods to rapidly isolate and culture cells that are potent for use in adoptive immunotherapy. In one embodiment, the isolated cells of the invention are tumor infiltrating lymphocytes (TIL) that express CD137 (also known as 4-1BB and TNFSFR9).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
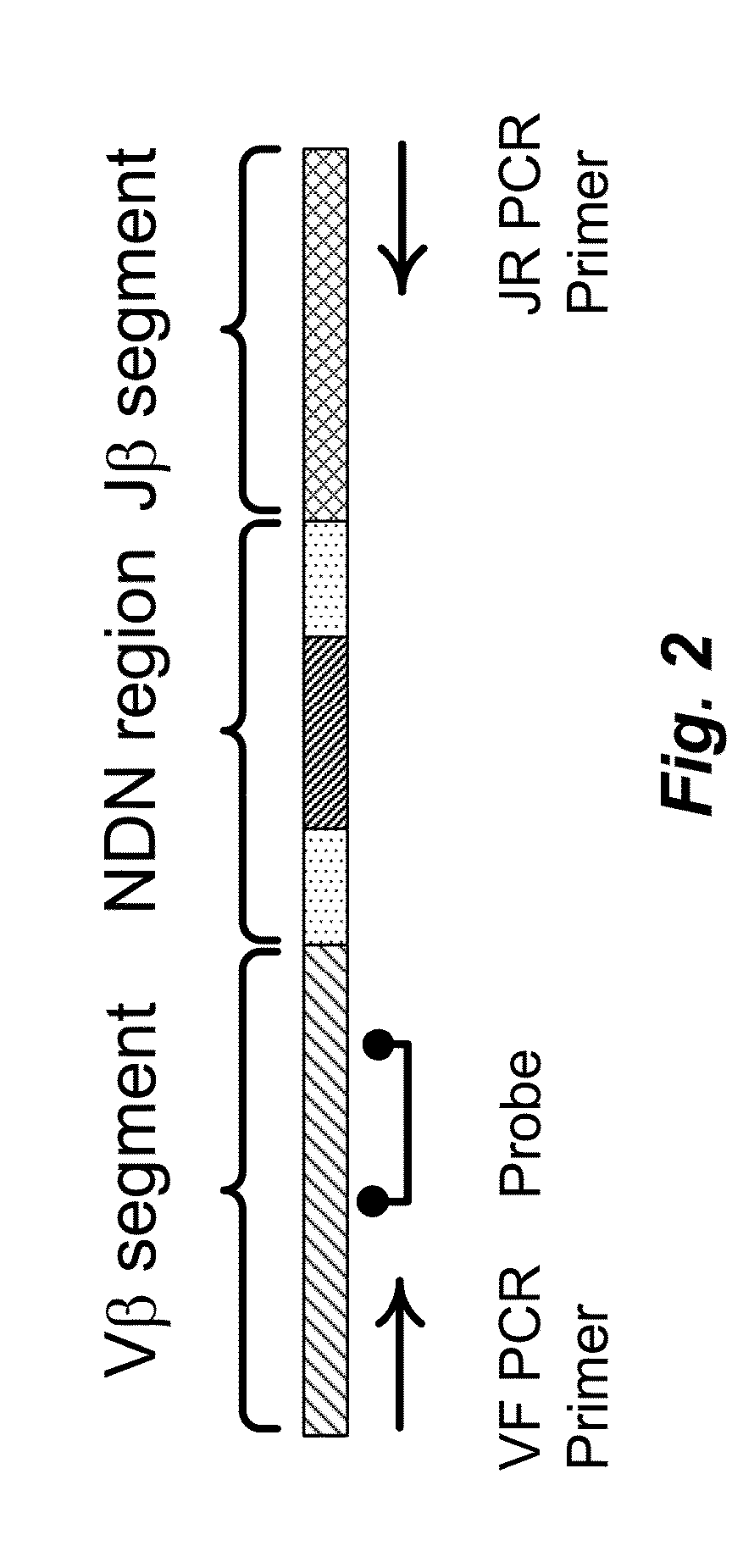
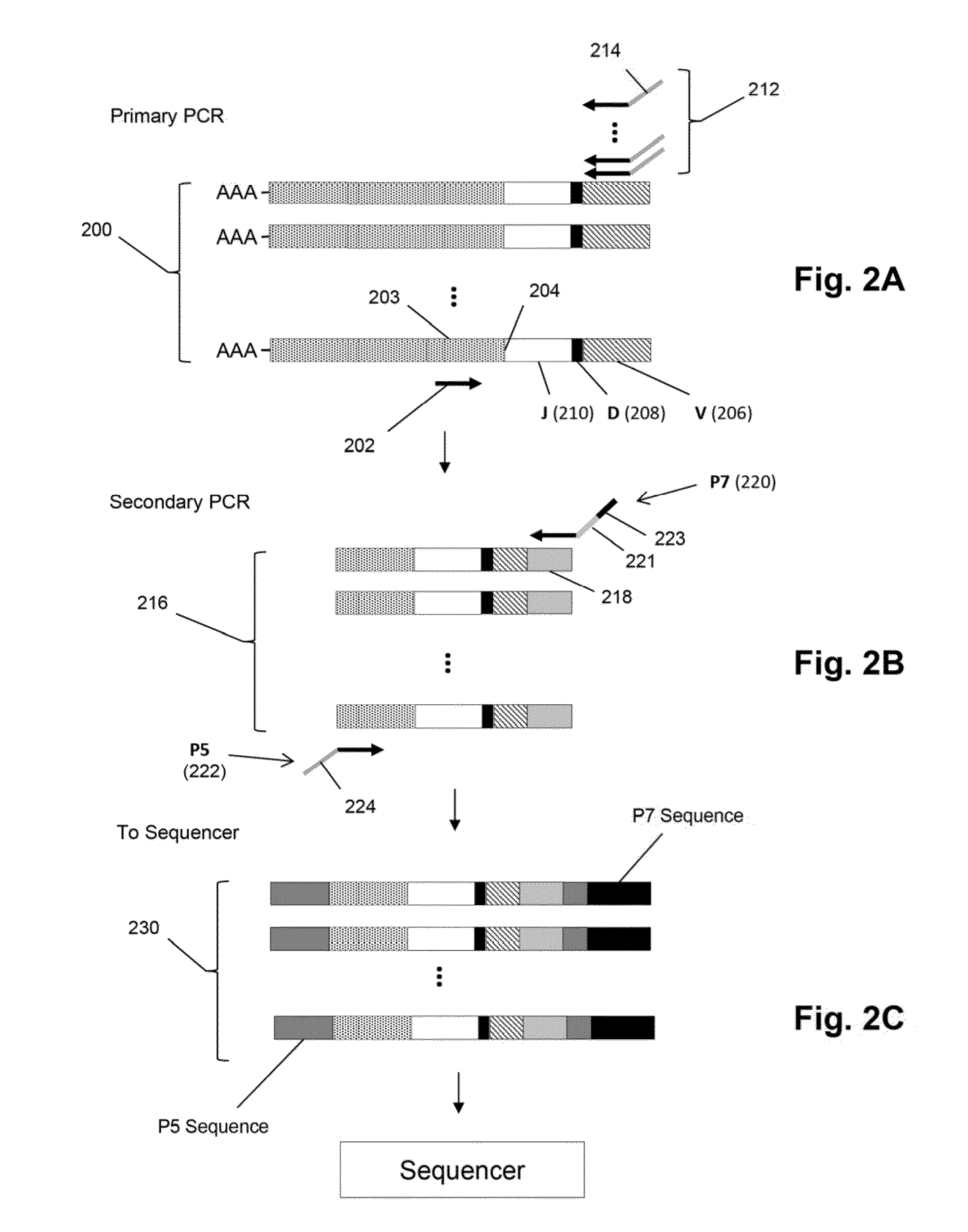
Quantification of adaptive immune cell genomes in a complex mixture of cells
Owner:ADAPTIVE BIOTECH +1
Detection and measurement of tissue-infiltrating lymphocytes
ActiveUS20130150252A1Microbiological testing/measurementLibrary member identificationDiseaseRegulatory T cell
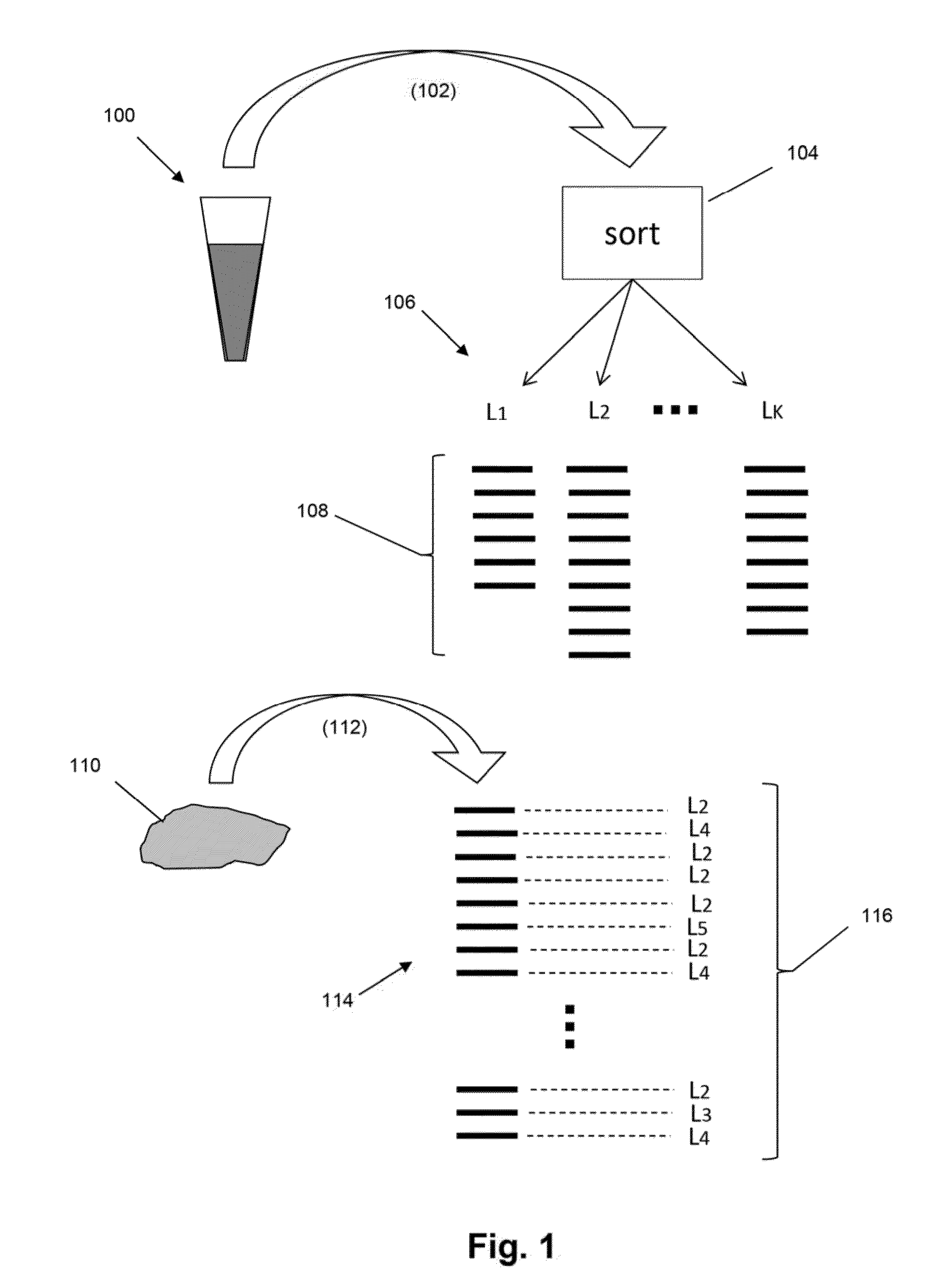
The present invention is drawn to methods for measuring numbers, levels, and / or ratios of cells, such as lymphocytes, infiltrated into a solid tissue, such as a tumor or a tissue affected by an autoimmune disease, and to methods for making patient prognoses based on such measurements. In one aspect, methods of the invention comprise sorting lymphocytes from an accessible tissue, such as peripheral blood, into functional subsets, such as cytotoxic T cells and regulatory T cells, and generating clonotype profiles of each subset. An inaccessible disease-affected tissue is sampled and one or more clonotype profiles are generated. From the latter clonotype profiles, levels lymphocytes in each of the functional subsets are determined in the disease-affected tissue by their clonotypes, which are identified from lymphocytes sorted into subsets from the accessible tissue.
Owner:ADAPTIVE BIOTECH
Quantification of Adaptive Immune Cell Genomes in a Complex Mixture of Cells
Compositions and methods are described for highly sensitive quantification of the relative representation of DNA from adaptive immune cells (e.g., T and / or B lymphocytes) in DNA extracted from complex mixtures of cells that include cells which are not adaptive immune cells. Included are methods for determining the relative presence in a tumor of tumor infiltrating lymphocytes (TIL), the relative presence of lymphocytes infiltrating a somatic tissue that is the target of an autoimmune disease, and the relative presence of lymphocytes infiltrating a transplanted organ.
Owner:ADAPTIVE BIOTECH +1
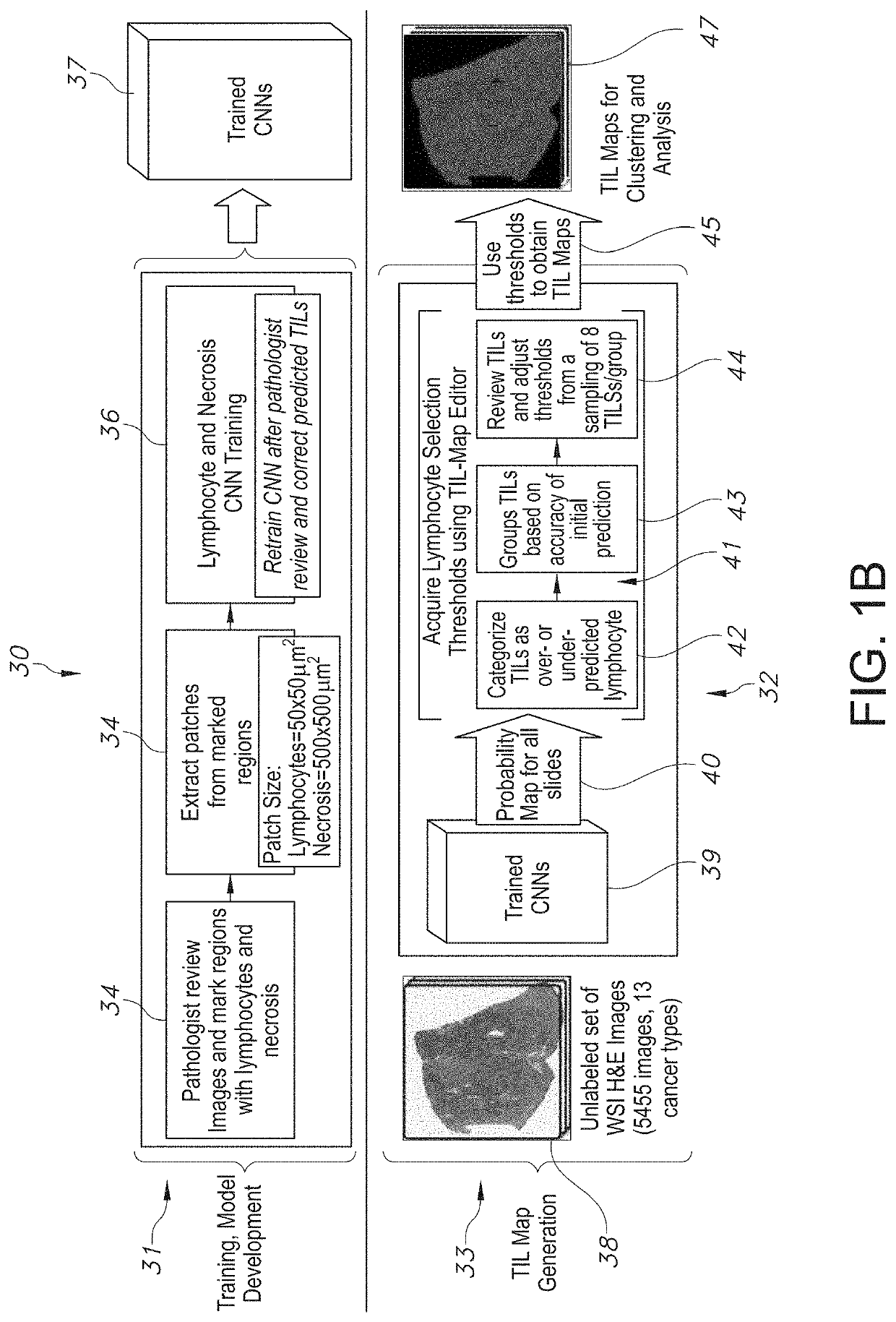
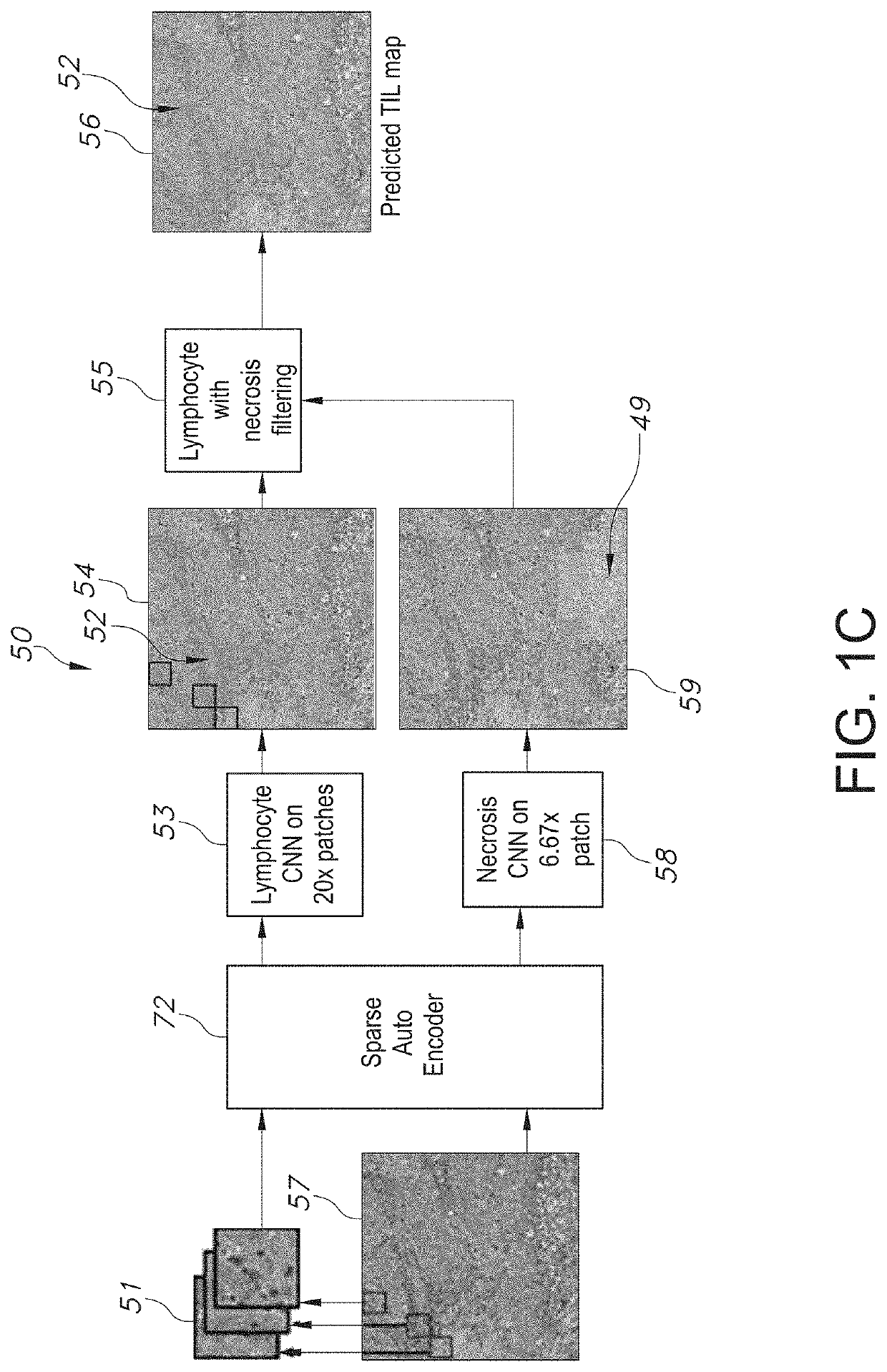
System and Method to Quantify Tumor-Infiltrating Lymphocytes (TILs) for Clinical Pathology Analysis Based on Prediction, Spatial Analysis, Molecular Correlation, and Reconstruction of TIL Information Identified in Digitized Tissue Images
A system associated with quantifying a density level of tumor-infiltrating lymphocytes, based on prediction of reconstructed TIL information associated with tumoral tissue image data during pathology analysis of the tissue image data is disclosed. The system receives digitized diagnostic and stained whole-slide image data related to tissue of a particular type of tumoral data. Defined are regions of interest that represents a portion of, or a full image of the whole-slide image data. The image data is encoded into segmented data portions based on convolutional autoencoding of objects associated with the collection of image data. The density of tumor-infiltrating lymphocytes is determined of bounded segmented data portions for respective classification of the regions of interest. A classification label is assigned to the regions of interest. It is determined whether an assigned classification label is above a pre-determined threshold probability value of lymphocyte infiltrated. The threshold probability value is adjusted in order to re-assign the classification label to the regions of interest based on a varied sensitivity level of density of lymphocyte infiltrated. A trained classification model is generated based on the re-assigned classification labels to the regions of interest associated with segmented data portions using the adjusted threshold probability value. An unlabeled image data set is received to iteratively classify the segmented data portions based on a lymphocyte density level associated with portions of the unlabeled image data set, using the trained classification model. Tumor-infiltrating lymphocyte representations are generated based on prediction of TIL information associated with classified segmented data portions. A refined TIL representation based on prediction of the TIL representations is generated using the adjusted threshold probability value associated with the classified segmented data portions. A corresponding method and computer-readable device are also disclosed.
Owner:THE RES FOUND TOR THE STATE UNIV OF NEW YORK +2
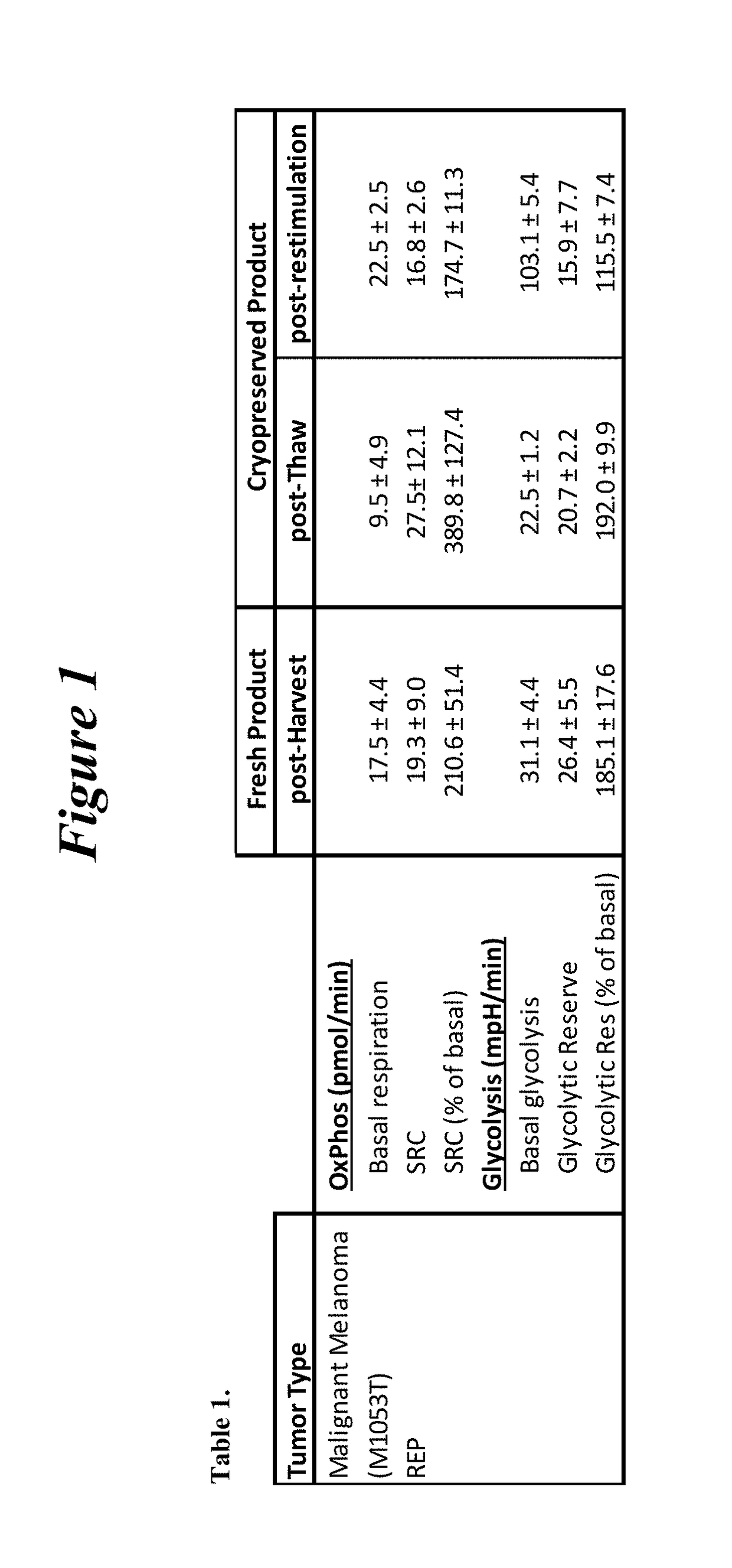
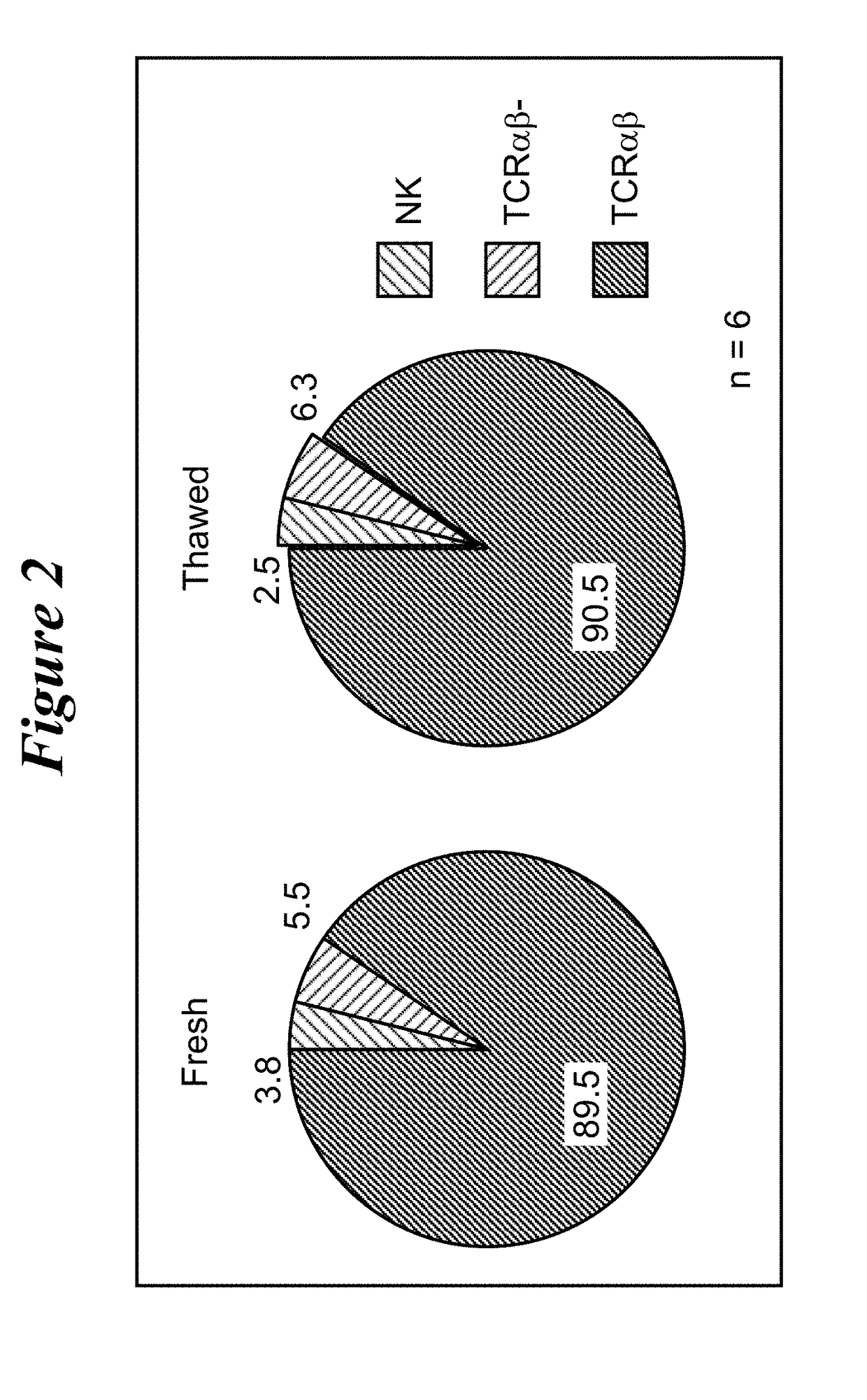
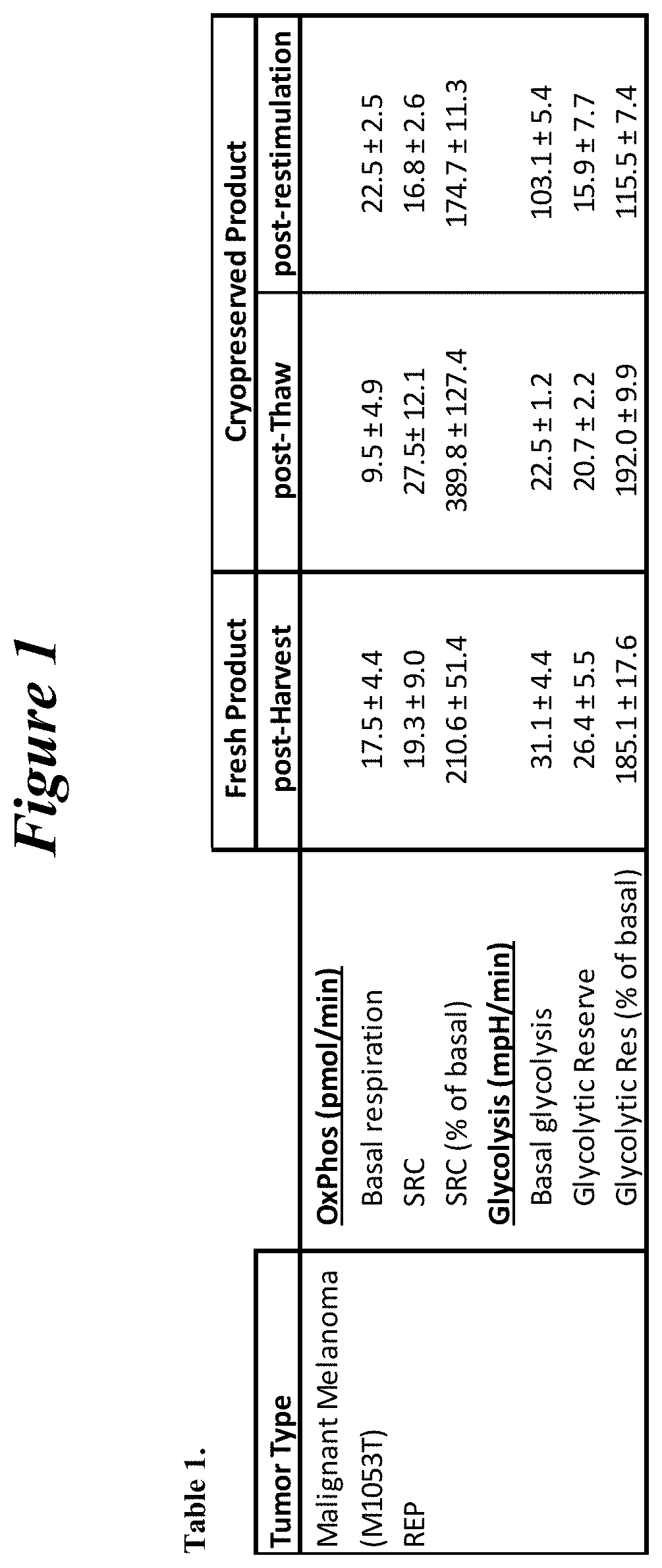
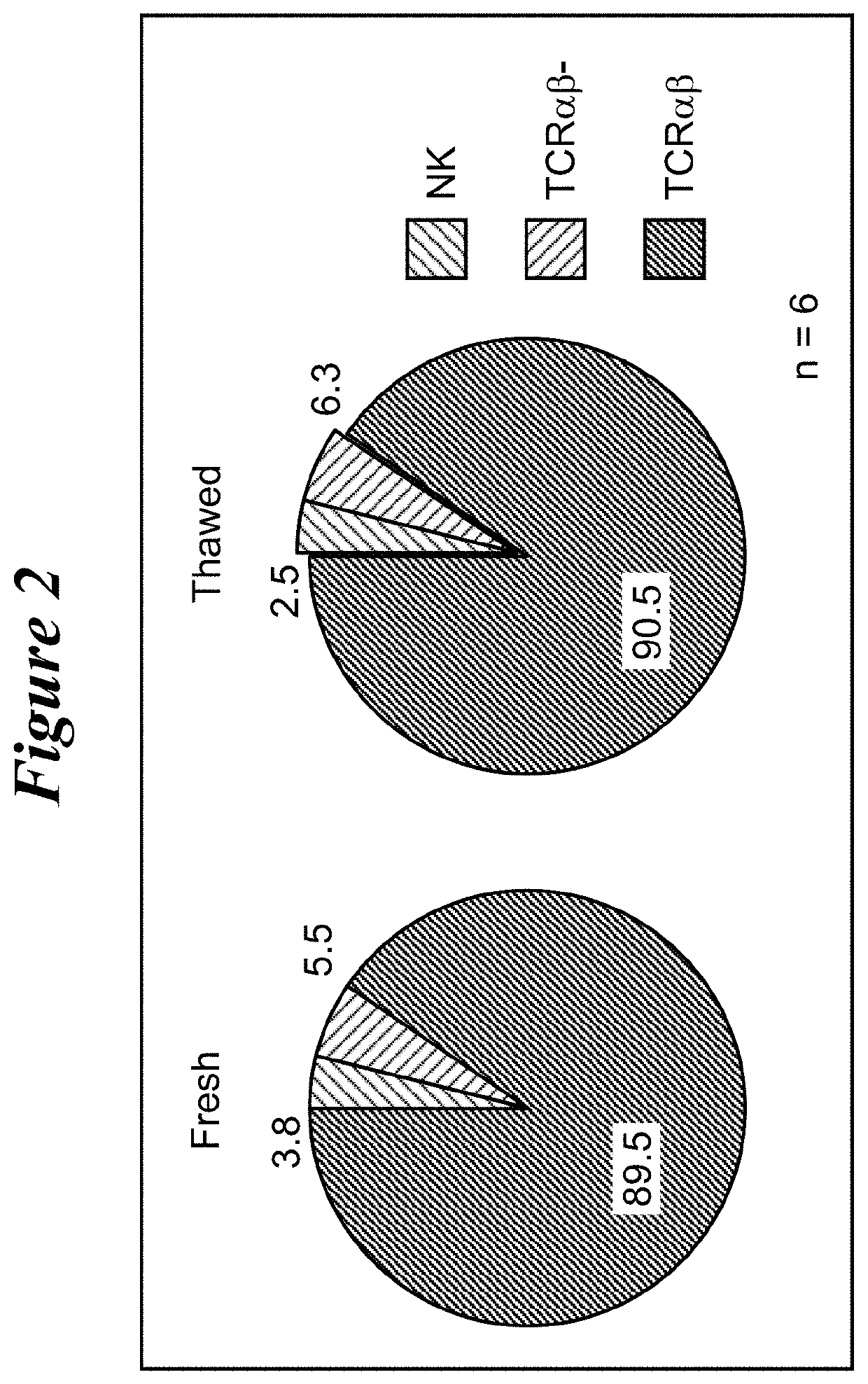
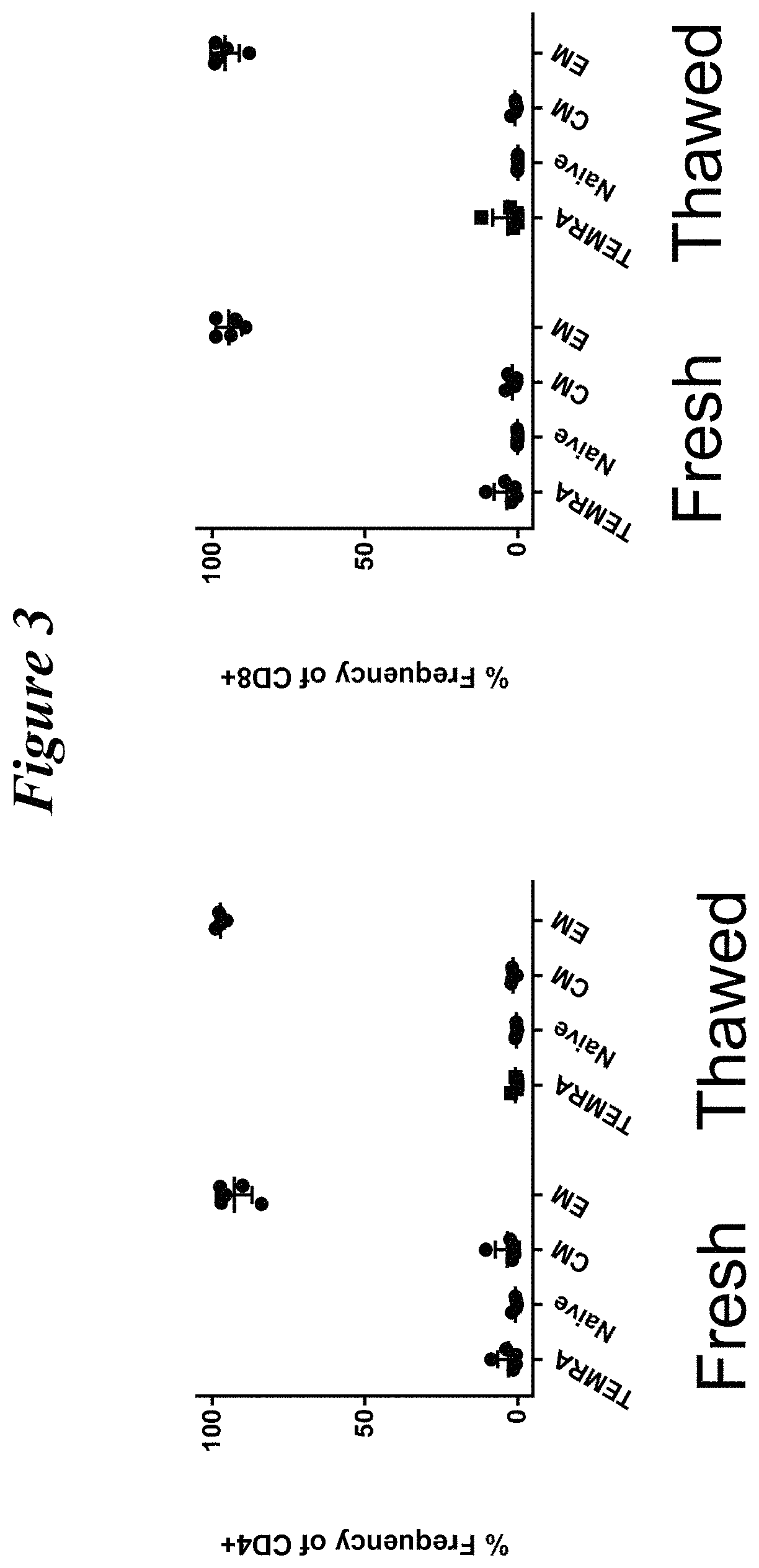
Restimulation of cryopreserved tumor infiltrating lymphocytes
ActiveUS20180228841A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyLymphocyteCryopreservation
The present disclosure provides methods for re-stimulating TIL populations that lead to improved phenotype and increased metabolic health of the TILs and provides methods of assaying for TIL populations to determine suitability for more efficacious infusion after re-stimulation.
Owner:IOVANCE BIOTHERAPEUTICS INC
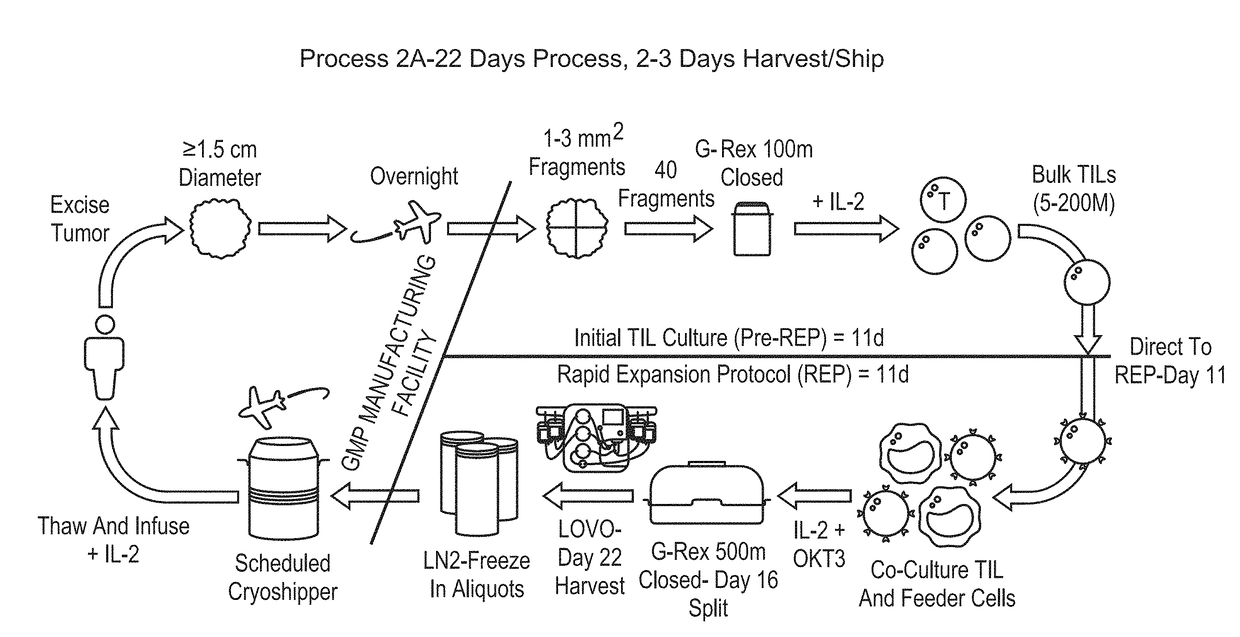
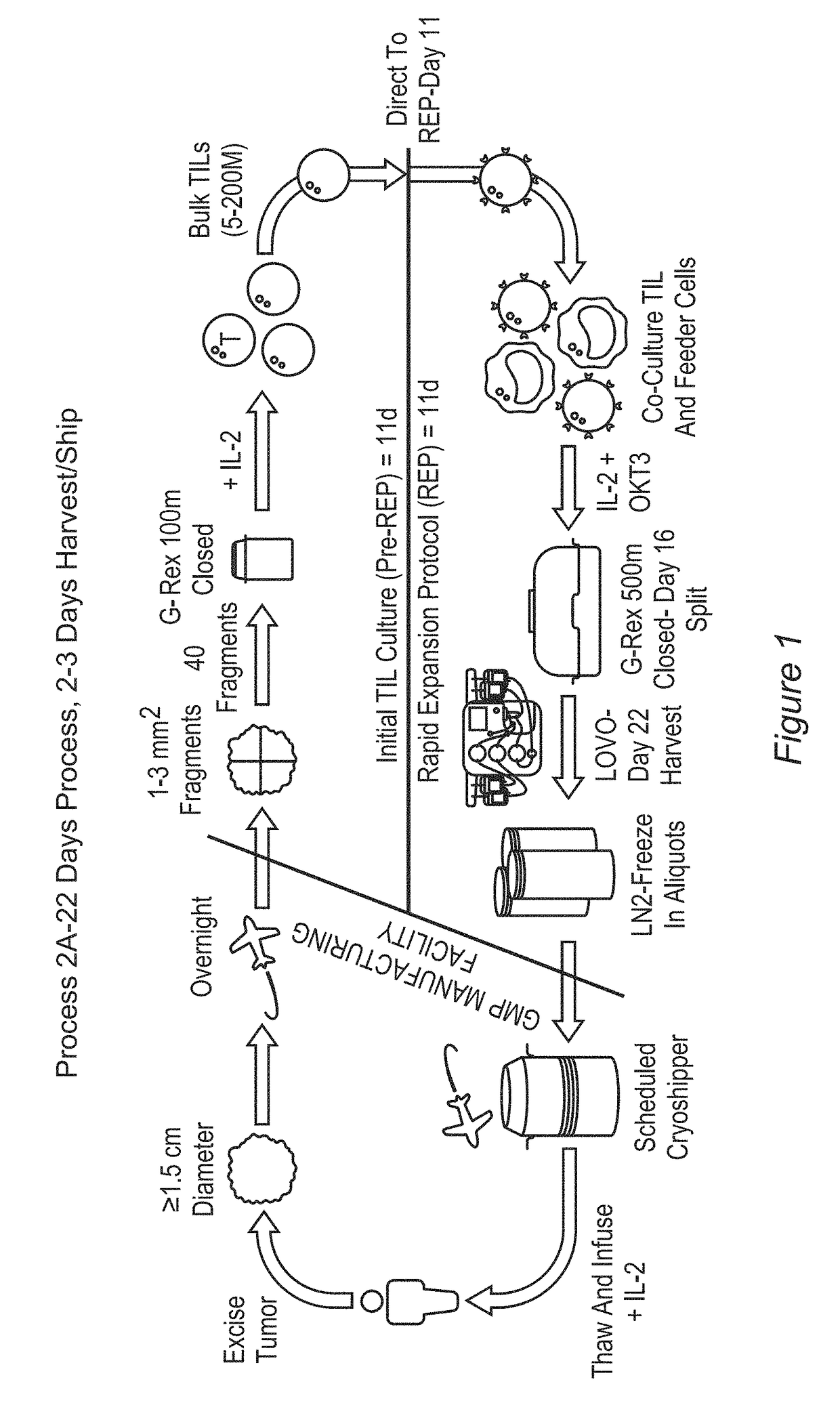
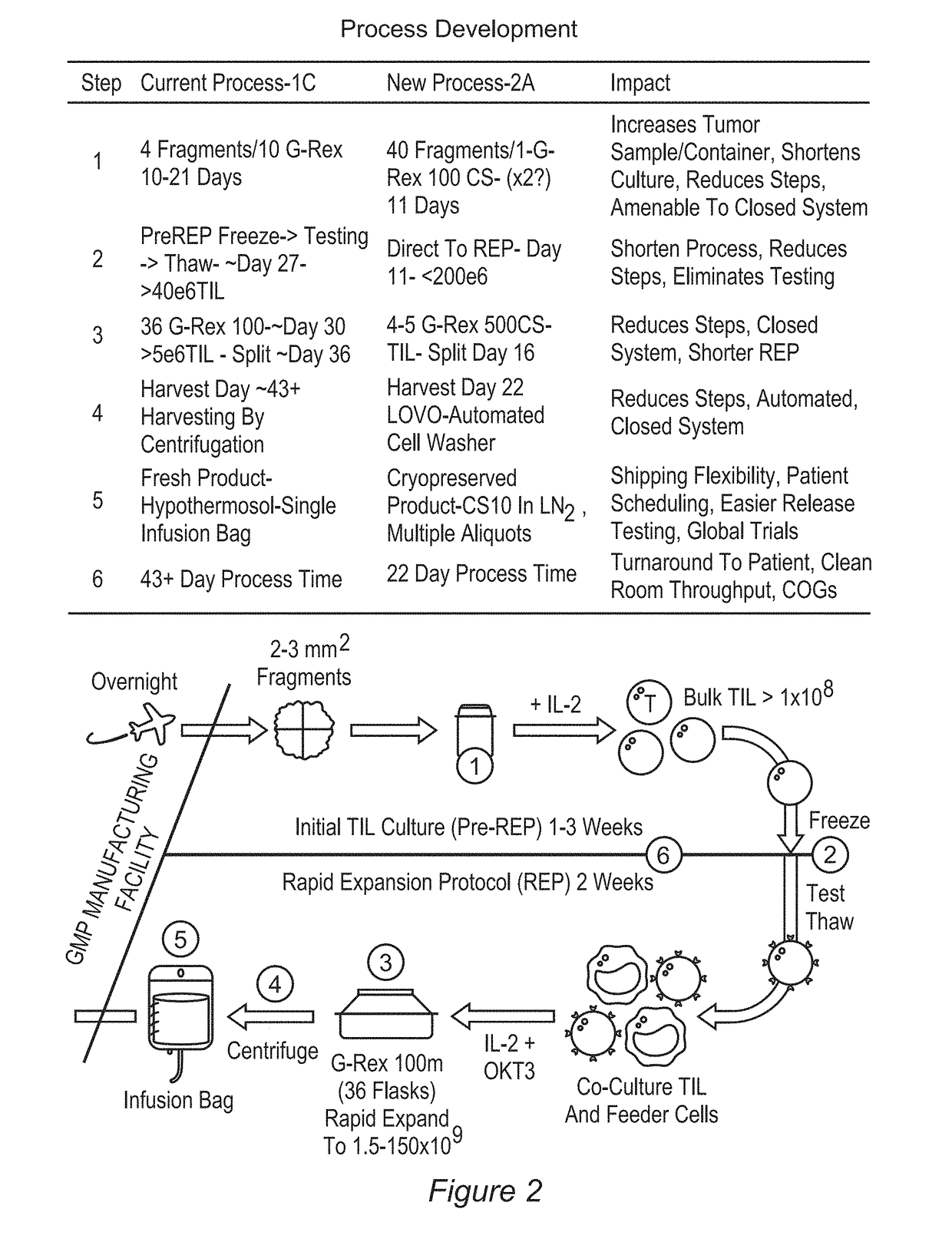
Processes for production of tumor infiltrating lymphocytes and uses of same in immunotherapy
ActiveUS20180207201A1Increase percentageGood curative effectOrganic active ingredientsPeptide/protein ingredientsRegimenTumor-infiltrating lymphocytes
The present invention provides improved and / or shortened methods for expanding TILs and producing therapeutic populations of TILs, including novel methods for expanding TIL populations in a closed system that lead to improved efficacy, improved phenotype, and increased metabolic health of the TILs in a shorter time period, while allowing for reduced microbial contamination as well as decreased costs. Such TILs find use in therapeutic treatment regimens.
Owner:IOVANCE BIOTHERAPEUTICS INC
Processes for production of tumor infiltrating lymphocytes and uses of same in immunotherapy
ActiveUS20180280436A1Good curative effectIncrease productionOrganic active ingredientsPeptide/protein ingredientsRegimenTherapeutic treatment
The present invention provides improved and / or shortened methods for expanding TILs and producing therapeutic populations of TILs, including novel methods for expanding TIL populations in a closed system that lead to improved efficacy, improved phenotype, and increased metabolic health of the TILs in a shorter time period, while allowing for reduced microbial contamination as well as decreased costs. Such TILs find use in therapeutic treatment regimens.
Owner:IOVANCE BIOTHERAPEUTICS INC
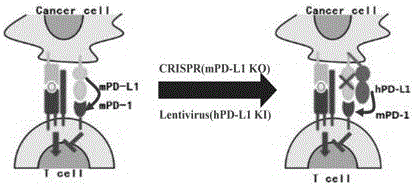
Humanized PD-L1 tumor cell line, animal model with same and application of humanized PD-L1 tumor cell line and animal model
ActiveCN105950560ASpeed up the processLethalCompounds screening/testingCell receptors/surface-antigens/surface-determinantsPD-L1Wilms' tumor
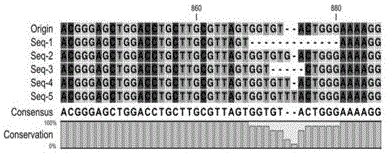
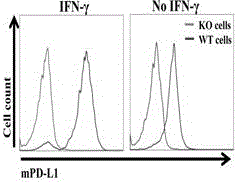
The invention provides a humanized PD-L1 tumor cell line MC-38-hPD-L1, a builtanimal tumor model with the same and a method for constructing the humanized PD-L1 tumor cell line. The method particularly includes knocking out animal-origin PD-L1 by the aid of CRISPR-CAS9; carrying out amplification and cultivation to obtain knocked-out cell banks; extracting DNA (deoxyribonucleic acid) and carrying out PCR (polymerase chain reaction) amplification; recycling and cloning amplification products; carrying out over-expression on human-origin PD-L1 in MC-38 cell lines of mPD-L1 KO by the aid of lentivirus systems; packaging lentivirus and screening Puromycin to obtain the humanized MC-38 cell line of PD-L1. The humanized PD-L1 tumor cell line, the animal tumor model and the method have the advantages that as shown by results, high killing efficiency and multiplication capacity are obviously presented by tumor infiltration CD8 T lymphocytes after antibody treatment is carried out, tumor infiltration Treg cells can be obviously inhibited after antibody treatment is carried out, and accordingly the method is proved to be effective and feasible from the aspect of molecular mechanisms.
Owner:SUZHOU INST OF SYST MEDICINE
Method of preparing adenosine-resistant anti-tumor T lymphocytes for adoptive immunotherapy
In the past, adoptive immunotherapy often failed because the transferred immune cells were inactive in vivo. This disclosure provides a method of producing immune cells that are highly active in vivo. The immune cells may be expanded in vitro in the presence of an adenosine receptor agonist or an antisense nucleic acid that downregulates expression of an adenosine receptor, for example. The immune cells may be tumor-infiltrating lymphocytes (TIL), cytotoxic T lymphocytes (CTL), natural killer (NK) cells, or lymphokine-activated killer (LAK) cells, for example. The methods described herein may be used to treat a number of diseases including cancer, infectious diseases, and immunodeficiencies.
Owner:NORTHEASTERN UNIV
CEACAM1 Based Cancer Therapy and Diagnosis
ActiveUS20070071758A1Good curative effectCompounds screening/testingImmunoglobulin superfamilyTumor-infiltrating lymphocytesCancer therapy
The invention relates to methods and compositions for the treatment and diagnosis of cancers. At least one aspect of the present invention relates to methods and compositions for enhancing the efficacy of tumor-infiltrating lymphocyte (TIL) therapy in the treatment of cancer by negatively modulating the activity of the CEACAM1 protein.
Owner:MARKEL GAL
Restimulation of cryopreserved tumor infiltrating lymphocytes
ActiveUS10517894B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyMetabolic healthTumor-infiltrating lymphocytes
The present disclosure provides methods for re-stimulating TIL populations that lead to improved phenotype and increased metabolic health of the TILs and provides methods of assaying for TIL populations to determine suitability for more efficacious infusion after re-stimulation.
Owner:IOVANCE BIOTHERAPEUTICS INC
Expansion Of Peripheral Blood Lymphocytes (PBLS) From Peripheral Blood
InactiveUS20200347350A1Organic active ingredientsPeptide/protein ingredientsDiseaseHaematological malignancy
Methods of expanding tumor infiltrating lymphocytes (TILs), including peripheral blood lymphocytes (PBLs) and marrow infiltrating lymphocytes (MILs), from blood and / or bone marrow of patients with hematological malignancies, such as liquid tumors, including lymphomas and leukemias, and genetic modifications of expanded TILs, PBLs, and MILs to incorporate chimeric antigen receptors, genetically modified T-cell receptors, and other genetic modifications, and uses of such expanded and / or modified TILs, PBLs, and MILs in the treatment of diseases such as cancers and hematological malignancies are disclosed herein.
Owner:IOVANCE BIOTHERAPEUTICS INC
T cell subpopulations capable of treating cancer
InactiveUS20100310534A1BiocideMicrobiological testing/measurementTumor-infiltrating lymphocytesCancer therapy
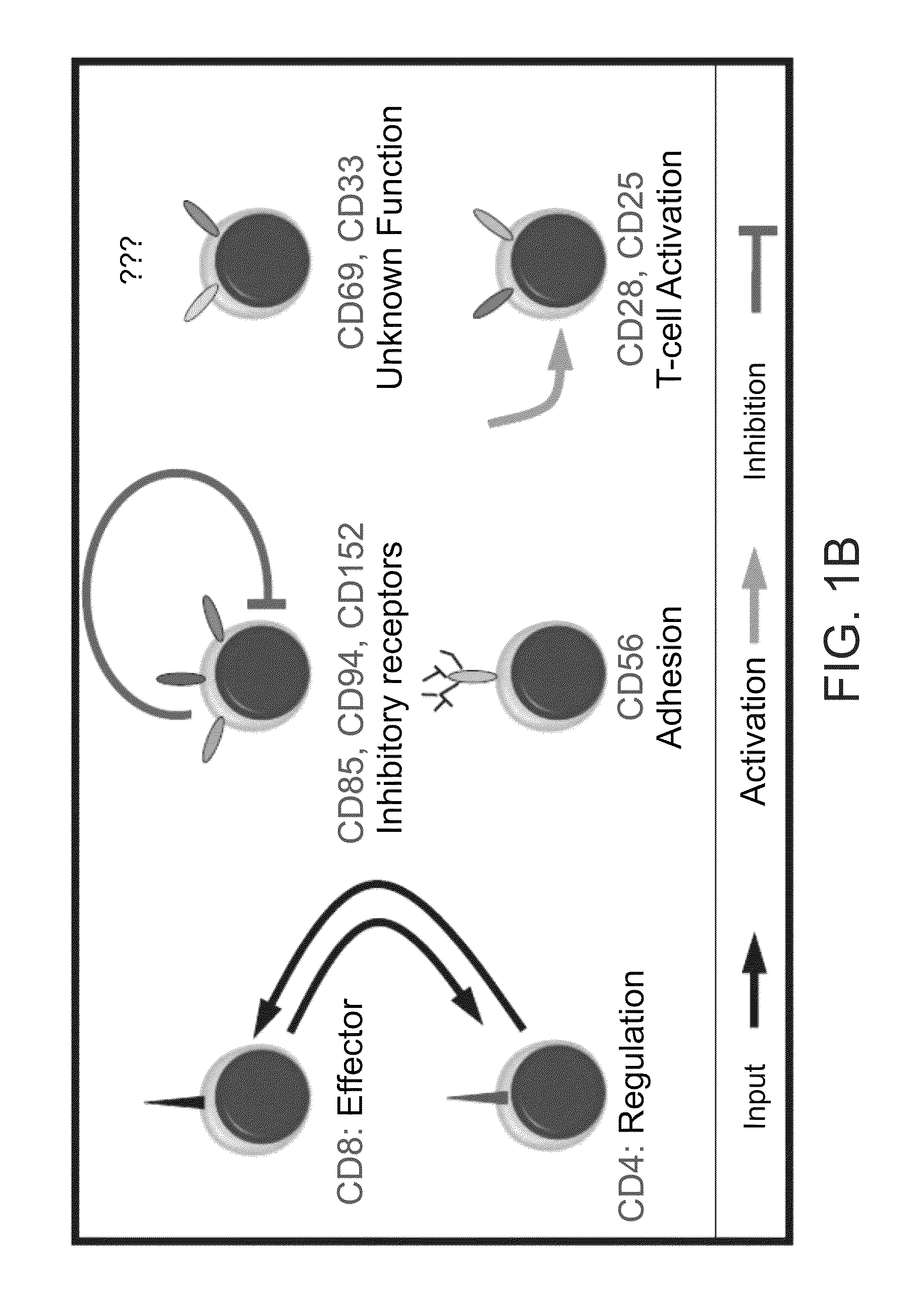
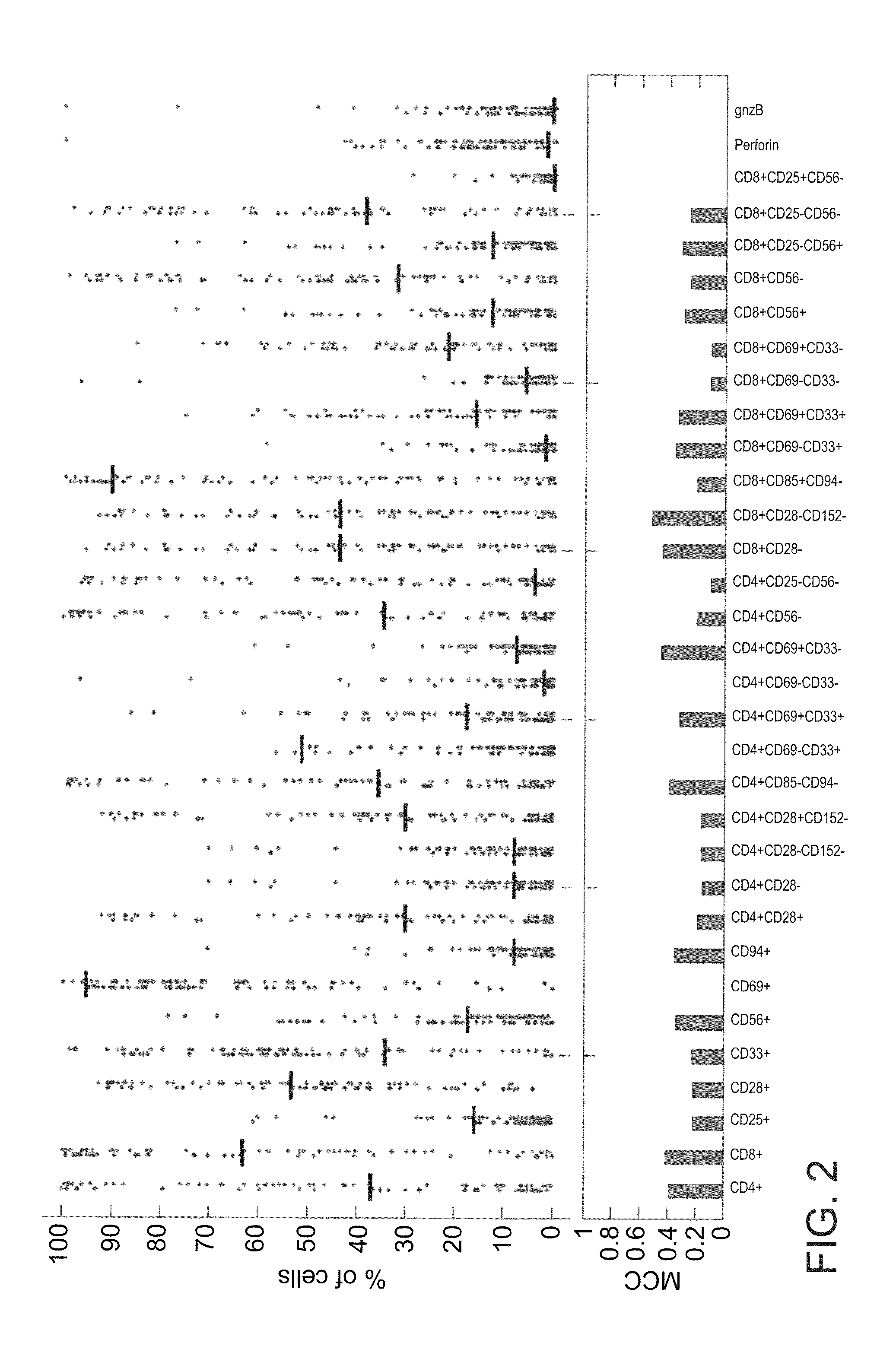
A method of determining responsiveness to cancer treatment is disclosed. The method comprises analyzing a frequency of tumor infiltrating lymphocytes (TILs) having a CD8+CD28−CD152− signature in a sample of the subject, wherein a frequency of TILs having the CD8+CD28−CD152− signature above a predetermined level is indicative of a positive responsiveness to cancer treatment. Other signatures reflecting responsiveness to cancer treatment are also disclosed. In addition, methods of treating cancer based on these signatures are also disclosed.
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES +1
Adoptive immunotherapy for treating cancer
ActiveUS20170349880A1Cancer antigen ingredientsBlood/immune system cellsTumor-infiltrating lymphocytesCancer therapy
The present invention provides methods for producing and / or expanding tumor-infiltrating lymphocytes (TILs) that can be used in adoptive immunotherapy in cancer treatment.
Owner:UNIVERSITY OF LAUSANNE
Method for in-vitro expansion of CD8<+>T cells
PendingCN105713878AIncrease the amplification factorInhibition of negative regulationBlood/immune system cellsCell culture active agentsPeritoneal EffusionAscitic fluid
The invention provides a method for in-vitro expansion of CD8<+>T cells. The method comprises steps as follows: TIL (tumor infiltrating lymphocyte) cells are extracted from a malignant pleural and peritoneal effusion source, cytokines IL-2, CD3McAb and PD-1 monoclonal antibodies are added to a TIL cell culture medium, the concentration of the IL-2 is 300-1,000 U / ml, the concentration of the CD3McAb is 10-50 ng / ml, and the concentration of the PD-1 monoclonal antibodies is 50-200 ng / ml. The cell factor combination adopted by the method can increase the TIL cell expansion multiple, effectively inhibit the negative regulation effect of Treg cells on the CD8<+>T cells and promote the anti-tumor effect.
Owner:杭州特马赛生物技术有限公司
Method for effectively culturing tumor infiltrating lymphocytes (TILs)
InactiveCN102174469AShorter hospital stayReduce the burden of hospitalizationBlood/immune system cellsAntineoplastic agentsClinical efficacyApoptosis Regulatory Proteins
The invention discloses a method for effectively culturing tumor infiltrating lymphocytes (TILs). The method comprises the following steps: extracting mononuclear cells from the pectoral ascite or tumor tissue of a patient, washing, inoculating into a culture bottle coated with recombinant human fibrin and cluster-of-differentiation-3 antibody, culturing for 24 hours, adding interleukin II, then using a serum-free culture medium containing the interleukin II and the supernatant pectoral ascite to enlarge and subculture every two or three days, and adding apoptosis-regulated protein survivin and mucoprotein-1 on the twelveth or thirteenth day to activate the killing activity; and harvesting cells on the fourteenth day. By adopting the method, the culture time, which is only 14 days, is greatly shortened, so that the hospital stays of the patient can be greatly shortened and the hospital burden of the patient can be reduced. Meanwhile, the problems that the cell proliferation speed is low and the proliferation ratio is low can be solved, thus the number of the cultured cells can meet the clinical requirement. The cultured TILs has strong cell specificity so as to enhance the clinical curative effect.
Owner:宋鑫 +1
Tumor tissue tumor infiltrating lymphocyte (TIL) cell preparation method and dedicated culture medium
ActiveCN107384867AImprove scalabilityIncrease the amount of amplificationCell dissociation methodsCulture processAdditive ingredientSodium phosphates
The invention relates to a tumor tissue tumor infiltrating lymphocyte (TIL) cell preparation method and a dedicated culture medium. The method comprises the following steps of tumor surrounding tissue obtaining, cell digestion, cell primary culture, cell subculture and cell collection, wherein a primary culture medium is based on a RPMI 1640 culture medium and prepared from the following concentration ingredients of 10% volume of human-derived serum, 20 to 45ng / ml of basic fibroblast growth factor (bFGF), 1 to 5mg / ml of riboflavin, 70 to 90ng / ml of cortisol, 10 to 25mg / ml of sodium dihydrogen phosphate monohydrate, 47 to 62ng / ml of recombinant human leukaemia inhibitory factor (LIF) and 500 to 800U / ml of IL-2; a subculture medium is based on the RPMI 1640 culture medium and prepared from the following concentration ingredients of 10% volume of the human-derived serum, 20 to 40mmol / L of HEPES, 1000 to 2000U / ml of the IL-2, 0.03 to 0.07mmol / L of beta-mercaptoethanol and 5 to 15ng / ml of sodium phosphate. According to the preparation method, an existing culture medium is improved, different culture mediums are utilized to culture the TIL cells in pertinence, TIL cell expansion capacity is improved, meanwhile a culture period is reduced, a culture complexity degree is reduced, a use amount of the IL-2 is reduced, and toxic reaction is reduced.
Owner:CENTURY BIOSTRENGTH BEIJING PTY LTD
Culture method for functionally enhanced TILs
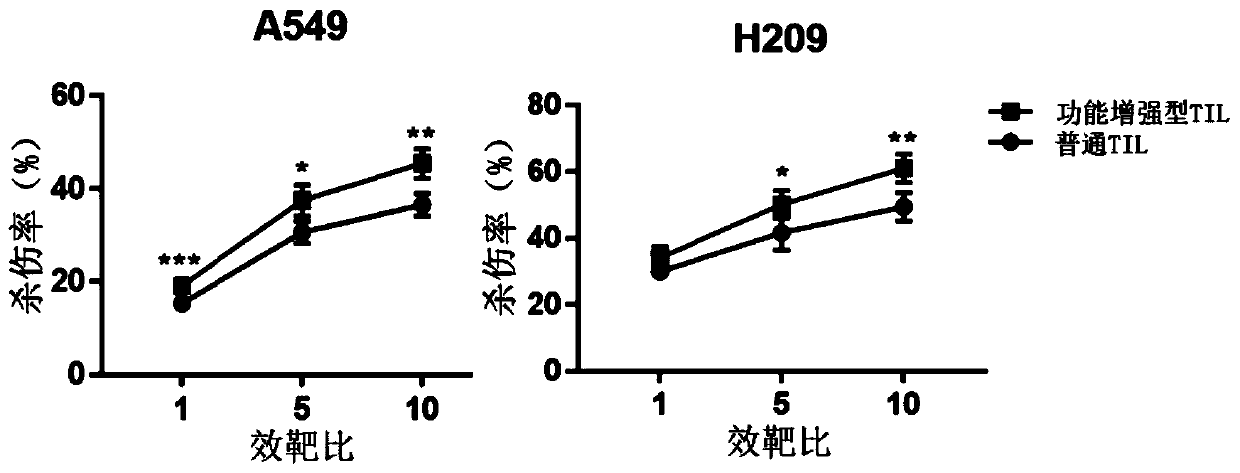
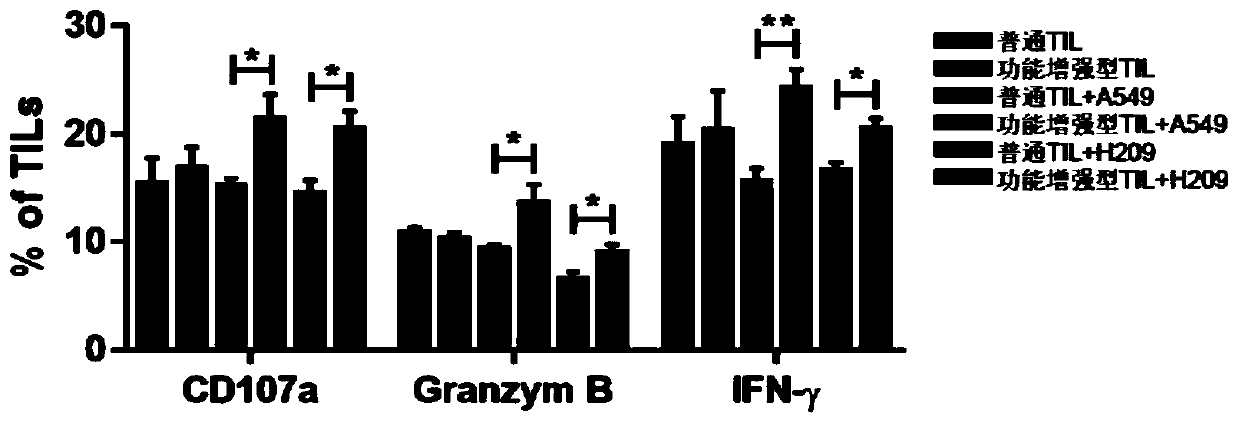
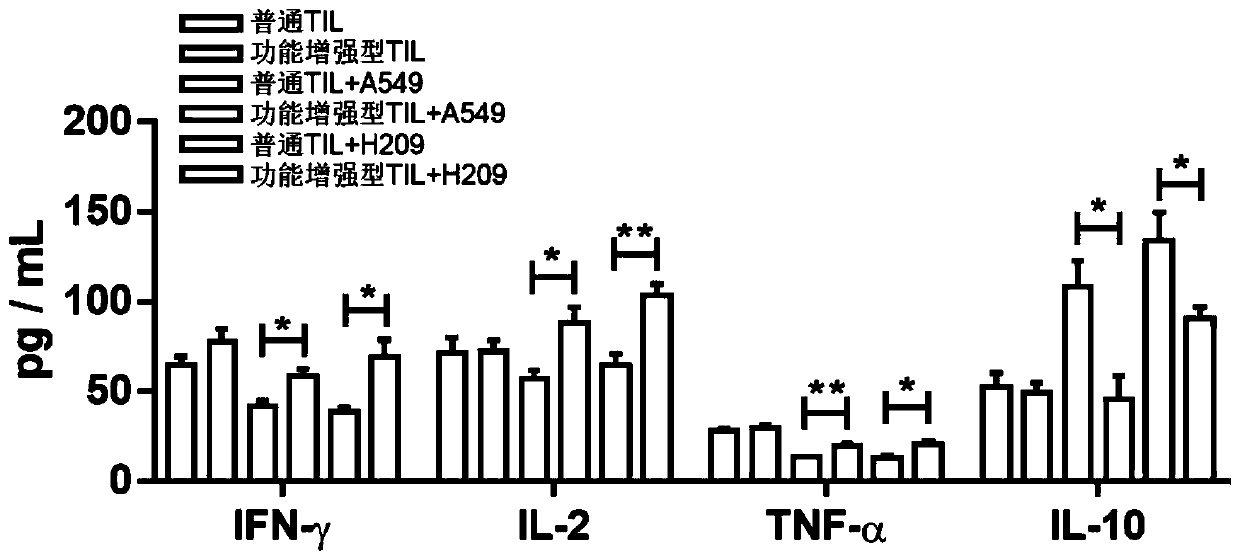
PendingCN110938594AHigh activityPromote secretionBlood/immune system cellsCell culture active agentsLymphocyte cultureTumor cells
The invention discloses a culture method for functionally enhanced tumor infiltrating lymphocytes (TILs). The method includes the following steps: separating lymphocytes from tumor tissue, adding a start culture medium, performing inoculation in a 12-well culture plate (4 ml / well), performing start lymphocyte culture for 10 days or 14 days to obtain start TILs, and performing cryopreservation on the obtained start TILs for standby application; suspending the lymphocytes in a 25 cm<2> culture flask (20 ml / flask) by using an induction culture medium, placing the culture flask in an incubator having 5% CO2 at 37 DEG C, and performing TIL induction culture for 1 day; performing half quantity change by using an expansion culture medium, and performing expansion flask culture and expansion bag culture for 13 days or 14 days; on the 14th or 15th day of culture, collecting TILs, performing washing by using normal saline, and resuspending the TILs by using a function enhancement culture medium,and performing incubation for 30 min; and collecting TILs, and performing functional test to obtain the functionally enhanced TILs. The culture method described in the invention can obtain the functionally enhanced TILs with stronger tumor cell killing activity and higher-level anti-tumor cytokine secretion ability.
Owner:SUN YAT SEN UNIV CANCER CENT
Method of predicting responsiveness to autologous adoptive cell transfer therapy
A method of determining responsiveness to cancer treatment is disclosed. The method comprises analyzing a frequency of tumor infiltrating lymphocytes (TILs) having a CD8+CD28−CD152− signature in a sample of the subject, wherein a frequency of TILs having the CD8+CD28−CD152− signature above a predetermined level is indicative of a positive responsiveness to cancer treatment. Other signatures reflecting responsiveness to cancer treatment are also disclosed. In addition, methods of treating cancer based on these signatures are also disclosed.
Owner:TEL HASHOMER MEDICAL RES INFRASTRUCTURE & SERVICES +1
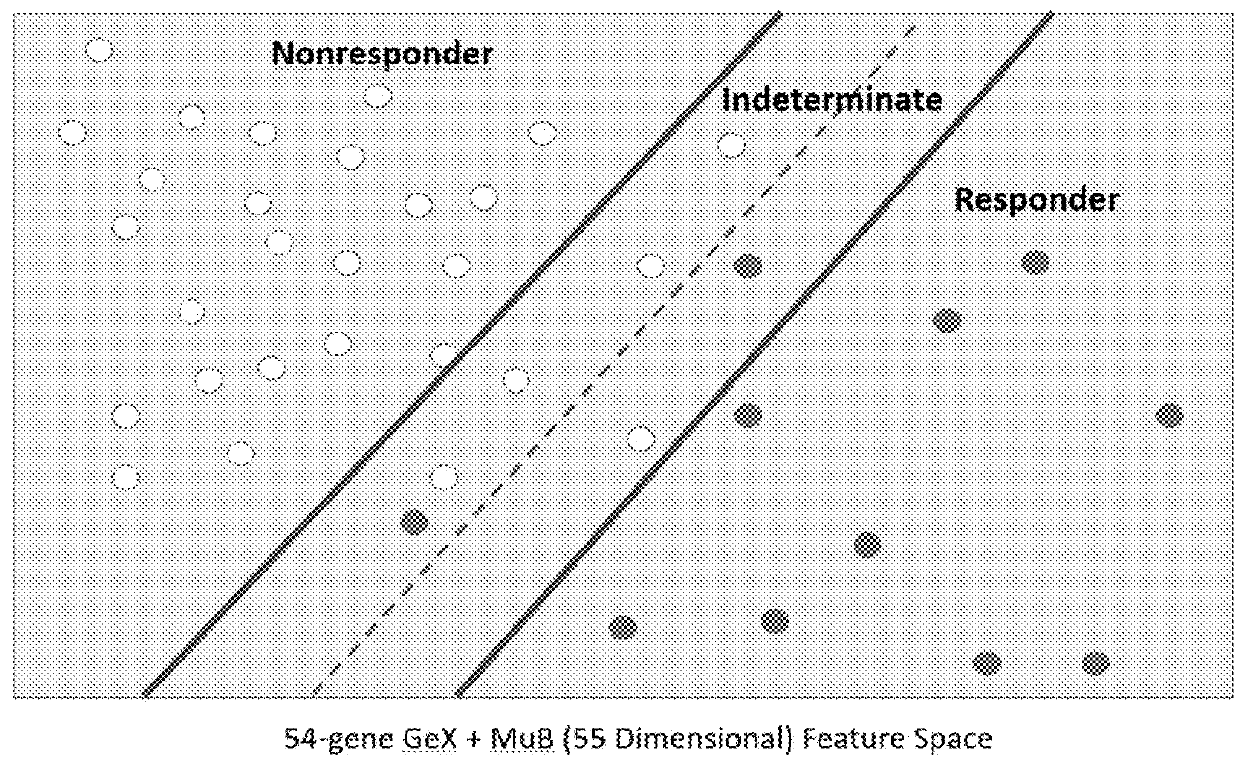
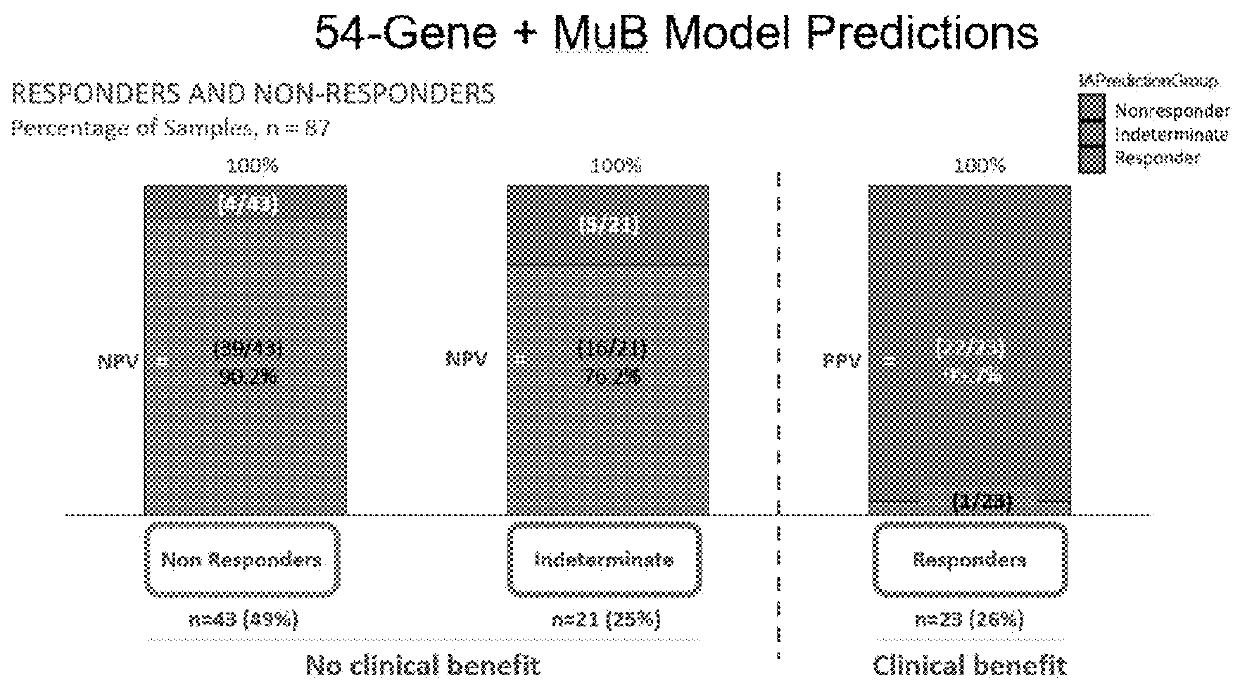
Methods and systems for determining personalized therapies
A method for generating an immune score, the method comprising the steps of: (i) determining a qualitative and / or quantitative assessment of tumor infiltrating lymphocytes in a sample; (ii) determining a qualitative and / or quantitative assessment of T-cell receptor signaling in the sample; (iii) determining a qualitative and / or quantitative assessment of mutation burden in the sample; (iv) generating, using a predictive algorithm, an immune score based on the determined qualitative and / or quantitative assessment of tumor infiltrating lymphocytes, the determined qualitative and / or quantitative assessment of T-cell receptor signaling, and the determined qualitative and / or quantitative assessment of mutation burden.
Owner:OMNISEQ INC
Dominant sequence of delta 1 chain complementary determining region (CDR) 3 in gamma delta T lymphocytes, and T cell receptor (TCR) transfected cells and application thereof
The invention discloses a dominant sequence GTM of a delta 1 chain complementary determining region (CDR) 3 in human gastric cancer tissue-derived tumor-infiltrating gamma delta T lymphocytes, a T cell receptor (TCR) containing the sequence, and TCR transfected cells. The amino acid residue sequence of the GTM is shown as SEQ ID NO.1 in a sequence table. Experiments prove that GTM peptide can be specifically combined with tumor cells, tumor tissue and V delta 1 T cell ligand MICA protein; and a J.RT3-T3.5 cell platform for expressing the TCR containing the dominant sequence GTM of the delta 1 chain CDR3 in the human gastric cancer tissue-derived tumor-infiltrating gamma delta T lymphocytes on a J.RT3-T3.5 surface is established by a lentivirus expression system, gamma delta 1 tumor-infiltrating lymphocytes (TIL) and a tumor identifying and killing mechanism thereof are simulated in the cell level, the killing activity of the transfected cells TCR gamma 4 delta 1 on the tumor cells is obviously increased, and the killing effect is TCR-dependent. The invention plays an important role in the development of medicines for treating malignant tumors and the adoptive treatment of malignant tumors, and has wide application prospects.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Culture and application of oligoclonal tumor-infiltrating lymphocytes for liver cancer
InactiveCN103396992ANo damageHigh purityUnknown materialsBlood/immune system cellsTumor-infiltrating lymphocytesLiver cancer
The invention relates to culture and an application of oligoclonal tumor-infiltrating lymphocytes for liver cancer, and provides an oligoclonal cultural method of tumor-infiltrating lymphocytes (TIL), wherein TIL cell population with strong antitumor activity is selectively amplified from liver cancer tissues, and is applied to adoptive immunotherapy of liver cancer.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Remnant Tumor Infiltrating Lymphocytes and Methods of Preparing and Using the Same
ActiveUS20190276802A1Organic active ingredientsPeptide/protein ingredientsLymphocyteTumor-infiltrating lymphocytes
In some embodiments, methods of delivering a therapeutically effective amount of an expanded number of tumor infiltrating lymphocytes obtained from tumor remnants to a patient in need thereof, for the treatment of a cancer, are disclosed.
Owner:IOVANCE BIOTHERAPEUTICS INC
Method for rapidly and efficiently amplifying tumor infiltrating lymphocytes
The invention discloses a method for rapidly and efficiently amplifying tumor infiltrating lymphocytes. The method is mainly used for efficiently and rapidly amplifying tumor infiltrating lymphocytes (TIL) within a short period of time and basically realizing no influences on the phenotypes of the TIL. The method comprises: carrying out tumor tissue treatment, culturing liquid use, and IL-2 and OKT3 dosage and culturing treatment; taking 1-2mm<3> tissues from different areas of a liver cancer specimen, putting the tissues in the pores of a 24-pore plate, placing the pore plate in a culturing case, culturing, observing every other day, carrying out semis liquid replacement of the pore plate culturing liquid after the 5-6 day culture, carrying out semis liquid replacement of the pore plate culturing liquid every one or two days according to the TIL growth condition, collecting the TIL in all the pores once the 24-pore plate is full of the TIL, and culturing the TIL to form young TIL; and carrying out rapid in-vitro rapid amplification of the young TIL above 4000 times.
Owner:爱瑞康医疗投资管理(北京)有限公司
Engineered artificial antigen presenting cells for tumor infiltrating lymphocyte expansion
In some embodiments, compositions and methods relating to isolated artificial antigen presenting cells (aAPCs) are disclosed, including aAPCs comprising a myeloid cell transduced with one or more viral vectors, such as a MOLM-14 or a EM-3 myeloid cell, wherein the myeloid cell endogenously expresses HLA-AB / C, ICOS-L, and CD58, and wherein the one or more viral vectors comprise a nucleic acid encoding CD86 and a nucleic acid encoding 4-1BBL and / or OX40L and transduce the myeloid cell to express CD86 and 4-1BBL and / or OX40L proteins. In some embodiments, methods of expanding tumor infiltrating lymphocytes (TILs) with aAPCs and methods of treating cancers using TILs after expansion with aAPCs are also disclosed.
Owner:IOVANCE BIOTHERAPEUTICS INC
Detection and measurement of tissue-infiltrating lymphocytes
ActiveUS9499865B2Microbiological testing/measurementLibrary member identificationDiseaseRegulatory T cell
The present invention is drawn to methods for measuring numbers, levels, and / or ratios of cells, such as lymphocytes, infiltrated into a solid tissue, such as a tumor or a tissue affected by an autoimmune disease, and to methods for making patient prognoses based on such measurements. In one aspect, methods of the invention comprise sorting lymphocytes from an accessible tissue, such as peripheral blood, into functional subsets, such as cytotoxic T cells and regulatory T cells, and generating clonotype profiles of each subset. An inaccessible disease-affected tissue is sampled and one or more clonotype profiles are generated. From the latter clonotype profiles, levels lymphocytes in each of the functional subsets are determined in the disease-affected tissue by their clonotypes, which are identified from lymphocytes sorted into subsets from the accessible tissue.
Owner:ADAPTIVE BIOTECH
Expansion of tumor infiltrating lymphocytes from liquid tumors and therapeutic uses thereof
InactiveUS20200224161A1Organic active ingredientsPeptide/protein ingredientsDiseaseHaematological malignancy
Methods of expanding tumor infiltrating lymphocytes (TILs), including peripheral blood lymphocytes and marrow infiltrating lymphocytes, from blood and / or bone marrow of patients with hematological malignancies, such as liquid tumors, including lymphomas and leukemias, and uses of such expanded TILs in the treatment of diseases such as cancers and hematological malignancies are disclosed herein.
Owner:IOVANCE BIOTHERAPEUTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com