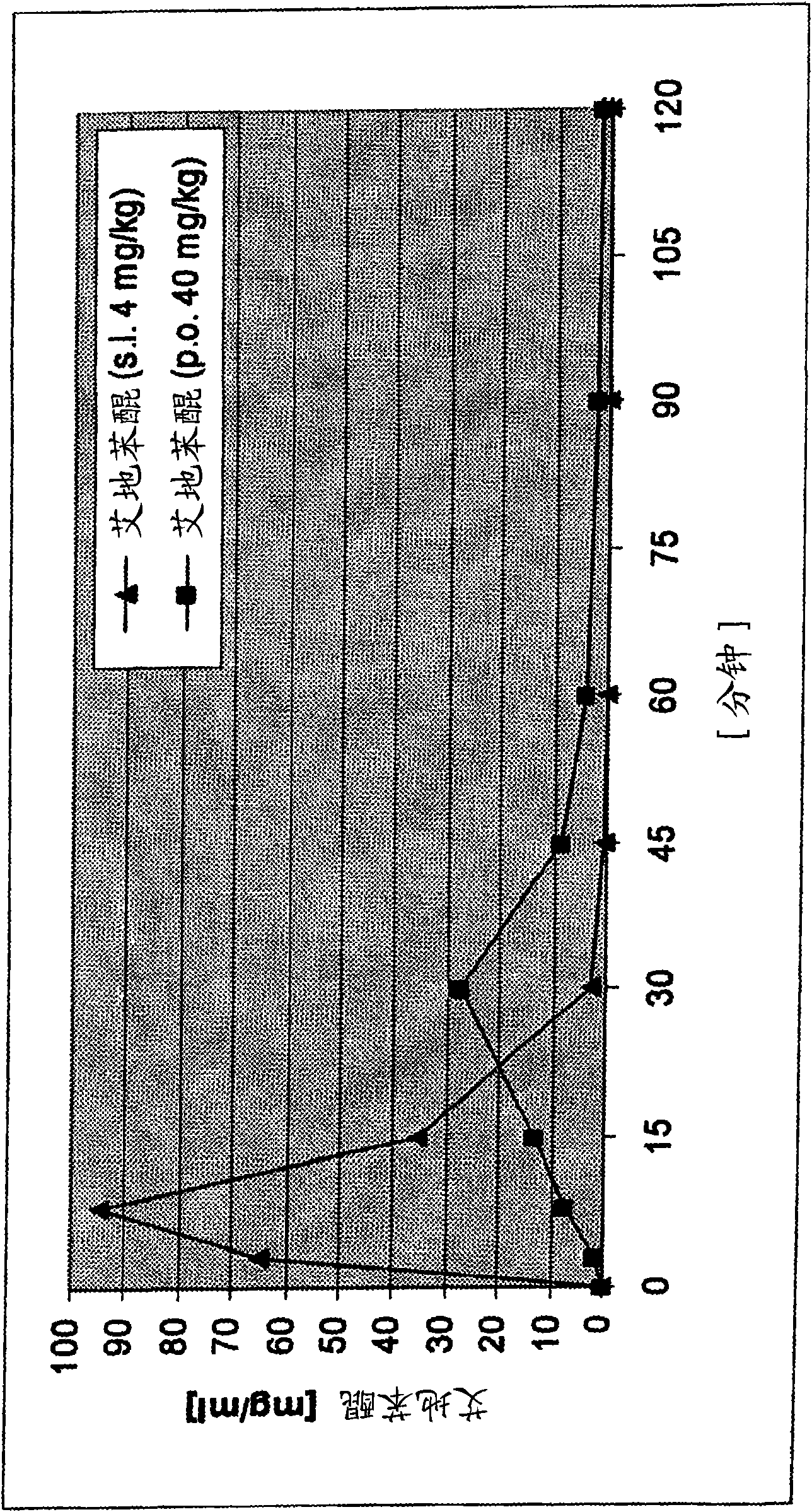
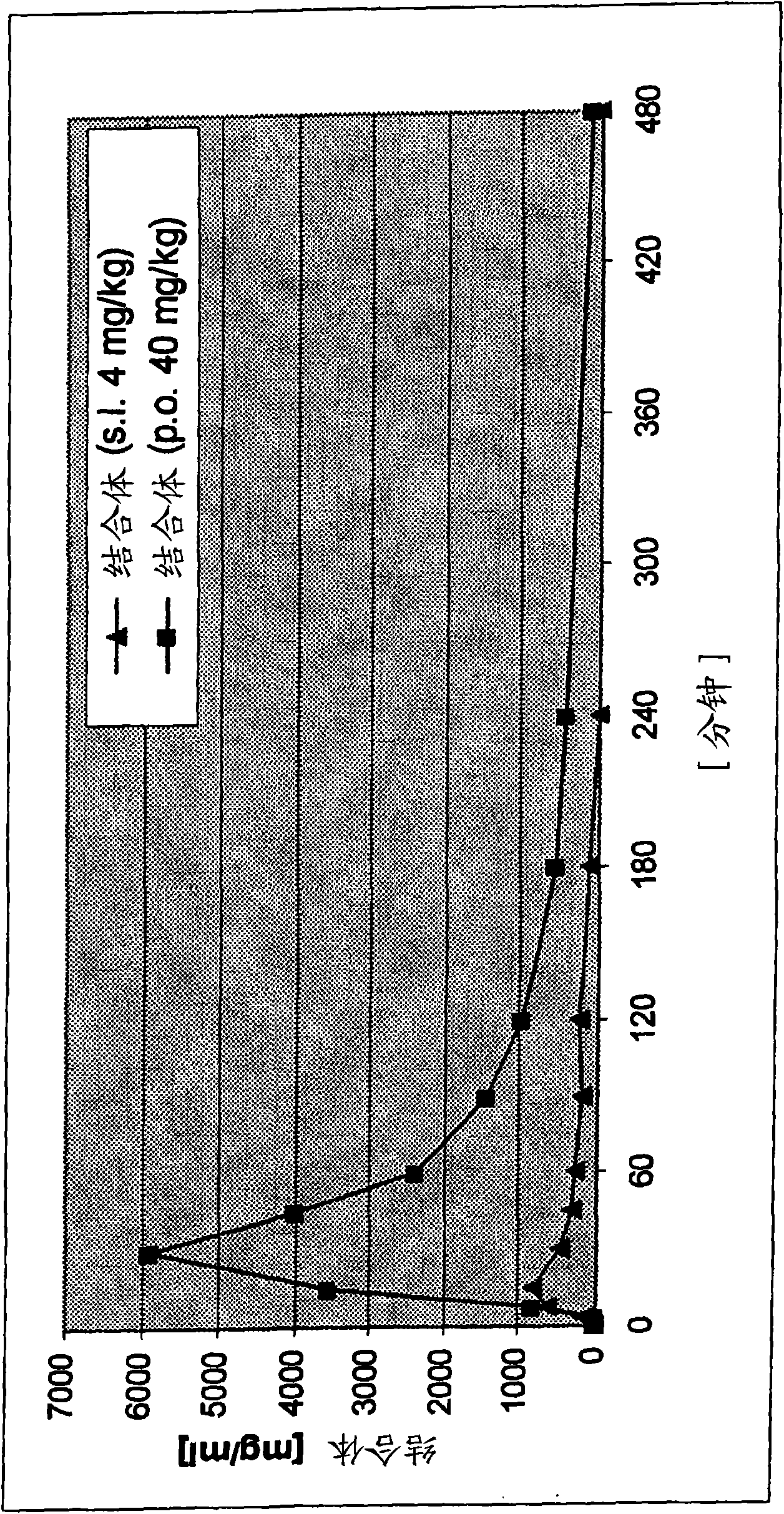
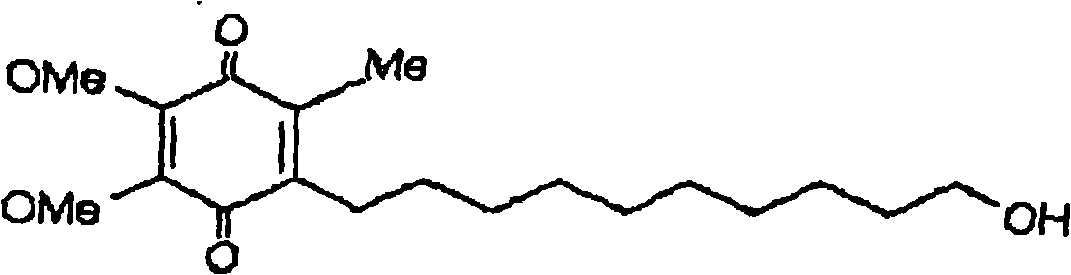
Transmucosal administration of 2,3-dimethoxy-5-methyl-6-(10- hydroxydecy l)-1,4-benzoquinone
A technique for mucosal administration of idebenone, applied in the field of mucosal administration of 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] microemulsion
[0035] Table 1
[0036] Element
[0037] Preparation: Preheat TPGS (tocopherol-polyethylene glycol-400-succinate) to about 60°C. Miglyol 812N and idebenone were mixed and sonicated for approximately 10 minutes until the material was completely dissolved (clear orange solution). Molten TPGS was added to the Miglyol / idebenone solution and stirred. The mixture is diluted and homogenized until a homogeneous emulsion is obtained.
Embodiment 2
[0039] sublingual lozenge
[0040] Table 2
[0041] Element
[0042] Preparation: Idebenone, lactose monohydrate, flavor and aspartame were mixed in a high shear mixer until a homogeneous mixture was obtained. Povidone was dissolved in water (approximately 8-10% solution) and added to the dry mix and granulated. The wet granulation is dried in a fluid bed drier, sieved and added to a premix of microcrystalline cellulose, magnesium stearate and talc. The final blend is compressed into tablets.
Embodiment 3
[0044] spray preparation
[0045] table 3
[0046] Element
[0047] Preparation: 10 g of idebenone was dissolved in 500 ml of ethanol (96%) and mixed with 400 ml of pure water. Methylparaben, propylparaben and aspartame were dissolved in the mixture. Adjust the final volume to 1000ml with purified water. The solution is filtered and filled into a suitable spray device.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com