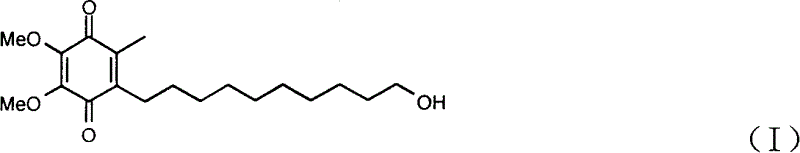
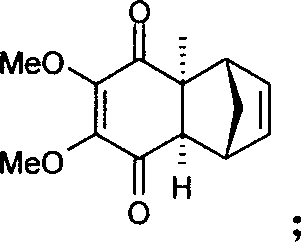
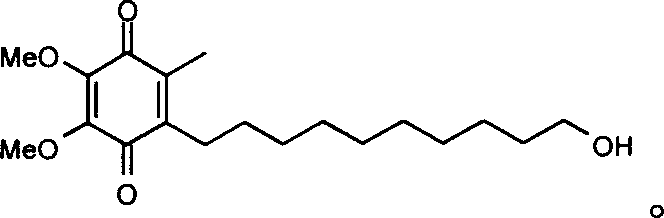
Method for synthesizing Idebenone
A technology of idebenone and its synthesis method, which is applied in the field of synthesis of central nervous system drug compounds, can solve the problems of high oxidant cost, low yield, unsatisfactory industrialization effect, etc., and achieve the effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of [2-(10-bromo-decyloxy)-tetrahydrofuran] (c)
[0022] Add 23.7g (0.1mol) 10-bromodecan-1-alcohol and 200ml methylene chloride in a 500ml three-necked flask, add 10.5g (0.15mol) 2,3-dihydrofuran and a catalytic amount of p-toluenesulfonic acid under ice water cooling , naturally rose to room temperature and stirred for 18 hours. After the reaction was finished, the crude product of (c) was obtained through aftertreatment, and 28.5 g of (c) with a purity greater than 99% was obtained by column chromatography. (The eluent is petroleum ether: ether = 10:1)
[0023] [4,5-Dimethoxy-7-(10'oxo-R group)decyl-2-methyltricyclo[6.2.1.0 2.7 ] undeca-4,9-diene-3,6-dione] (d) preparation
[0024] Under the protection of argon, the compound, 5-dimethoxy-2-methyltricyclo [6.2.1.0 2.7 ] Undecyl-4,9-diene-3,6-dione (b) 15g (0.06mol) and 300ml of anhydrous ether, cooled, and 7.5g (0.066mol) of potassium tert-butoxide was added at -20°C, Raise to 0°C and stir for 30 minute...
Embodiment 2
[0030] Preparation of [2-(10-bromo-decyloxy)-tetrahydropyran] (c)
[0031] Add 23.7g (0.1mol) 10-bromodecan-1-alcohol and 200ml ether in the 500ml there-necked flask, add 16.8g (0.2mol) 2,3-dihydropyran and the p-toluenesulfonic acid of catalytic amount under ice water cooling, Naturally raised to room temperature and stirred for 18 hours, after the reaction was completed, the crude product of (c) was obtained after post-treatment, and 28.5 g of (c) with a purity greater than 99% was obtained by column chromatography. (The eluent is petroleum ether: ether = 10:1)
[0032] [4,5-dimethoxy-7-(10'pyranyloxy)decyl-2-methyltricyclo[6.2.1.0 2.7 ] undeca-4,9-diene-3,6-dione] (d) preparation
[0033] Under argon protection, add 15g (0.06mol) and 300ml of anhydrous ether to a 500ml three-necked flask, cool, add 12.5g (0.108mol) of potassium tert-butoxide at -20°C, raise the temperature to 0°C and stir for 30 minutes, then cool down to -20°C, add dropwise the diethyl ether dissolved i...
Embodiment 3
[0039] Other steps are identical with embodiment 1, just in [4,5-dimethoxy-7-(10' hydroxyl) decyl-2-methyltricyclo[6.2.1.0 2.7 ] Undecane-4,9-diene-3,6-dione] The hydrochloric acid in the preparation step of (e) was replaced by tetrabutylammonium fluoride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com