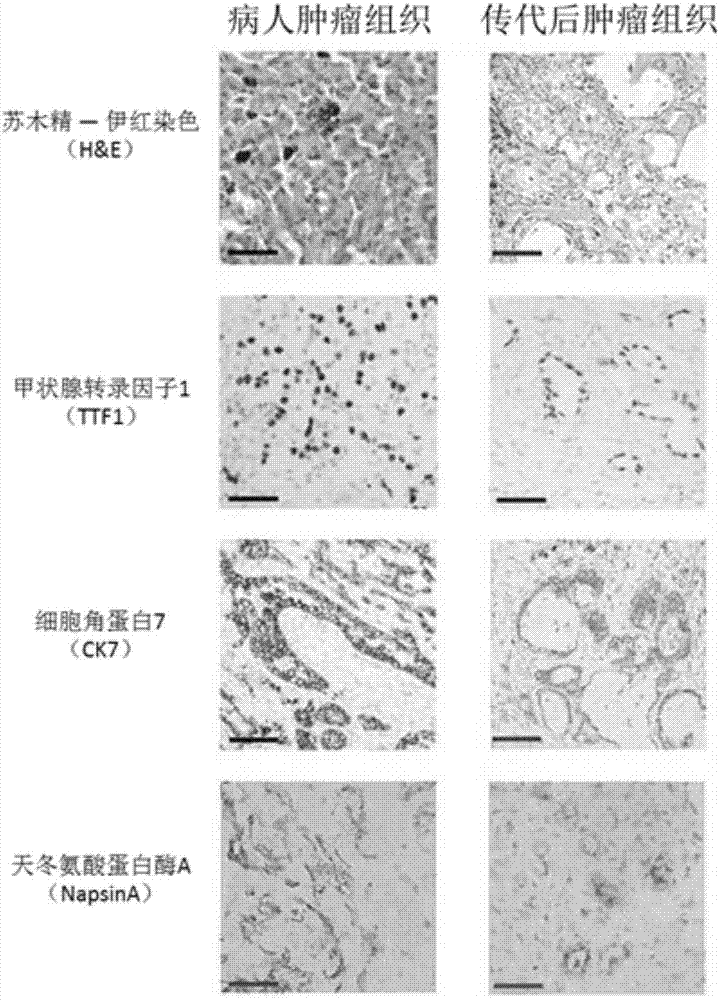
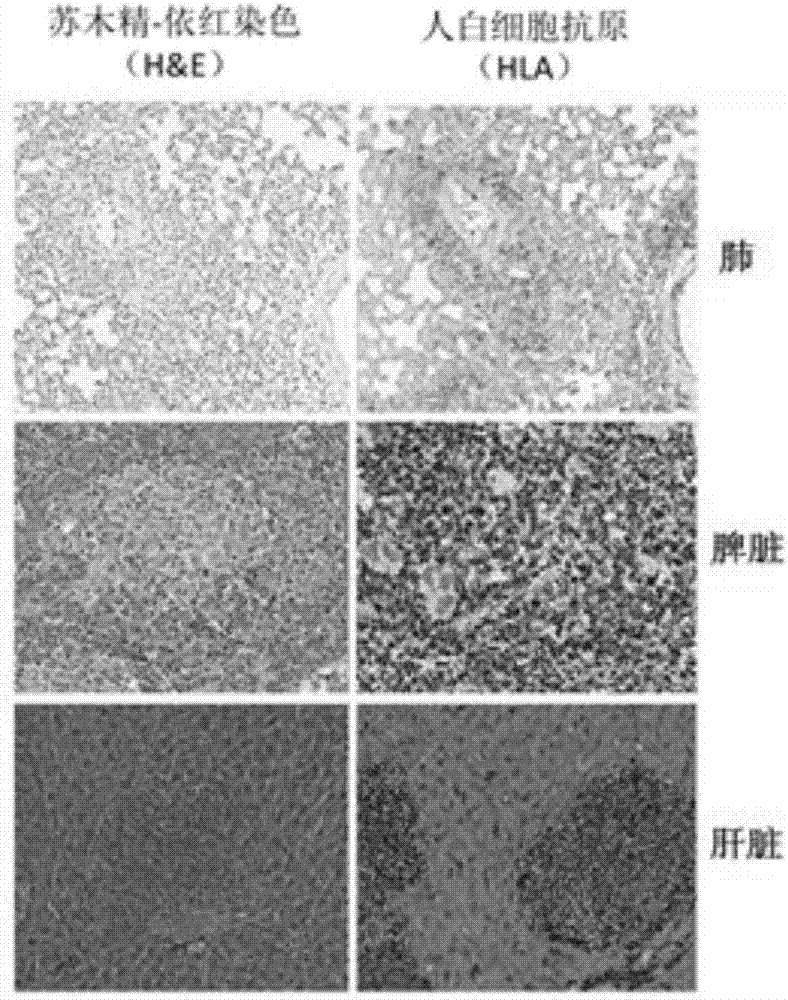
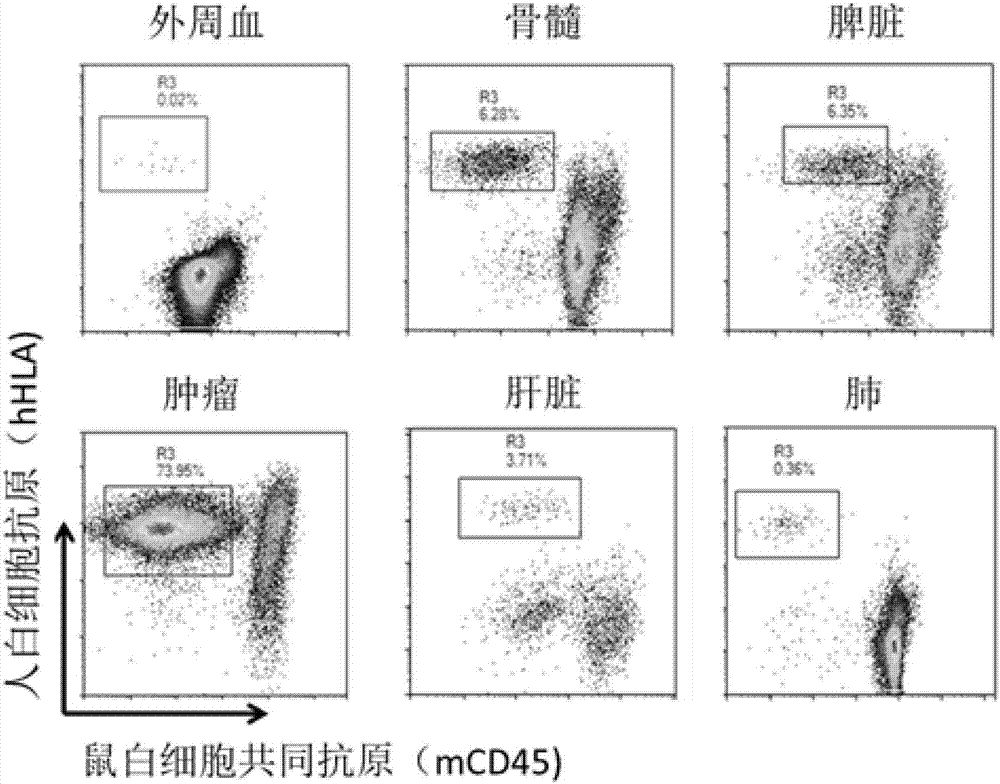
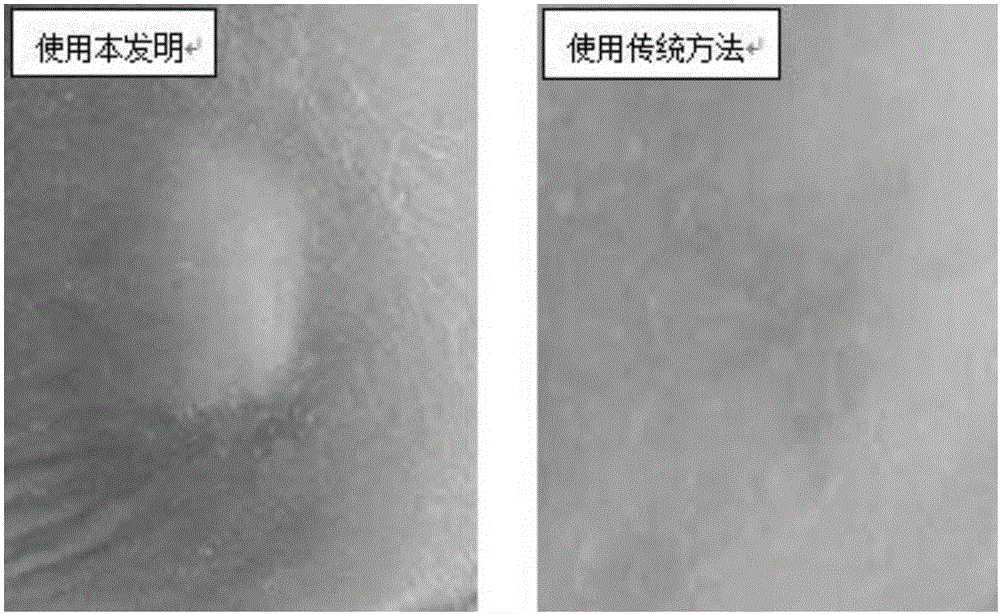
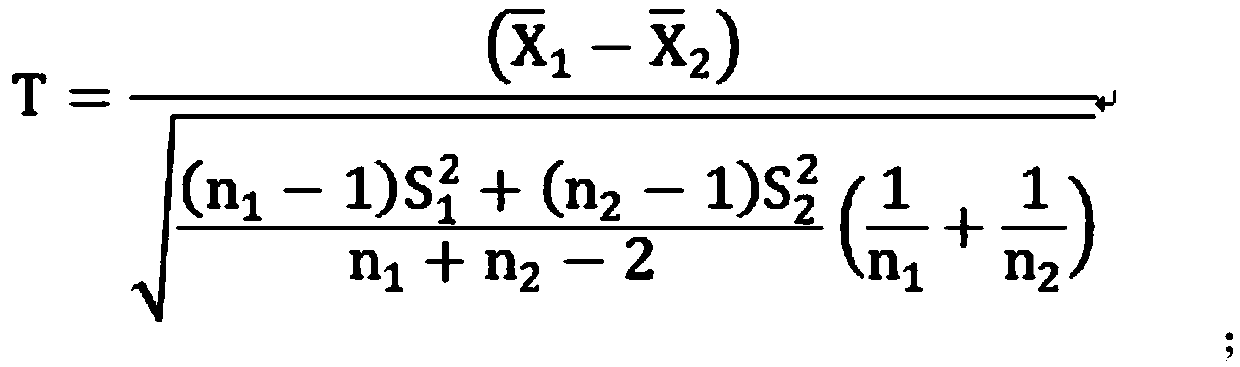Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
107 results about "Tumor tissue sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of growing tumor infiltrating lymphocytes in gas-permeable containers
InactiveUS20120244133A1Increase the number ofBiocideArtificial cell constructsTumour tissueCell culture media
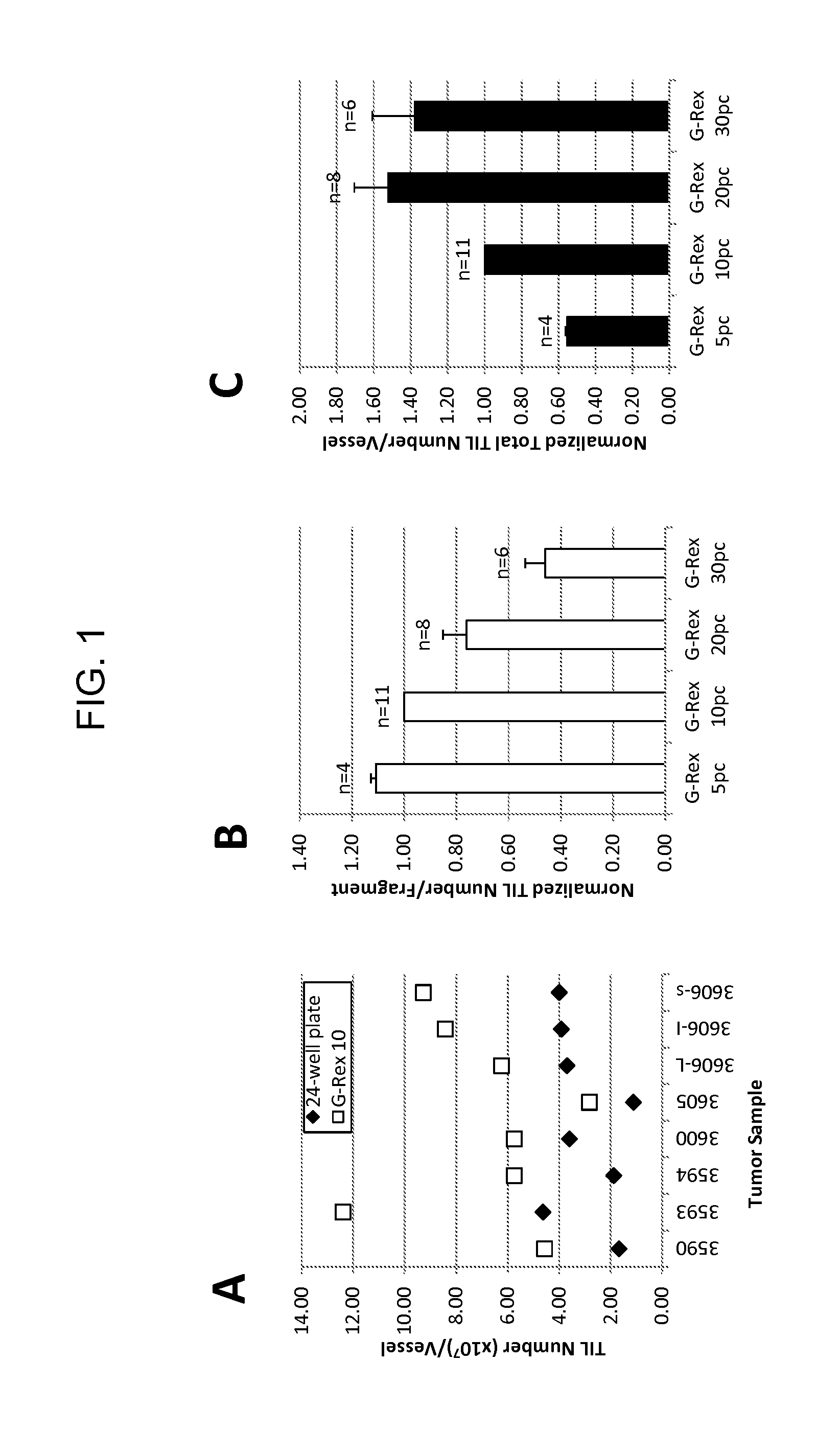
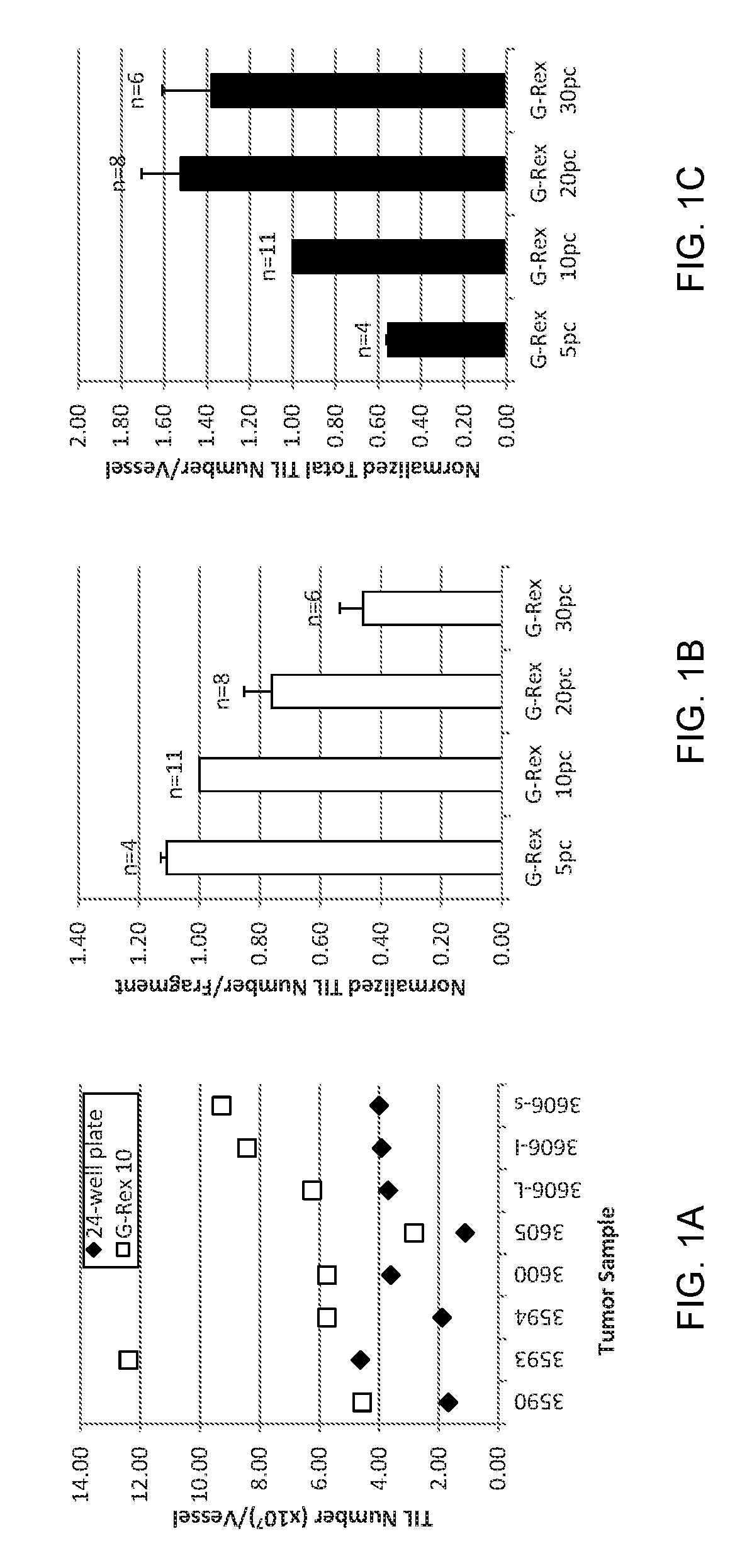
An embodiment of the invention provides a method of promoting regression of cancer in a mammal comprising obtaining a tumor tissue sample from the mammal; culturing the tumor tissue sample in a first gas permeable container containing cell medium therein; obtaining tumor infiltrating lymphocytes (TIL) from the tumor tissue sample; expanding the number of TIL in a second gas permeable container containing cell medium therein using irradiated allogeneic feeder cells and / or irradiated autologous feeder cells; and administering the expanded number of TIL to the mammal. Methods of obtaining an expanded number of TIL from a mammal for adoptive cell immunotherapy are also provided.
Owner:UNITED STATES OF AMERICA +1
Methods of growing tumor infiltrating lymphocytes in gas-permeable containers
InactiveUS20170152478A1Mammal material medical ingredientsCancer antigen ingredientsAbnormal tissue growthTumour tissue
An embodiment of the invention provides a method of promoting regression of cancer in a mammal comprising obtaining a tumor tissue sample from the mammal; culturing the tumor tissue sample in a first gas permeable container containing cell medium therein; obtaining tumor infiltrating lymphocytes (TIL) from the tumor tissue sample; expanding the number of TIL in a second gas permeable container containing cell medium therein using irradiated allogeneic feeder cells and / or irradiated autologous feeder cells; and administering the expanded number of TIL to the mammal. Methods of obtaining an expanded number of TIL from a mammal for adoptive cell immunotherapy are also provided.
Owner:UNITED STATES OF AMERICA +1
Molecular markers predicting response to adjuvant therapy, or disease progression, in breast cancer
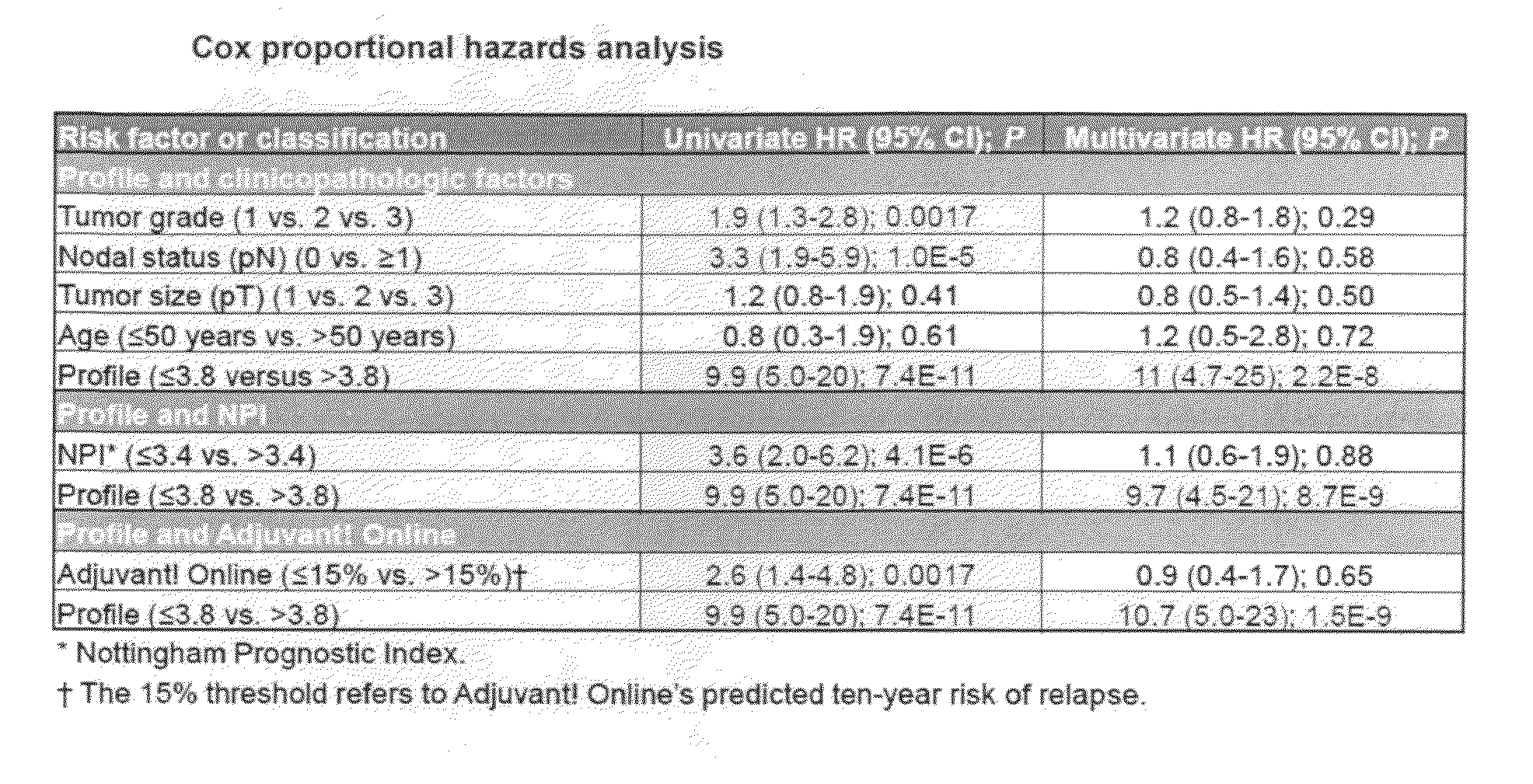
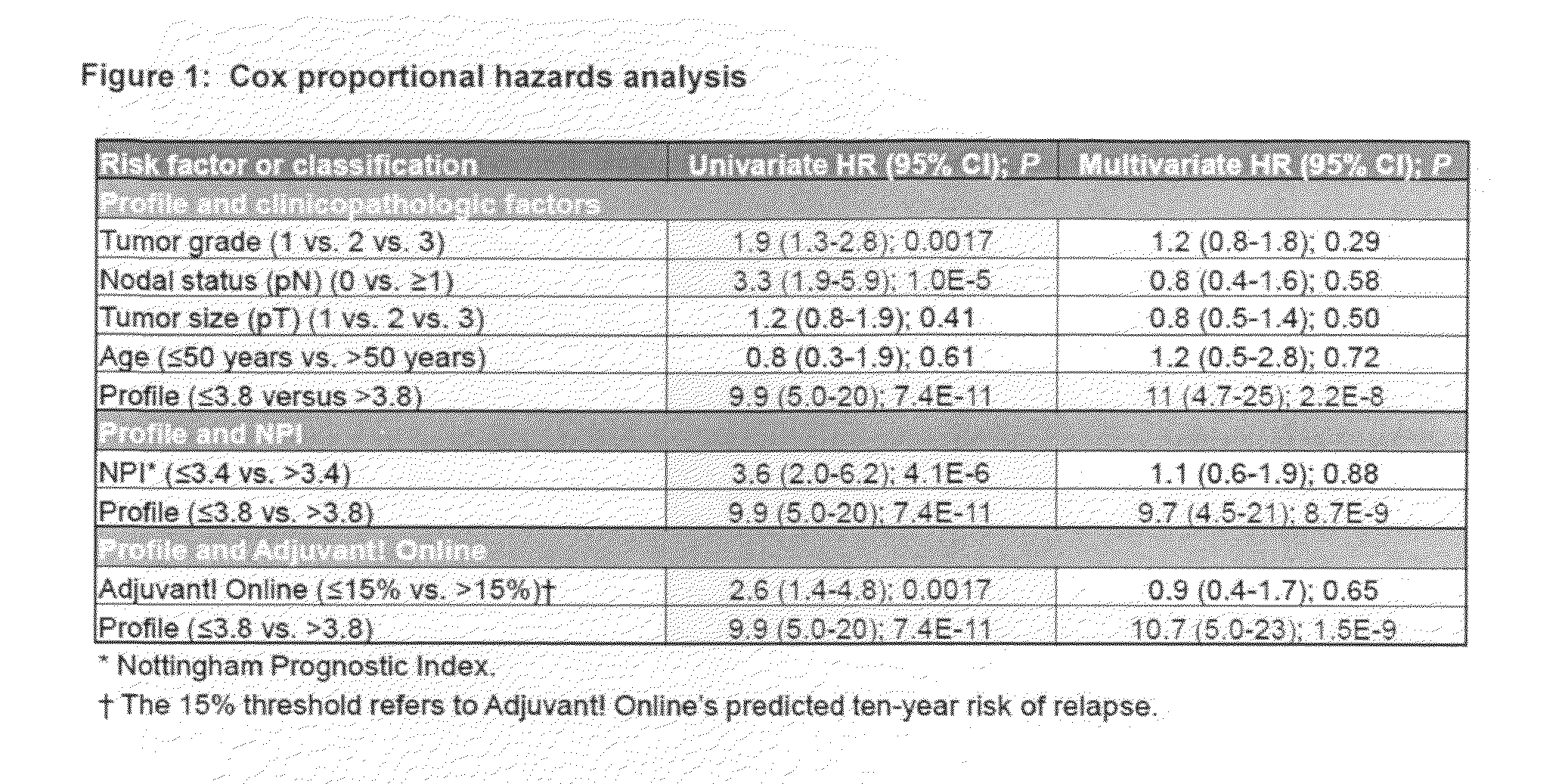
Predicting response to adjuvant therapy or predicting disease progression in breast cancer is realized by (1) first obtaining a breast cancer test sample from a subject; (2) second obtaining clinicopathological data from said breast cancer test sample; (3) analyzing the obtained breast cancer test sample for presence or amount of (a) one or more molecular markers of hormone receptor status, one or more growth factor receptor markers, (b) one or more tumor suppression / apoptosis molecular markers; and (c) one or more additional molecular markers both proteomic and non-proteomic that are indicative of breast cancer disease processes; and then (4) correlating (a) the presence or amount of said molecular markers and, with (b) clinicopathological data from said tissue sample other than the molecular markers of breast cancer disease processes. A kit of (1) a panel of antibodies; (2) one or more gene amplification assays; (3) first reagents to assist said antibodies with binding to tumor samples; (4) second reagents to assist in determining gene amplification; permits, when applied to a breast cancer patient's tumor tissue sample, (A) permits observation, and determination, of a numerical level of expression of each individual antibody, and gene amplification; whereupon (B) a computer algorithm, residing on a computer can calculate a prediction of treatment outcome for a specific treatment for breast cancer, or future risk of breast cancer progression.
Owner:LINKE STEVEN +2
Therapeutic and diagnostic methods for cancer
ActiveUS20190219586A1Conducive to survivalImprove survivalCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsTissues tumorMelanoma
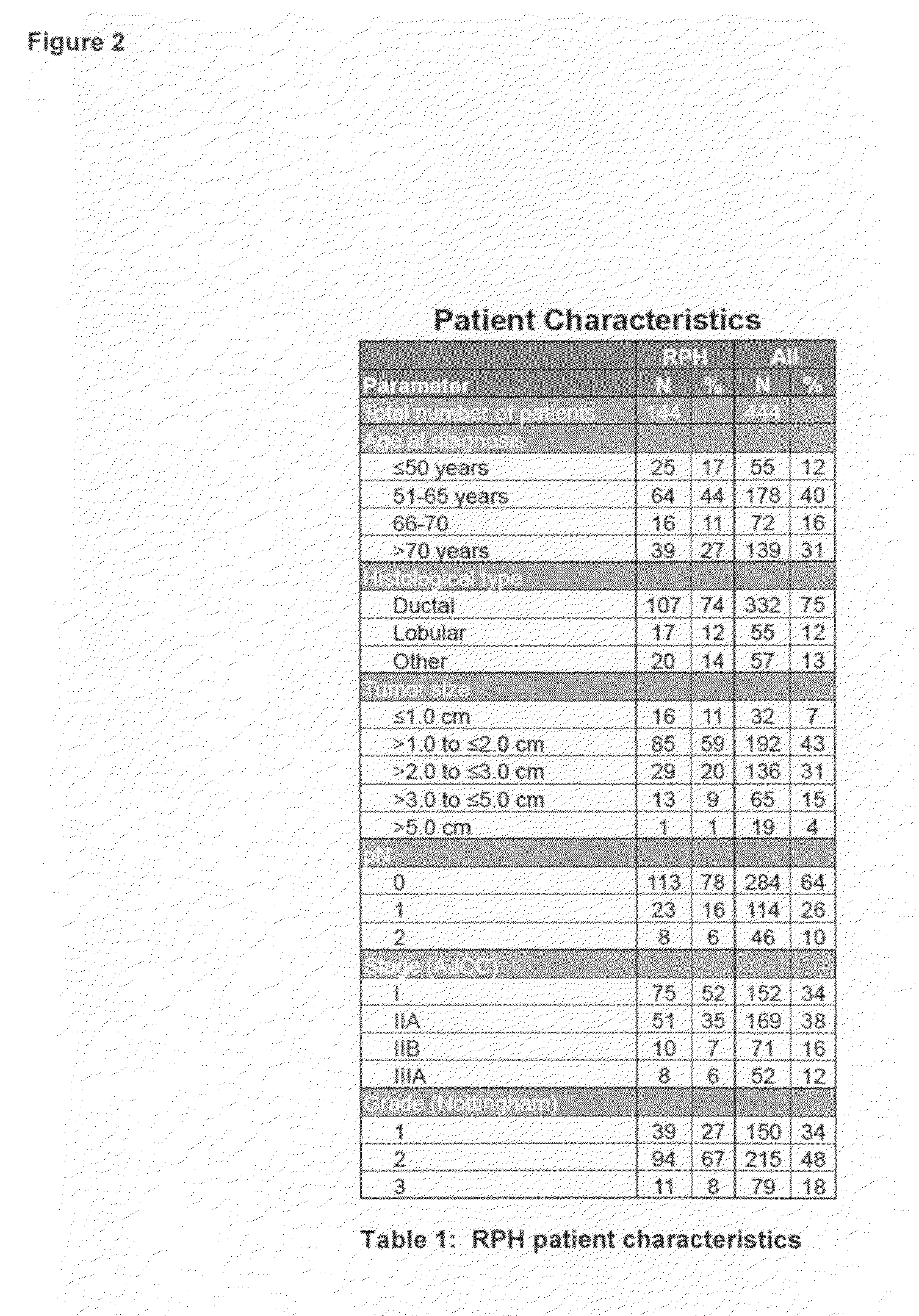
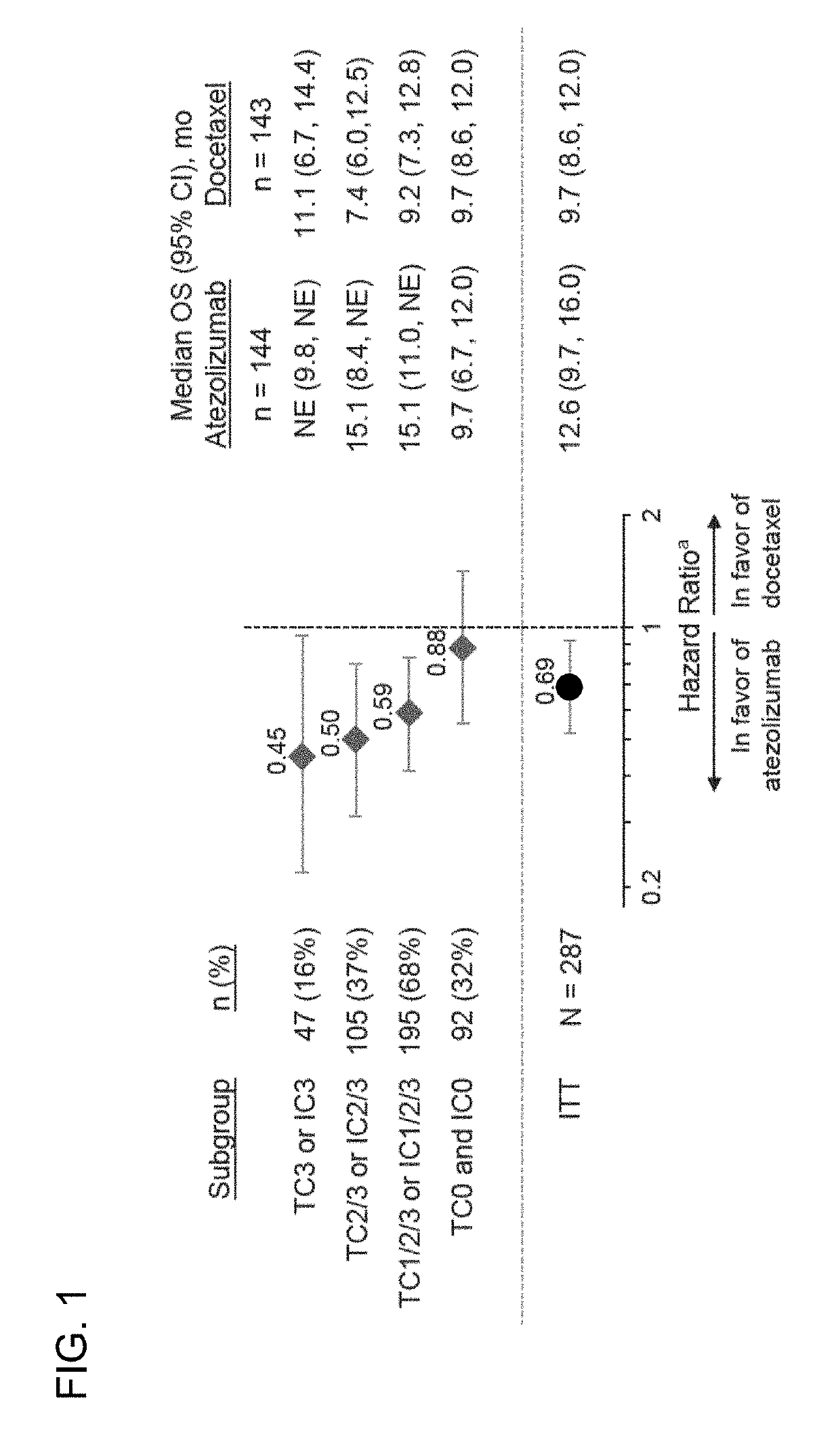
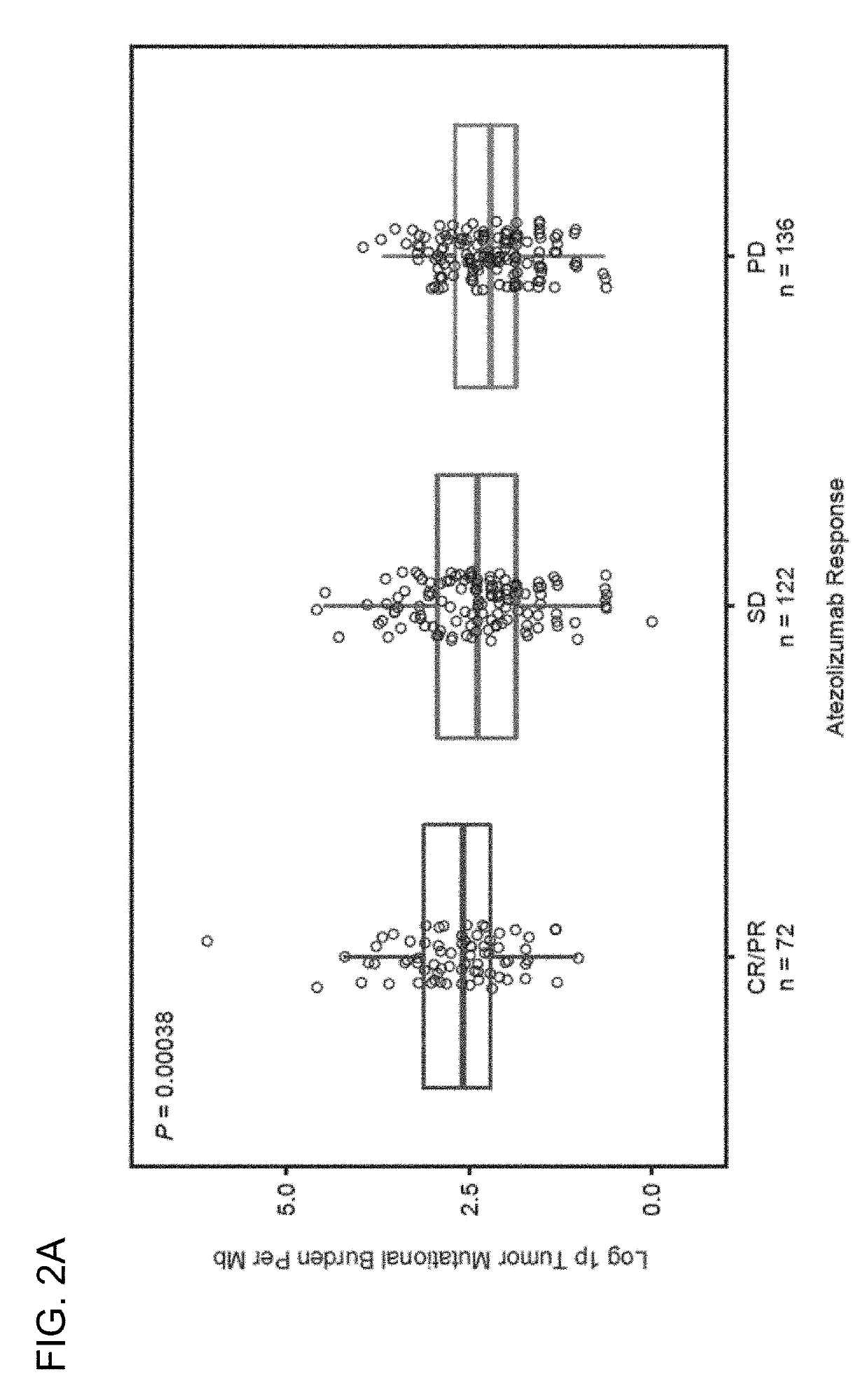
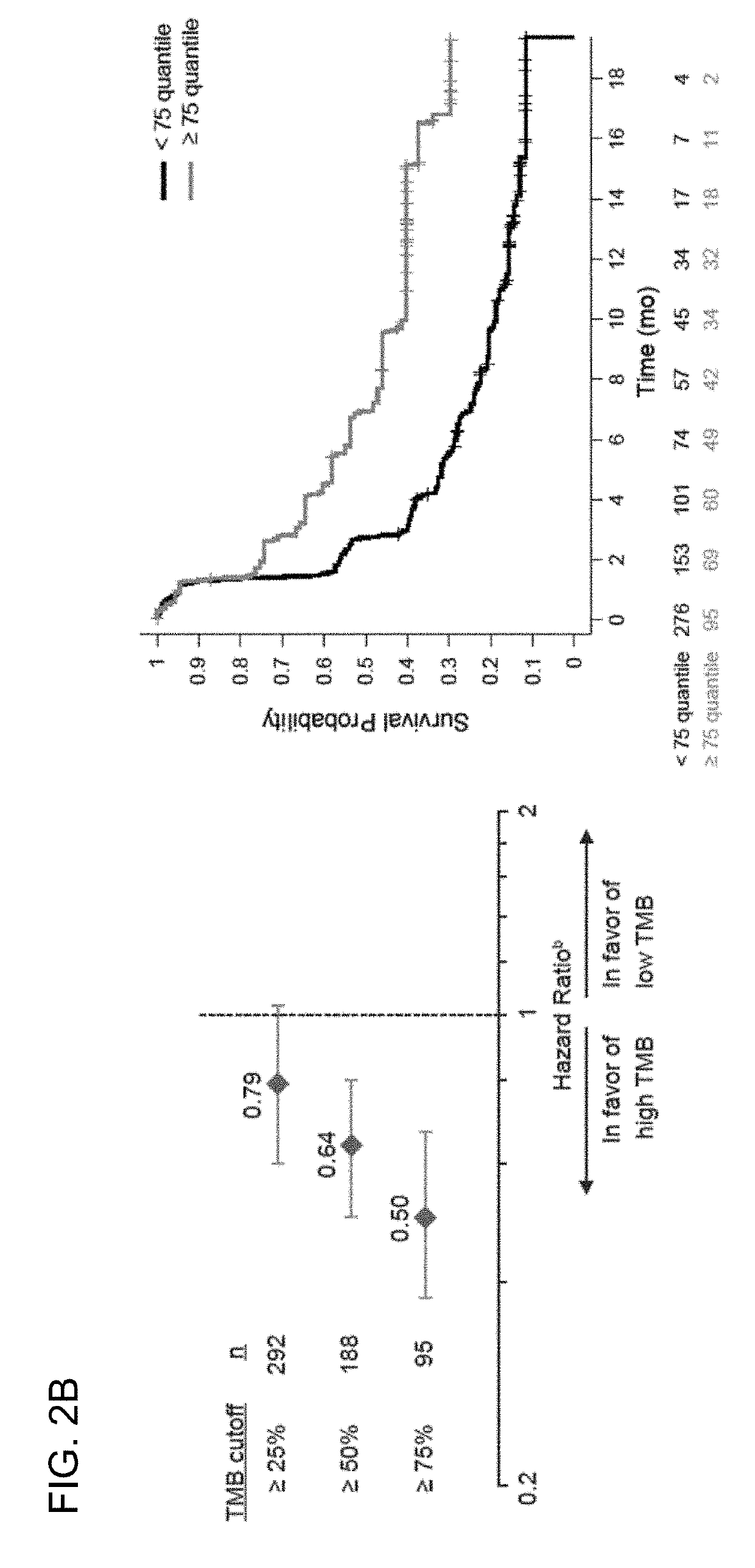
The present invention provides therapeutic and diagnostic methods and compositions for cancer, for example, lung cancer (e.g., NSCLC), bladder cancer (e.g., UC), kidney cancer (e.g., RCC), breast cancer (e.g., TNBC), or melanoma. The invention provides methods of treating cancer (e.g., lung cancer (e.g., NSCLC), bladder cancer (e.g., UC), kidney cancer (e.g., RCC), breast cancer (e.g., TNBC), or melanoma), methods of determining whether a patient suffering from cancer (e.g., lung cancer (e.g., NSCLC), bladder cancer (e.g., UC), kidney cancer (e.g., RCC), breast cancer (e.g., TNBC), or melanoma) is likely to respond to treatment comprising a PD-L1 axis binding antagonist, methods of predicting responsiveness of a patient suffering from cancer (e.g., lung cancer (e.g., NSCLC), bladder cancer (e.g., UC), kidney cancer (e.g., RCC), breast cancer (e.g., TNBC), or melanoma) to treatment comprising a PD-L1 axis binding antagonist, and methods of selecting a therapy for a patient suffering from cancer (e.g., lung cancer (e.g., NSCLC), bladder cancer (e.g., UC), kidney cancer (e.g., RCC), breast cancer (e.g., TNBC), or melanoma), based on a tissue tumor mutational burden (tTMB) score, which reflects somatic mutation levels of genes in a tumor tissue sample obtained from the patient, alone or in combination with PD-L1 expression levels (e.g., PD-L1 expression levels in tumor or tumor-infiltrating immune cells in a tumor sample (tumor area) obtained from the patient).
Owner:GENENTECH INC +1
Primer probe combination for detection of EGFR gene mutation and application of same
ActiveCN108611412AGrowth inhibitionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMutation detectionEGFR Gene Mutation
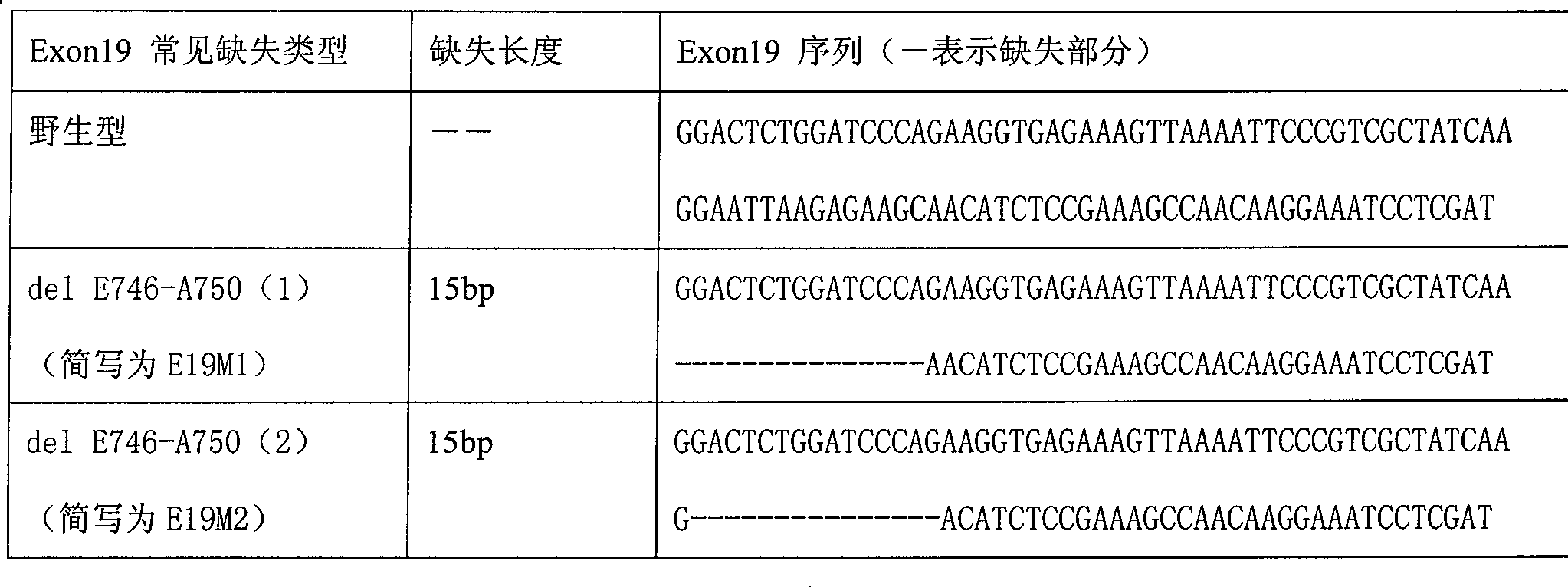
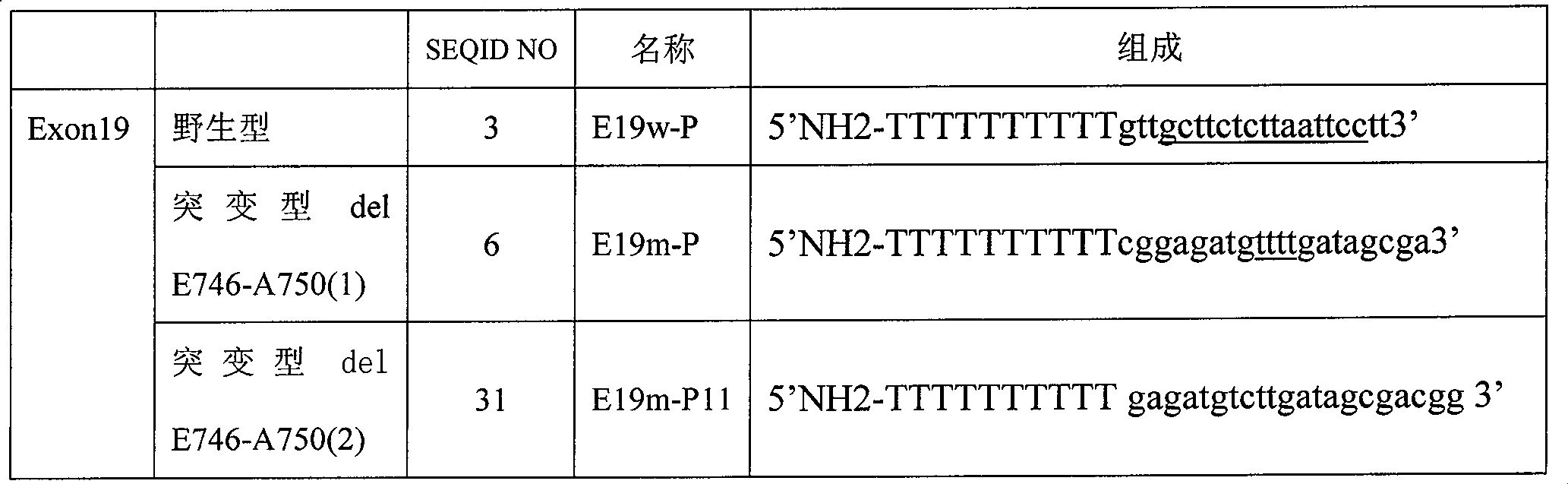
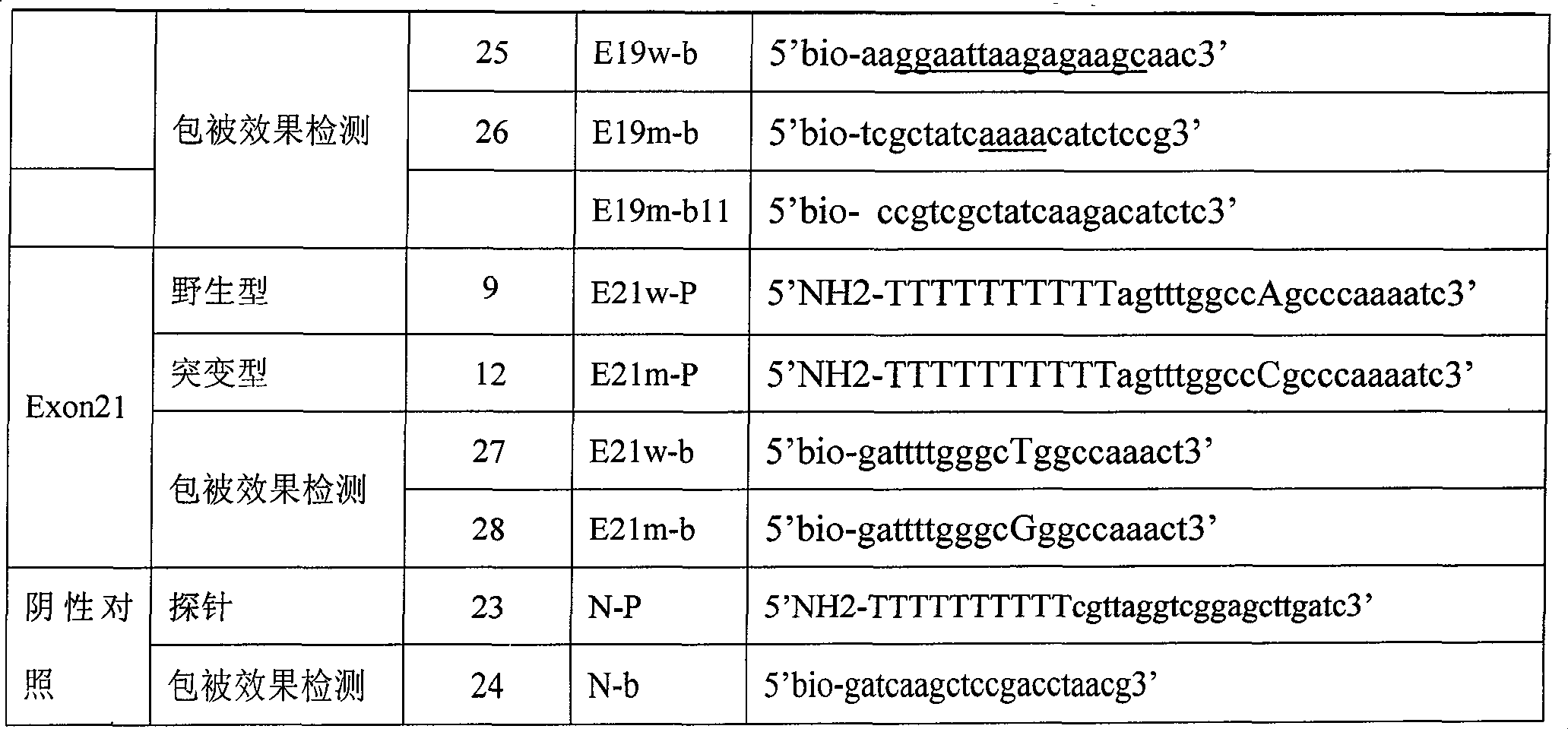
The invention relates to a detection product for gene mutation and particularly relates to a primer probe combination for detection of EGFR gene mutation and an application of the same. The primer probe combination includes a detection primer probe, which includes an EGFR gene mutation detection specific primer pair, an EGFR gene specific probe, an amplification blocking probe, and an internal reference system, wherein the loci of mutation detection of the EGFR gene are the mutation loci of 18-21 exons. The primer probe combination has high specificity and sensitivity on the EGFR gene mutationand simple and quick operation. The detection result has great accuracy and repeatability. The primer probe combination can detect a tumor tissue sample and can help clinical treatment, so that the primer probe combination has important value.
Owner:WUXI SHENRUI BIO PHARMA
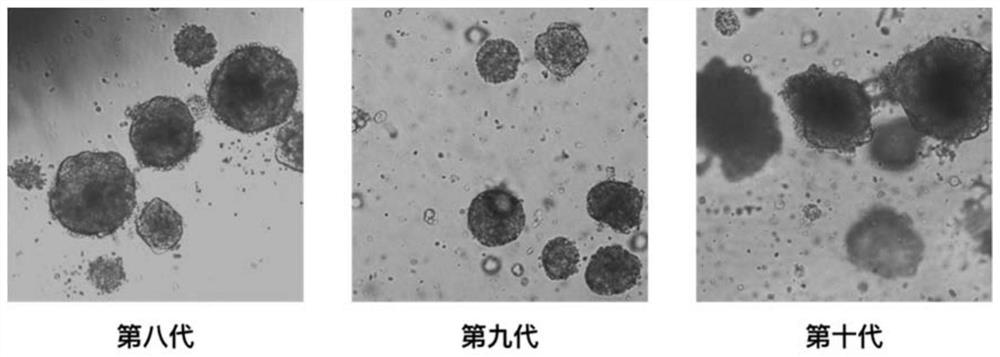
Tumor organoid preparation method and device and application thereof
ActiveCN111607495AHigh activityIncrease the number ofBioreactor/fermenter combinationsBiological substance pretreatmentsPore diameterOncology
The invention provides a tumor organoid preparation method and device and application thereof. The method comprises the following steps: obtaining a tumor tissue sample, cutting the tumor tissue sample into tissue blocks suitable for mechanical grinding; grinding the tissue blocks through a mechanical grinding method, performing first-stage filtration through a first-stage filter screen with a pore diameter of 80-200 [mu]m to form first-stage filtrate; and carrying out second-stage filtration on the first-stage filtrate by a second-stage filter screen with a pore diameter of 30-60 [mu]m to obtain a solid on the second-stage filter screen, thereby obtaining the tumor organoid. The tumor organoid is obtained by a simple method, the operation is easy and convenient, the survival rate and number of the organoid are remarkably increased, and the organoid culture time is greatly shortened.
Owner:奥格诺德生物科技(北京)有限公司
Specific primer probe composition, kit and method for detecting T790M site of EGFR gene
InactiveCN107868828AGood choiceHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationFluoProbesWild type
The invention discloses a specific primer probe composition, a kit and a method for detecting a T790M site of an EGFR gene. The kit comprises an upstream primer, a downstream primer, a fluorescent probe I for detecting T790M mutation and a fluorescent probe II for detecting a wild type. For the kit, the primer pair with the specific sequences and the probes with the specific sequences are designedfor the human EGFR gene T790M mutation and reaction systems are optimized. According to the invention, through a method of a Raindropdigital PCR platform, high sensitivity detection on the T790M mutation of the EGFR gene is achieved and the abundance of the mutation is obtained simultaneously; the detection limit can be low to one in 100000. Furthermore, the invention can be used for detecting multi-source samples, comprising tumor tissue samples and ctDNAs, so that the application scope of the kit, reaction systems and method are expanded, and a medication guidance for treatment of patientswith lung cancer T790M mutation is provided.
Owner:中源协和基因科技有限公司
Method for establishing human tumor xenotransplantation model cultured in vitro
ActiveCN109090039AOptimize inoculation conditionsReduce apoptosisAnimal husbandryHuman tumorApoptosis
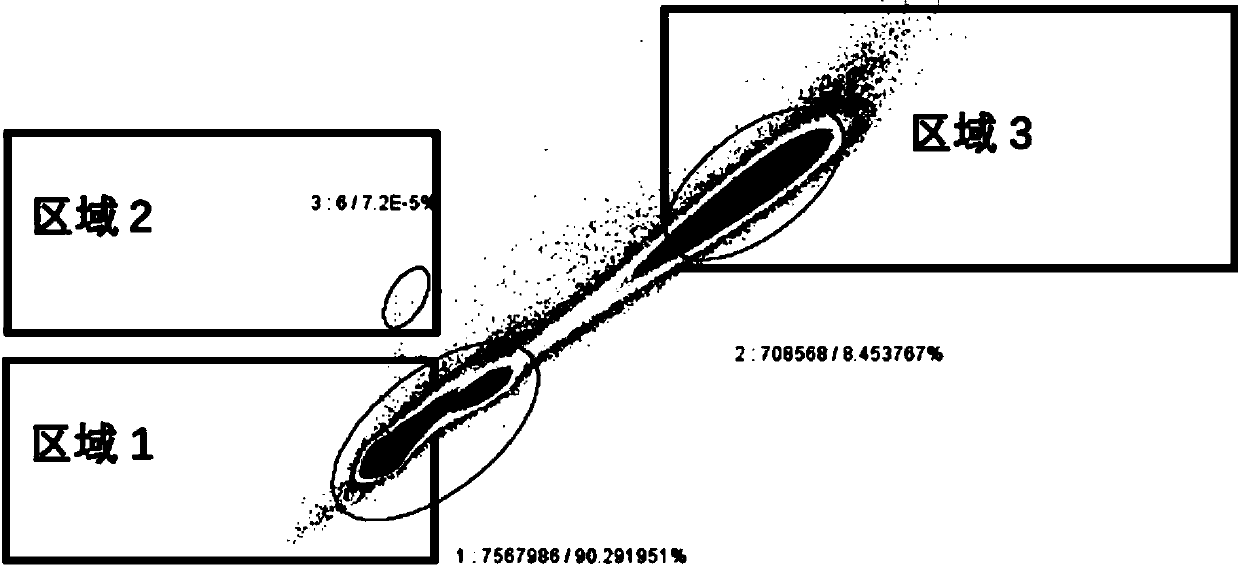
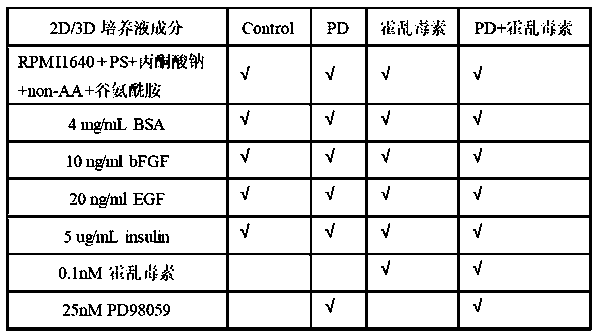
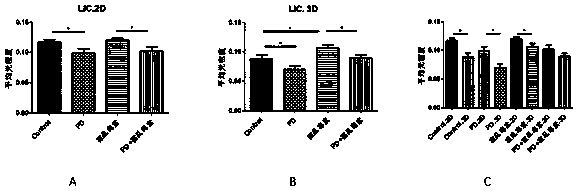
The invention relates to the field of human xenotransplantation model research, in particular to a method for establishing a human tumor xenotransplantation model cultured in vitro. The establishing method includes the following steps: (1) collecting a tumor tissue sample; (2) placing a tumor tissue in a culture solution; (3) completing the preparation for low-temperature transportation and treatment; (4) cutting the tumor tissue into grain size in a sterile operating table; (5) cleaning the tumor tissue and then placing the tumor tissue in a hole plate 48 for culture; (6) detecting tissue apoptosis by in situ end labeling method; (7) selecting immunocompromised NCG mice to perform anesthesia and skin treatment; (8) implanting the cultured tumor tissue into the renal capsule of the mice; (9) completing modeling after 3 months. According to the invention, tumor tissue blocks are selected for modeling for the xenotransplantation model, which is beneficial to preserving the histopathological and genetic characteristics of primary tumors; and moreover, the obtained tumor tissue are cultured in vitro, which is beneficial to tumor typing and clinical practice; the addition of PD 98059 and artificial matrix glue can greatly reduce tissue apoptosis, which is beneficial to improving the success rate of transplantation.
Owner:广州长峰生物技术有限公司
Method for detecting multiple mutation types of genome
InactiveCN109337957AHigh precisionIncrease linearlyMicrobiological testing/measurementLinear growthSingle nucleotide mutation
The invention relates to a method for detecting multiple mutation types of a genome. A BAM file is used as an input file, a tumor tissue sample is detected, multiple mutation information of differentsample types is detected, and detection of the tumor tissue sample comprises the steps as follows: 1) detection of mononucleotide mutation; 2) detection of insertion and deletion; 3) detection of structural variants; 4) detection of complex variants. According to the method for detecting multiple mutation types of the genome, insertion and deletion are detected with a GeneReader algorithm obtainedthrough combination of a supervised method and an unsupervised method, the local comparison precision is optimized, linear growth with increase of the sequencing depth is realized in the aspect of speed, the detection effect is good, the detection result is accurate, and actual application demands can be well met.
Owner:江苏医联生物科技有限公司
Circulating tumor cell mouse model and construction method and application thereof
ActiveCN107137425AConsistent biological backgroundEasy to carry outCell dissociation methodsMammal material medical ingredientsPrimary tumorLymphatic Spread
The invention relates to a circulating tumor cell mouse model and a construction method and application thereof. The method includes the following steps of 1, subcutaneous transplantation, wherein a primary tumor tissue sample is transplanted into the body of an immunodeficient mouse to construct a primary cancer heterotransplantation model PDX; 2, collection of circulating tumor cells, wherein the circulating tumor cells in peripheral blood in the primary cancer heterotransplantation model obtained in the step 1 are collected; 3, renal sac membrane transplantation, wherein the circulating tumor cells collected in the step 2 are transplanted into the renal sac membrane of the immunodeficient mouse, and then the circulating tumor cell mouse model is successfully constructed. According to the method, the mode of transplantation with two or more times of passages is adopted, the CTCs in the primary cancer heterotransplantation model are transplanted into the body of the immunodeficient mouse through renal sac membrane, and the circulating tumor cell mouse model is obtained; the circulating tumor cell mouse model can be applied to research on the in-vivo metastasis mechanism and proliferation condition of CTCs.
Owner:湖南昭泰生物医药有限公司
Tumor tissue cryopreservation and resuscitation kit and treatment method adopted by same
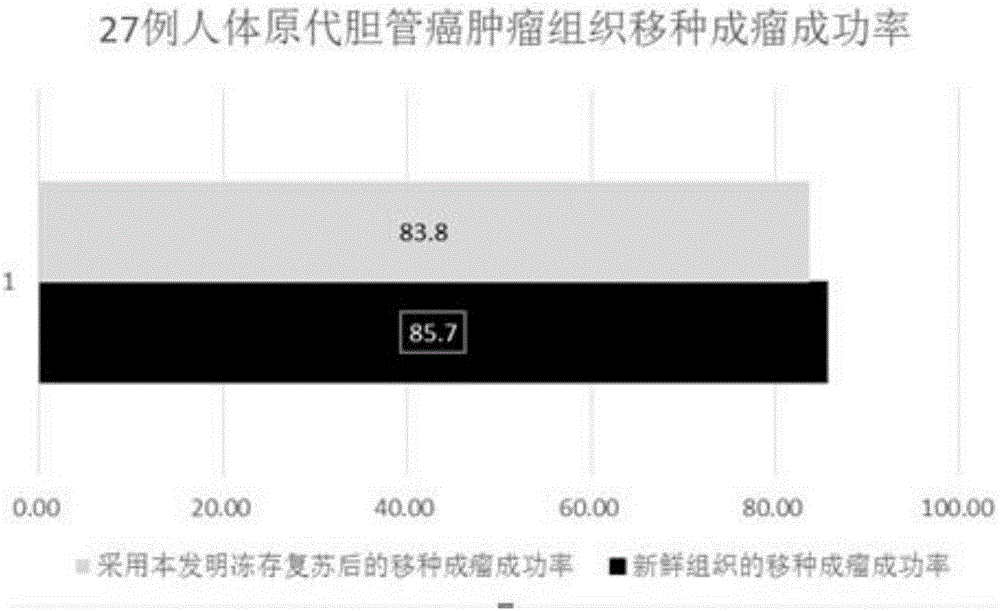
ActiveCN106047812AExtended shelf lifeImprove stabilityWithdrawing sample devicesCulture processPrimary tumorVitrification
The invention relates to the technical field of treatment for living tumor tissues, and provides a living tumor tissue cryopreservation and resuscitation kit and a treatment method adopted by the living tumor tissue cryopreservation and resuscitation kit. The cryopreservation and resuscitation kit comprises a tissue vitrification solution and a resuscitation solution, the contained various ingredients are completely clear as well as stable and controllable, no serum substances are added, the quality guarantee period is long, the batch-to-batch stability is good, the cryopreservation and resuscitation kit can be directly applied to living tissue cryopreservation and resuscitation, dilution or self-made preparation is not needed, and therefore, the operation is simplified. With the adoption of the living tumor tissue cryopreservation and resuscitation kit and the treatment method, the cryopreservation and resuscitation of the tumor tissue have relatively high motility rate, the original characteristics of the tumor tissue are preserved, after transplantation, the tumor tissue can be subjected to multiplication or passage in the form of the primary tumor, in addition, the original gene mutation of the tumor is preserved, the application range of the tumor tissue sample is expanded, and finally, good services are provided for the clinical scientific research.
Owner:SHANGHAI CRYOWISE MEDICAL TECH CO LTD
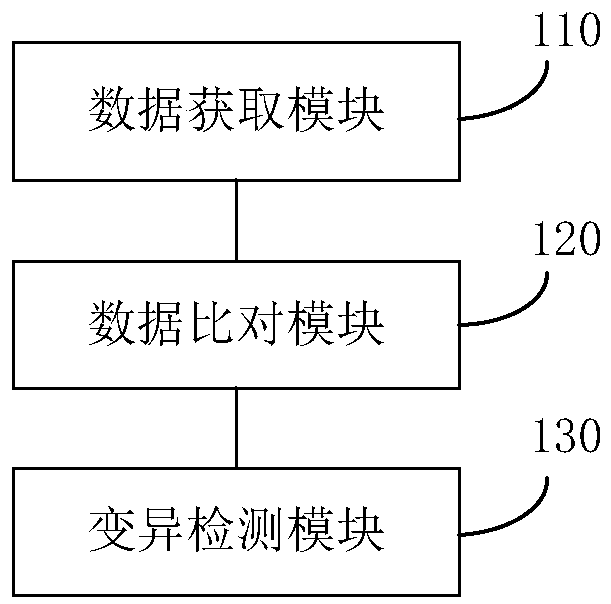
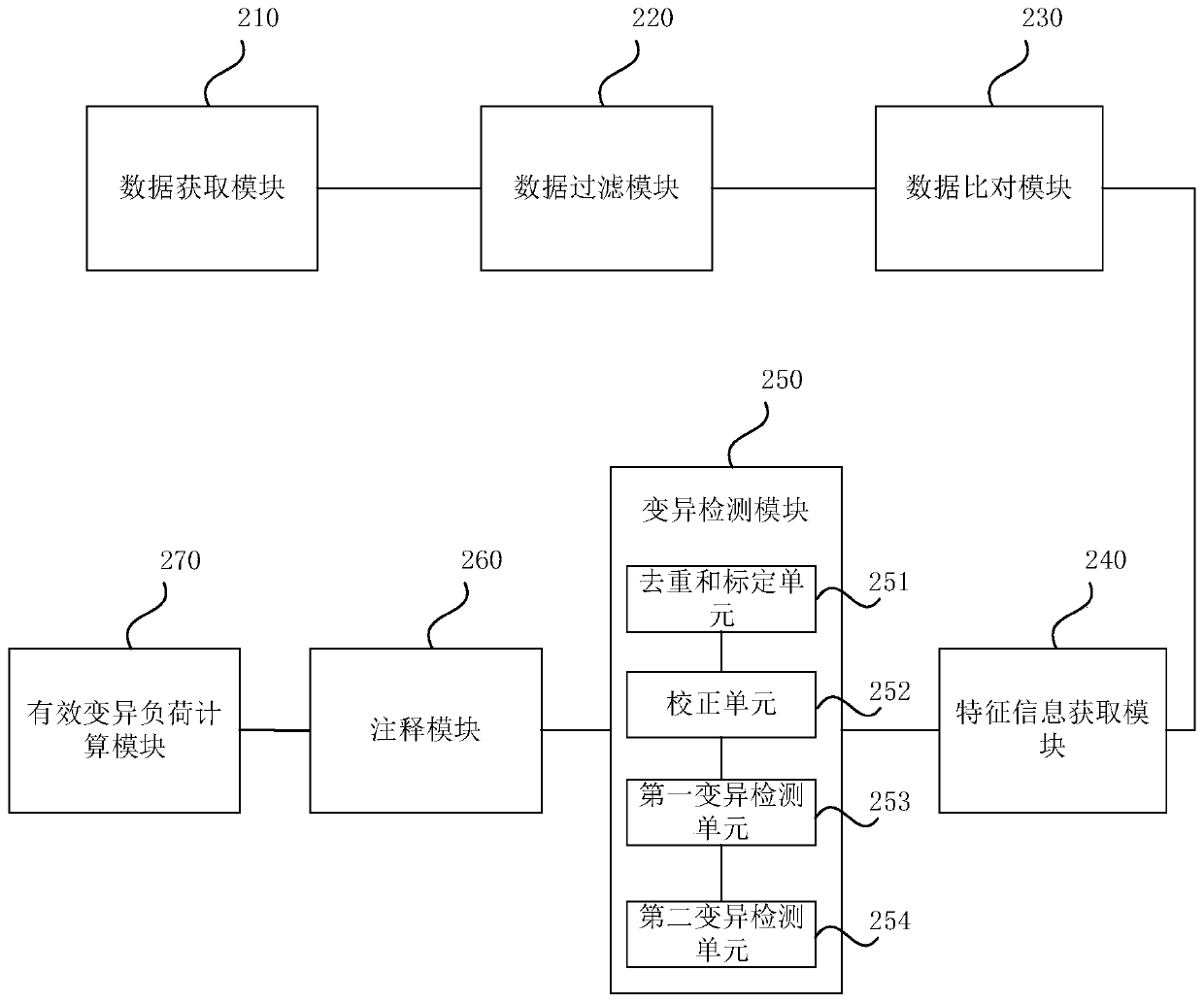
Single-sample-based next-generation sequencing tumor somatic cell variation detection device
ActiveCN110060733AMutation is fast and correctVariation detection is fast and accurateBiostatisticsProteomicsSingle samplePaired samples
One embodiment of the invention provides a single-sample-based next-generation sequencing tumor somatic cell variation detection device. The single-sample-based next-generation sequencing tumor somatic cell variation detection device includes a data acquisition module for acquiring sequencing data, the sequencing data comprising two blood samples of at least the same tumor and at least one tumor tissue sample; a data comparison module for comparing the sequencing data with a preset reference genome to obtain compared data; and a variation detection module for carrying out variation detection on the compared data and determining a somatic cell variation site and base variation of the tumor tissue sample. The technical scheme of the single-sample-based next-generation sequencing tumor somatic cell variation detection device carries out variation detection on the at least two blood samples so as to be combined into a variation background library, then filters reproductive variation by applying the variation background library in tumor tissue sample variation detection to obtain tumor-specific somatic variation, and detects the somatic variation of the tumor tissue sample under the condition that no paired sample exists, so that the false positive of tumor somatic variation detection is reduced.
Owner:SHANGHAI BIOTECAN PHARMA +1
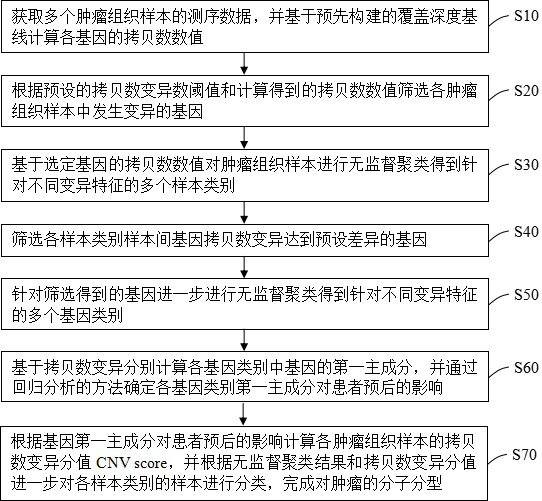
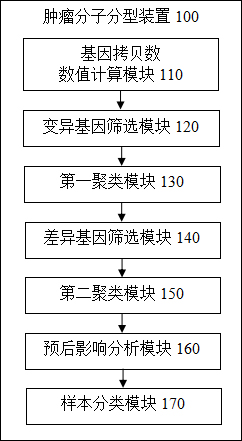
Tumor molecule typing method and device, terminal equipment and readable storage medium
ActiveCN112766428AEffectively discriminate between prognostic differencesWide range of clinical applicationsBiostatisticsCharacter and pattern recognitionMolecular typingTumor patient
The invention provides a tumor molecular typing method and device, terminal equipment and a readable storage medium, and the method comprises the following steps: obtaining sequencing data of a plurality of tumor tissue samples, and calculating a copy number value; screening mutated genes in each tumor tissue sample; performing unsupervised clustering on the mutated genes to obtain a plurality of sample categories; screening genes with significant gene copy number variation among samples of each sample category, and performing unsupervised clustering to obtain a plurality of gene categories; calculating a first principal component based on the copy number variation, and determining the influence of the first principal component on the prognosis of the patient through regression analysis; and calculating copy number variation scores of the tumor tissue samples according to the influence of the first principal component on the prognosis of the patient, and classifying the samples of the sample categories according to the copy number variation scores to complete the molecular typing of the tumor. Molecular typing is carried out based on copy number variation of each gene, the resolution ratio is high, typing is accurate, and prognosis of tumor patients with different molecular types can be remarkably distinguished.
Owner:臻和(北京)生物科技有限公司 +1
DNA markers for management of cancer
ActiveUS7718364B2Stage increaseMinimally invasiveSugar derivativesMicrobiological testing/measurementBlastomaSpecific chromosome
A method is provided for assessing allelic losses and hypermethylation of genes in CpG tumor promotor region on specific chromosomal regions in cancer patients, including melanoma, neuroblastoma breast, colorectal, and prostate cancer patients. The method relies on the evidence that free DNA and hypermethylation of genes in CpG tumor promotor region may be identified in the bone marrow, serum, plasma, and tumor tissue samples of cancer patients. Methods of melanoma, neuroblastoma, colorectal cancer, breast cancer and prostate cancer detection, staging, and prognosis are also provided.
Owner:JOHN WAYNE CANCER INST
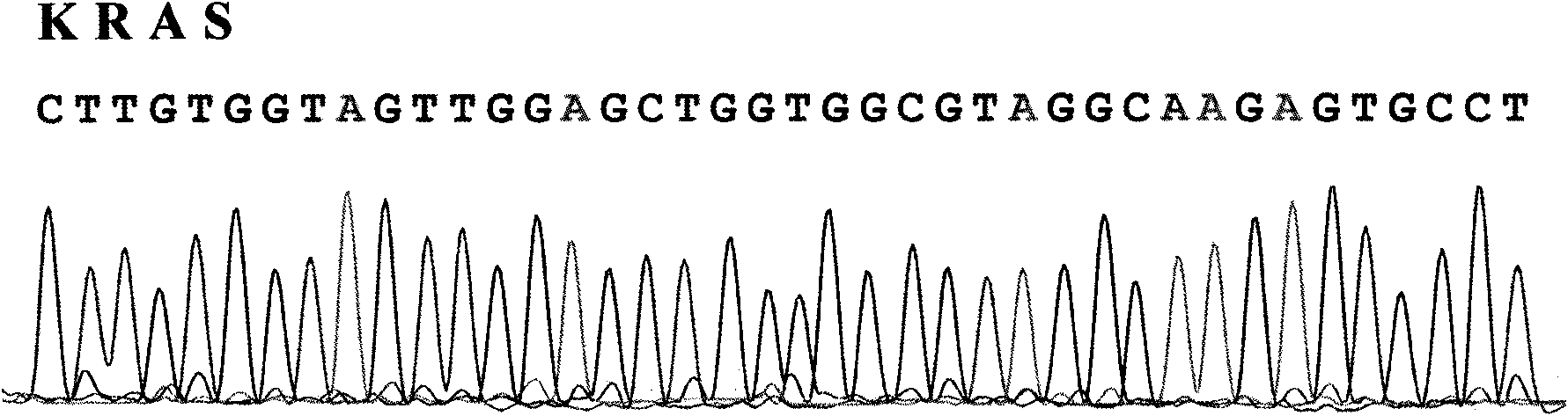
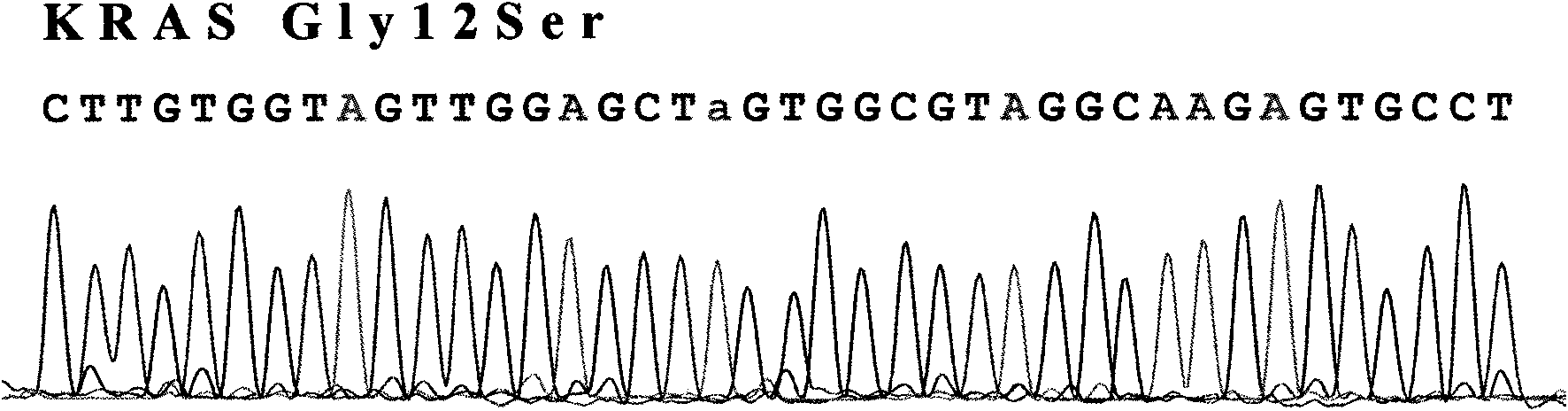
Test method of KRAS gene mutation for screening and assessing therapeutic effects of molecular targeted agents
InactiveCN101654702AHigh puritySmall amount of sampleMicrobiological testing/measurementMaterial analysis by electric/magnetic meansEGFR Tyrosine Kinase InhibitorsGenomic DNA
The invention relates to a test method of KRAS gene mutation for screening and assessing therapeutic effects of molecular targeted agents, which is characterized by the following specific steps: acquiring serum or tumor tissue samples of testees, extracting genomic DNA, testing a No.2 exon of the KRAS gene, designing corresponding site-specific primers and adopting experimental schemes, reagents and report generation systems for sequence determination and assessing the effectiveness of EGFR tyrosine kinase inhibitors used for treating cancer individuals by simultaneously testing and analyzingwhether mutation exists on the No.2 exon of the individual KRAS gene. The method has the advantage of being used for application of the EGFR tyrosine kinase inhibitors used for treating cancer individuals.
Owner:上海中优医药高科技股份有限公司
Kits for survival and prognosis of glioblastoma related to SCOS3
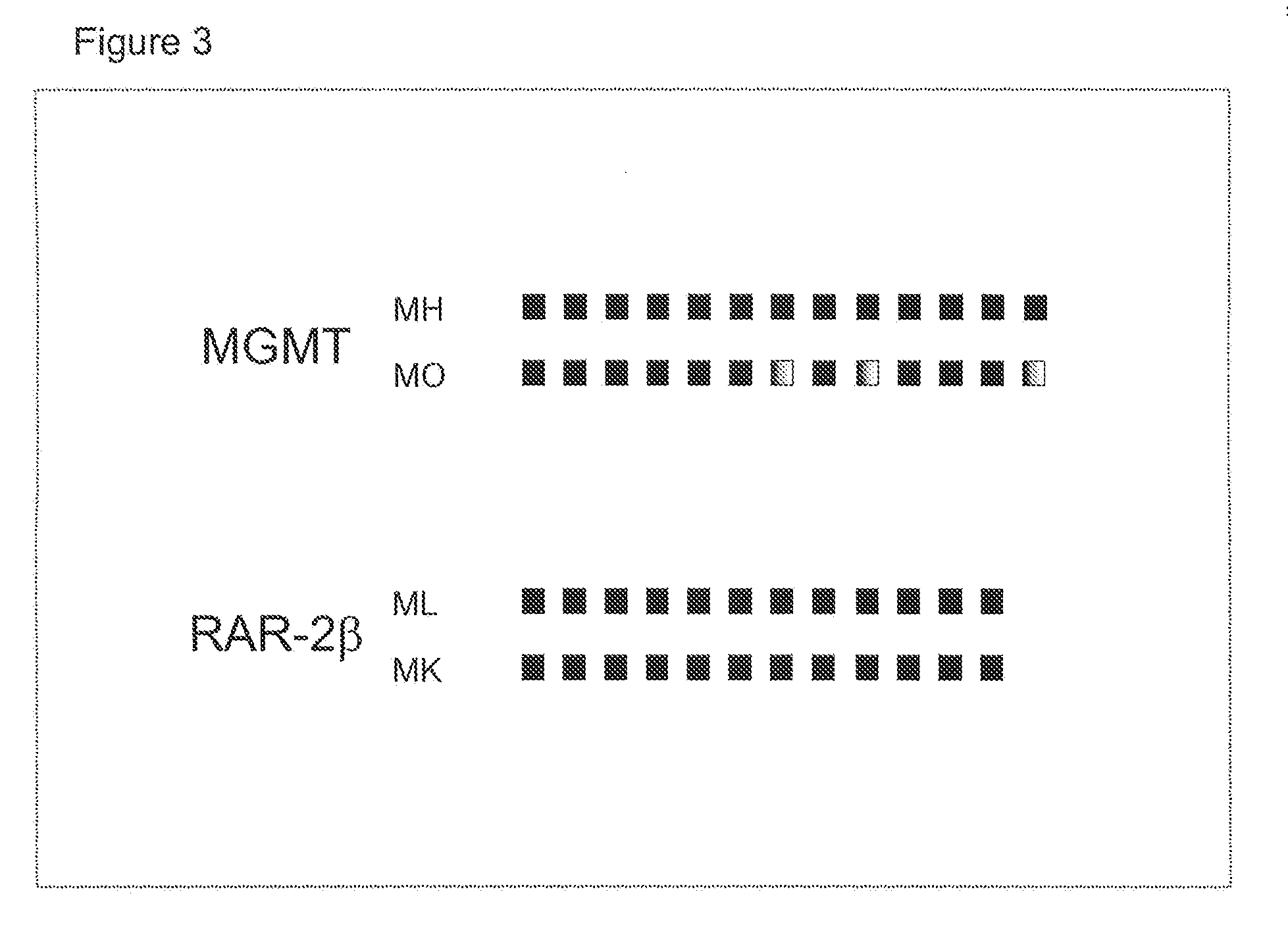
The invention provides kits for the survival and the prognosis of glioblastoma related to SCOS3. The kits comprise a kit for extracting DNA from a tumor tissue sample of a patient with primary glioblastoma, an optional instruction manual for the DNA extracting kit, a kit for analyzing the methylation level of gene SOCS3 in the extracted DNA and an optional instruction manual for the kit for analyzing the methylation level of the gene SOCS3. The invention also provides a molecular marker SOCS3 gene for the survival and the prognosis of glioblastoma.
Owner:江涛
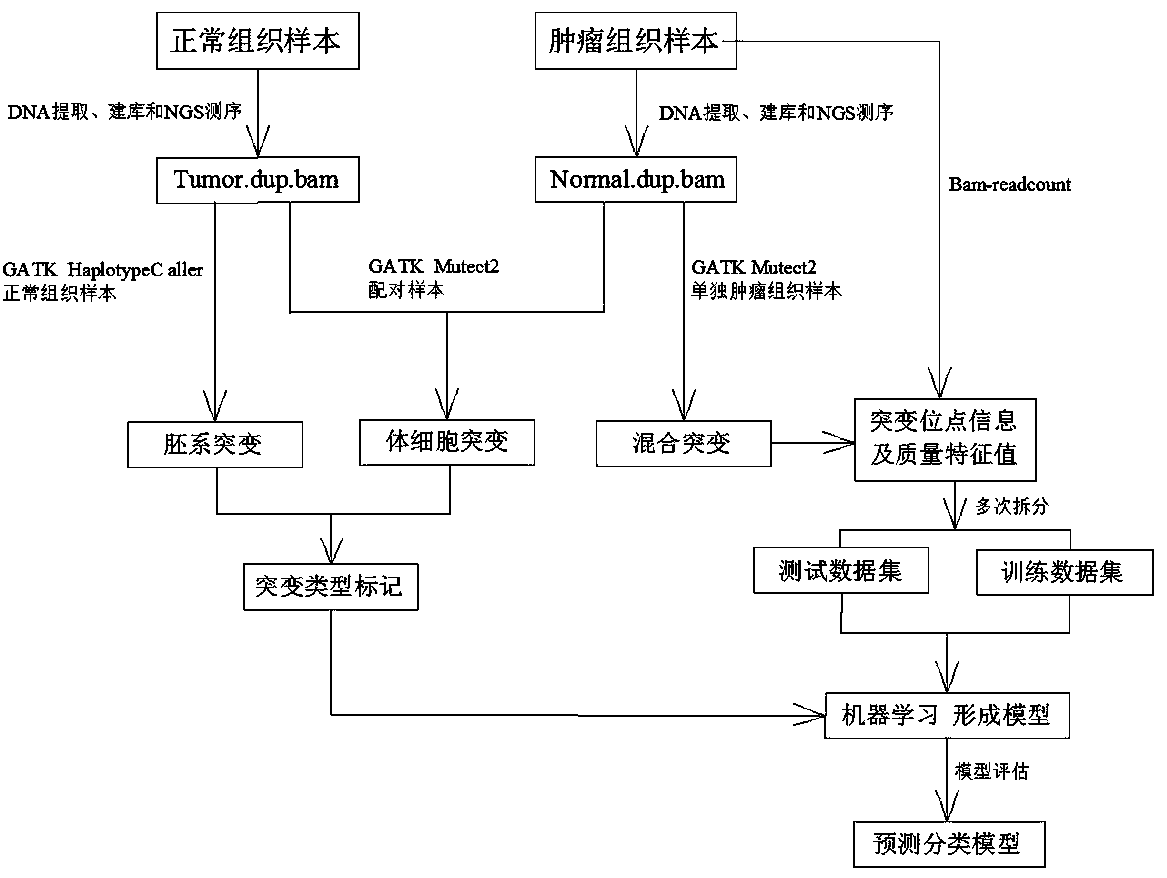
Method for distinguishing gene mutation type from individual tumor sample based on second-generation sequencing
ActiveCN110846411ALow detection costImprove detection efficiencyMicrobiological testing/measurementProteomicsGermline mutationSomatic cell
The invention relates to a method for distinguishing gene mutation types an individual tumor sample based on second-generation sequencing. A tumor tissue sample and a normal tissue sample are used forlibrary construction and NGS sequencing respectively, strand bias and different types of base frequencies of mutation sites stored in an intermediate file BAM for biological information analysis of the tumor tissue sample are analyzed, the quality of base comparison and the frequency of noise are used as the training characteristics of machine learning, meanwhile, type information of corresponding mutation sites of the normal tissue sample is paired to serve as a prediction mutation type, a classification prediction model is constructed to distinguish somatic mutation from germline mutation,the model is used to distinguish somatic mutation from germline mutation, the detection efficiency is high, the specificity is high, and after the model is established, the individual tumor sample canbe used for NGS sequencing and mutation detection, the detection cost of a normal or cancer sample can be well saved, and meanwhile, the problem that normal tissues of tumor patients with specific types are difficult to obtain can be solved.
Owner:上海仁东医学检验所有限公司 +1
Method for detecting mutation and HRD scoring of tumor patient to guide medication
PendingCN112820351ACalculation speedEasy to detectDrug and medicationsBiostatisticsAllele ImbalanceWhite blood cell
The invention relates to a method for for detecting mutation and HRD scoring of tumor patient to guide medication, which comprises the following steps: detecting a tumor tissue sample and a control leukocyte sample thereof on the basis of taking a BAM file as an input file, and detecting mutation information of an HRR pathway in panels of different sample types and large-fragment chromosome abnormality HRD caused by gene recombination repair defects, the method for detecting the tumor tissue sample comprises the following steps: 1) detecting mononucleotide mutation of an HRR pathway; 2) detecting the loss of heterozygosity (LOH), telomere allele imbalance (TAI) and large fragment state transition (LST) of the HRR region, wherein the HRD score is equal to the weighted sum of LOH, TAI and LST scores; the invention provides a mutation and HRD scoring medication guidance method for tumor patients. According to the method, a traditional method that the HRD value score is calculated through whole genome detection and is not beneficial to clinical actual effect is optimized, the calculation speed is increased, the detection effect is good, the detection result is accurate, and the requirements of actual application can be well met.
Owner:江苏医联生物科技有限公司
Sampling device for clinical biopsy sample in oncology
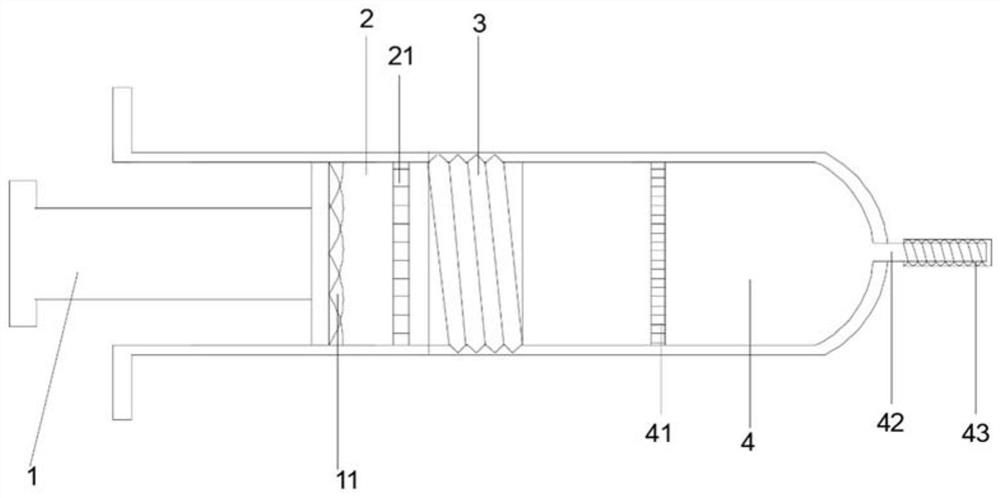
InactiveCN109431553AReasonable structural designAvoid shakingSurgical needlesVaccination/ovulation diagnosticsMuscle tissueGear wheel
The invention relates to the technical field of medical instruments, in particular to a sampling device for a clinical biopsy sample in oncology. The device comprises an outer box, a puncture needle,a sampling tube and a push rod, wherein the puncture needle is inserted into the outer box; the sampling tube is formed by buckling two semi-annular sampling needles and is inserted into the inner left end of the puncture needle; a first cutting line and a second cutting line are fixedly connected to the left and right ends of one sampling needle respectively; L-shaped rods inside the outer box are fixedly connected to the two connecting rods on the outer box; racks meshing with gear wheels on the puncture needle are fixedly connected to the L-shaped rods; the push rod is inserted into the puncture needle; and a push block is fixedly connected to the left end of the push rod. The structural design is reasonable, skin and muscle tissues enter into the right side of the sampling tube in thepuncture needle by inserting the puncture needle into a tumor tissue, and the tumor tissue enters into the sampling tube; holding rings are pressed to rotate the sampling tube; the tumor tissue can becut by using the first cutting line and the second cutting line; and a pure tumor tissue sample can be obtained by pushing out the sampling tube by using the push rod.
Owner:宋超
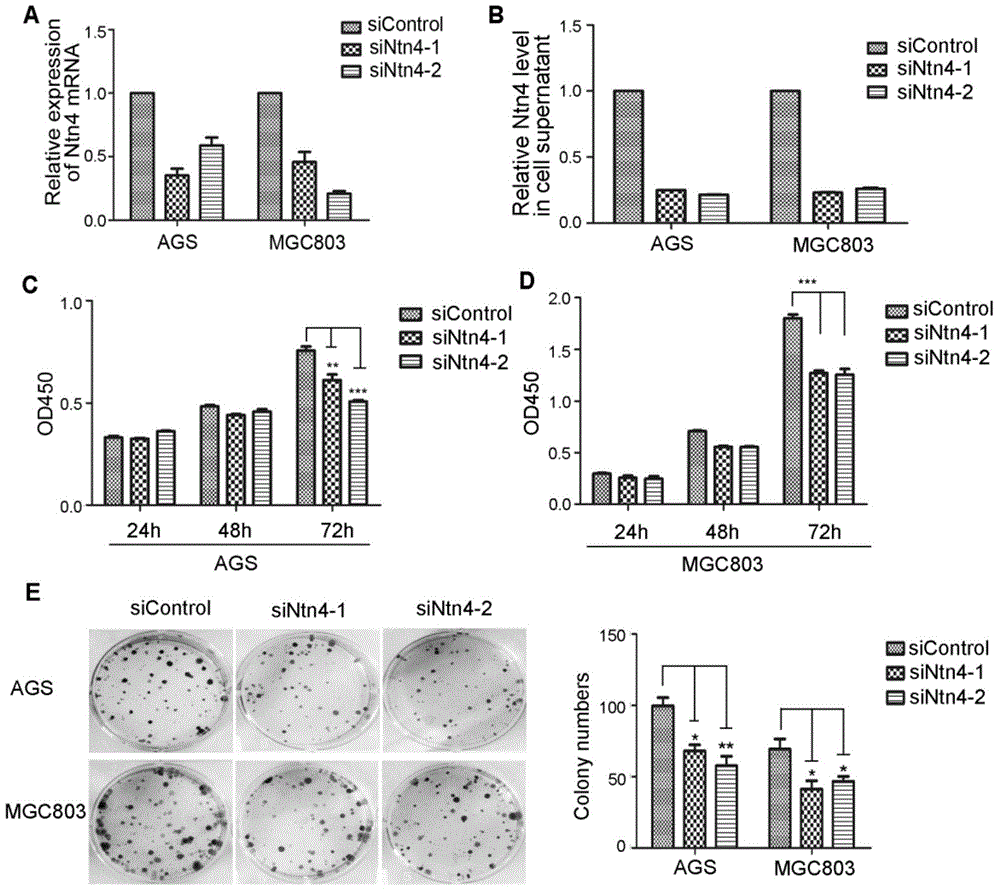
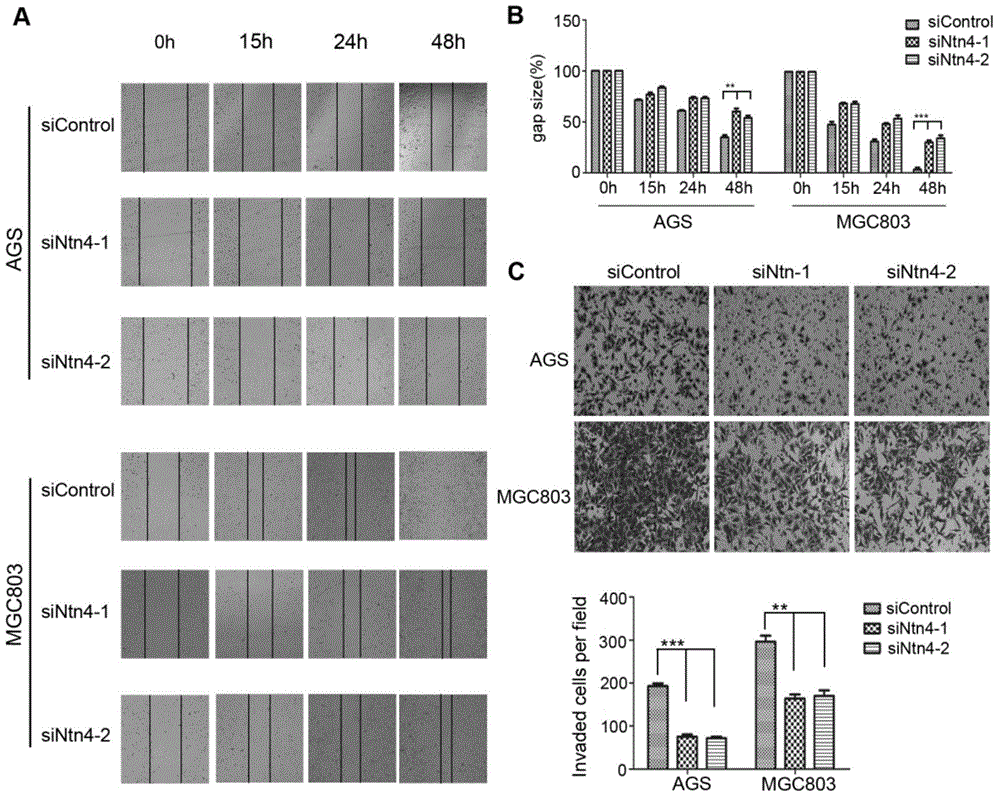
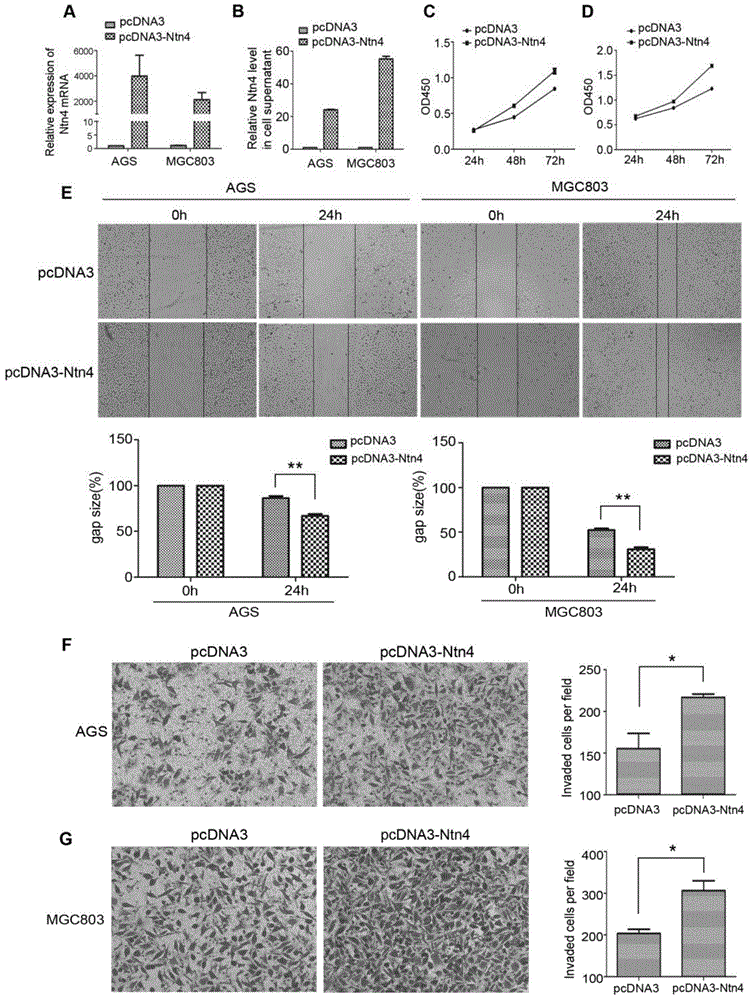
Application of Netrin-4 in preparing preparation for detecting stomach cancer and prognosis marker
The invention belongs to the technical field of cell biology, and relates to application of Netrin-4 in preparing a preparation for detecting stomach cancer and a prognosis marker. Experiments display that Ntn4 is combined with a ligand neogenin thereof to activate cancer promoting paths such as Jak / Stat, PI3K / Akt and ERK / MAPK, thereby achieving the effects of promoting gastric cancer cell proliferation and invasion. Experiment results show that Ntn4 has remarkable high expression in both a serum sample and a tumor tissue sample of a patient with gastric cancer, high expression is in negative correlation with the lifetime of the patient and in positive correlation with TNM staging, it shows that Ntn4 can be used as potential serological indicator for gastric cancer and prognosis and used for preparing the preparation for detecting stomach cancer and the prognosis marker. The preparation comprises a reagent and a kit which are used for detecting stomach cancer and the prognosis marker.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
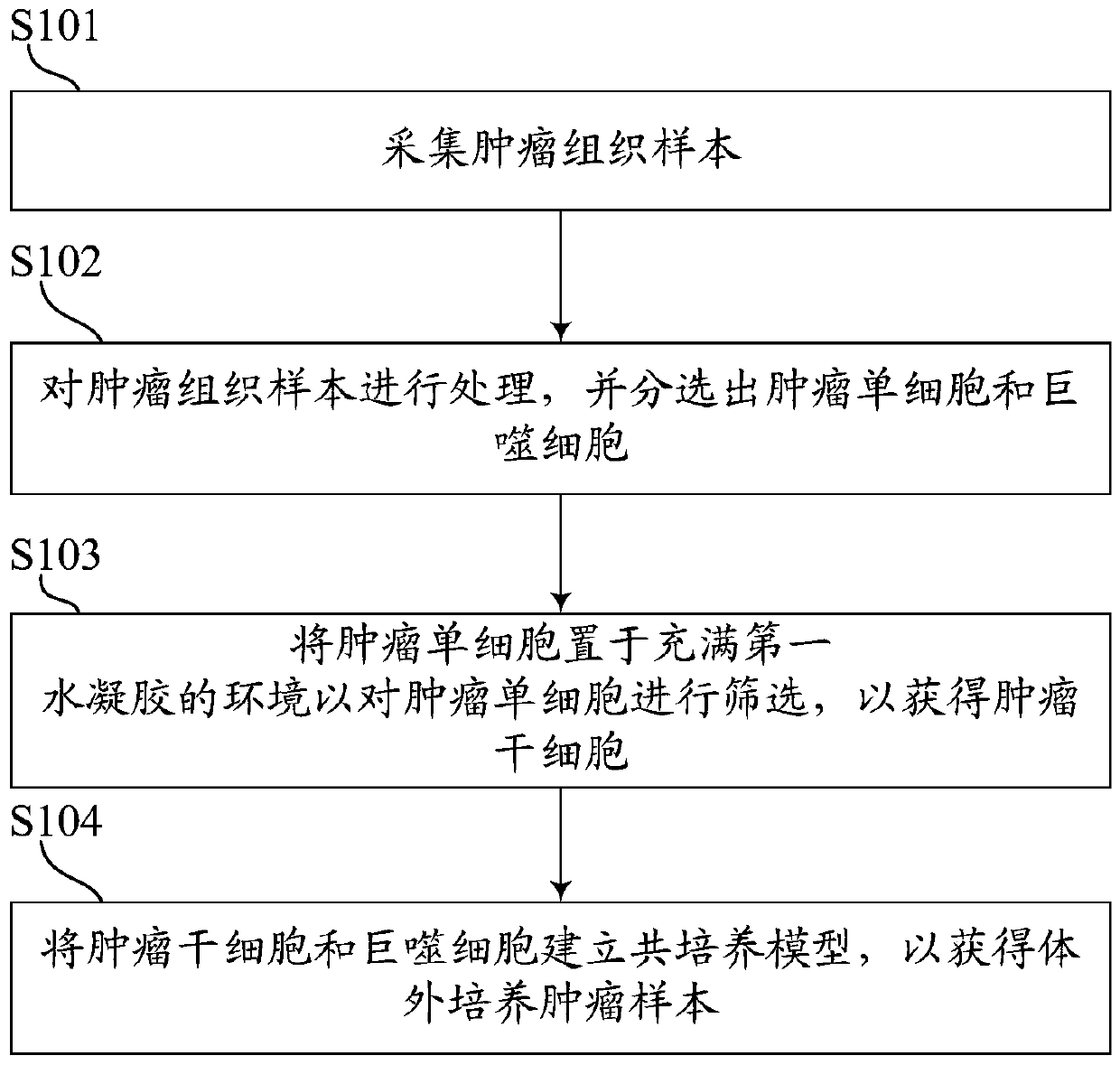
Tumor in-vitro culture method and clinical chemotherapeutic drug screening method
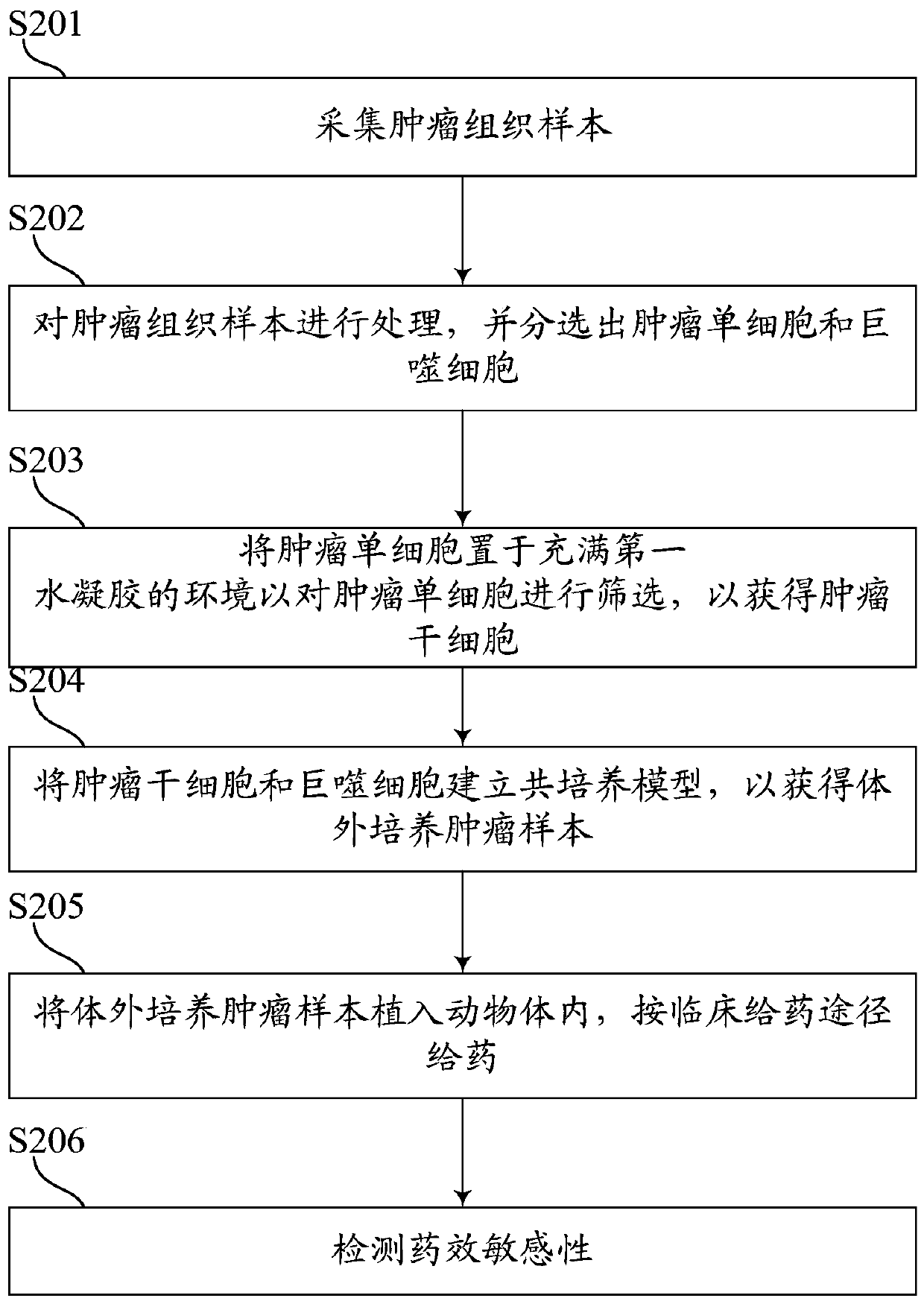
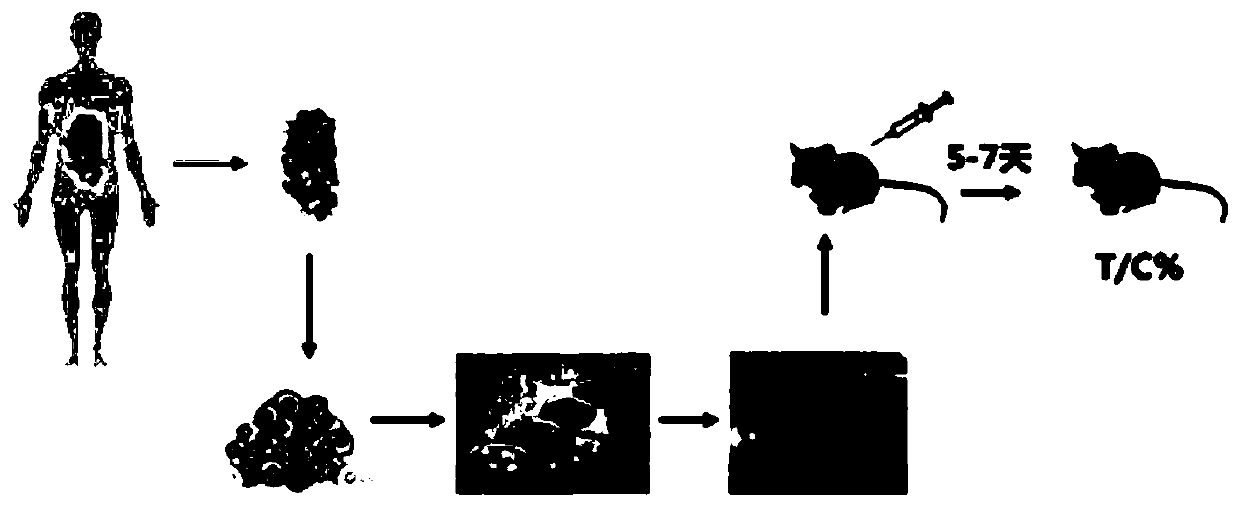
PendingCN109749999ALow costShorten tumor formation timeTumor/cancer cellsIn-vivo testing preparationsScreening methodWilms' tumor
The invention relates to the field of drug efficacy screening, and particularly discloses a tumor in-vitro culture method. The tumor in-vitro culture method includes the steps that tumor tissue samples are collected; the tumor tissue samples are treated, and single tumor cells and macrophages are sorted; the single tumor cells are placed in an environment full of hydrogel and then are screened toobtain tumor stem cells; the tumor stem cells and the macrophages are adopted to build a co-culture model to obtain in-vitro culture tumor samples. In this way, the tumor stem cells with high proliferation and differentiation capabilities can be screened out in vitro, therefore, the tumor stem cells are implanted into the animal body, the tumor formation time can be greatly shortened, the tumor formation rate is increased, and then susceptibility testing is conducted; therefore, the progress of susceptibility testing is speeded up, and time for treating patients is bought.
Owner:NANTONG UNIVERSITY
Detection probe, liquid phase chip and detection method thereof for EGFR gene mutation sites
ActiveCN101445829AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMicrosphereEGFR Gene Mutation
The invention discloses a detection probe, a liquid phase chip and a detection method thereof for EGFR gene mutation sites. The EGFR gene mutation detection liquid phase chip mainly comprises a microsphere for wrapping the probe; and a primer used for being amplified with a target sequence with 19 exons and / or 21 exons. By adopting the EGFR gene mutation detection liquid phase chip and the detection method, the gene mutation sites with higher relative frequency can be detected simultaneously, the 19 exons and the 21 exons can be detected separately, and also can be detected simultaneously, the reaction conditions during the detection are uniform, the specificity of the detected result is good, the sensitivity is high, the detection accuracy reaches above 90 percent, and the detection timeis short. The detection method not only can detect the EGFR gene mutation in the tumor tissue sample, but also can detect the EGFR gene mutation in the body fluid of the patient with tumor and also can perform dynamic detection.
Owner:广州益善医学检验所有限公司
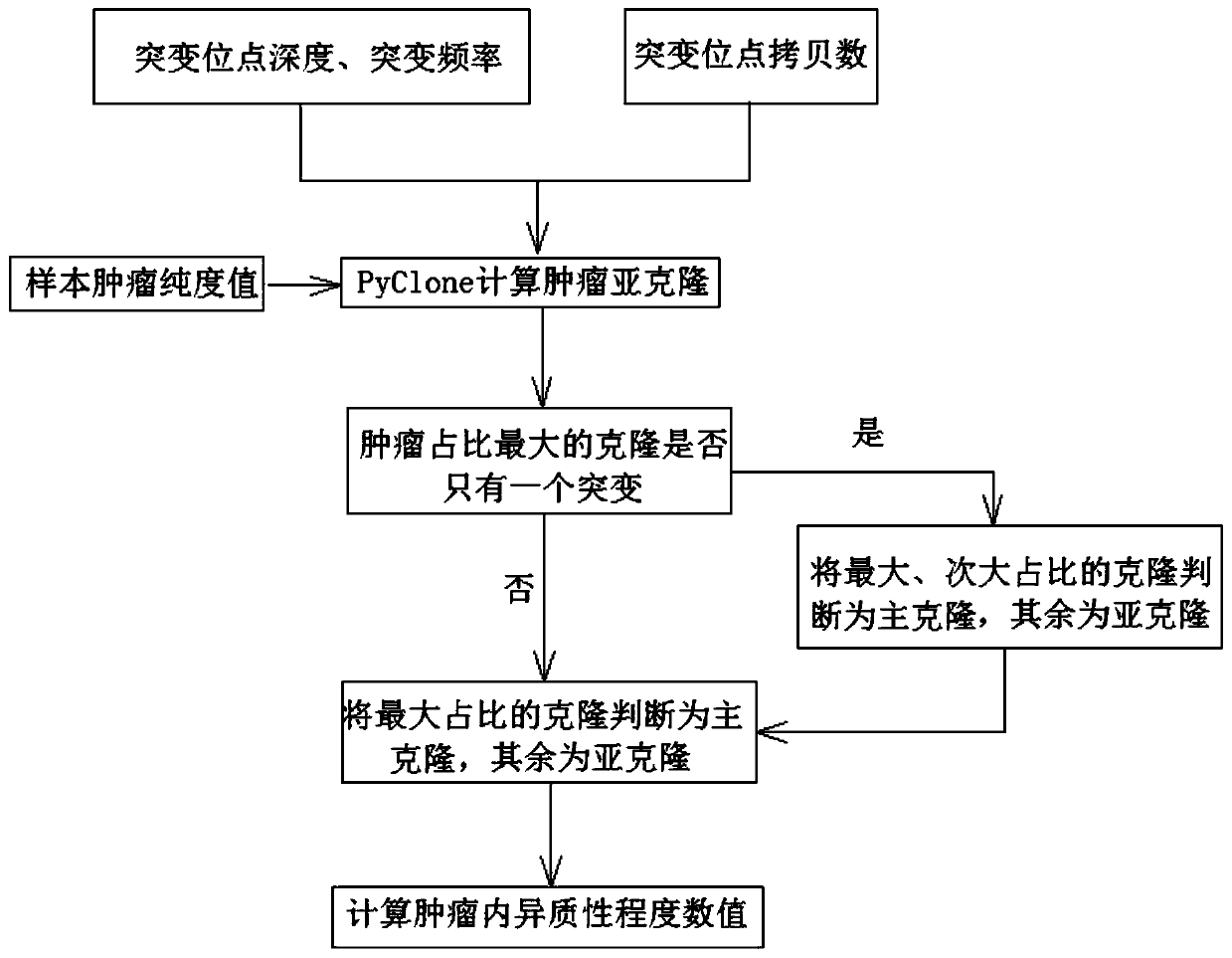
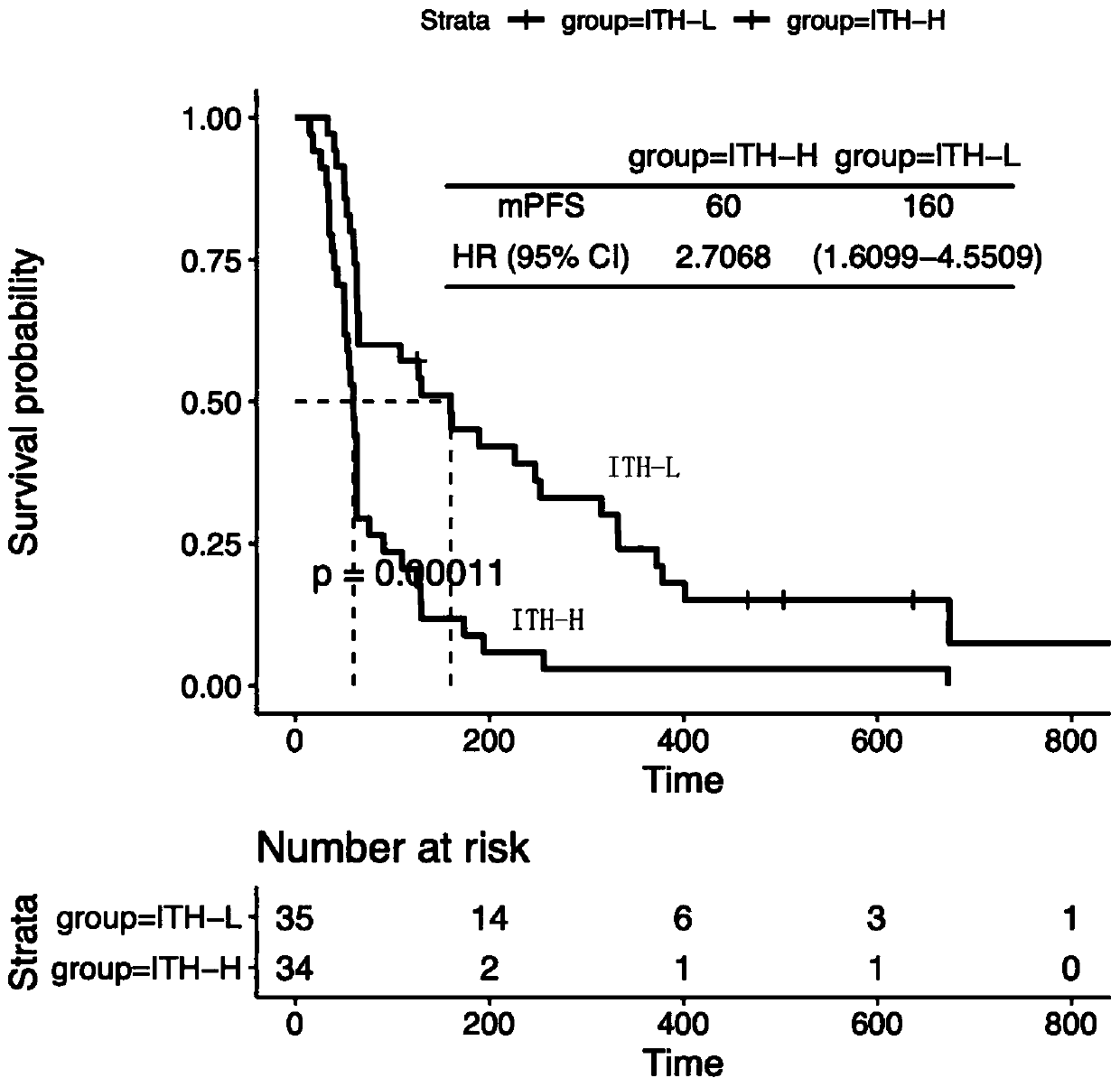
Method and system for detecting degree of tumor heterogeneity
The invention provides a method and system for detecting the degree of tumor heterogeneity. The method comprises the following steps: acquiring sequencing data of a tumor tissue sample and a control sample, carrying out somatic variation detection, and acquiring somatic variation sites; clustering the somatic cell variation sites according to clusters; judging a main cloning mutation cluster and asubcloning mutation cluster according to a clustering result; and calculating a ratio of the number of somatic cell variations in the subcloning mutation cluster in the tumor tissue sample to the number of all the somatic cell variations, wherein the ratio is the value of tumor heterogeneity. According to the method, mutation frequency detected at each mutation site of the sample is fully utilized to estimate the cell proportion of corresponding tumor cloning, so the specific numerical value of a tumor heterogeneity degree is calculated; and thus, a numerical reference basis is provided for subsequent prediction of immunotherapy effect.
Owner:深圳裕策生物科技有限公司
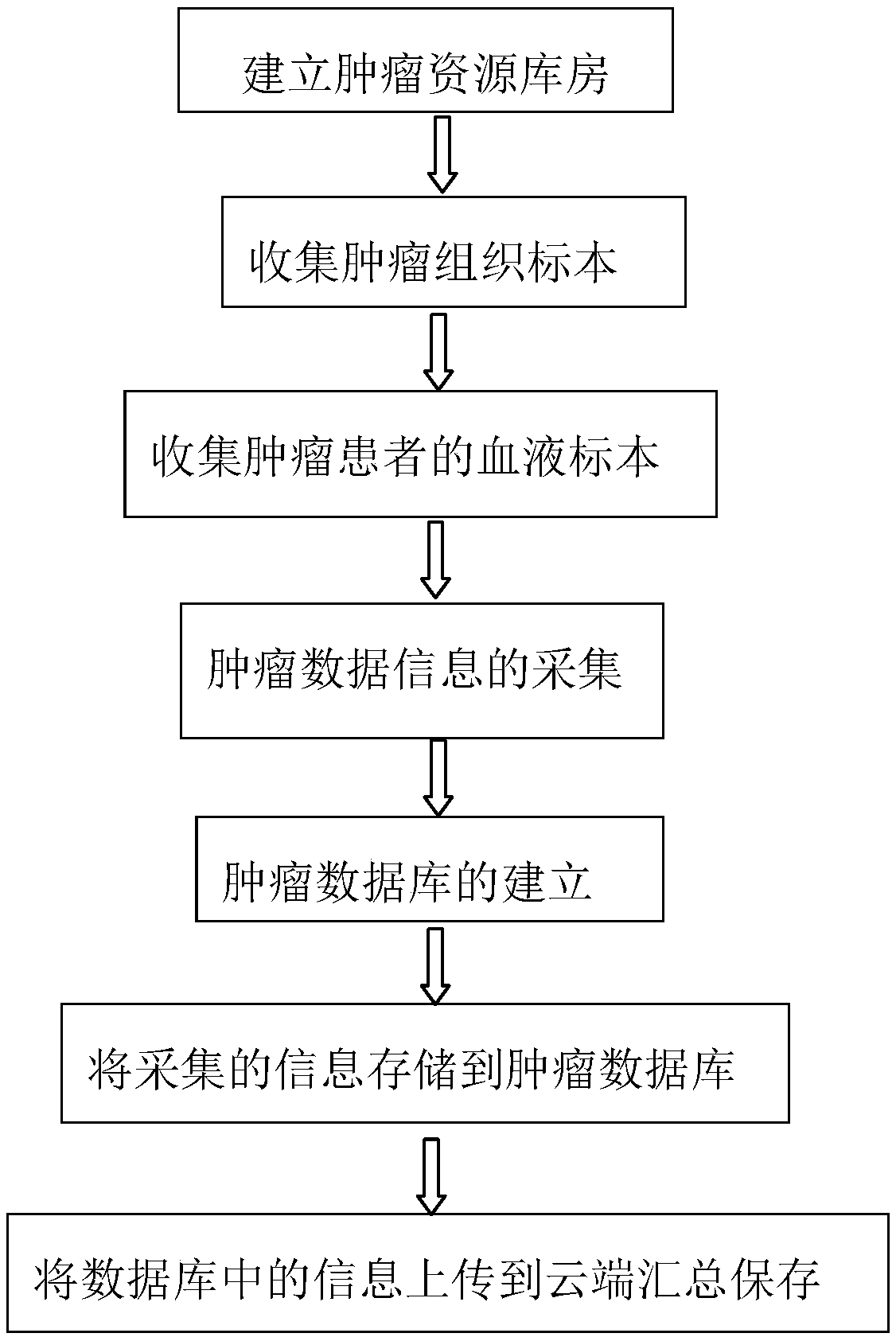
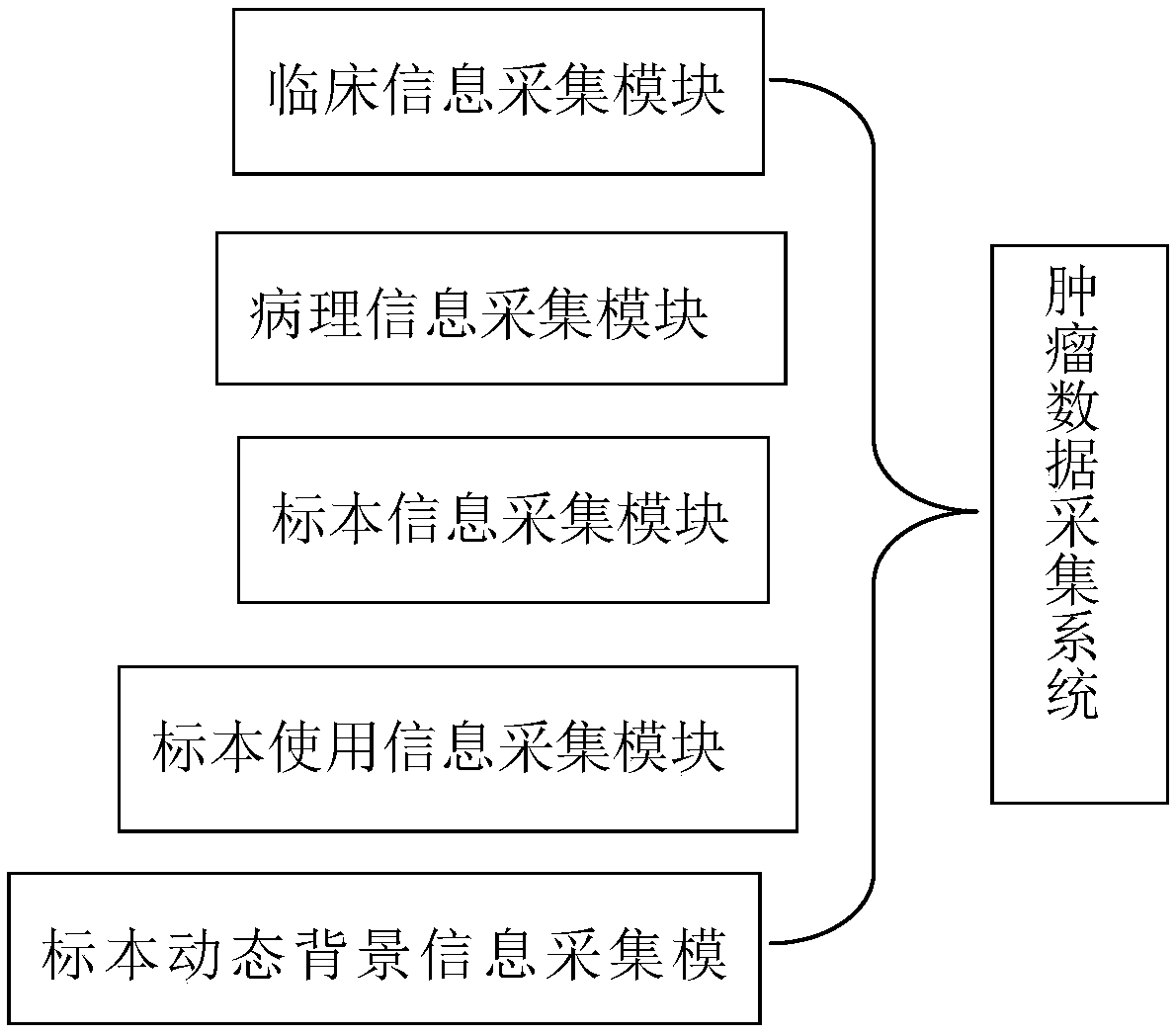
Tumor resource tissue library and method for establishing the same
InactiveCN108962396AFully protectedConvenient researchMedical referencesInstrumentsHigh risk populationsGenomics
The invention discloses a tumor resource tissue library and a method for establishing the same. The tumor resource tissue library comprises a tumor resource warehouse, tumor tissue samples and a tumordatabase. With the tumor resource tissue library, a problem of collecting fresh tumor tissues difficultly in the prior art is solved; the case resources are protected and utilized fully and reasonably; proper tumor samples are provided for the researchers; the research cycle is reduced, and the related researches on the lymphoma and other tumors are promoted. Therefore, research materials are provided for research workers in the fields of genetics, epigenetics, cell and molecular biology, genomics and proteomics and the like; and the bases are provided for tumor mutagenesis screening, geneticcounseling of high-risk populations, epidemiological characteristics of malignant tumors and tumor classification, early diagnosis, prognosis assessment and individualized treatment.
Owner:潘云
Multi-molecular marker and device for clinical evaluation of chemotherapy sensitivity of platinum-based drug for ovarian cancer and evaluation method thereof
InactiveCN108913773AEasy to getReal-time monitoring of drug resistanceMicrobiological testing/measurementSpecial data processing applicationsData setClinical trial
A multi-molecular marker and a device for clinical evaluation of chemotherapy sensitivity of a platinum-based drug for ovarian cancer and an evaluation method are disclosed, chemotherapy response genes simultaneously are screened from multiple data sets, and the predicting effect of the multi-molecular marker can be verified and comprehensively evaluated in dozens of independent data sets, so thatthe potential application value of the multi-molecular marker in evaluation of the chemotherapy sensitivity of the platinum-based drug can be ensured. The multi-molecular marker contains only 16 genes, is easy to use in tests in clinical trials and reduces the cost of the tests. A patient's tumor tissue sample is easily available, and the patient's tumor tissue sample can be directly obtained forgene expression measurement by a needle; the multi-molecular marker is not affected by the batch effect of experiments or the difference of detection platforms; before the multi-molecular marker is used, standardizing treatment of data of multiple samples is not necessary, and the multi-molecular marker is convenient to use. Patient's drug resistance is monitored in real time before and after chemotherapy, so that a clinical personalized treatment plan is performed in time by a doctor, and the situation that the patient's drug resistance is found after multiple rounds of highly toxic drug application can be avoided.
Owner:WENZHOU MEDICAL UNIV
In-vitro tissue preserving fluid and preserving method and application thereof
InactiveCN111066778APlay an antibacterial roleHigh activityDead animal preservationPenicillinNutrition
The invention discloses an in-vitro tissue preserving fluid as well as a preserving method and application thereof. The in-vitro tissue preserving fluid includes a penicillin-streptomycin solution, fetal calf serum and an improved L-15 culture medium. The obtained in-vitro tissue preserving fluid can provide nutrition for tumor tissues and has an antibacterial effect, the activity of the tumor tissues can be improved, the modeling success rate of clinical patient-derived tumor xenograft is further improved, in addition, tumor tissue samples can be preserved, a sample database can be established, secondary experiment needs are facilitated, and repeated material taking is reduced.
Owner:南京普恩瑞生物科技有限公司
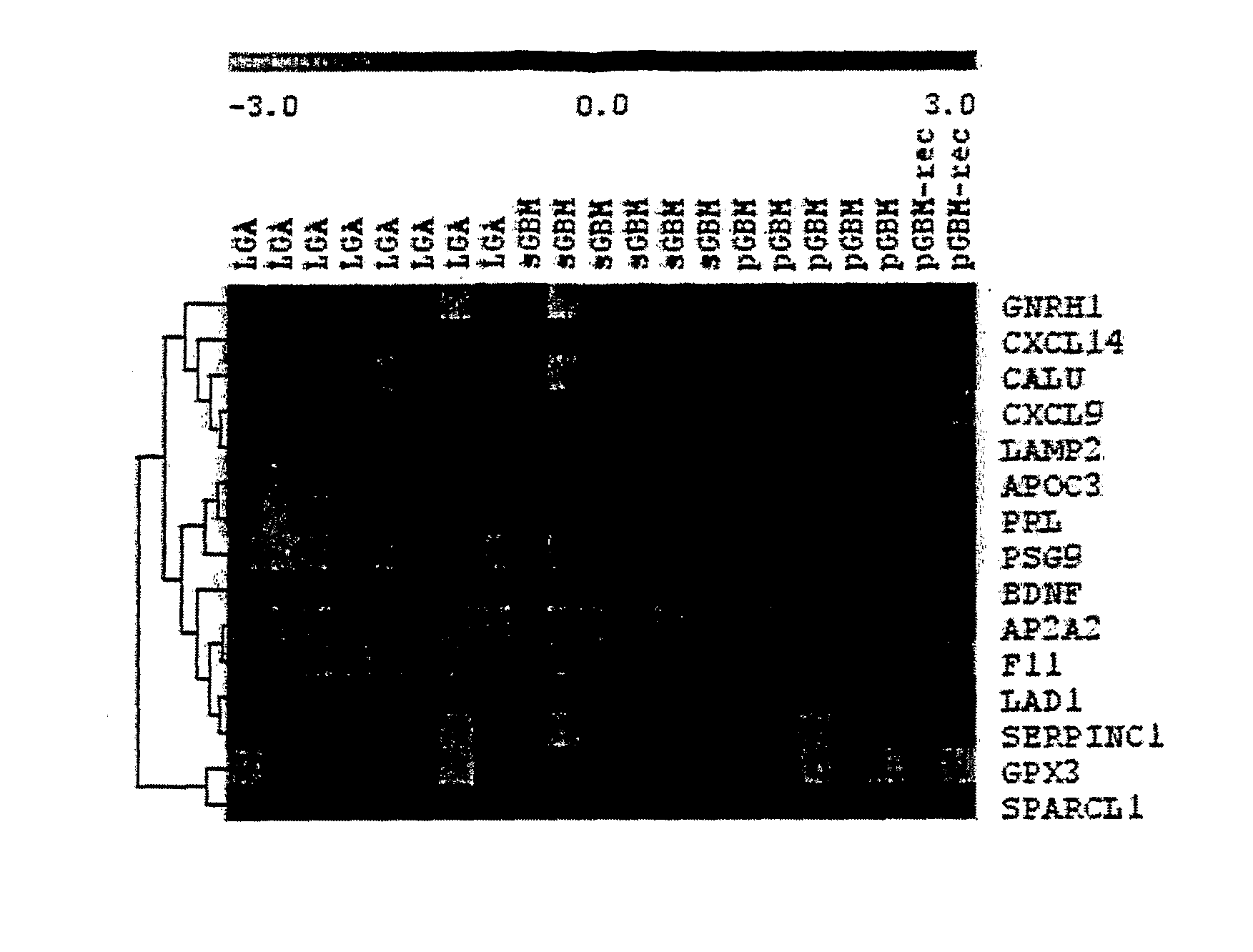
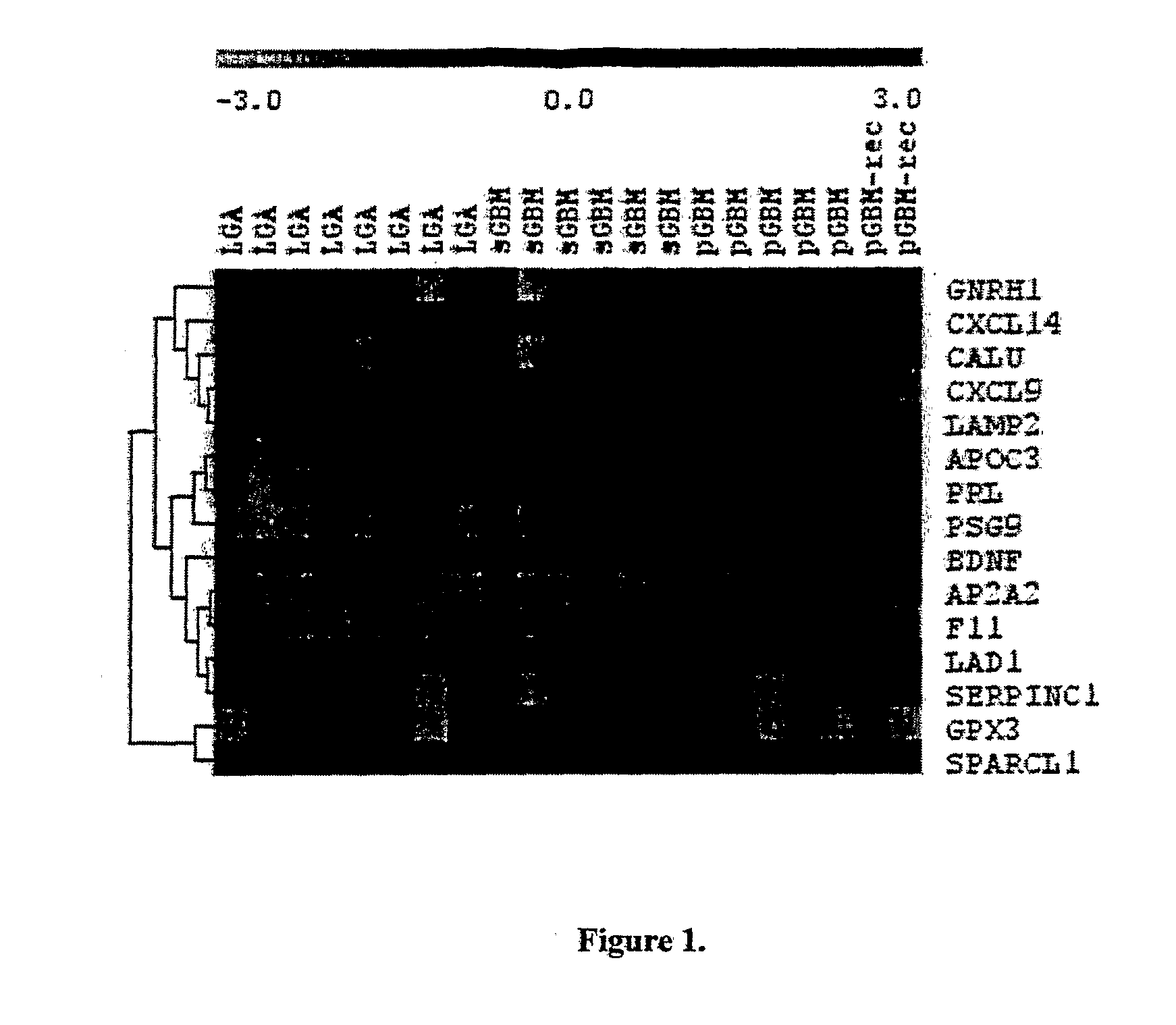
Method for the Diagnosis of Higher- and Lower-Grade Astrocytoma Using Biomarkers and Diagnostic Kit Thereof
Disclosed is a method of diagnosing the presence of higher grade astrocytoma / glioblastoma (GBM) or lower-grade grade astrocytoma (DA or AA) in a human subject using secreted or plasma membrane associated biomarkers, which involves the detection of the expression levels of said genes, alone or in combination, in either tumor tissue samples or body fluids and a diagnostic kit thereof.
Owner:COUNCIL OF SCI & IND RES
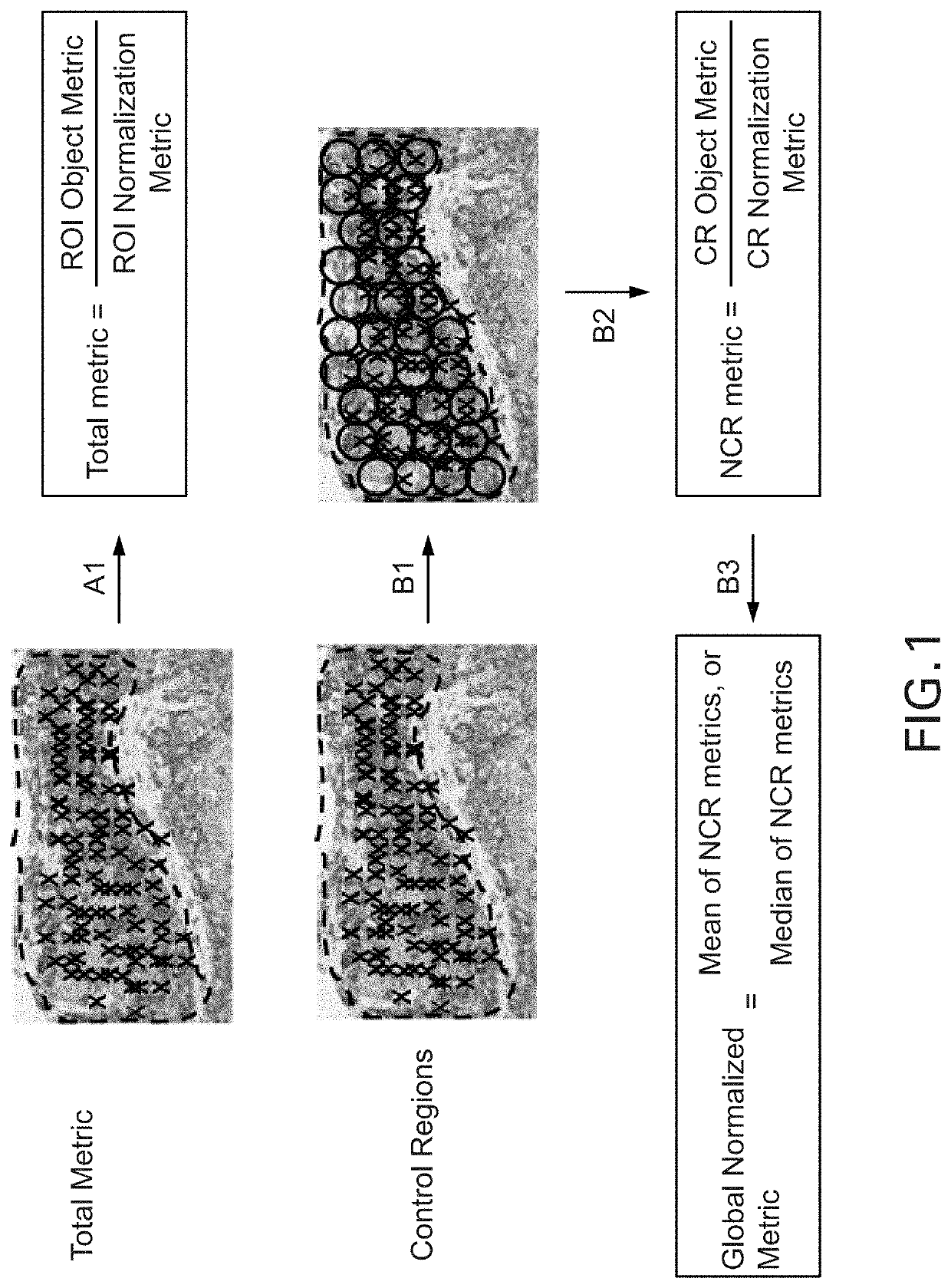
Methods and systems for evaluation of immune cell infiltrate in tumor samples
ActiveUS20200234442A1Improves prognosticGood prediction accuracyImage enhancementImage analysisProgression-free survivalCell marker
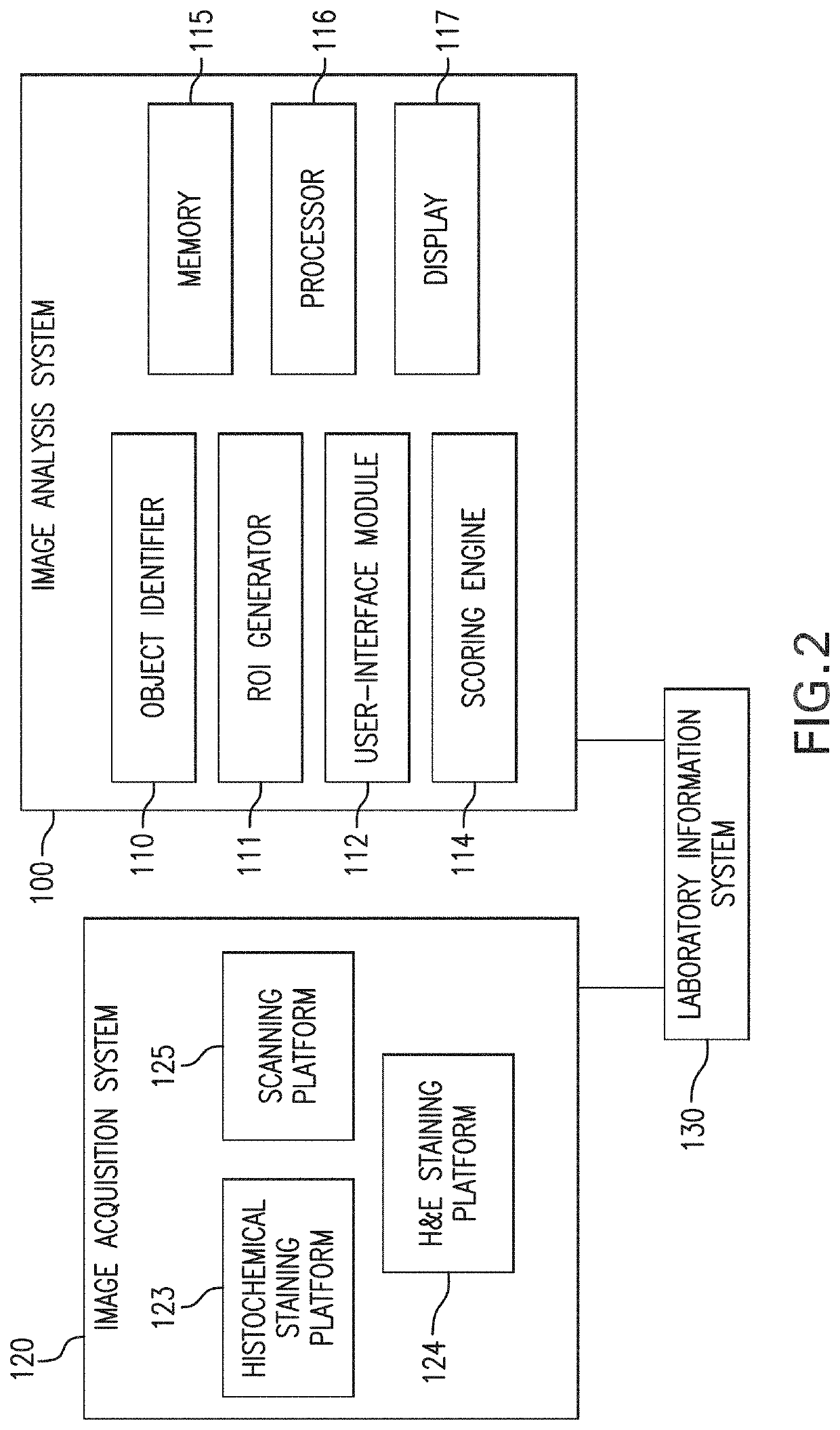
Immune context scores are calculated for tumor tissue samples using continuous scoring functions. Feature metrics for at least one immune cell marker are calculated for a region or regions of interest, the feature metrics including at least a quantitative measure of human CD3 or total lymphocyte counts. A continuous scoring function is then applied to a feature vector including the feature metric and at least one additional metric related to an immunological biomarker, the output of which is an immune context score. The immune context score may then be plotted as a function of a diagnostic or treatment metric, such as a prognostic metric (e.g. overall survival, disease-specific survival, progression-free survival) or a predictive metric (e.g. likelihood of response to a particular treatment course). The immune context score may then be incorporated into diagnostic and / or treatment decisions.
Owner:VENTANA MEDICAL SYST INC
DNA markers for management of cancer
InactiveUS20100330567A1Stage increaseInvasive detectionSugar derivativesMicrobiological testing/measurementMelanomaNeurocytoma
A method is provided for assessing allelic losses and hypermethylation of genes in CpG tumor promotor region on specific chromosomal regions in cancer patients, including melanoma, neuroblastoma breast, colorectal, and prostate cancer patients. The method relies on the evidence that free DNA and hypermethylation of genes in CpG tumor promotor region may be identified in the bone marrow, serum, plasma, and tumor tissue samples of cancer patients. Methods of melanoma, neuroblastoma, colorectal cancer, breast cancer and prostate cancer detection, staging, and prognosis are also provided.
Owner:HOON DAVE S B +1
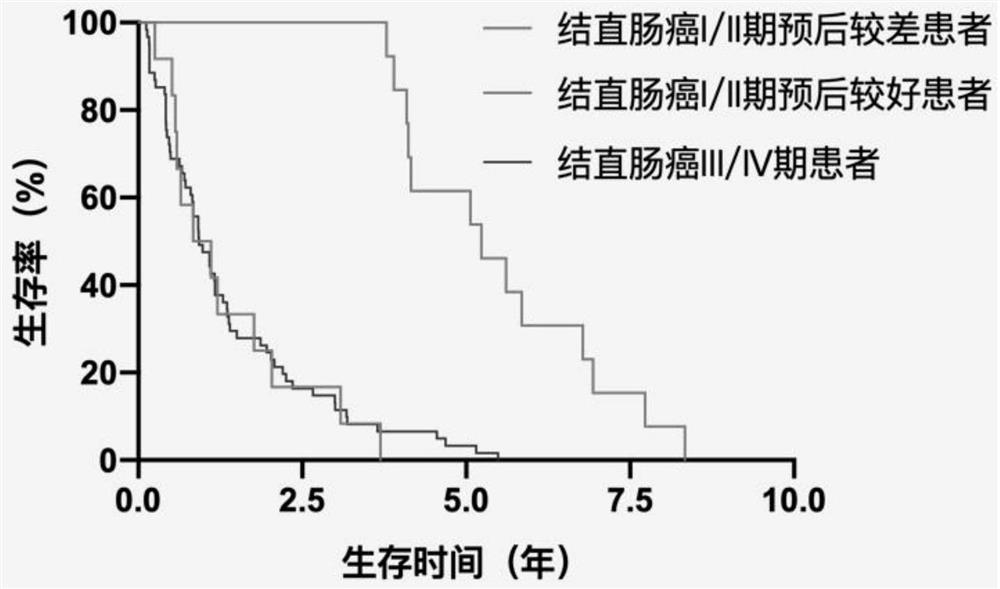
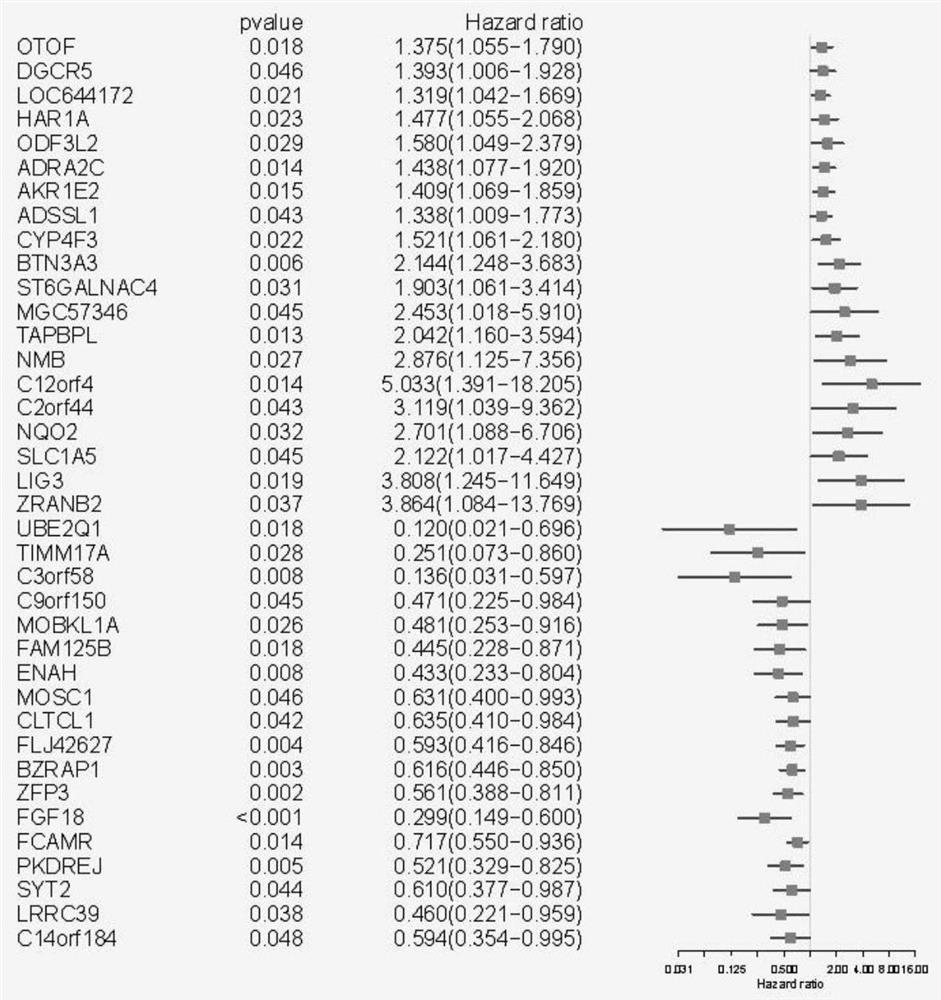
Model construction method for predicting prognosis risk of early-stage colon cancer patient
PendingCN113035358AImprove accuracyPrecision medicineHealth-index calculationMicrobiological testing/measurementOncologyBiology
The invention relates to a model construction method for predicting the prognosis risk of an early-stage colon cancer patient. The model construction method specifically comprises the following steps: firstly, obtaining a tumor tissue specimen of the patient through an operation; detecting the expression of the gene content in a tumor tissue sample of a patient by using difference analysis; then screening genes for constructing a risk model by using single-factor Cox regression analysis and LASSO regression analysis, and establishing a calculation formula of the risk model; finally, using Kaplan-Meier prognosis analysis and ROC analysis to verify the accuracy of the prognosis risk model for prognosis prediction in early-stage colon cancer patients. The method can better guide clinical design of a more targeted treatment scheme for a patient with poor early colon cancer prognosis, and realizes precise medical treatment; The number of genes for constructing the prediction model is large, the influence caused by tumor heterogeneity can be reduced to the maximum extent, and the accuracy is high; The gene detection method is simple and easy to implement.
Owner:NANJING FIRST HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com