Adoptive transfer of cd8 + t cell clones derived from central memory cells
a technology of central memory cells and t cells, which is applied in the field of adoptive immunotherapy, can solve the problems of limited efficacy of cultured t cells, especially cloned cd8sup>+/sup> t cells, and achieve the effect of increasing the proliferation of central memory t cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
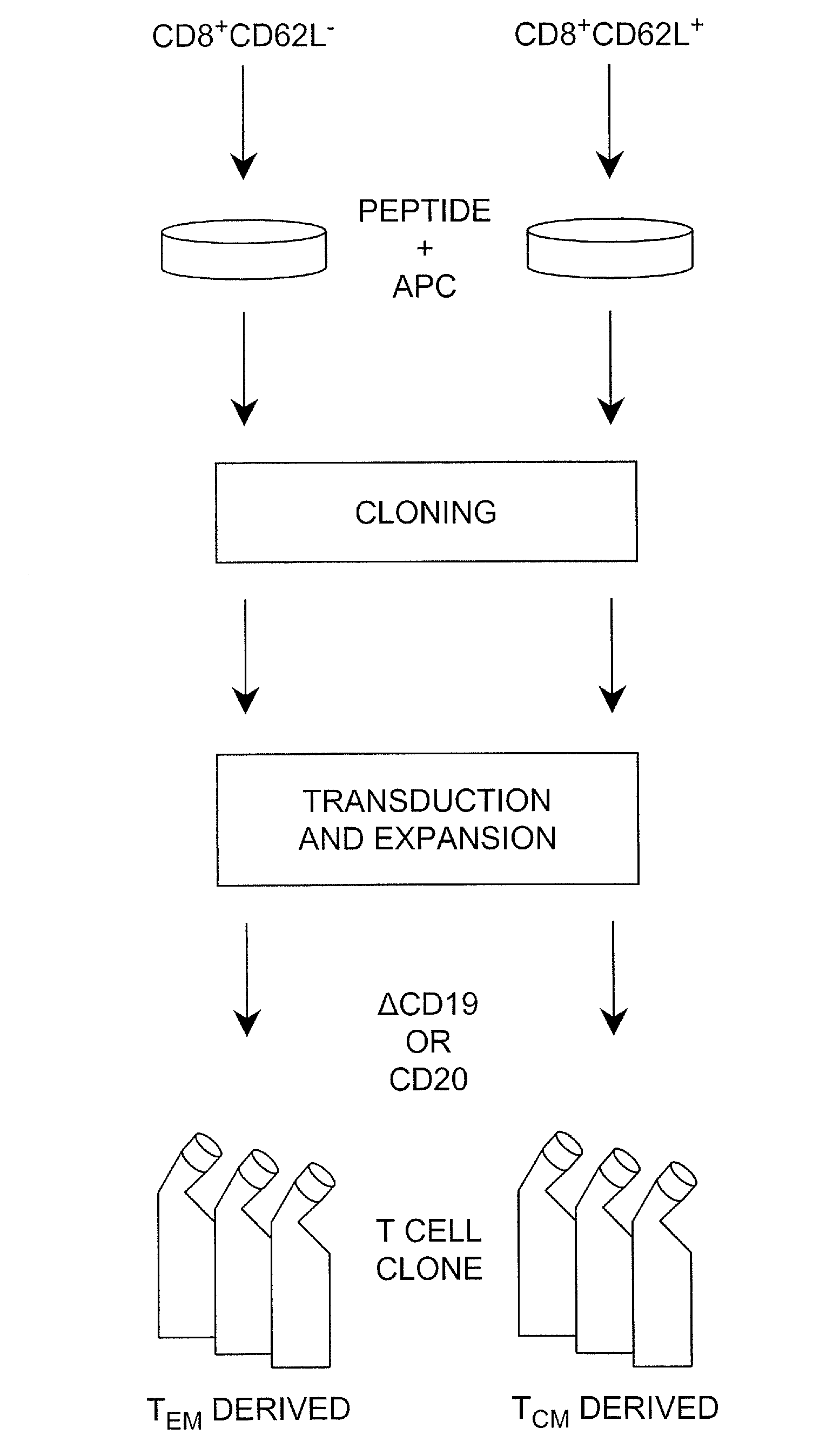
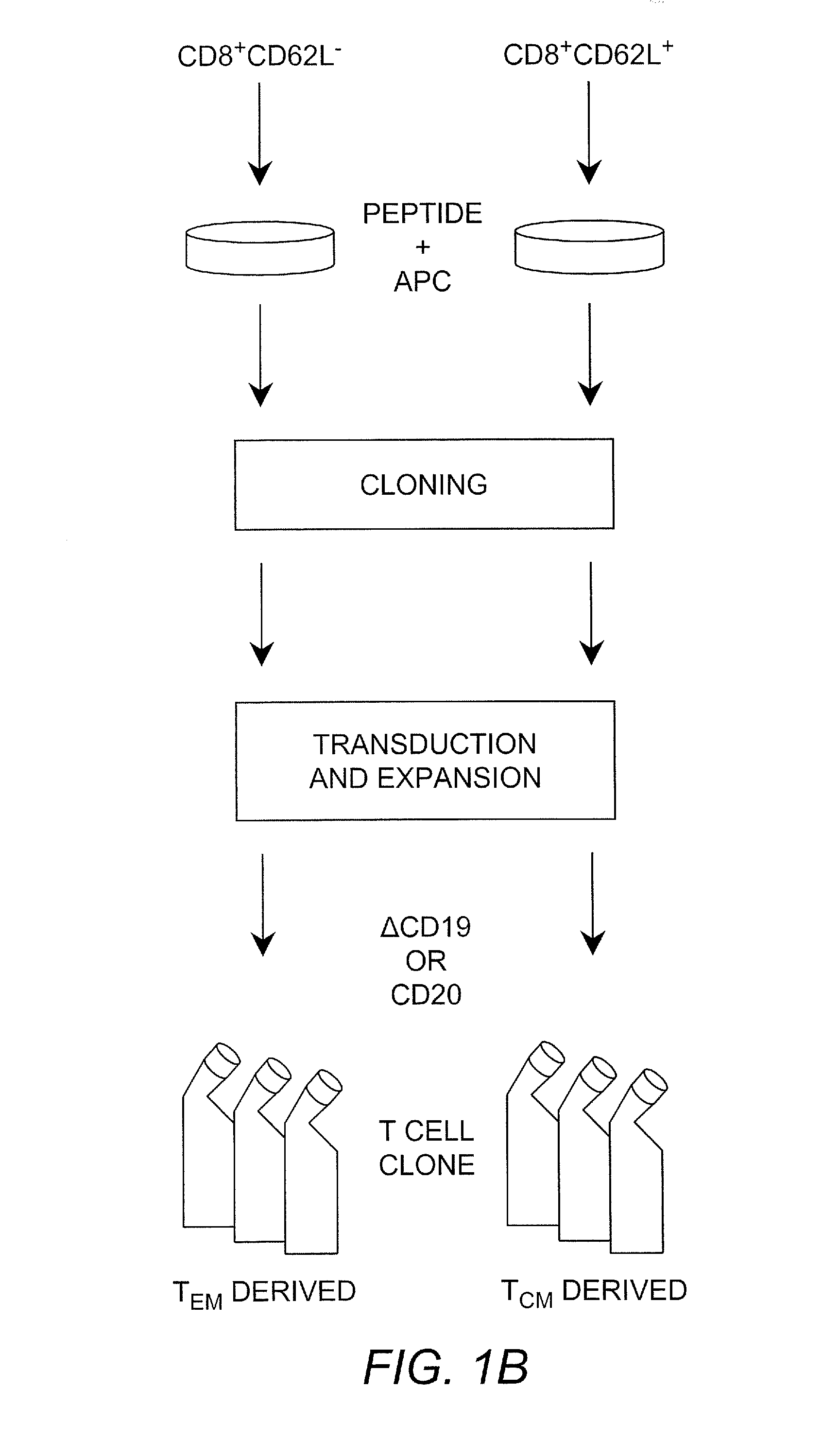
[0041]“T cells” or “T lymphocytes” as used herein may be from any mammalian, preferably primate, species, including monkeys, dogs, and humans. In some embodiments the T cells are allogenic (from the same species but different donor) as the recipient subject; in some embodiments the T cells are autologous (the donor and the recipient are the same); in some embodiments the T cells are syngeneic (the donor and the recipients are different but are identical twins).
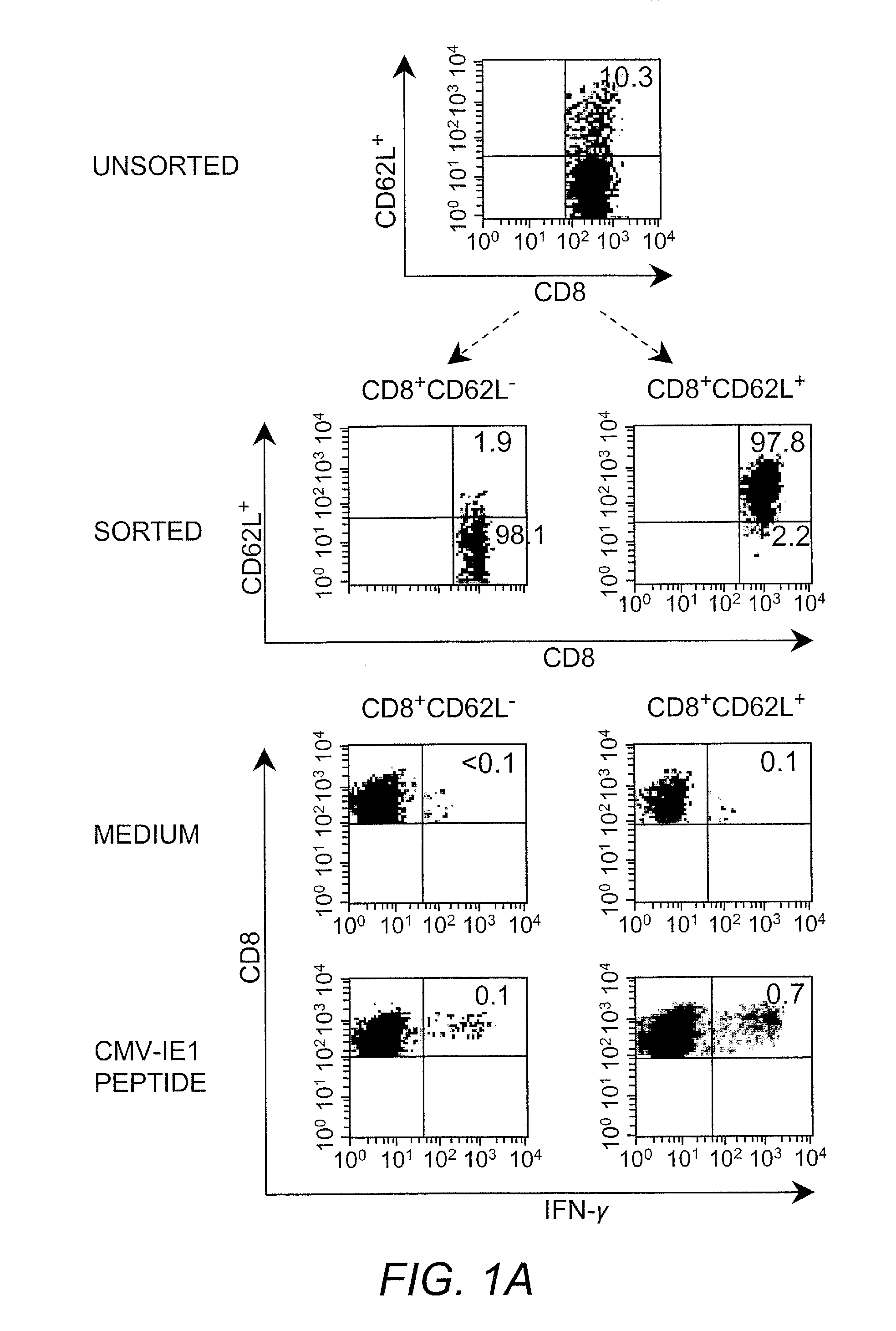
[0042]Cytotoxic T lymphocyte (CTL) as used herein refers to a T lymphocyte that expresses CD8 on the surface thereof (i.e., a CD8+ T cell). In some embodiments such cells are preferably “memory” T cells (TM cells) that are antigen-experienced. “Central memory” T cell (or “TCM”) as used herein refers to a CTL that expresses CD62L on the surface thereof (i.e., CD62L+CD8+ cells).
[0043]“Effector memory” T cell (or “TEM”) as used herein refers to a CTL that does not express CD62L on the surface thereof (i.e., CD62L−CD8+ cells).
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cytotoxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com