Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1768results about How to "Prevent crystallization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
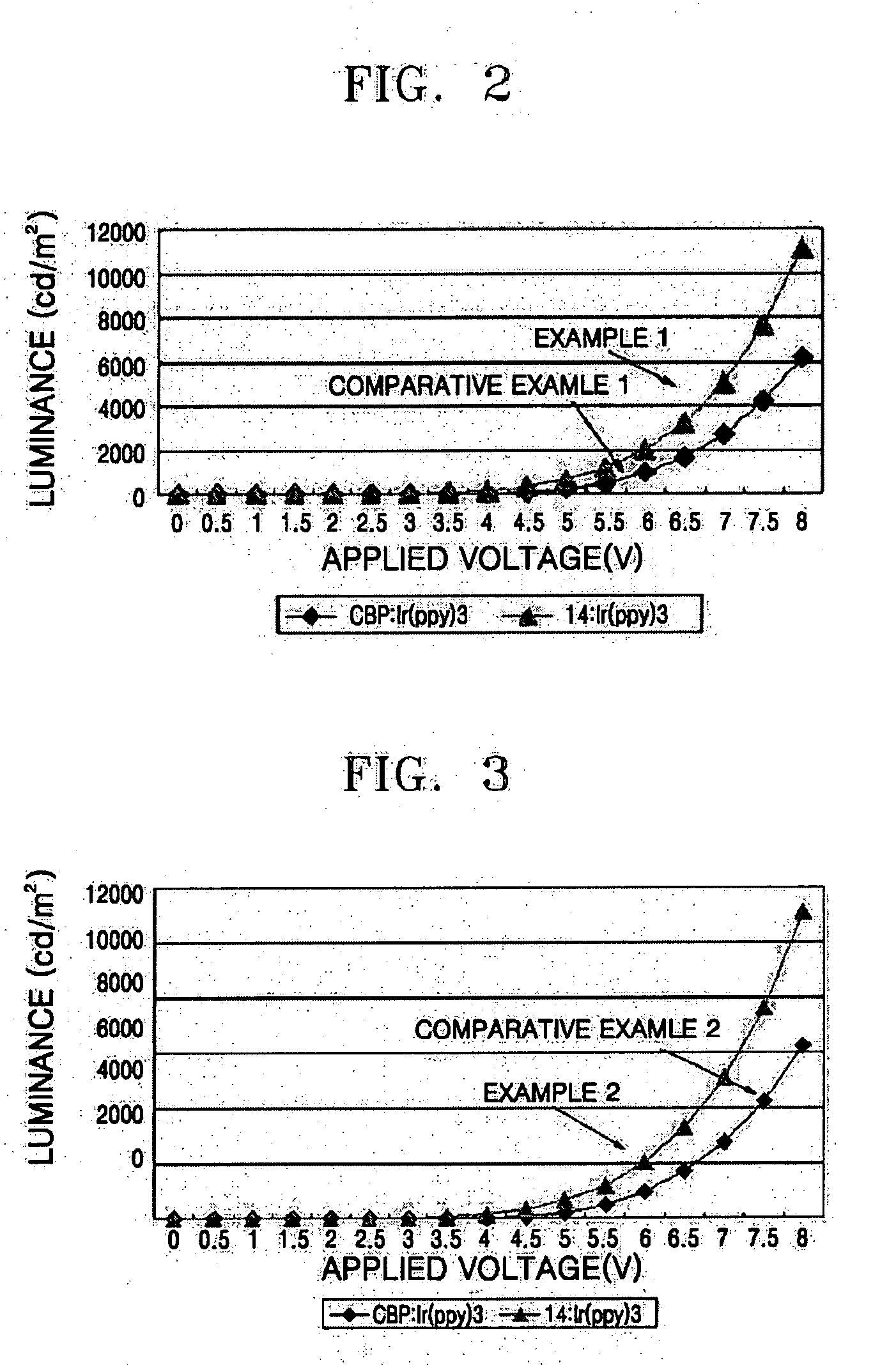
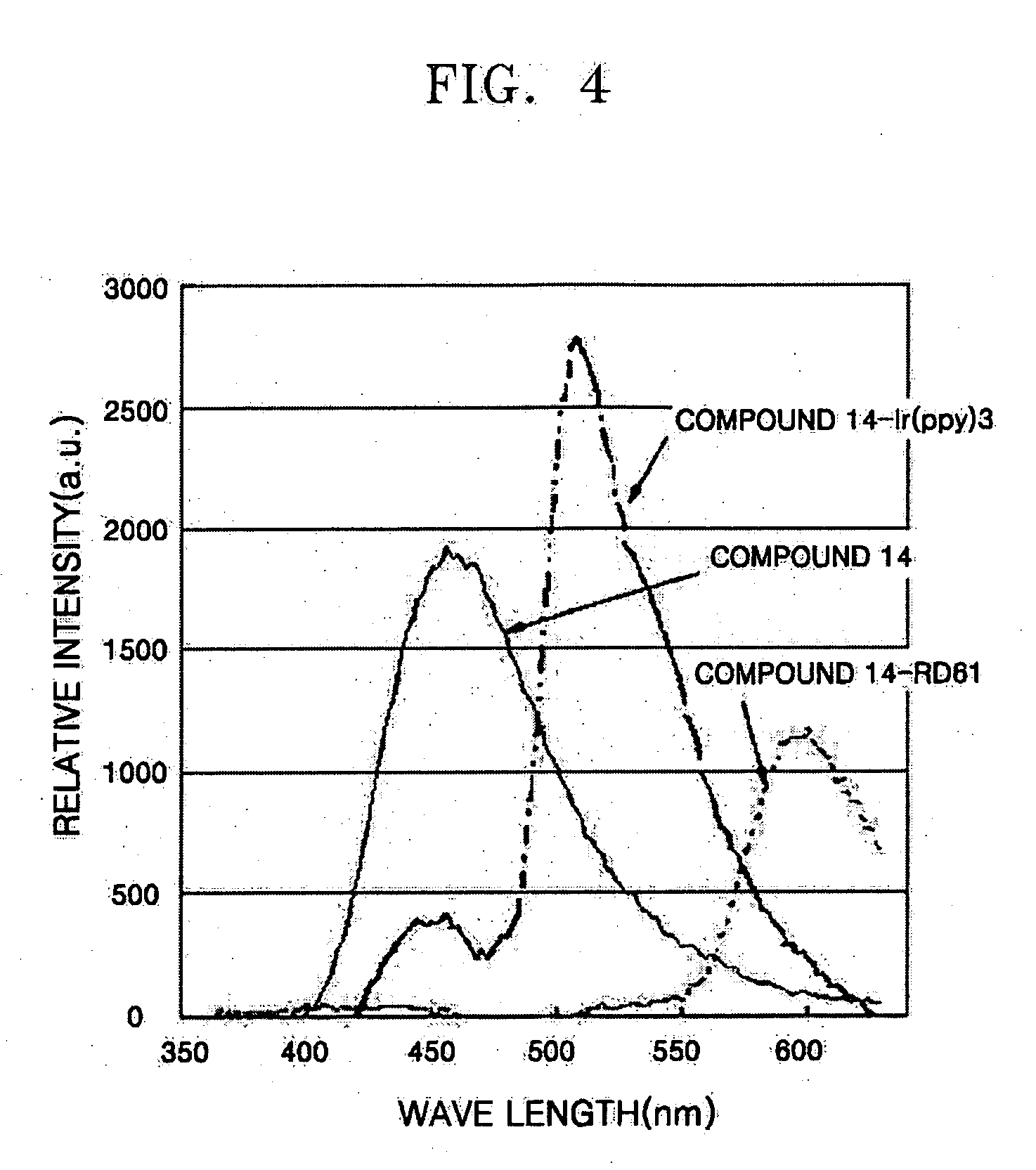
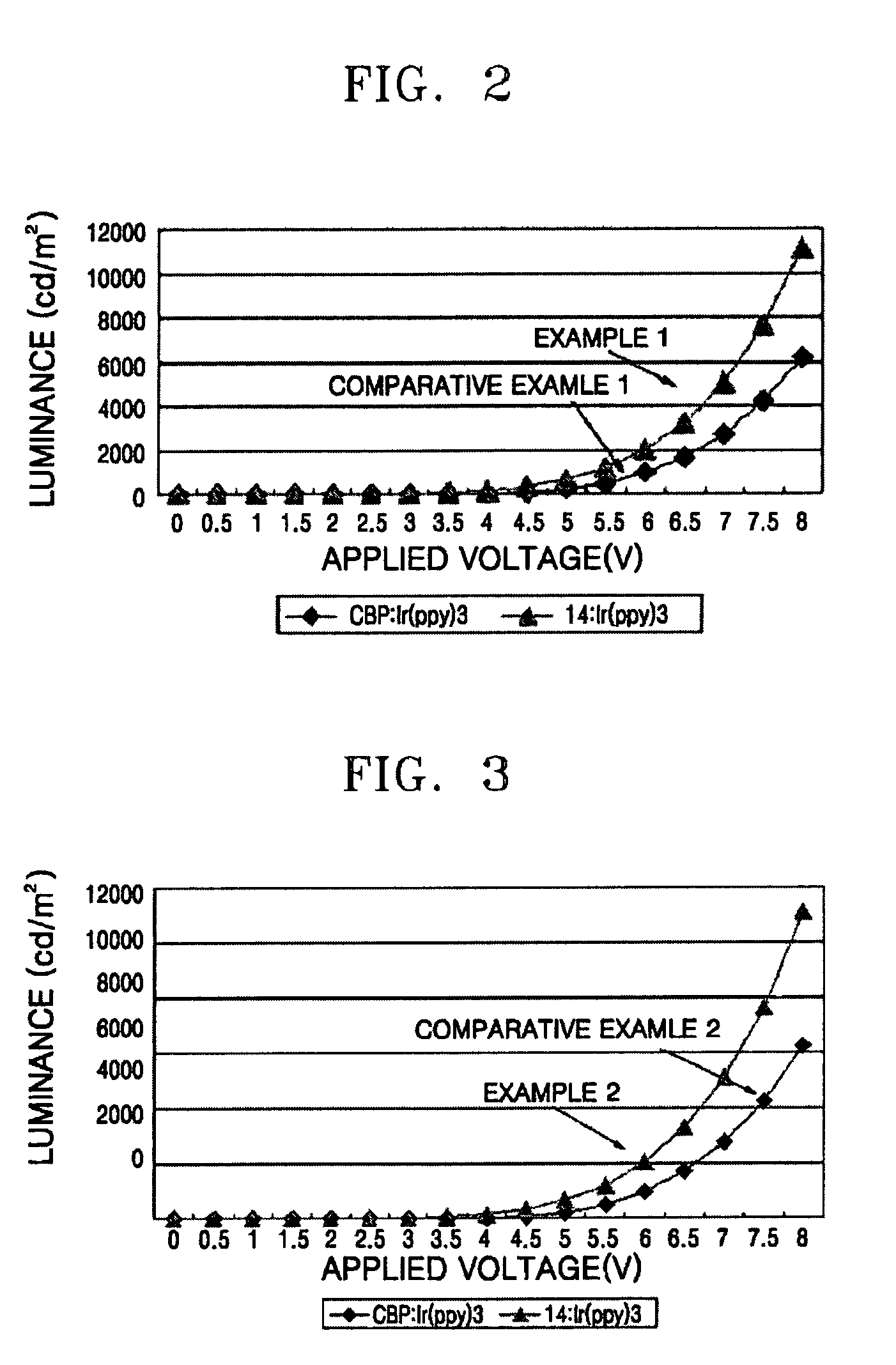
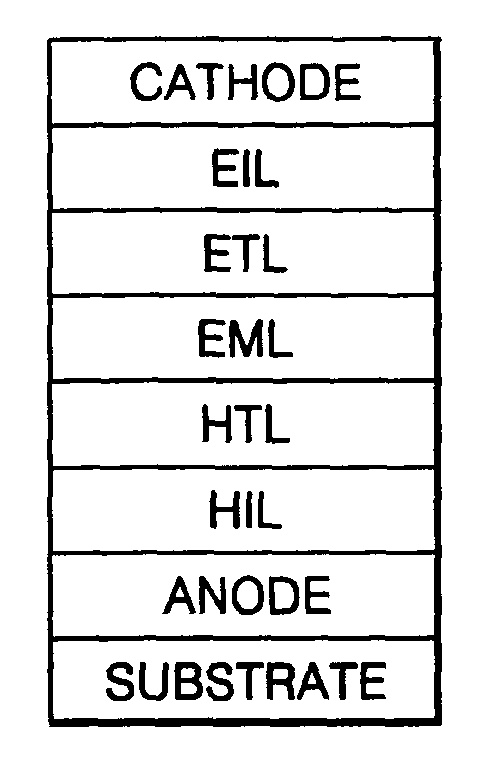
Phenylcarbazole-based compound and organic electroluminescent device employing the same
ActiveUS20080107919A1High glass transition temperatureElectric stabilityOrganic chemistryDischarge tube luminescnet screensCarbazoleLow voltage
A phenylcarbazole-based compound is represented by Formula 1, and has superior electric properties and charge transport abilities, and thus is useful as a hole injection material, a hole transport material, and / or an emitting material which is suitable for fluorescent and phosphorescent devices of all colors, including red, green, blue, and white colors. The phenylcarbazole-based compound is synthesized by reacting carbazole with diamine. The organic electroluminescent device manufactured using the phenylcarbazole-based compound has high efficiency, low voltage, high luminance, and a long lifespan.
Owner:SAMSUNG DISPLAY CO LTD
Fluorene-based compound and organic electroluminescent display device using the same
ActiveUS20050221124A1High charge transport performanceHigh glass transition temperatureDischarge tube luminescnet screensElectroluminescent light sourcesOrganic electroluminescenceFluorescence
The present invention relates to an organic electroluminescent (OEL) compound that comprises at least one fluorene derivative and at least one carbazole derivative. The compound has good electrical properties, light emitting properties and charge transport ability, and thus is suitable as a host material suitable for fluorescent and phosphorescent dopants of all colors including red, green, blue, white, etc., and as a charge transport material. An OEL display device that uses an organic layer that includes the OEL compound has a high efficiency, a low voltage, a high luminance, and a long lifespan because it has superior current density.
Owner:SAMSUNG DISPLAY CO LTD
Precursor compositions for the deposition of electrically conductive features
InactiveUS6951666B2Reduce settlementGood dispersionRadiation applicationsConductive materialElectrically conductiveCopper metal
A precursor composition for the deposition and formation of an electrical feature such as a conductive feature. The precursor composition advantageously has a viscosity of at least about 1000 centipoise and can be deposited by screen printing. The precursor composition also has a low conversion temperature, enabling the deposition and conversion to an electrical feature on low temperature substrates. A particularly preferred precursor composition includes silver and / or copper metal for the formation of highly conductive features.
Owner:CABOT CORP
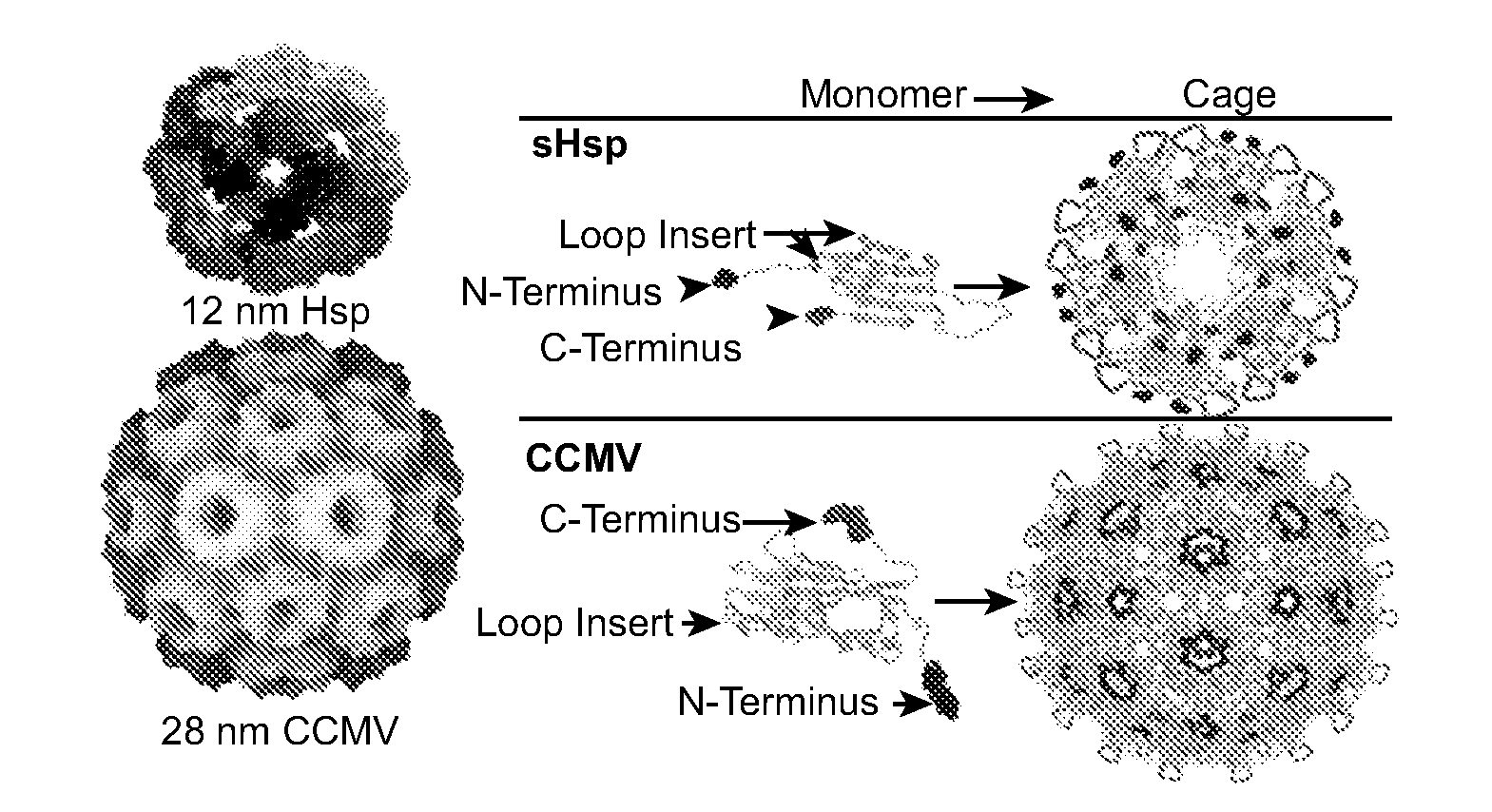
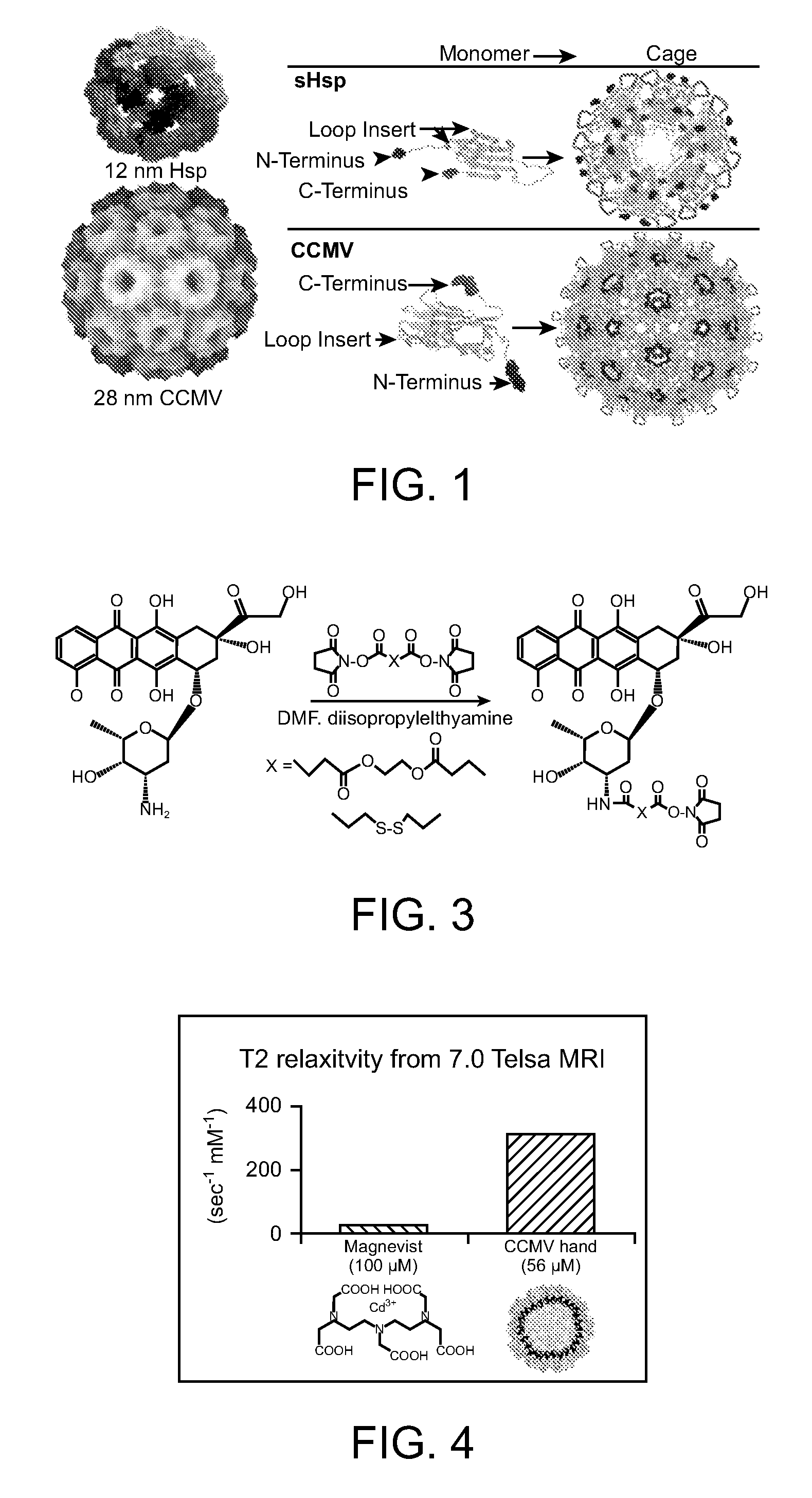
Novel nanoparticles and use thereof
InactiveUS20070258889A1Increase the number ofFacilitate aggregation and crystallizationPowder deliveryLuminescence/biological staining preparationImaging agentProtein cage
The present invention is directed to novel compositions and methods utilizing delivery agents or nanoparticles that include protein cages with modified and / or unmodified subunits and various agents, such as therapeutic and / or imaging agents located on the interior and / or exterior surface of the protein cages.
Owner:MONTANA STATE UNIVERSITY
Porous drug matrices and methods of manufacture thereof
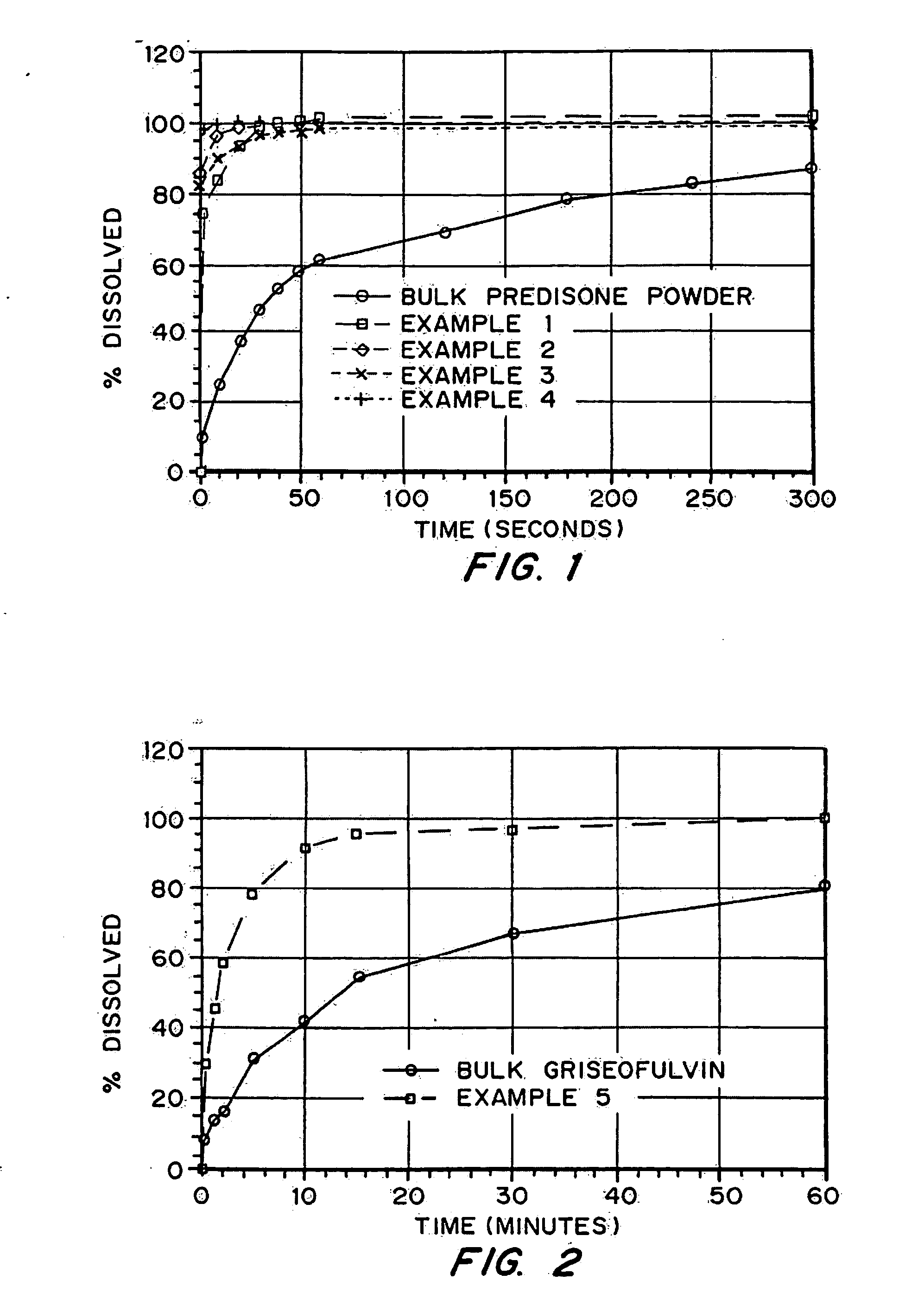
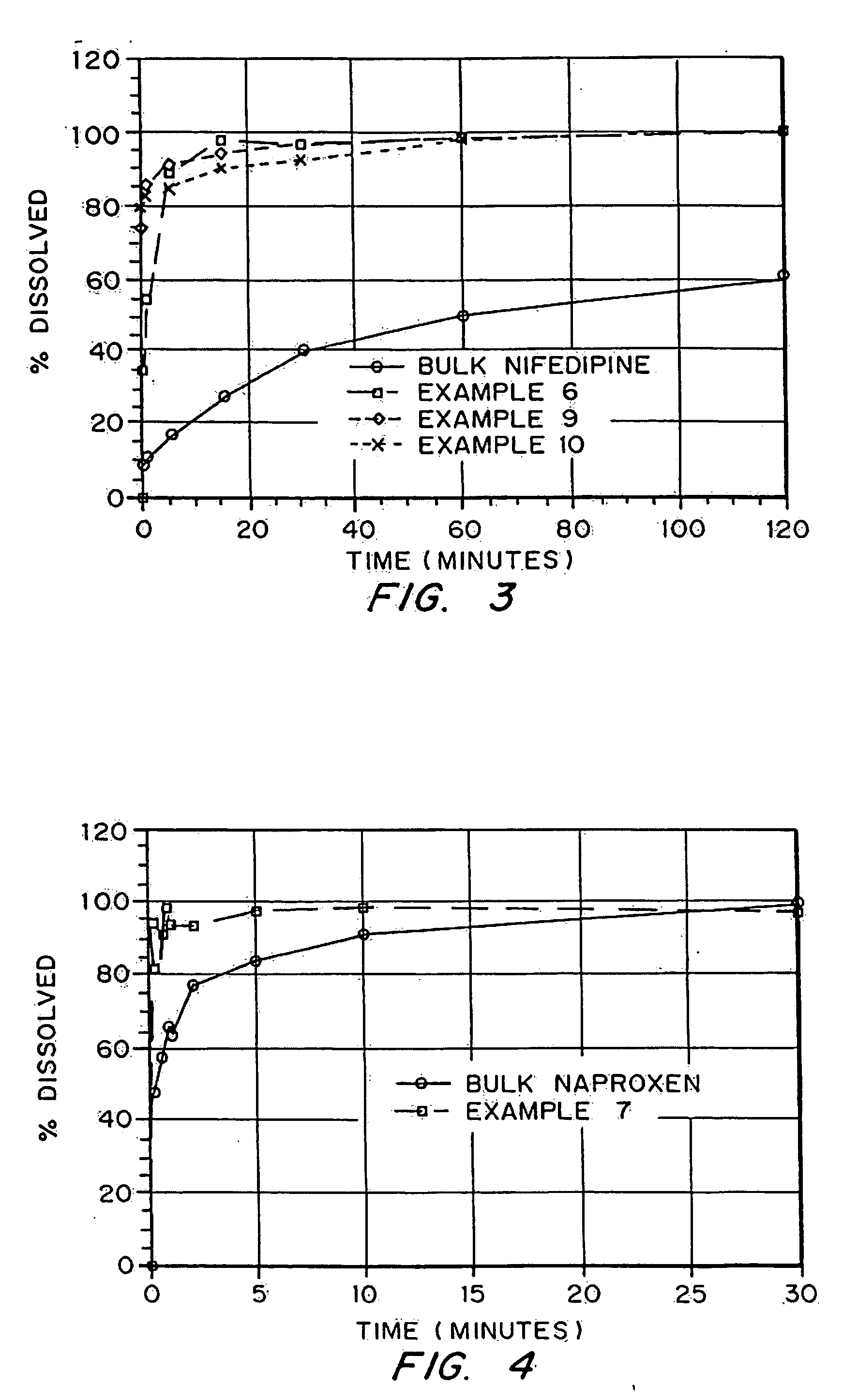
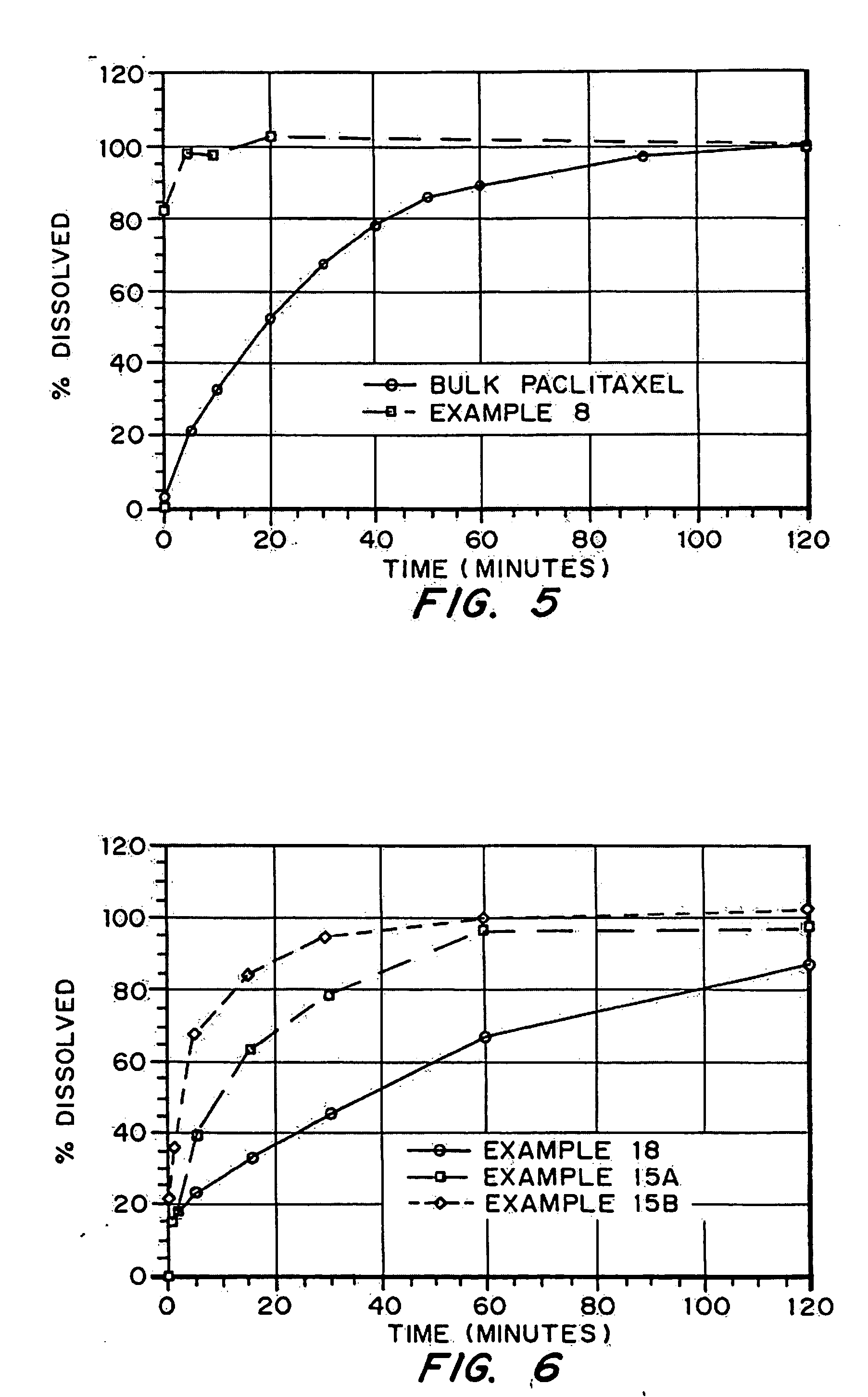
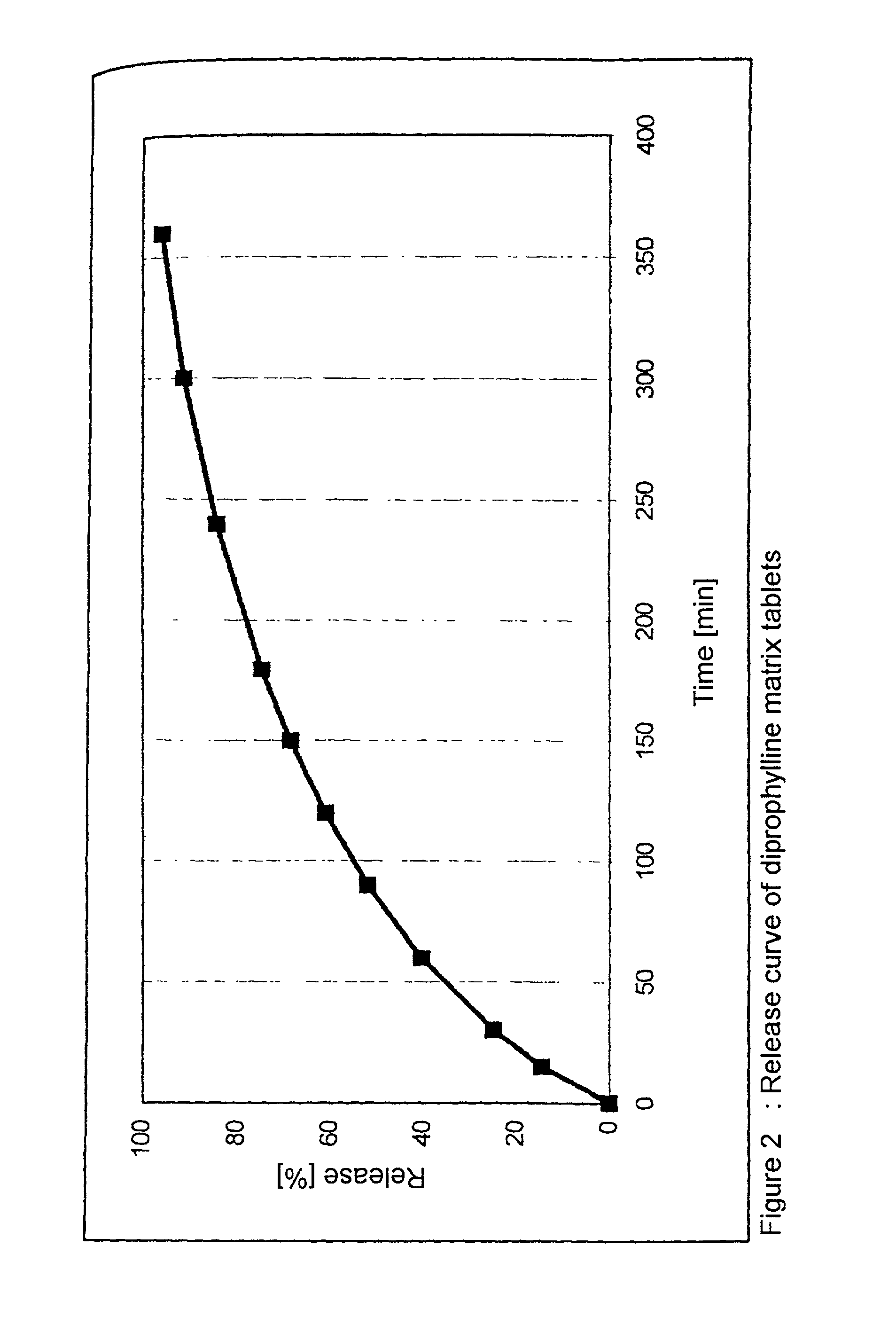
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
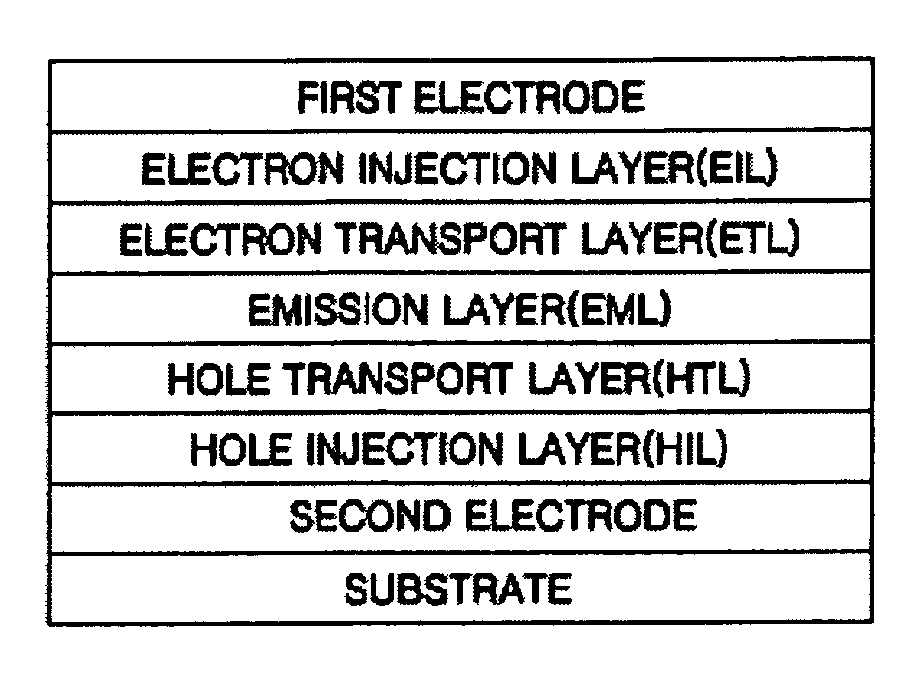
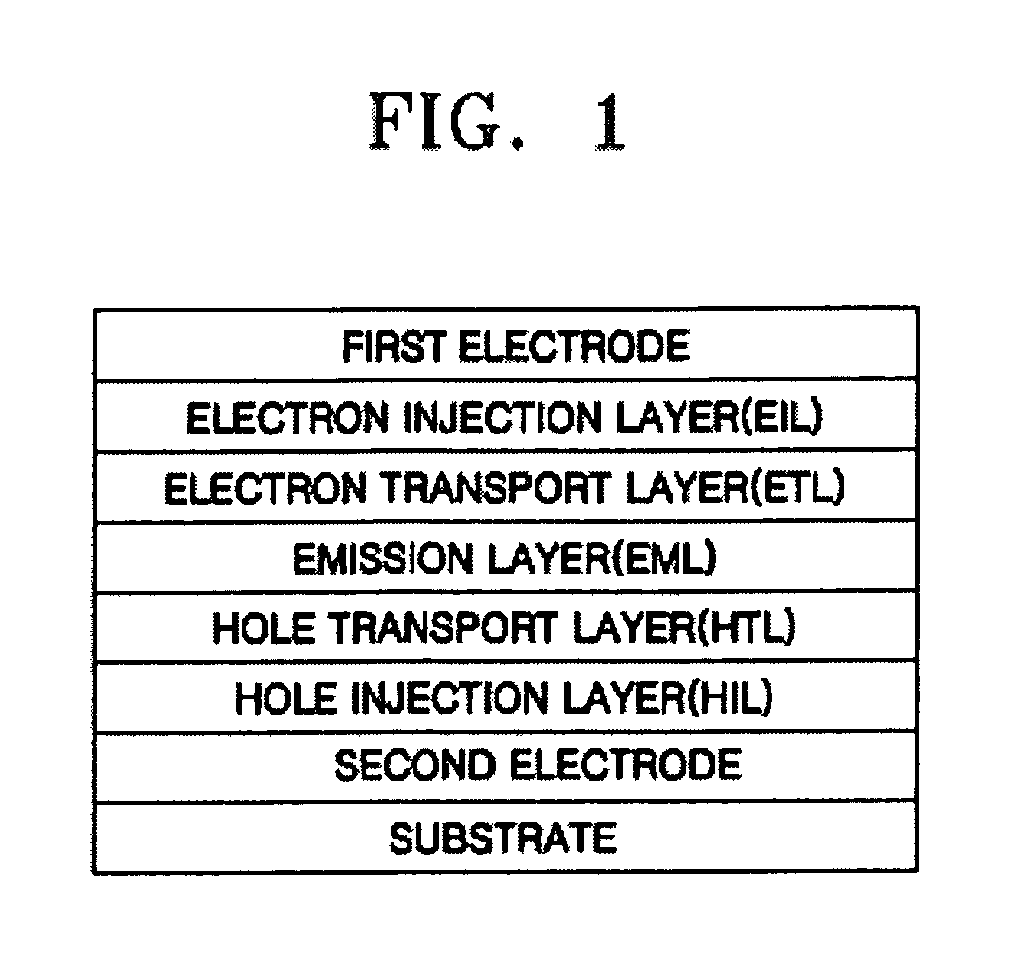
Light-Emitting Device And Method For Manufacturing The Same
ActiveUS20070200125A1Inhibit currentAvoid it happening againElectroluminescent light sourcesSolid-state devicesCompound (substance)Engineering
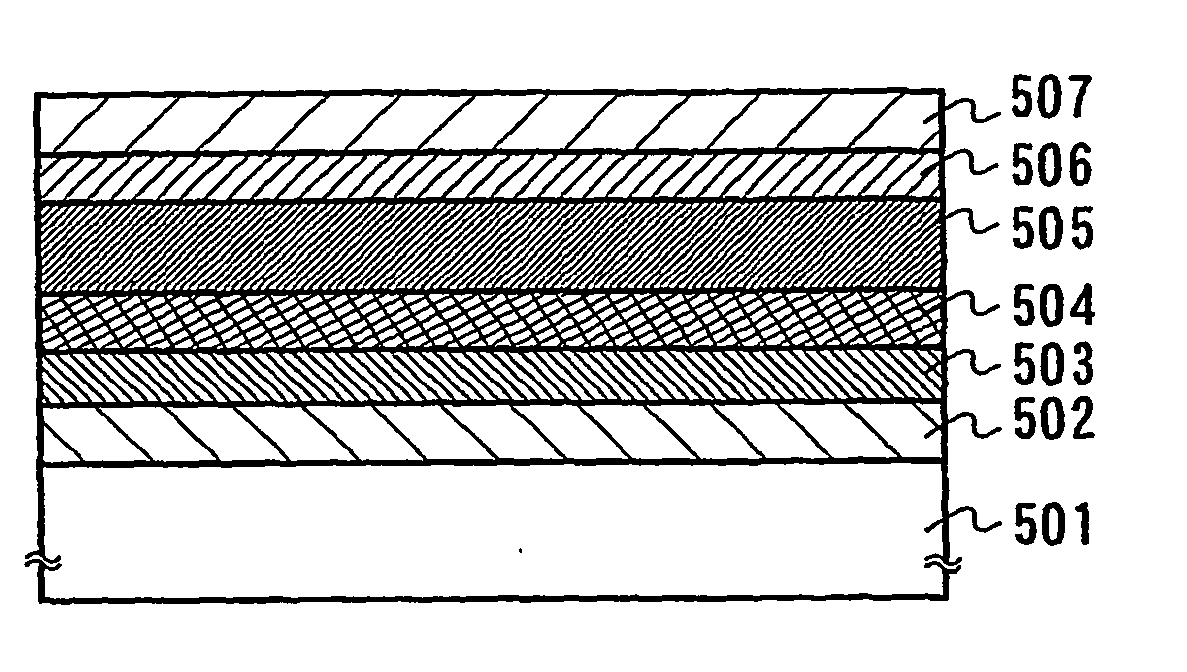
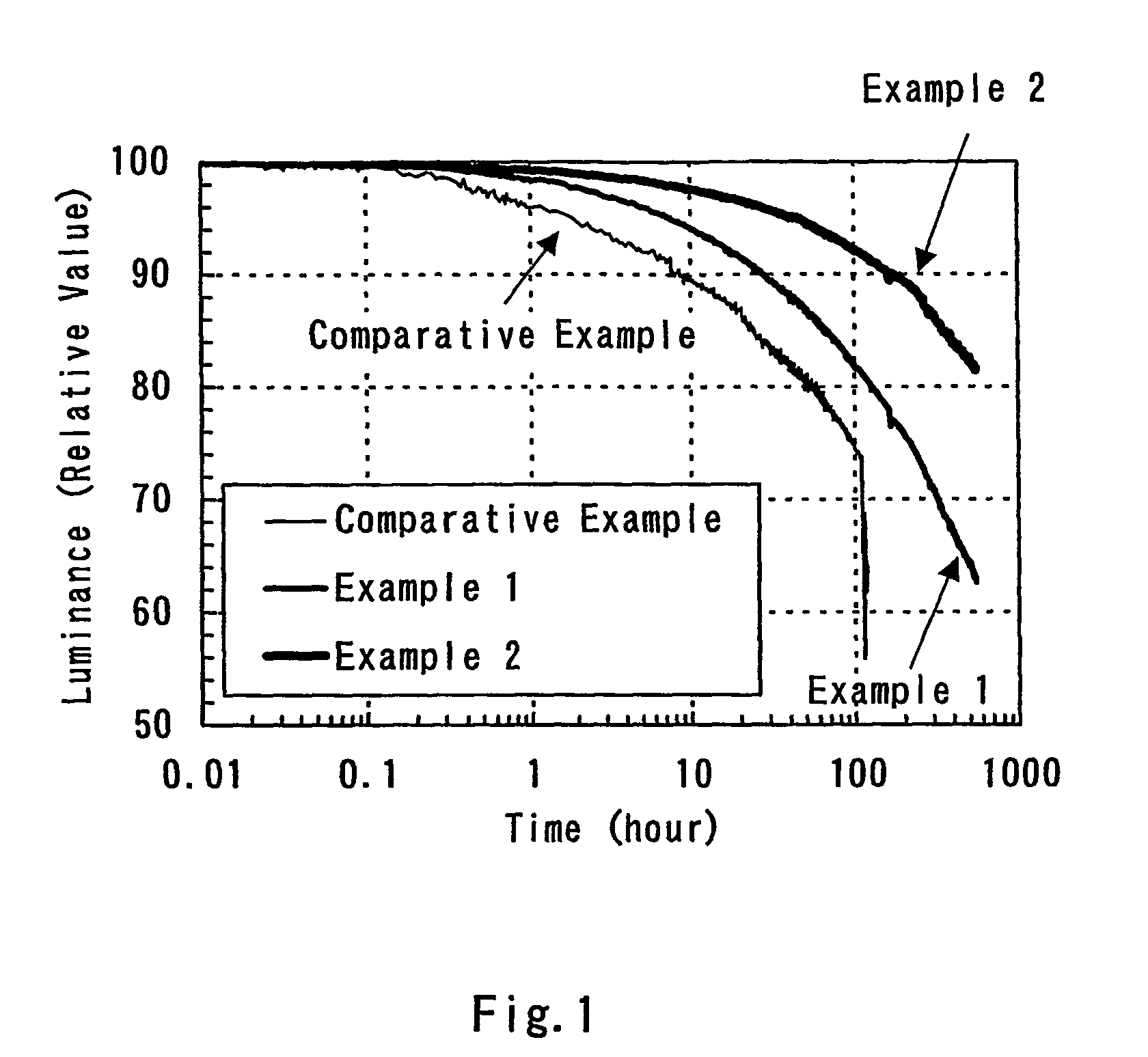
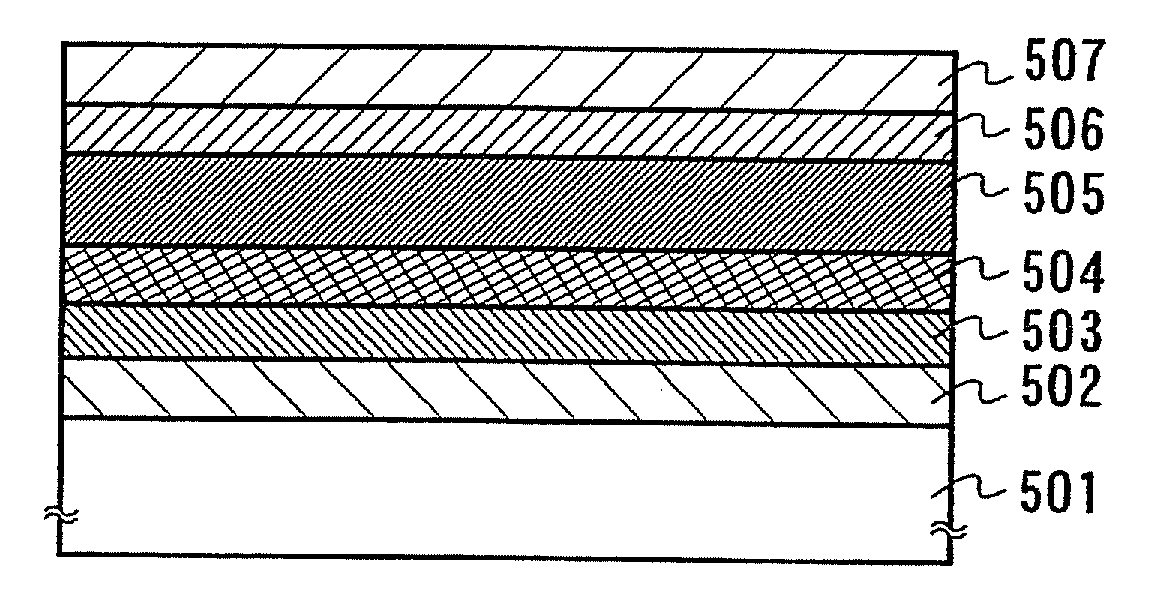
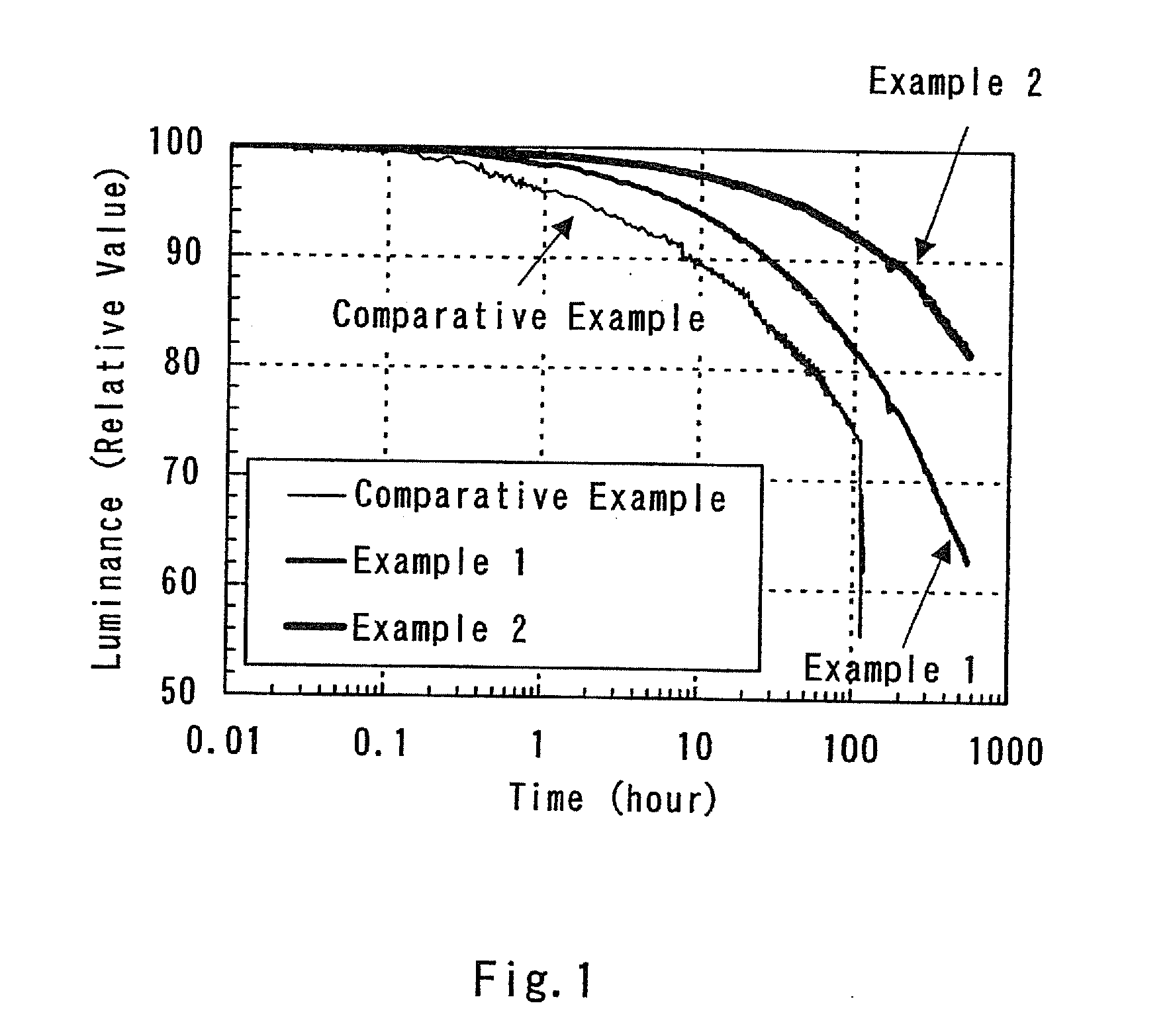
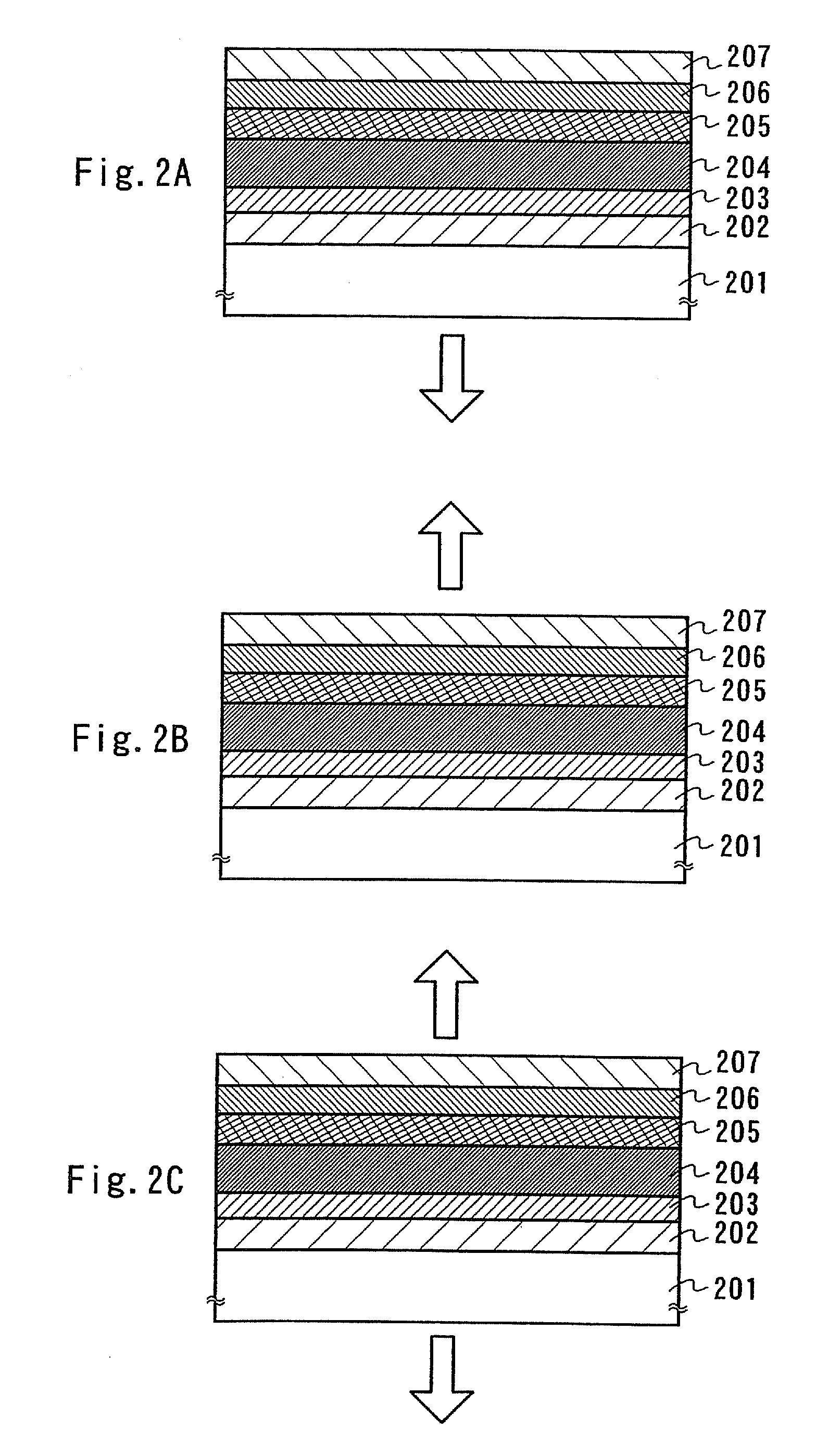
A light-emitting element is disclosed that can drive at a low driving voltage and that has a longer lifetime than the conventional light-emitting element, and a method is disclosed for manufacturing the light-emitting element. The disclosed light-emitting element includes a plurality of layers between a pair of electrodes; and at least one layer among the plurality of layers contains one compound selected from the group consisting of oxide semiconductor and a metal oxide, and a compound having high hole transportation properties. Such the light-emitting element can suppress the crystallization of a layer containing one compound selected from the group consisting of oxide semiconductor and a metal oxide, and a compound having high hole transportation properties. As a result, a lifetime of the light-emitting element can be extended.
Owner:SEMICON ENERGY LAB CO LTD
Method for the fabrication of conductive electronic features
InactiveUS20100112195A1Reduce settlementGood dispersionPretreated surfacesSemiconductor/solid-state device manufacturingElectronMaterials science
Owner:CABOT CORP
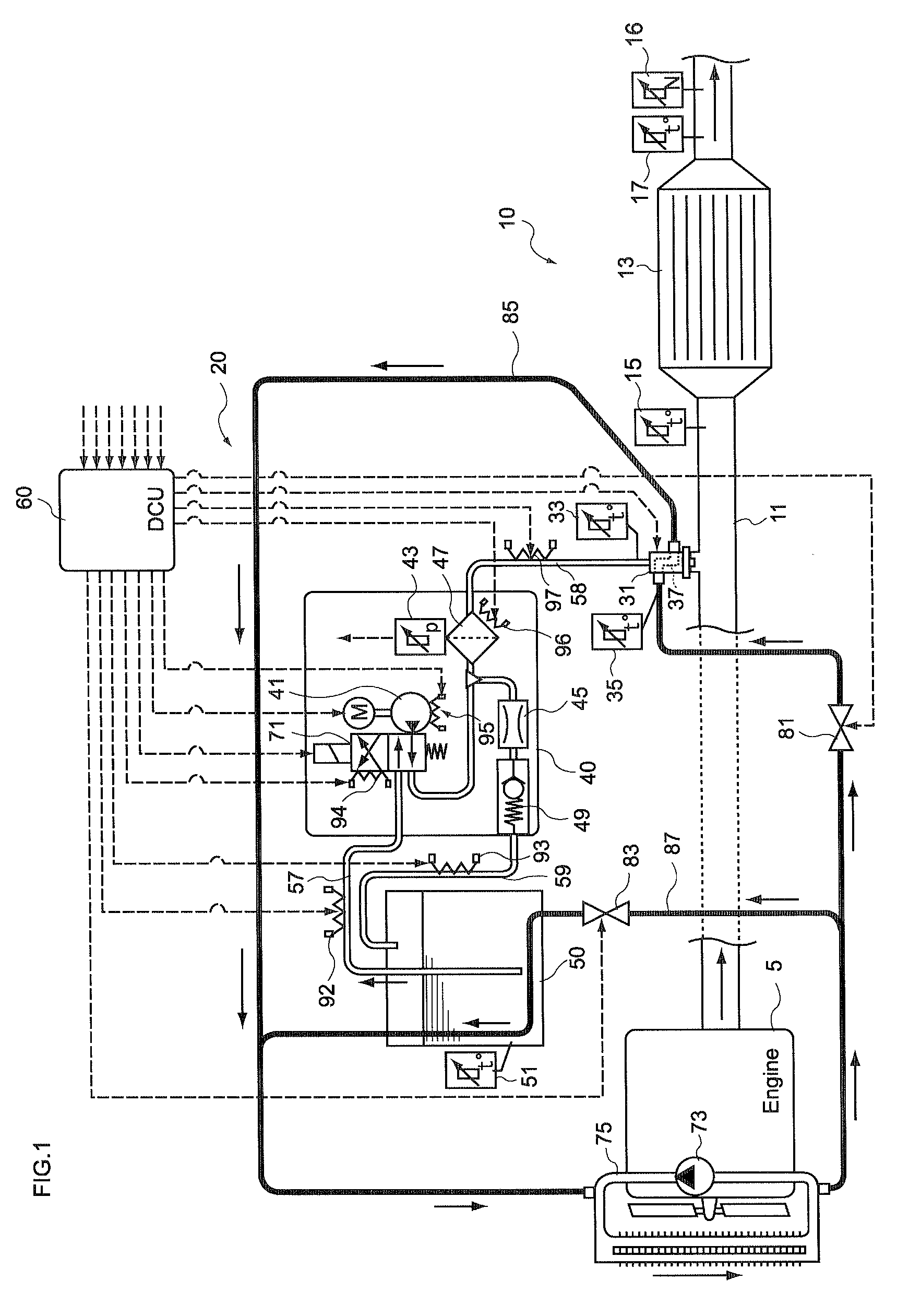
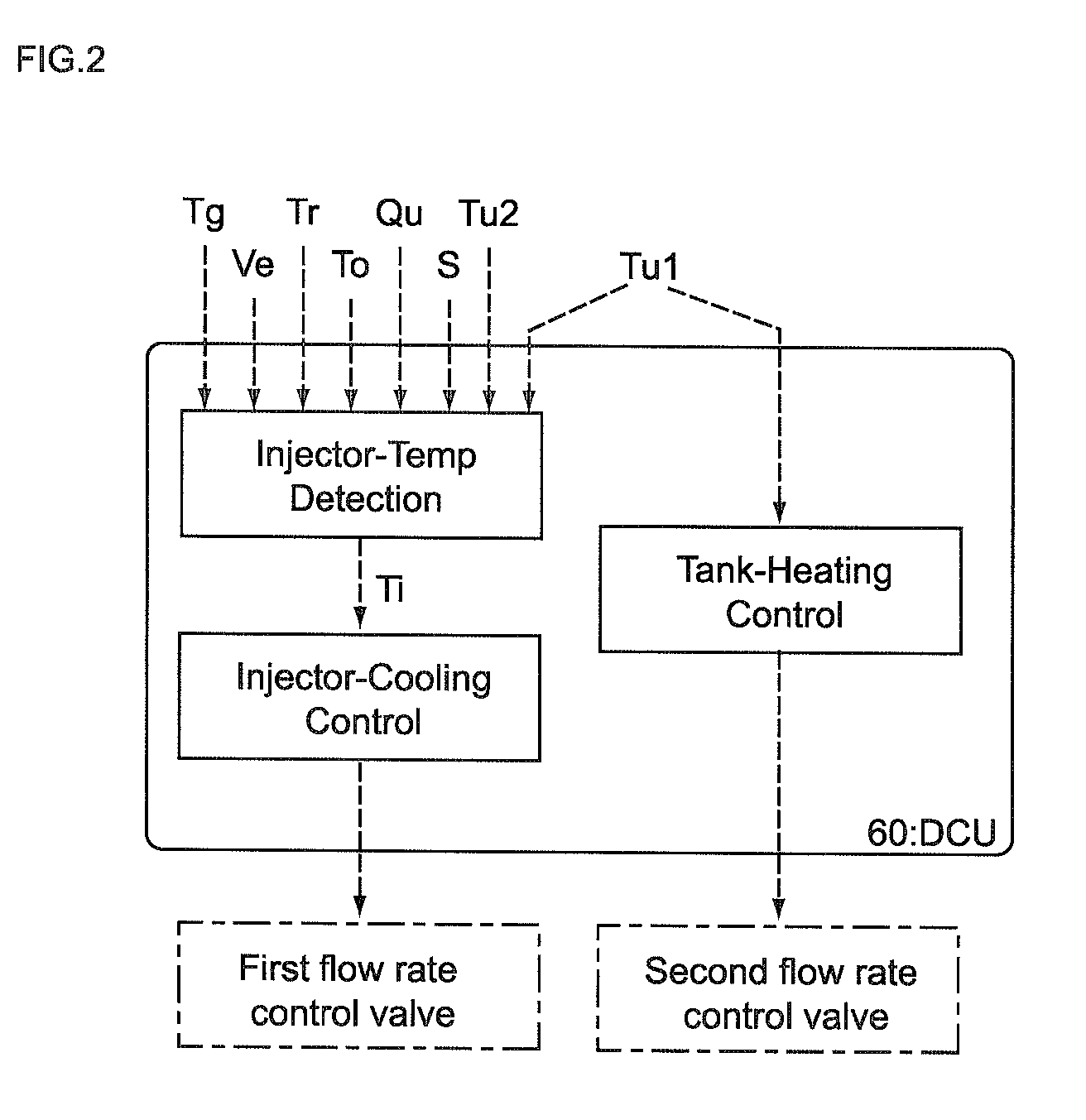
Control unit and control method for reductant supply device
InactiveUS20100242439A1Prevent overcoolingImprove cooling effectLiquid coolingInternal combustion piston enginesEngineeringWater circulation
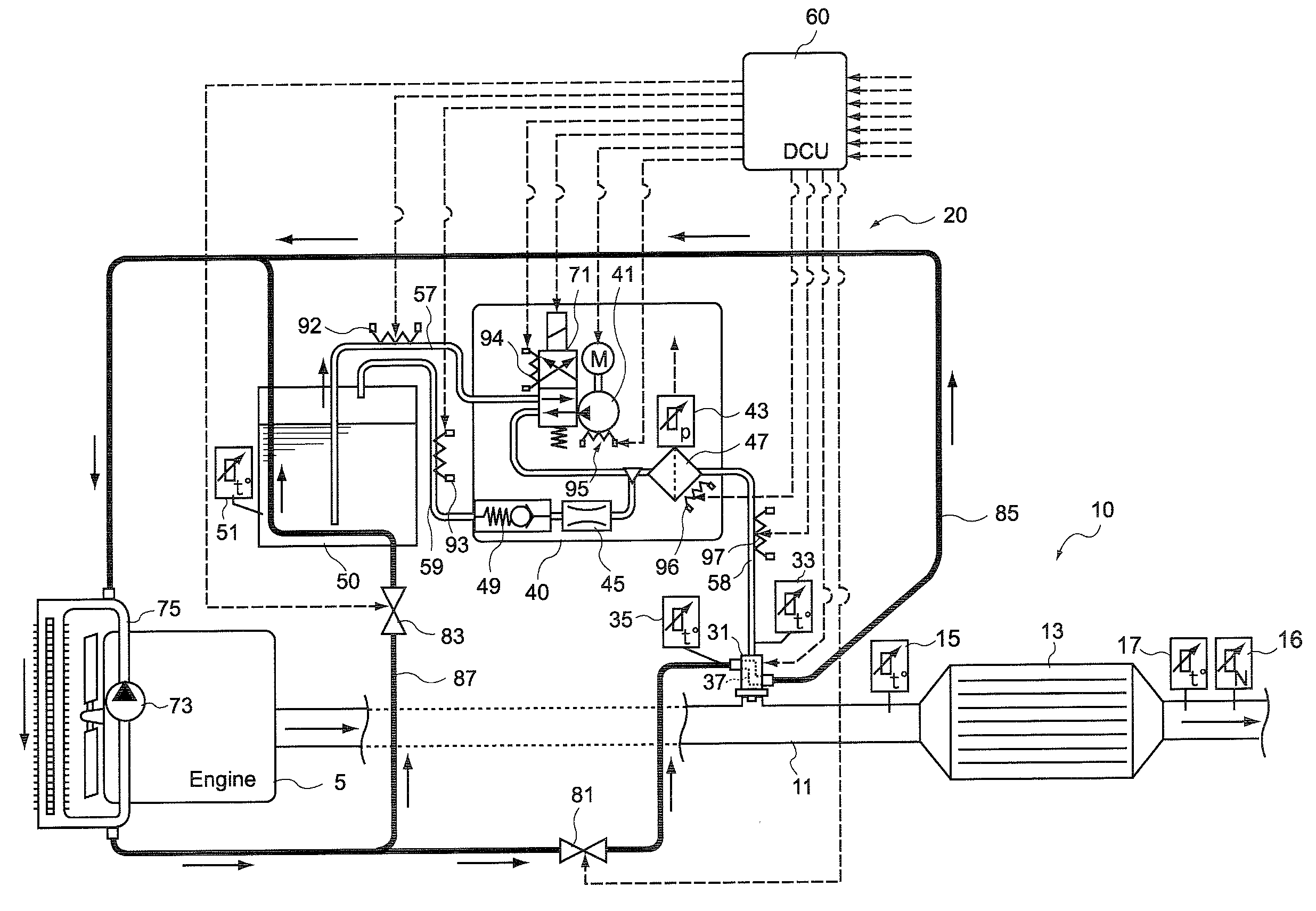
There are provided a reductant supply device and a control method for the reductant supply device, which can prevent heat damage of a reductant injection valve, and also prevent crystallization of urea solution due to excessive cooling of the solution reductant.The reductant supply device which is used in an exhaust gas purification device that injects and supplies, as a reductant, a urea solution to an exhaust gas upstream side of a reduction catalyst disposed in an exhaust gas passage of an internal combustion engine, and that reduces and purifies nitrogen oxides contained in exhaust gas using the reduction catalyst, the reductant supply device having a reductant injection valve that is fixed to an exhaust pipe on the exhaust gas upstream side of the reduction catalyst, includes: a cooling water circulation passage that circulates at least part of cooling water of the internal combustion engine to cool the reductant injection valve; flow rate control means for adjusting a flow rate of cooling water flowing through the cooling water circulation passage; temperature detection means for detecting a temperature of the reductant injection valve; and control means for controlling the flow rate control means based on the temperature of the reductant injection valve.
Owner:BOSCH CORP
Light-emitting device and method for manufacturing the same
ActiveUS7732808B2Improve hole transport abilityExtended service lifeElectroluminescent light sourcesSolid-state devicesEngineeringLight emitting device
A light-emitting element is disclosed that can drive at a low driving voltage and that has a longer lifetime than the conventional light-emitting element, and a method is disclosed for manufacturing the light-emitting element. The disclosed light-emitting element includes a plurality of layers between a pair of electrodes; and at least one layer among the plurality of layers contains one compound selected from the group consisting of oxide semiconductor and a metal oxide, and a compound having high hole transportation properties. Such the light-emitting element can suppress the crystallization of a layer containing one compound selected from the group consisting of oxide semiconductor and a metal oxide, and a compound having high hole transportation properties. As a result, a lifetime of the light-emitting element can be extended.
Owner:SEMICON ENERGY LAB CO LTD
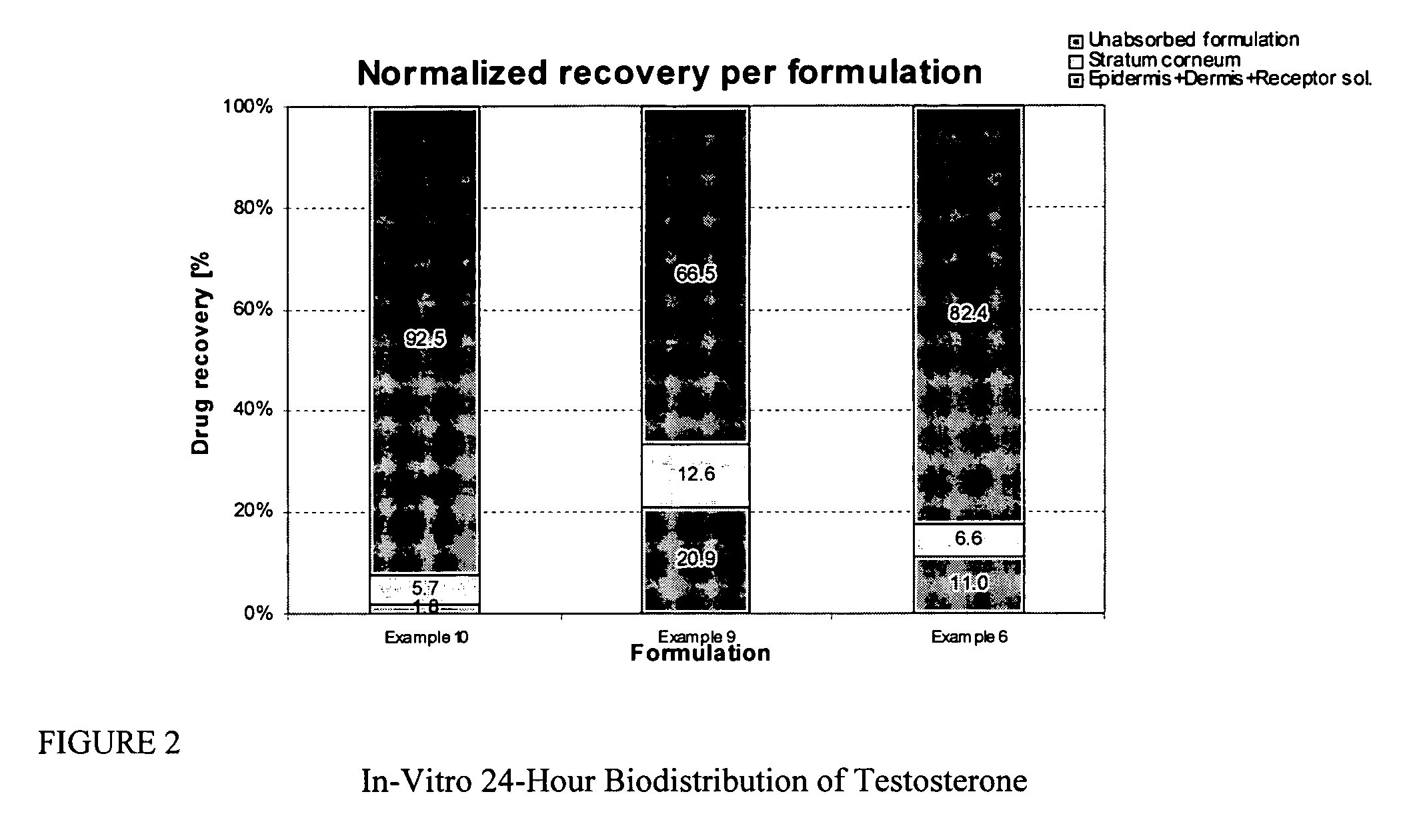
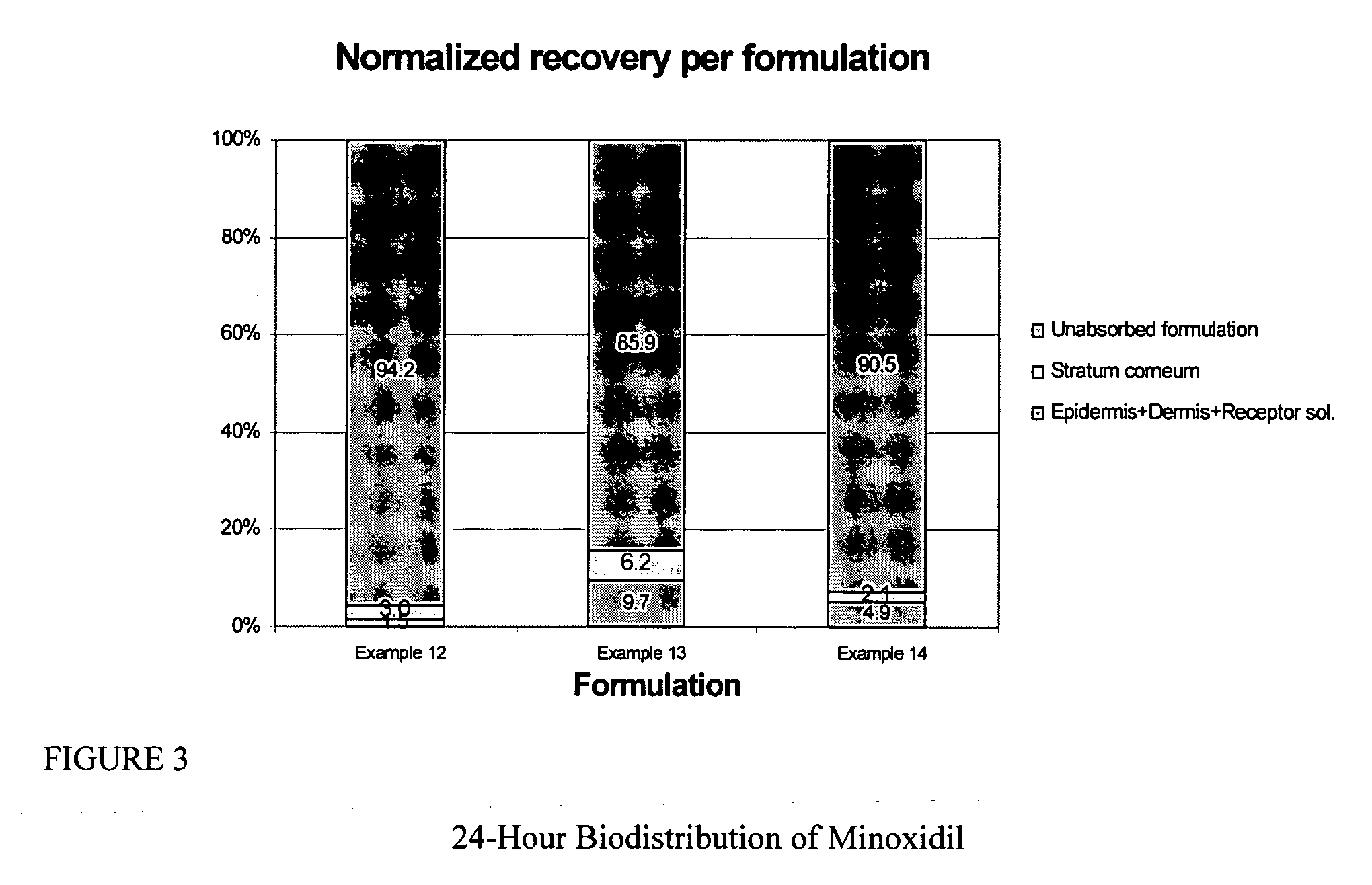
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS20060153905A1Reduce transferLoss of therapyOrganic active ingredientsNervous disorderActive agentBULK ACTIVE INGREDIENT
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
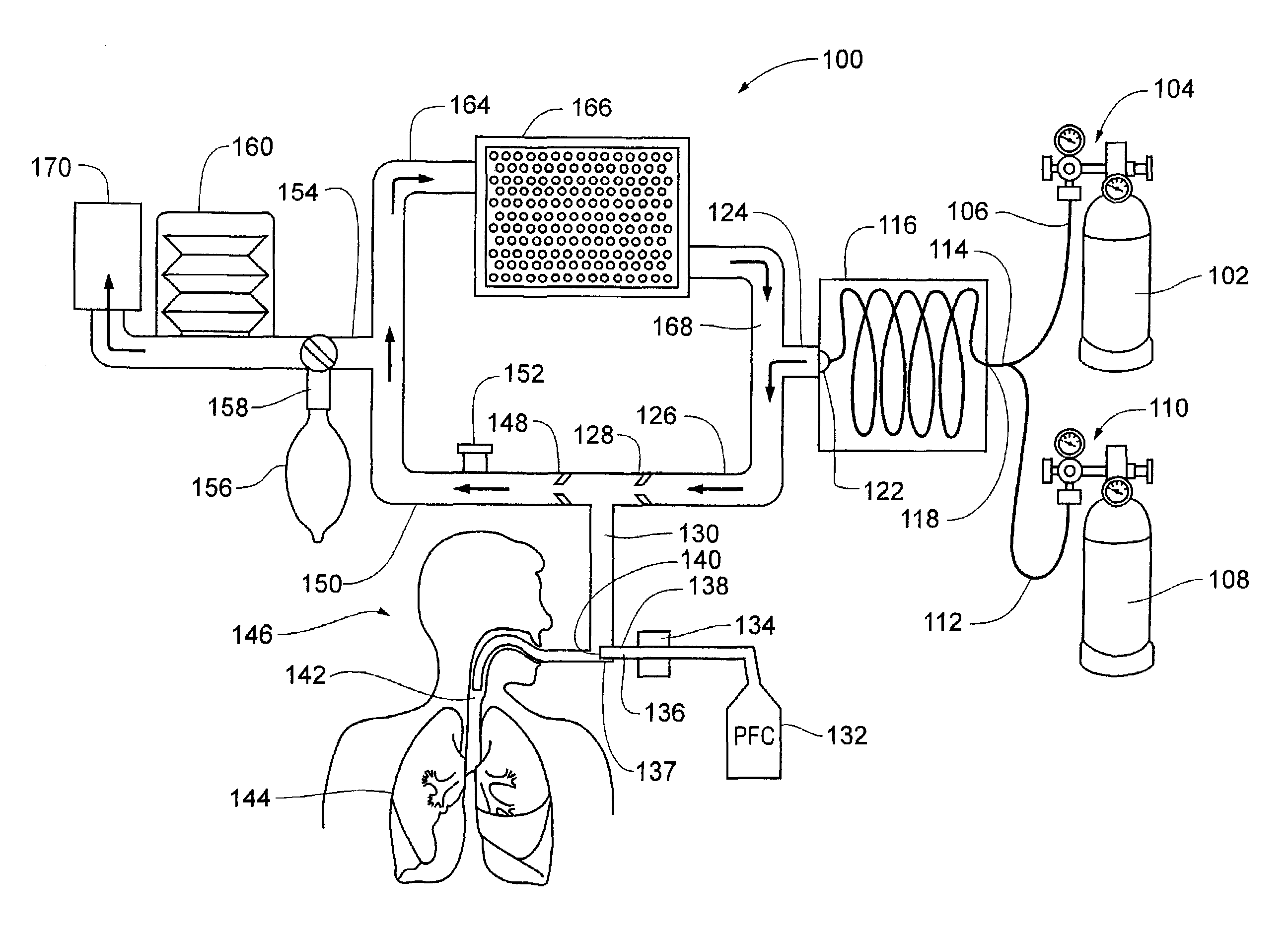
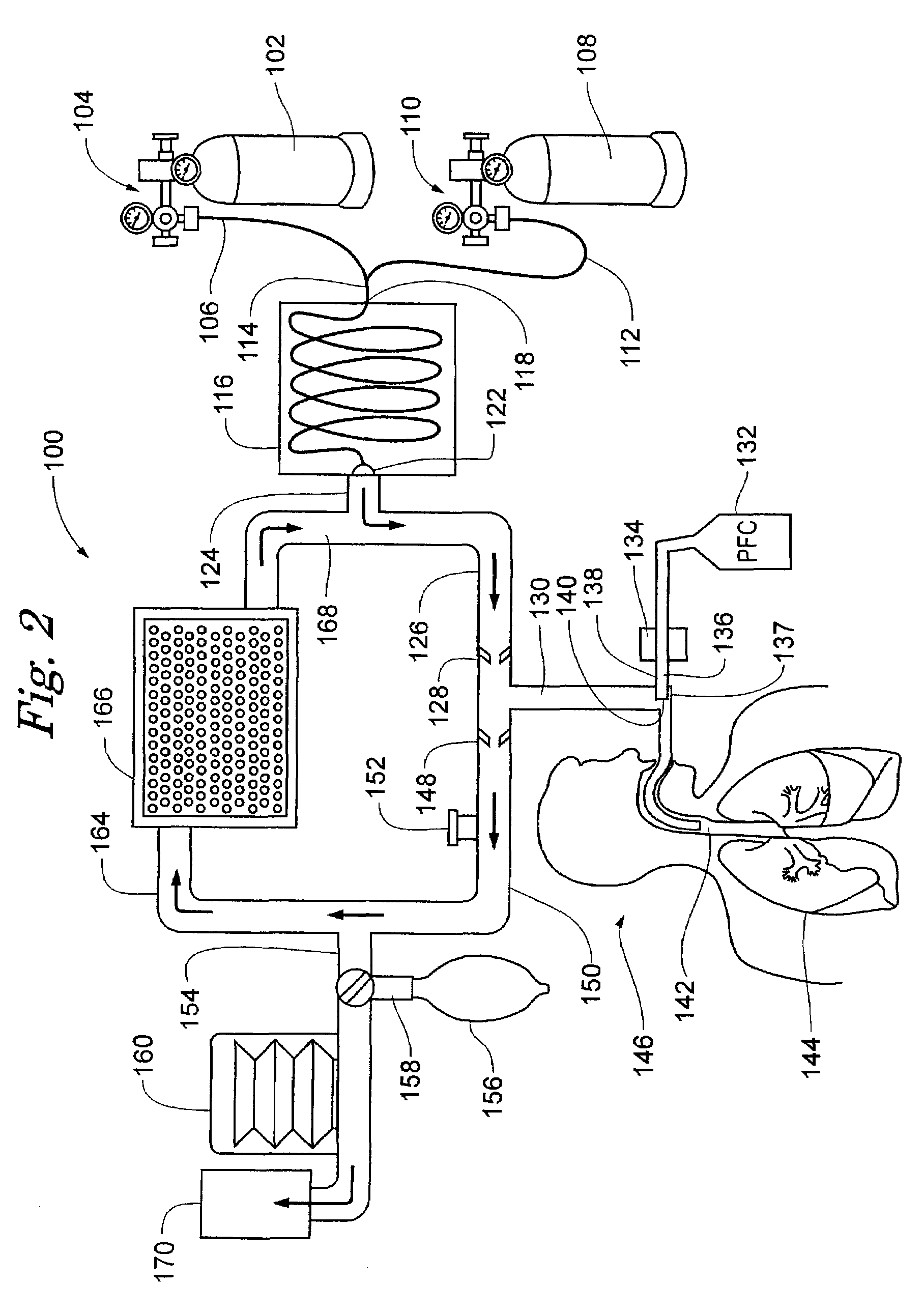
Inducing hypothermia and rewarming using a helium-oxygen mixture
ActiveUS6983749B2Mitigate warmingReduce coolingLighting and heating apparatusInorganic active ingredientsREFLEX DECREASEInspired gas temperature
Devices and methods to heat and cool human beings, including inducing and maintaining hypothermia in human patients. Methods include inducing hypothermia to treat ischemic events, including heart attack and stroke, to limit damage caused by the ischemic event. Methods can include: using the lungs for heat exchange; using cooled gases for ventilation; using helium in the ventilation gas mixture, using medications to control reflex heat production; and injecting a perfluorocarbon mist into the gas stream to increase the cooling rate. The high thermal conductivity and diffusivity of helium results in greater inspired gas temperature equalization toward body temperature. Due to the latent heat of vaporization, addition of even small quantity of phase-change perfluorocarbon dramatically increases the heat carrying capacity of the respiratory gases. Hypothermia may be terminated by discontinuing the medications and warming the patient using a warmed helium-oxygen mixture.
Owner:MINNESOTA HIGH TECH RESOURCES
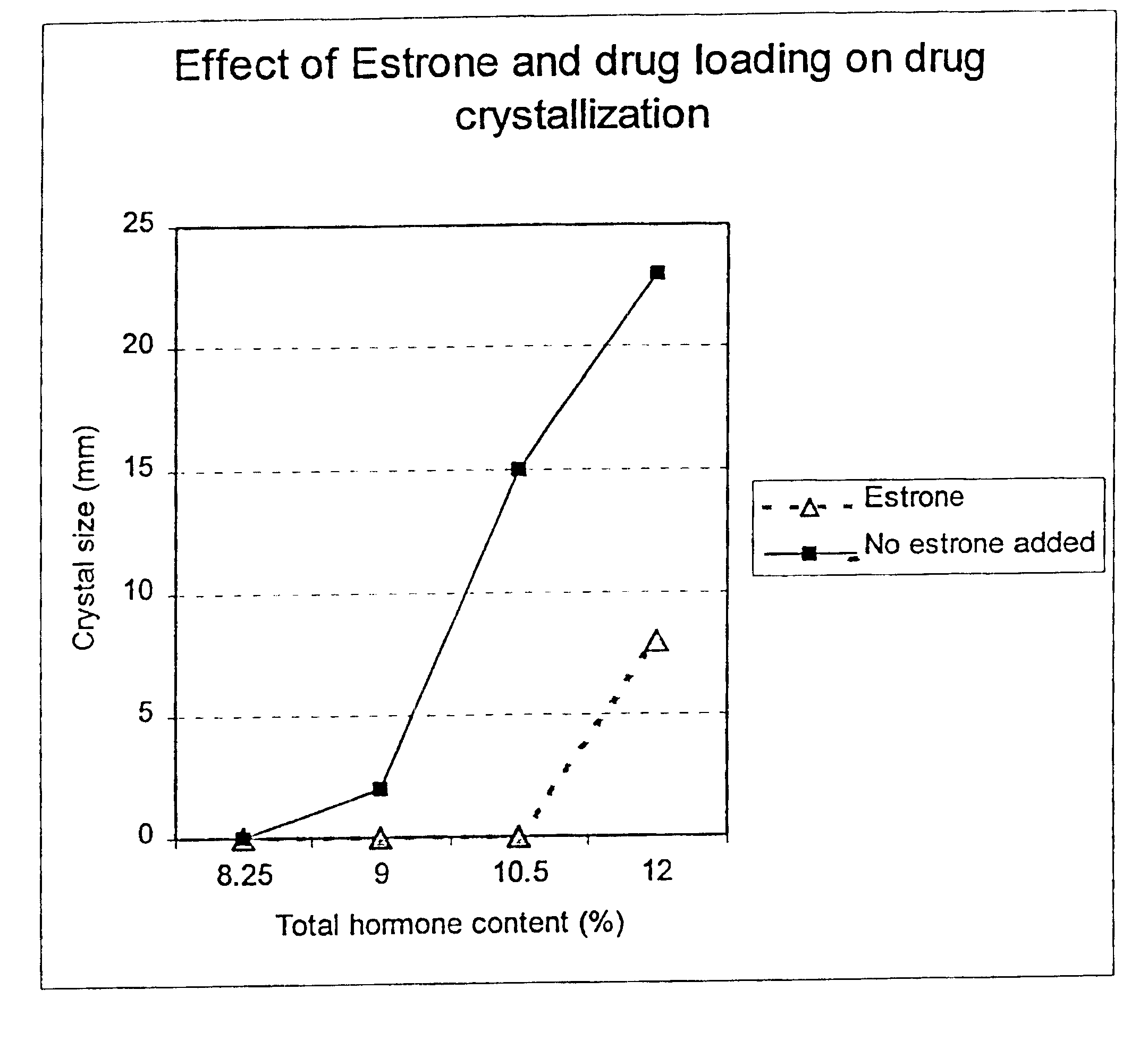
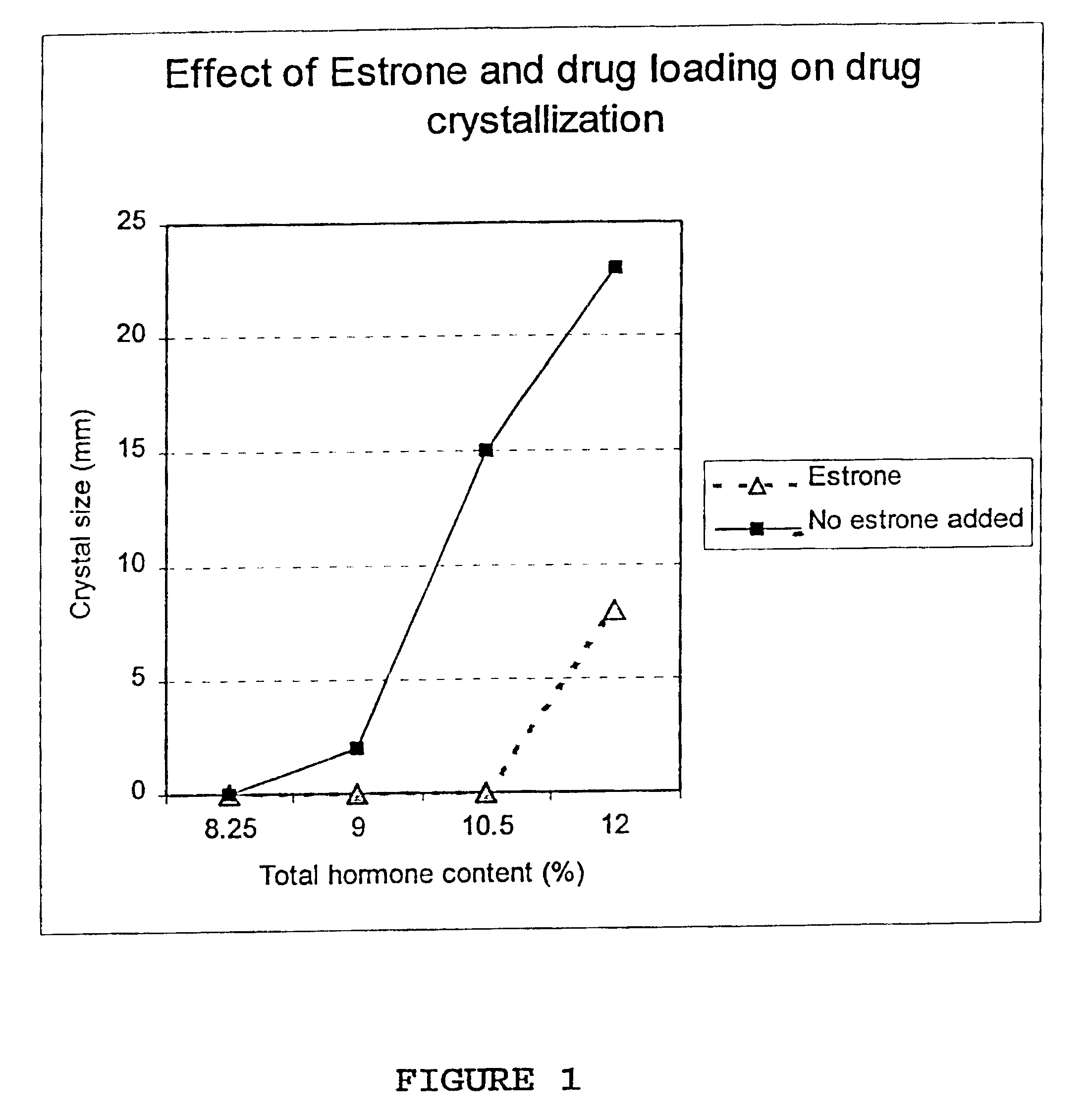
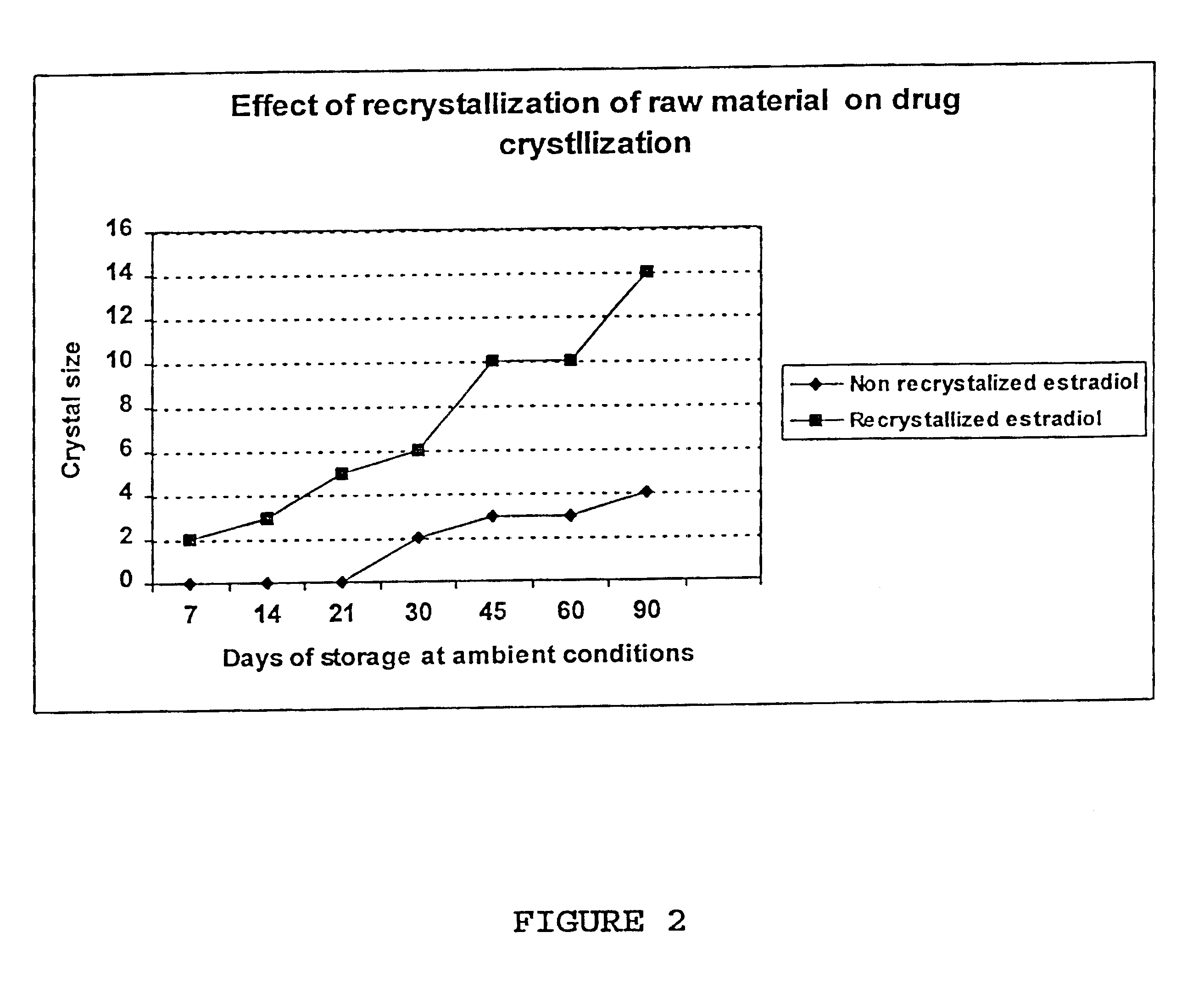
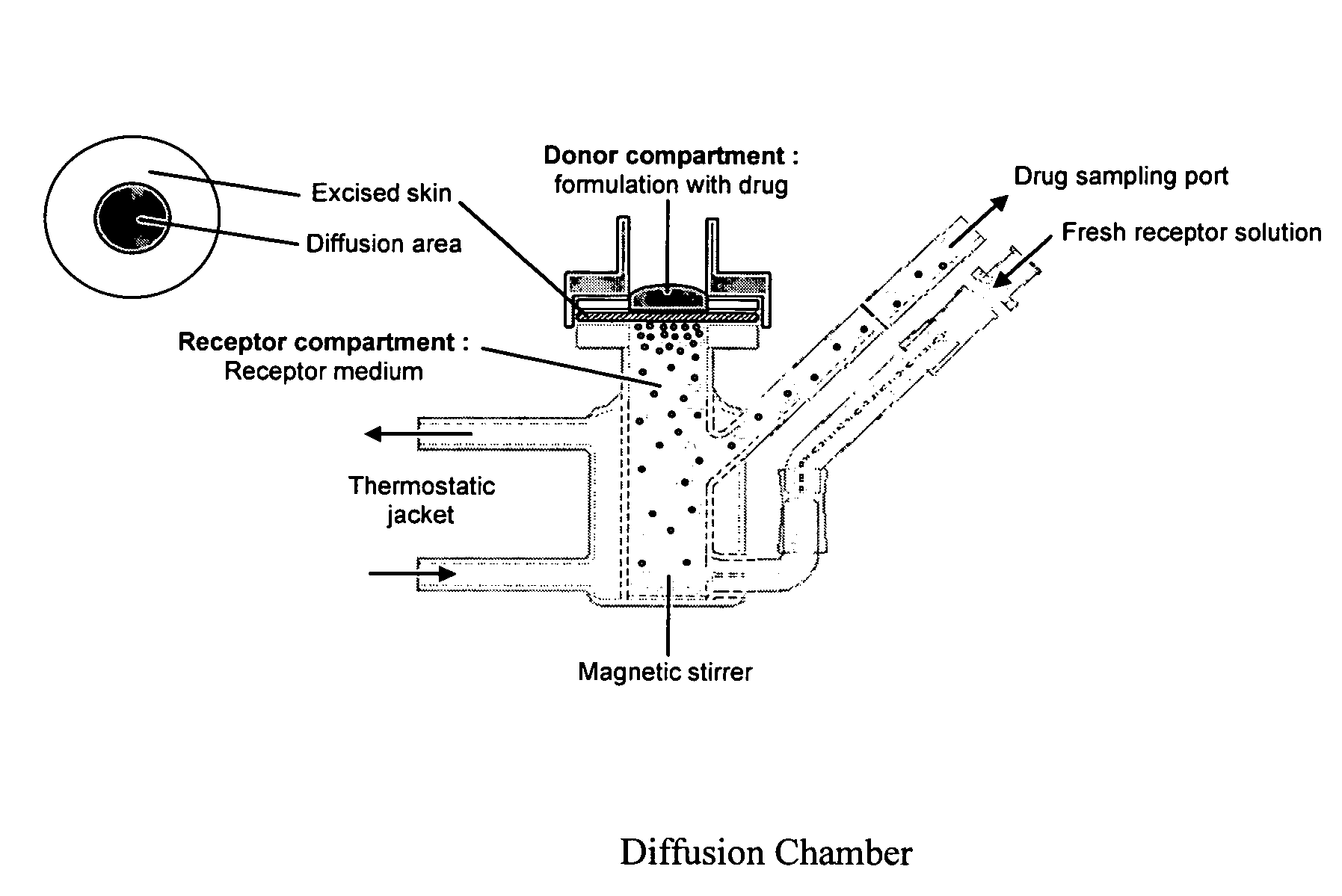
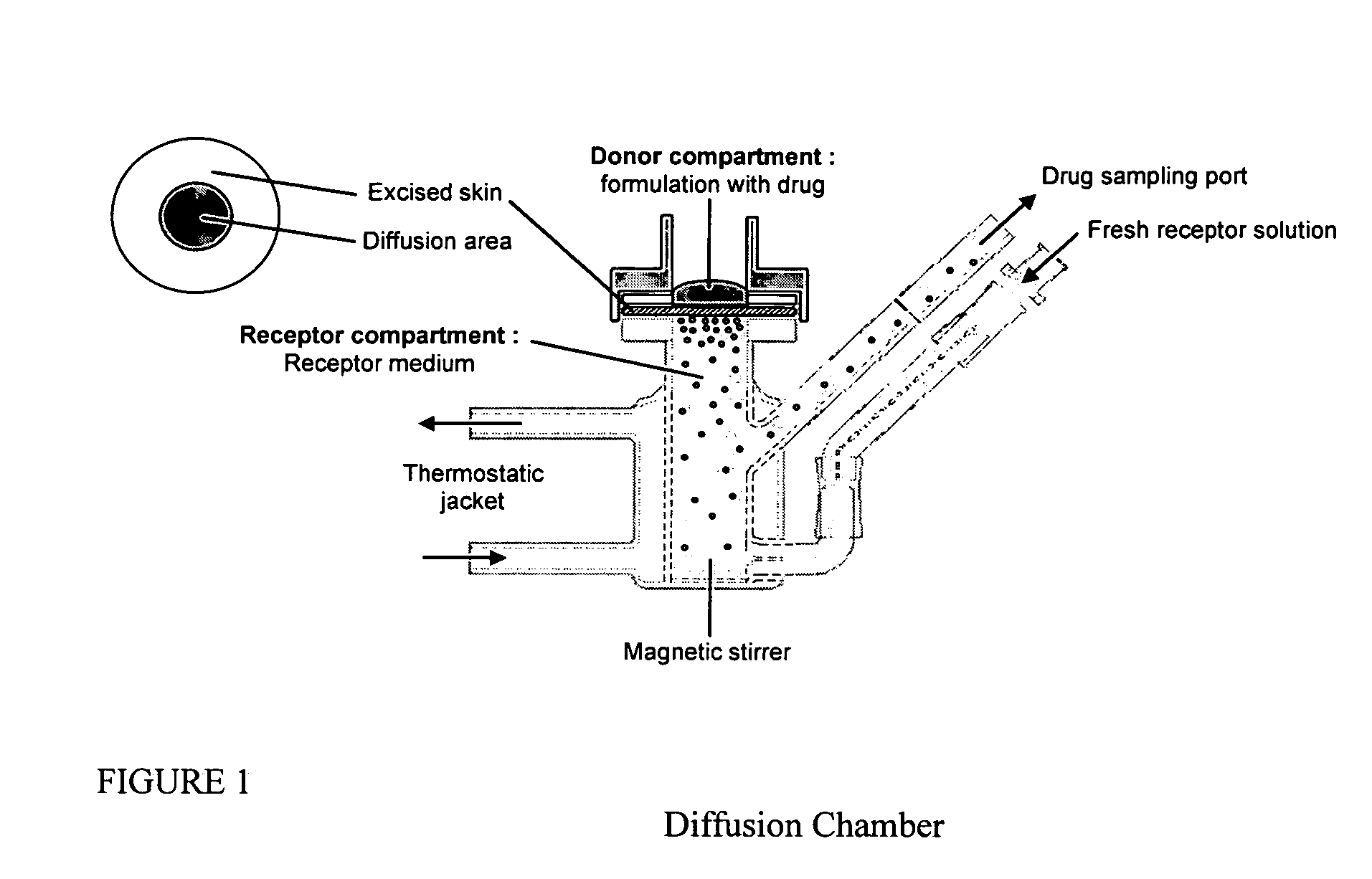
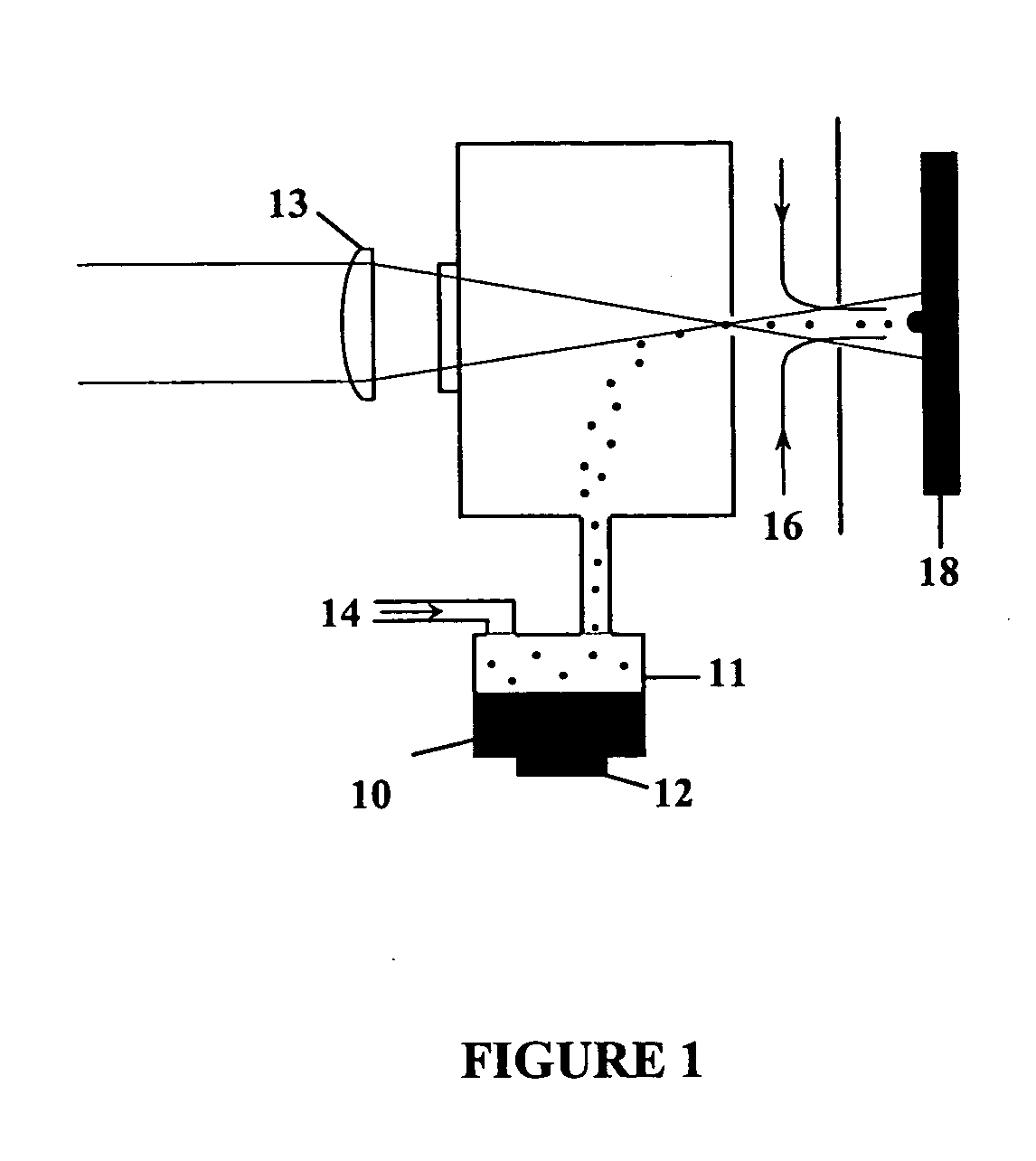
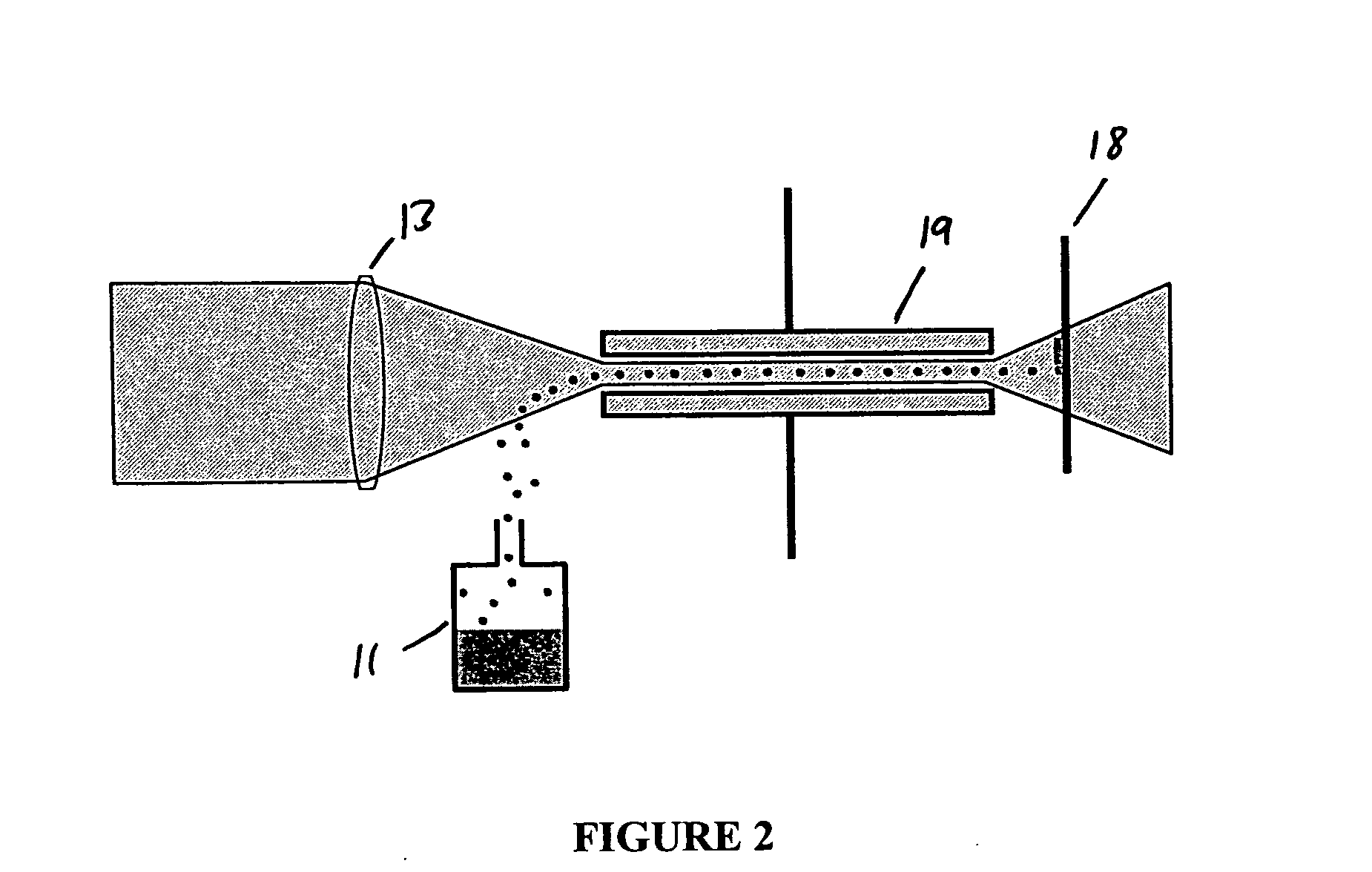
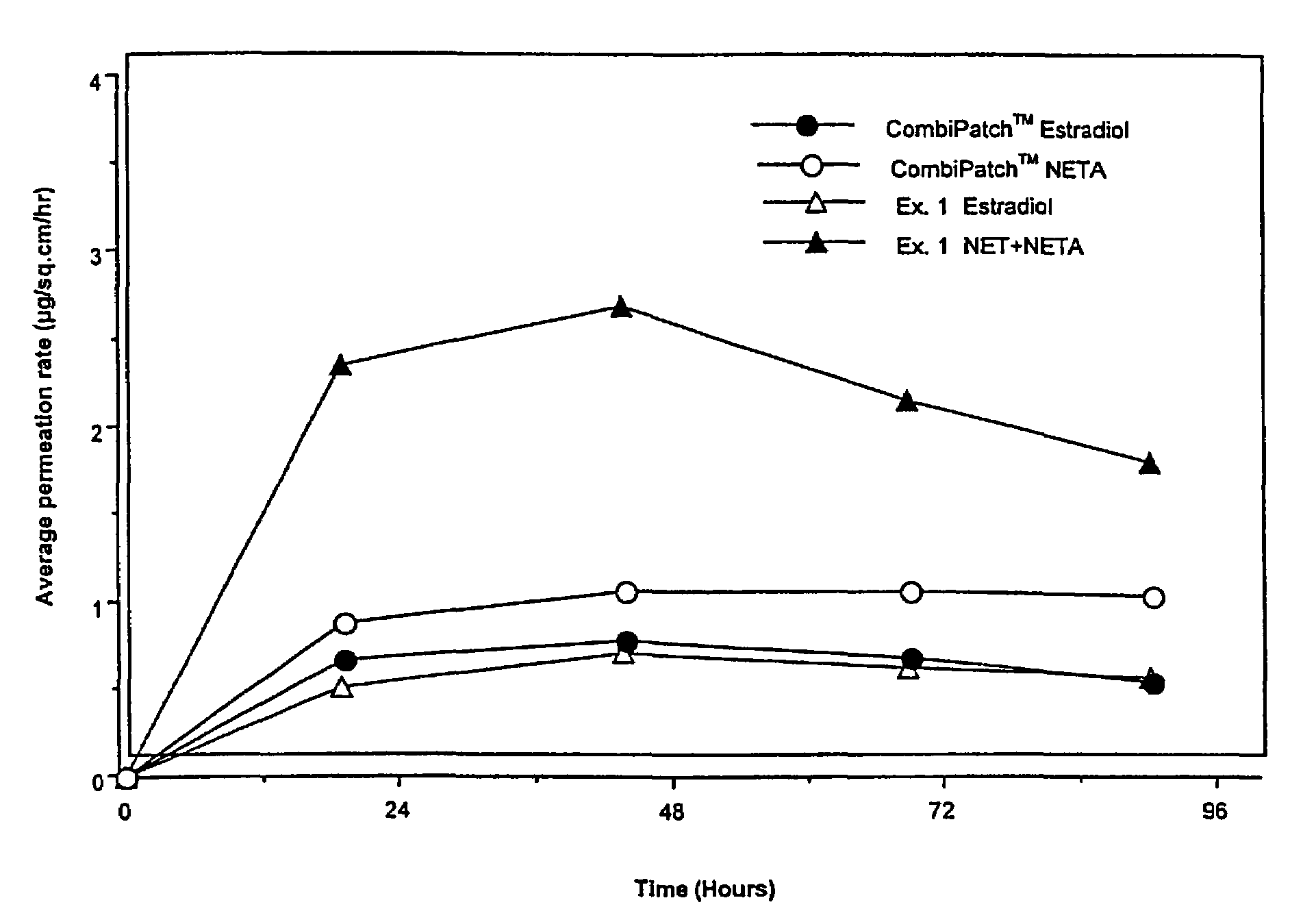
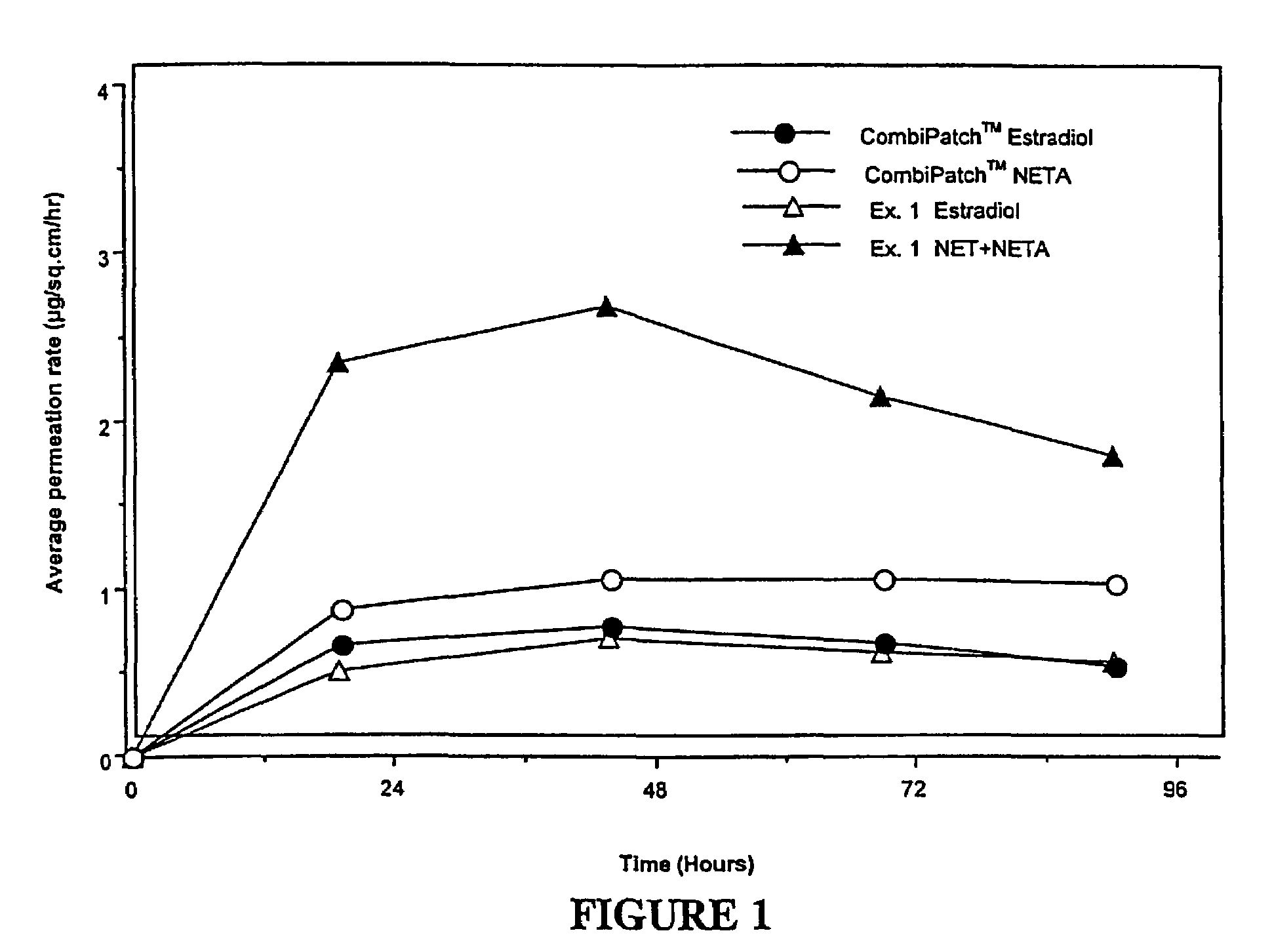
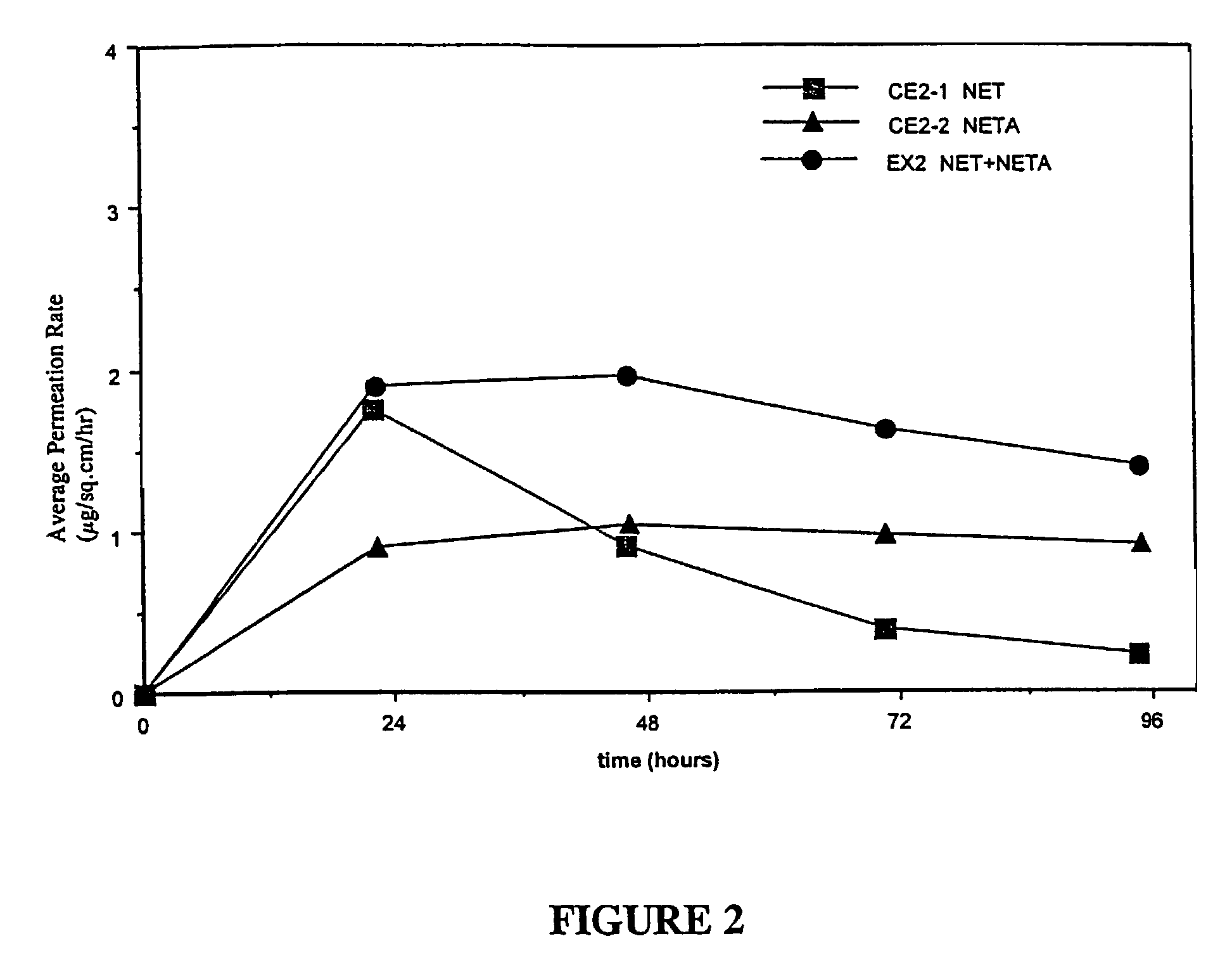
Inhibition of crystallization in transdermal devices
InactiveUS6465005B1Increase ratingsPrevent crystallizationPowder deliveryOrganic active ingredientsHormones regulationCrystallization
A steroid is used as an additive in manufacture of a transdermal drug delivery device, to act as a crystallization inhibitor inhibiting crystallization, during storage of the device, of an active drug in the form of a hormone which has a pharmaceutical or physiological effect in use of the device. The crystallization-inhibiting steroid is present in the device in an amount insufficient to provide significant pharmaceutical or physiological effect in use of the device.
Owner:AMARIN TECH
Tape compositions for the deposition of electronic features
InactiveUS20070178232A1Reduce settlementGood dispersionAdditive manufacturing apparatusSynthetic resin layered productsElectronMaterials science
Owner:CABOT CORP
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS7335379B2Reducing and preventing transferMinimize contaminationOrganic active ingredientsNervous disorderAlcoholActive agent
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Microbial detection and quantification
InactiveUS20060134728A1Increase brightnessGood colorBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismCell membrane
A method for semi-quantitatively or quantitatively detecting the presence of a microbe in a sample is provided. The method utilizes a test dye that undergoes a detectable color change in the presence of one or more microbes. For example, in one embodiment, the test dye is a solvatochromic dye (e.g., Reichardt's dye) that responds to differences in polarity between microbe components (e.g., cell membrane, cytoplasm, etc.) and the environment outside the cell. Alternatively, other mechanisms may be wholly or partially responsible for the interaction between the dye and the microbe, such as acid-base reactions, redox reactions, and so forth. Regardless, the color of the test dye may be compared to the color of a control dye, wherein the color of the control dye corresponds to a known microbe concentration.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Light-emitting organic compound and EL display device utilizing the same
InactiveUS20070007870A1Improve reliabilityHighly reliable display portionIncadescent screens/filtersDischarge tube luminescnet screensSimple Organic CompoundsDisplay device
By repeating a purification process of a light-emitting organic compound several times, a thin film made of the light-emitting organic compound to be used in an EL display device contains ionic impurities at the concentration of 0.1 ppm or lower and has a volume resistivity in the range of 3×1010 Ωcm or larger. By using such a thin film as a light-emitting layer in the EL device, a current caused by reasons other than the carrier recombination can be prevented from flowing through the thin film, and deterioration caused by unnecessary heat generation can be suppressed. Accordingly, it is possible to obtain an EL display device with high reliability.
Owner:SEMICON ENERGY LAB CO LTD
EL display device utilizing light-emitting organic compounds and method for forming the same
InactiveUS7112374B2Improve reliabilityHighly reliable display portionTelevision system detailsElectroluminescent light sourcesCharge carrierDisplay device
Owner:SEMICON ENERGY LAB CO LTD
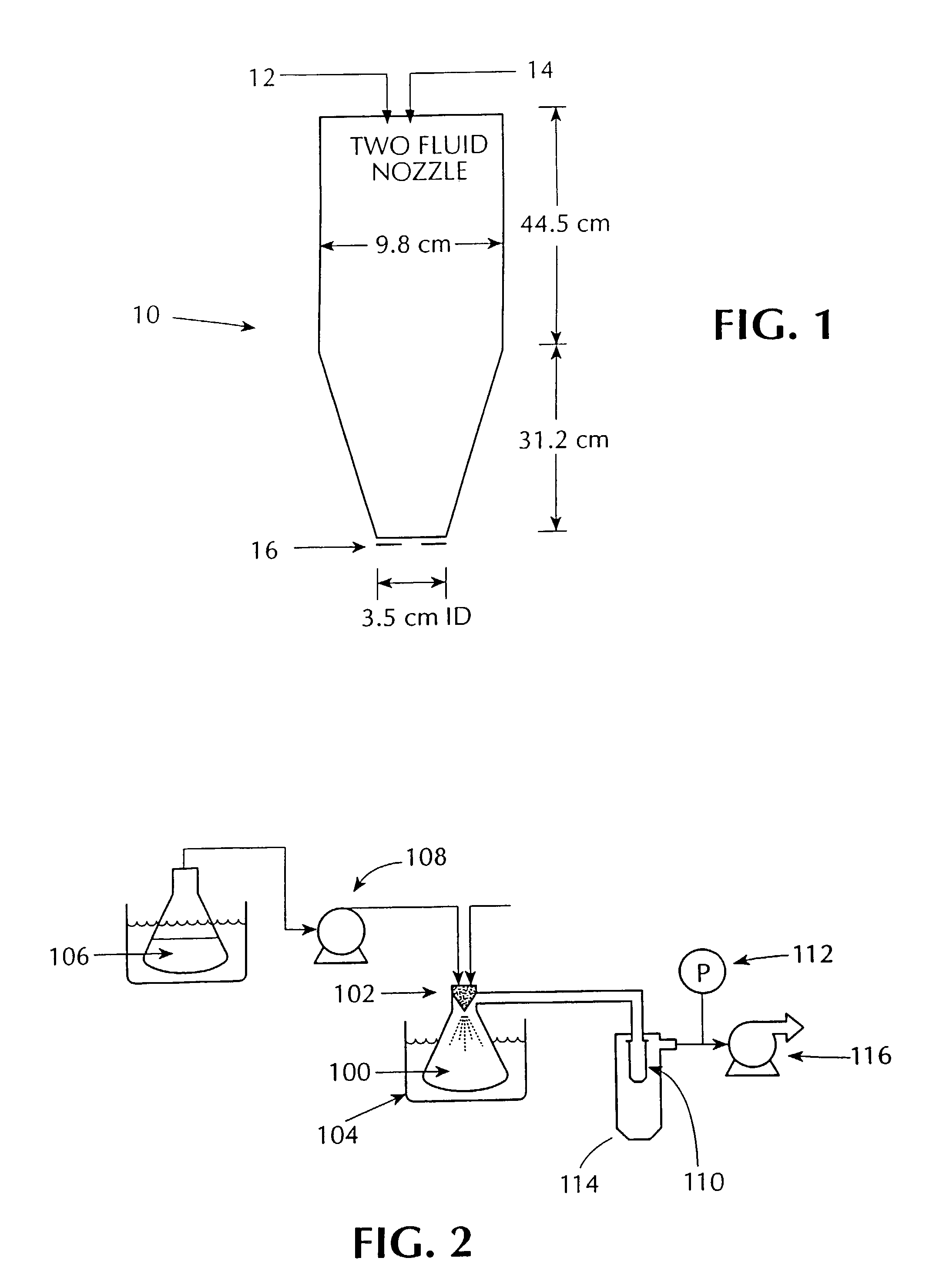
Maskless direct write of copper using an annular aerosol jet
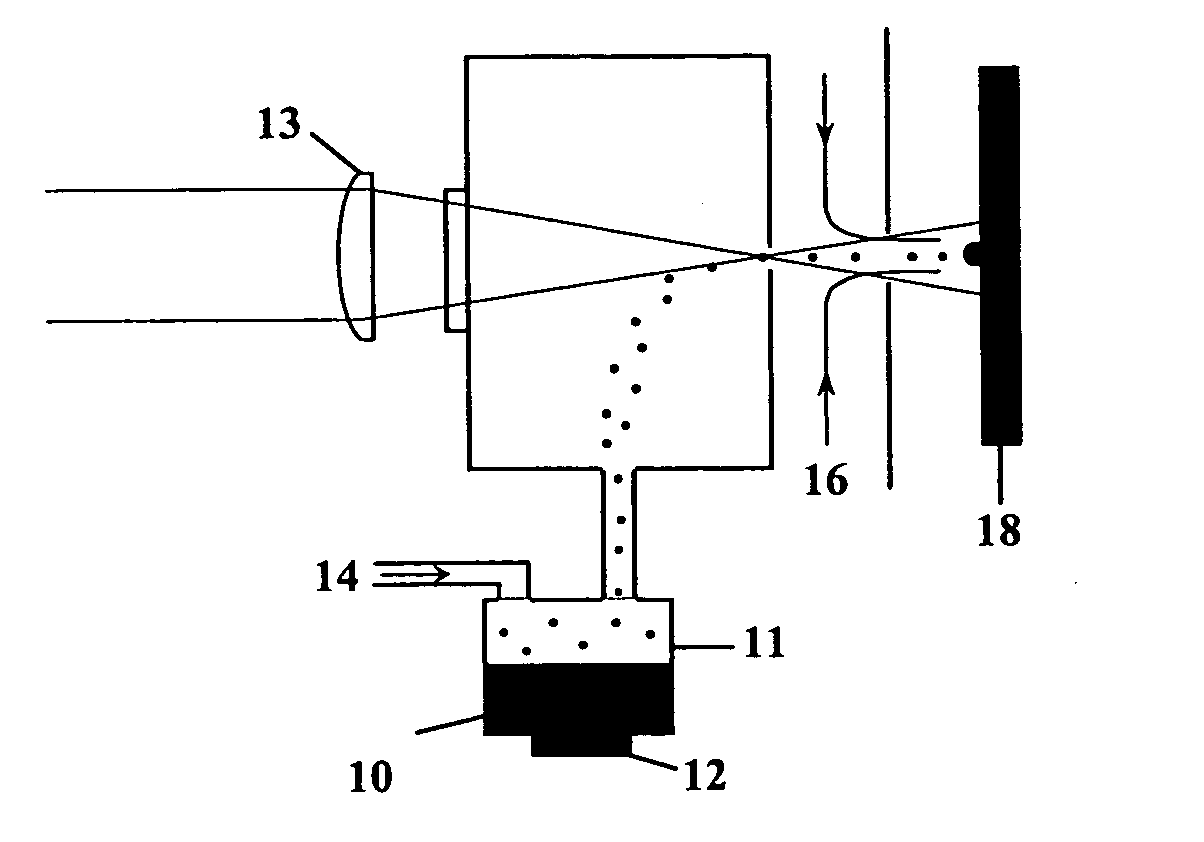
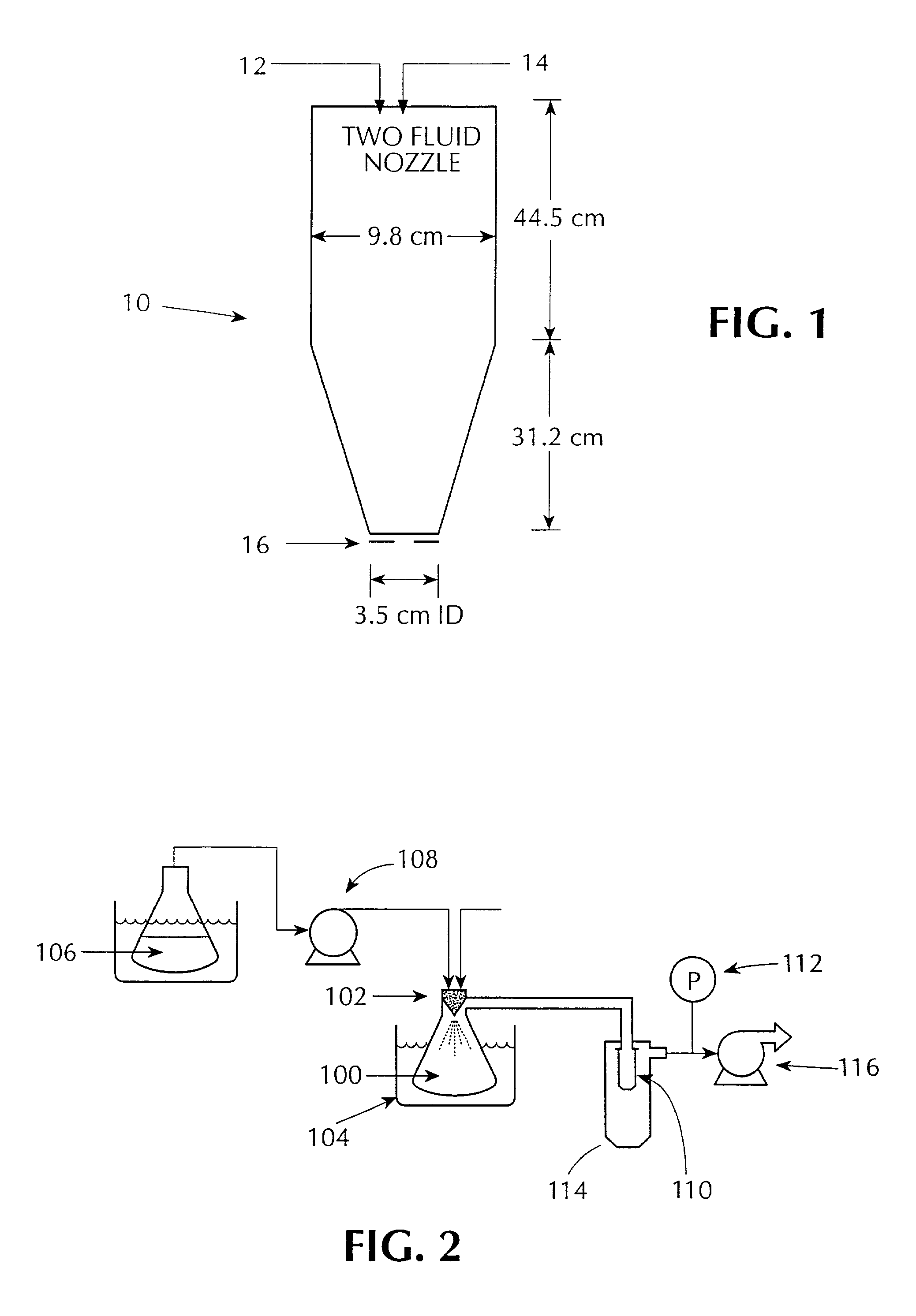
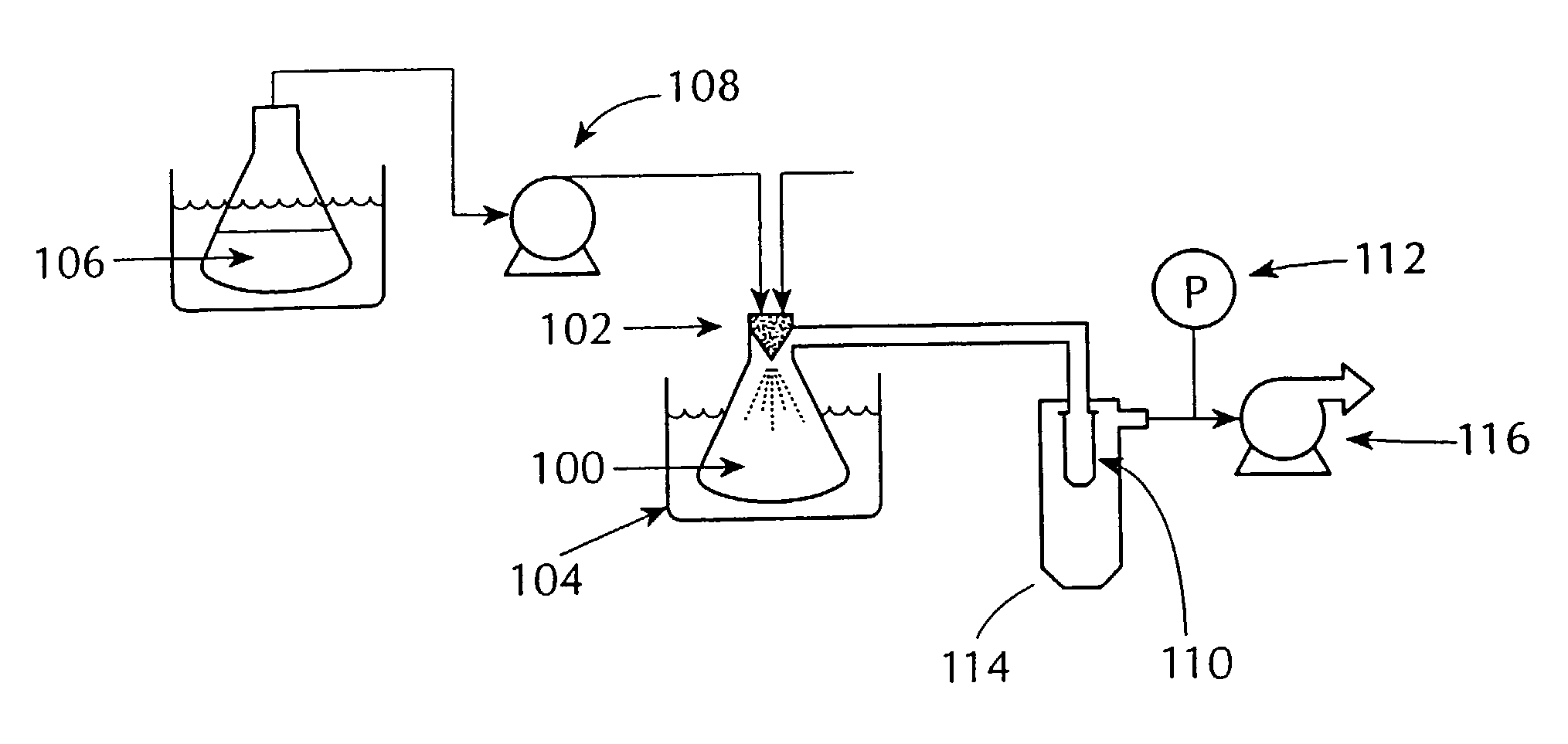
InactiveUS20050156991A1Prevent crystallizationLower decomposition temperatureSolid-state devicesLiquid/solution decomposition chemical coatingLaser processingSource material
Methods and apparatus for the deposition of a source material (10) are disclosed. An atomizer (12) renders a supply of source material (10) into many discrete particles. A force applicator (14) propels the particles in continuous, parallel streams of discrete particles. A collimator (16) controls the direction of flight of the particles in the stream prior to their deposition on a substrate (18). In an alternative embodiment of the invention, the viscosity of the particles may be controlled to enable complex depositions of non-conformal or three-dimensional surfaces. The invention also includes a wide variety of substrate treatments which may occur before, during or after deposition. In yet another embodiment of the invention, a virtual or cascade impactor may be employed to remove selected particles from the deposition stream. Also a method and apparatus for maskless deposition of copper lines on a target, specifically relating to localized solution-based deposition of copper using an annular aerosol jet and subsequent material processing using conventional thermal techniques or laser processing.
Owner:OPTOMEC DESIGN CO
Enhanced drug delivery in transdermal systems
InactiveUS7456159B2Convenient amountGood synergyOrganic active ingredientsBiocideActive agentEthyl ester
A composition for transdermal administration resulting from an admixture includes: a therapeutically effective amount of a drug that includes a parent drug and a prodrug; and and a pharmaceutically acceptable carrier, wherein the parent drug and prodrug are individually present in an amount sufficient for a pharmacological effect. In a preferred embodiment, the admixture includes: a therapeutically effective amount of a pharmaceutically active agent that includes a corresponding steroid and a steroid derivative; and a carrier for the pharmaceutically active agent. The steroid and the corresponding steroid derivative are present in a weight ratio of 10:1 to 1:10 steroid: corresponding steroid derivative. In a preferred embodiment ratio is 6:1 to 1:6. In a preferred embodiment, the corresponding steroid derivative is a steroid ester. In another preferred embodiment, the carrier is a polymer that includes a pressure-sensitive adhesive. In another preferred embodiment, the parent drug is an ACE inhibitor such as ramipril and the prodrug is an ACE inhibitor prodrug such as ramipril ethyl and / or methyl ester.
Owner:NOVEN PHARMA
Fluorene-based compound and organic electroluminescent display device using the same
ActiveUS7737627B2High charge transport performanceHigh glass transition temperatureDischarge tube luminescnet screensElectroluminescent light sourcesDopantFluorescence
Owner:SAMSUNG DISPLAY CO LTD
Phenylcarbazole-based compound and organic electroluminescent device employing the same
ActiveUS8021764B2High glass transition temperatureElectric stabilityDischarge tube luminescnet screensOrganic compound preparationCarbazoleLow voltage
A phenylcarbazole-based compound is represented by Formula 1, and has superior electric properties and charge transport abilities, and thus is useful as a hole injection material, a hole transport material, and / or an emitting material which is suitable for fluorescent and phosphorescent devices of all colors, including red, green, blue, and white colors. The phenylcarbazole-based compound is synthesized by reacting carbazole with diamine. The organic electroluminescent device manufactured using the phenylcarbazole-based compound has high efficiency, low voltage, high luminance, and a long lifespan.
Owner:SAMSUNG DISPLAY CO LTD
Precursor compositions for the deposition of electrically conductive features
InactiveUS20060043346A1Reduce settlementGood dispersionConductive materialSemiconductor/solid-state device manufacturingScreen printingViscosity
A precursor composition for the deposition and formation of an electrical feature such as a conductive feature. The precursor composition advantageously has a viscosity of at least about 1000 centipoise and can be deposited by screen printing. The precursor composition also has a low conversion temperature, enabling the deposition and conversion to an electrical feature on low temperature substrates. A particularly preferred precursor composition includes silver and / or copper metal for the formation of highly conductive features.
Owner:CABOT CORP
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8263128B2Improve bioavailabilityPreventing and retarding ratePowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
Electrolytic capacitor and a fabrication method therefor
InactiveUS6876083B2Avoid excessive leakage currentAvoid excessive currentTransistorSolid electrolytic capacitorsElectrolytic capacitorNiobium alloy
An electrolytic capacitor including one type of electrode selected from a group consisting of an electrode of at least one type of alloy selected from a group consisting of niobium alloy, titanium alloy, and tungsten alloy, an electrode of mixed sinter of niobium and aluminum, or a fluorine-doped electrode of niobium or niobium alloy and on a surface of each electrode a dielectric layer is formed by anodizing the electrode.
Owner:SANYO ELECTRIC CO LTD
Solid pharmaceutical dispersions with enhanced bioavailability
InactiveUS8257741B2Improve solubilityEffective dispersionPowder deliveryBiocideAcetic acidHydroxypropylmethylcellulose acetate succinate
Spray dried solid dispersions comprising a sparingly soluble drug and hydroxypropylmethylcellulose acetate succinate (HPMCAS) provide increased aqueous solubility and / or biavailability in a use environment.
Owner:BEND RES
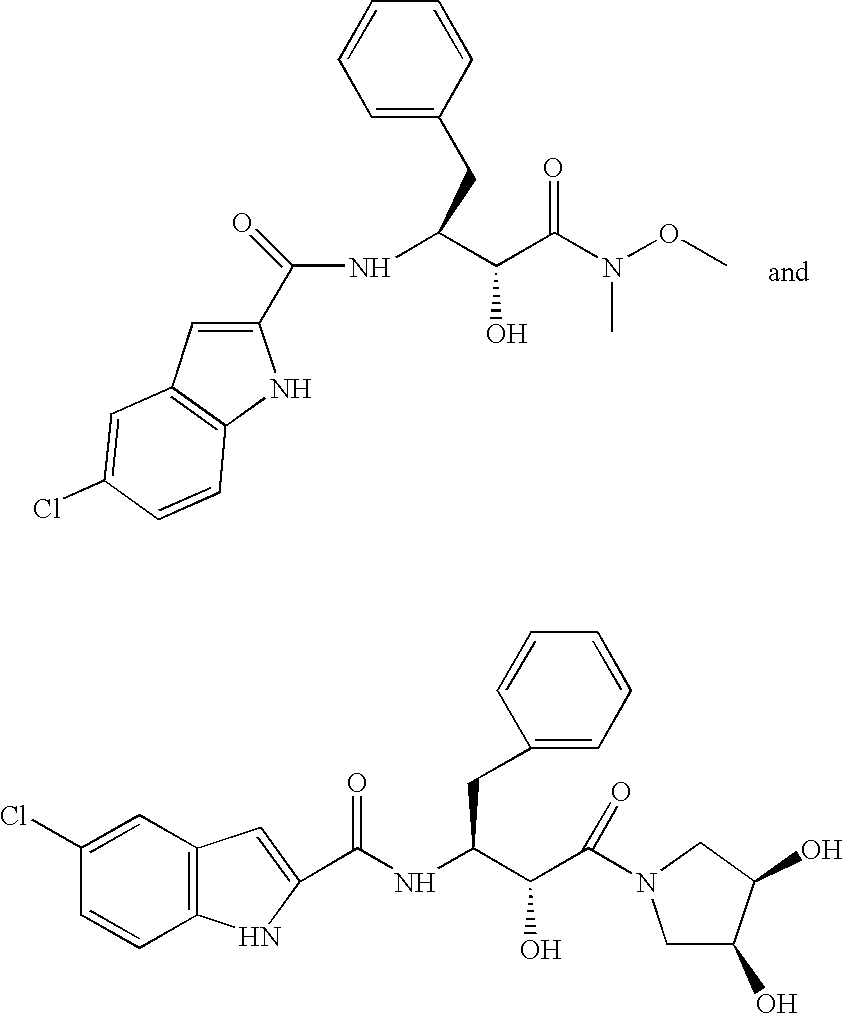
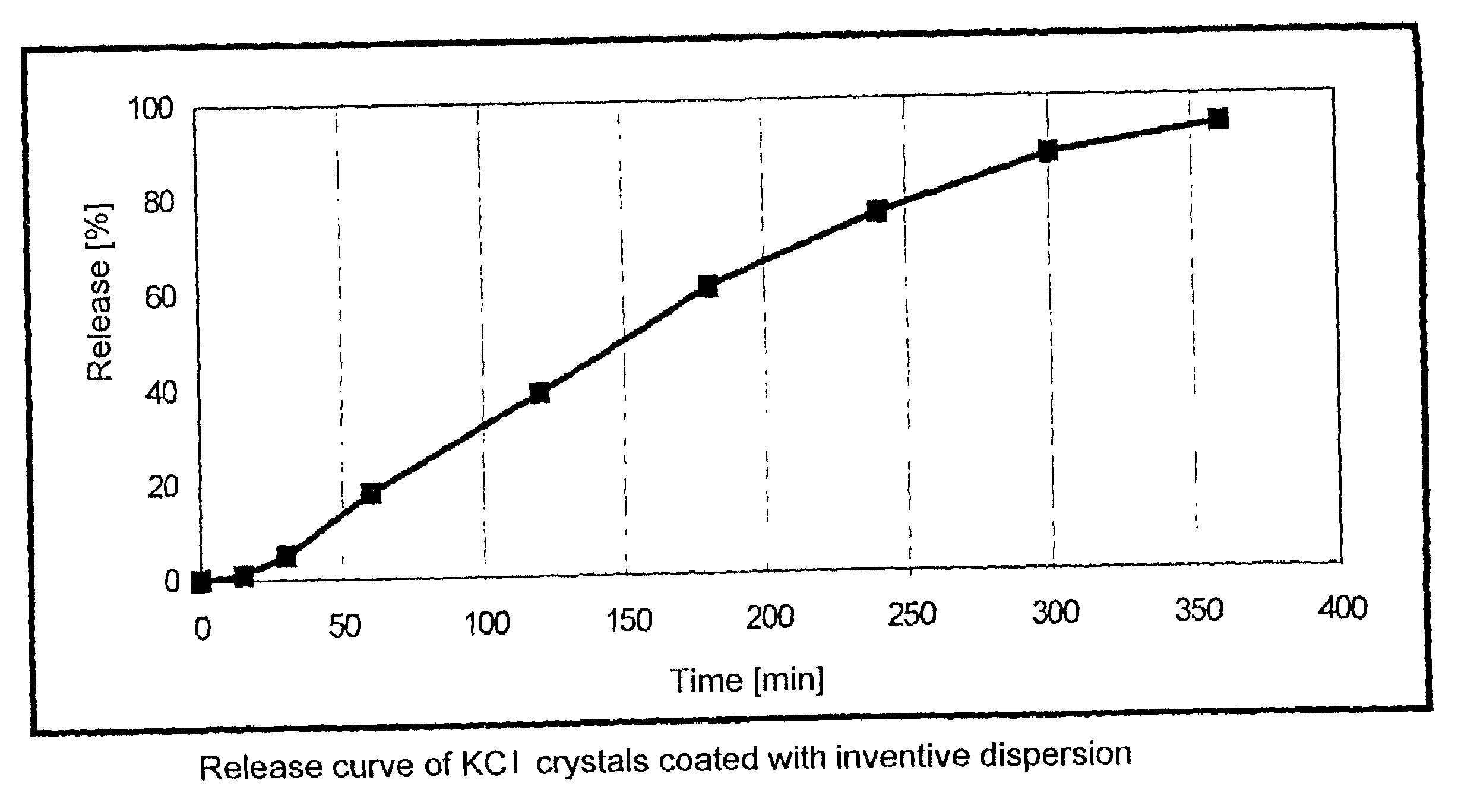
Dispersion comprising a non-ionic emulsifier
InactiveUS6998140B2Quality improvementPrevent crystallizationPowder deliveryTransportation and packagingVitrificationNon ionic
The invention relates to a dispersion suitable for use as coating agent and binder for pharmaceutical forms, having a solids content of 10–70% by weight consisting ofa) from 90 to 99% by weight of a methacrylate copolymer consisting of at least 90% by weight of (meth)acrylate monomers containing neutral radicals and having a glass transition temperature Tg of from −20° C. to +20° C. as determined by the DSC method, andb) 1–10% by weight of a nonionic emulsifier having an HLB of from 15.2 to 17.3.
Owner:EVONIK OPERATIONS GMBH
Composite magnetic material and method of manufacturing the same
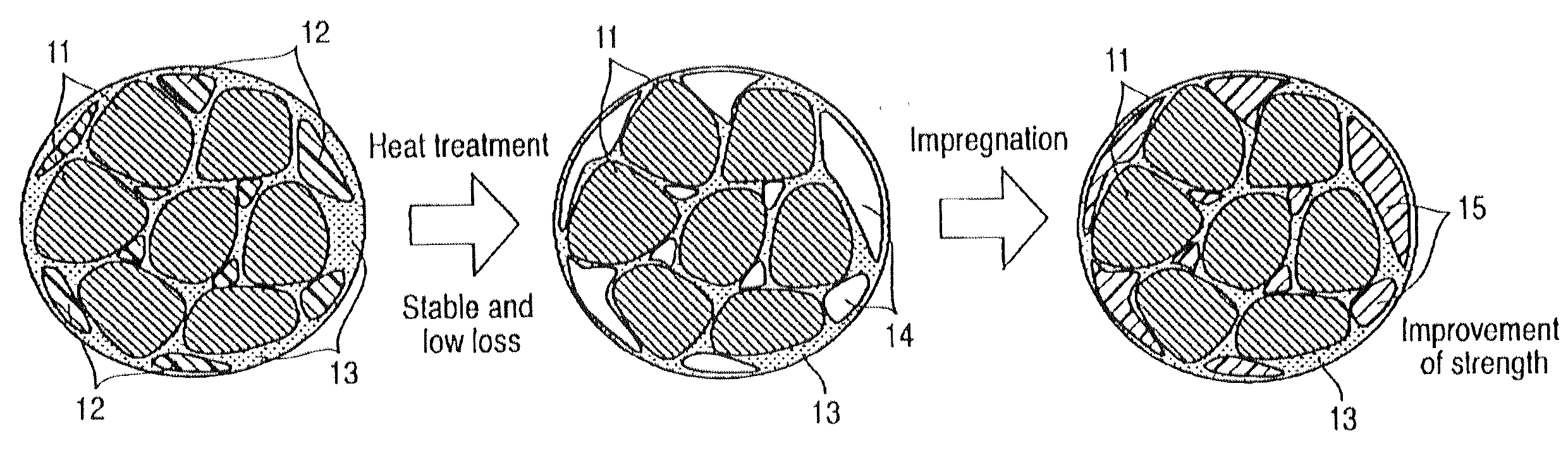
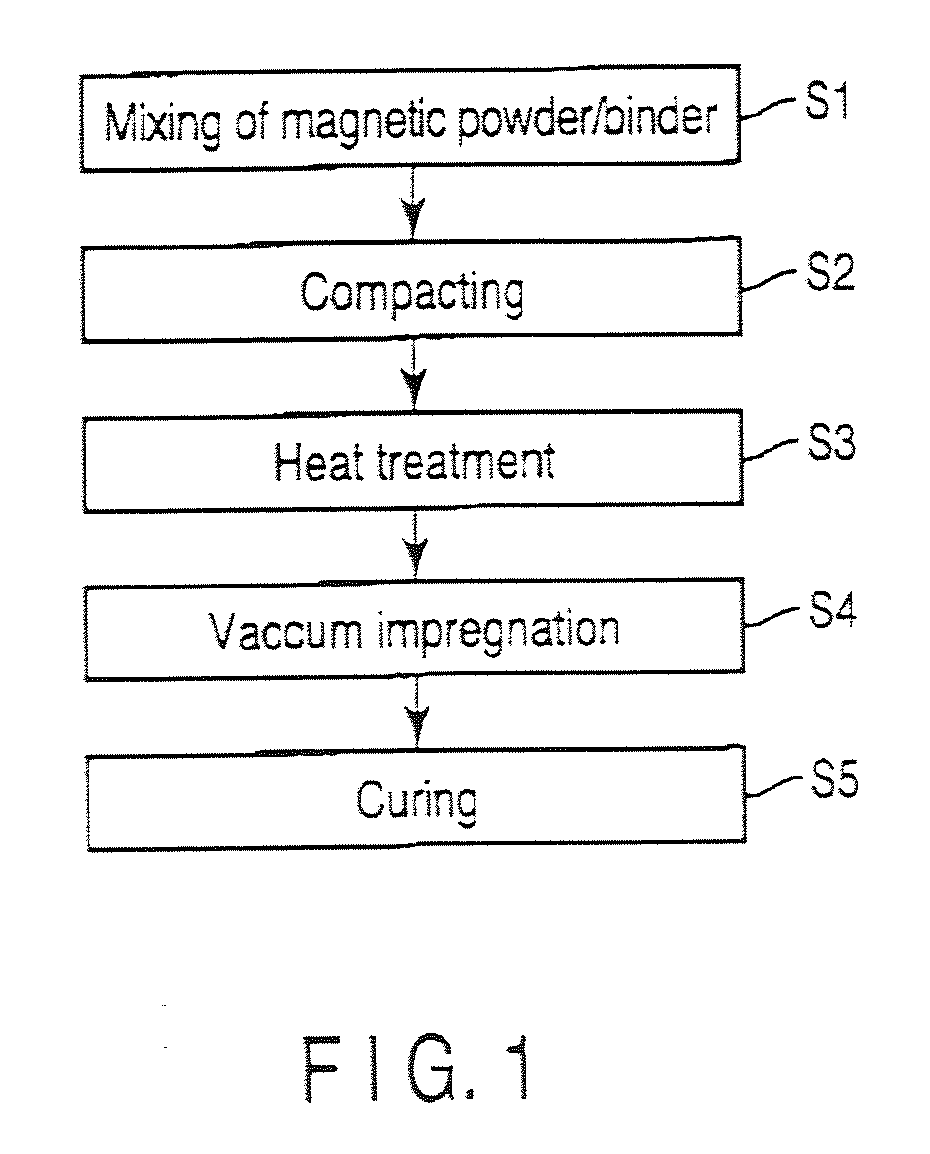
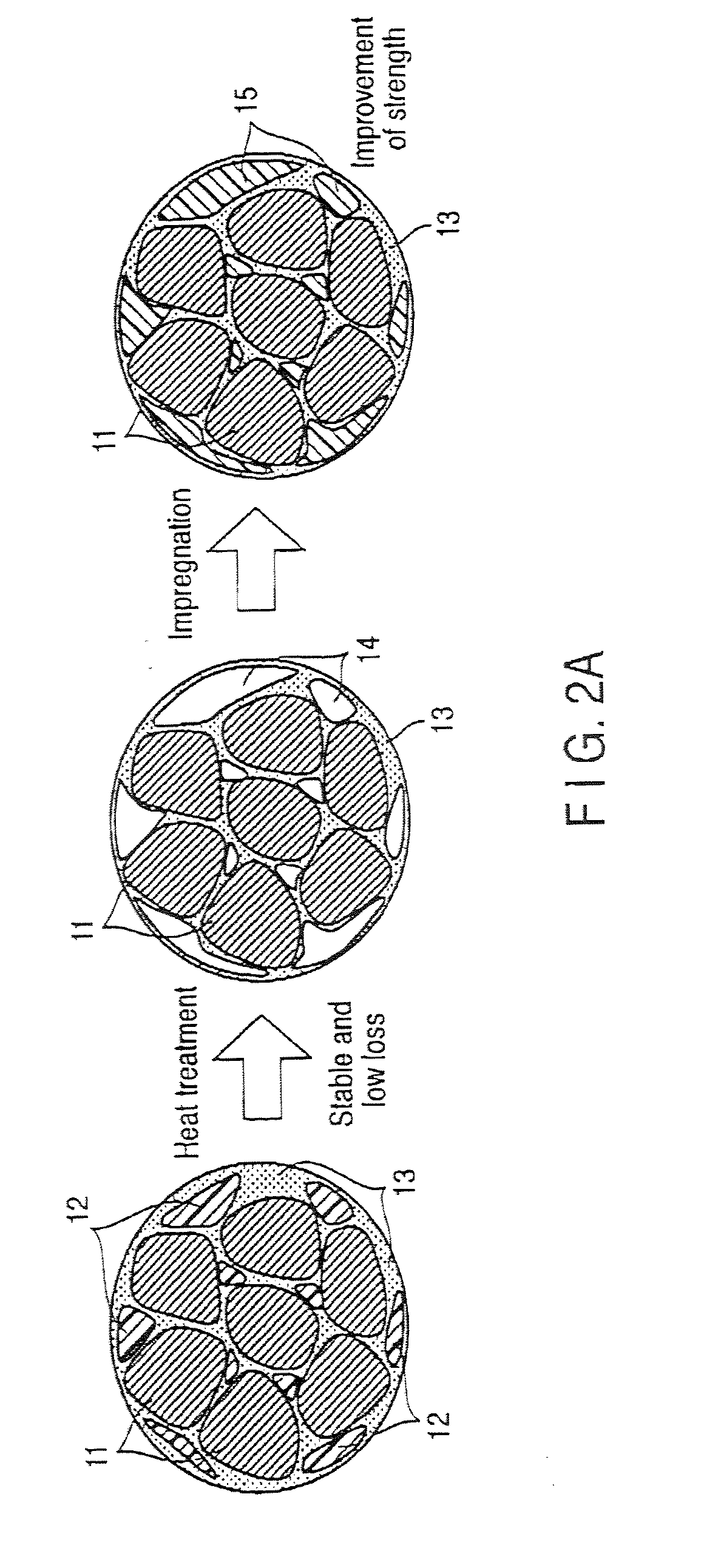
InactiveUS20110024670A1Good molding effectReduce the amount requiredTransportation and packagingMetal-working apparatusSpherical shapedInductor
Certain embodiments provide a composite magnetic material for an inductor, wherein a non-magnetic material contains a first binder as a compacting additive, and is added to and mixed with the soft magnetic metal powder, and a second binder that is impregnated to a compact as a binder after the heat treatment of the compact obtained by adding the first binder to the soft magnetic metal powder and compacting it, and the soft magnetic metal powder contains 40% by mass or more (including 100%) of spherical particles of which the ratio L2 / L1 between a perimeter L1 of a particle cross-section in the two dimensional plane view and a perimeter L2 of a circle having equivalent cross-sectional area is 0.5 or more.
Owner:TOHO ZINC
Light-emitting device and method for manufacturing the same
ActiveUS20100096627A1Improve hole transport abilityExtended service lifeElectroluminescent light sourcesSolid-state devicesEngineeringLight emitting device
A light-emitting element is disclosed that can drive at a low driving voltage and that has a longer lifetime than the conventional light-emitting element, and a method is disclosed for manufacturing the light-emitting element. The disclosed light-emitting element includes a plurality of layers between a pair of electrodes; and at least one layer among the plurality of layers contains one compound selected from the group consisting of oxide semiconductor and a metal oxide, and a compound having high hole transportation properties. Such the light-emitting element can suppress the crystallization of a layer containing one compound selected from the group consisting of oxide semiconductor and a metal oxide, and a compound having high hole transportation properties. As a result, a lifetime of the light-emitting element can be extended.
Owner:SEMICON ENERGY LAB CO LTD
Amorphous alloy thermoforming apparatus and technique
ActiveCN101468370APrevent crystallizationImprove processing efficiencyShaping toolsForging/hammering/pressing machinesNitrogen gasAmorphous metal
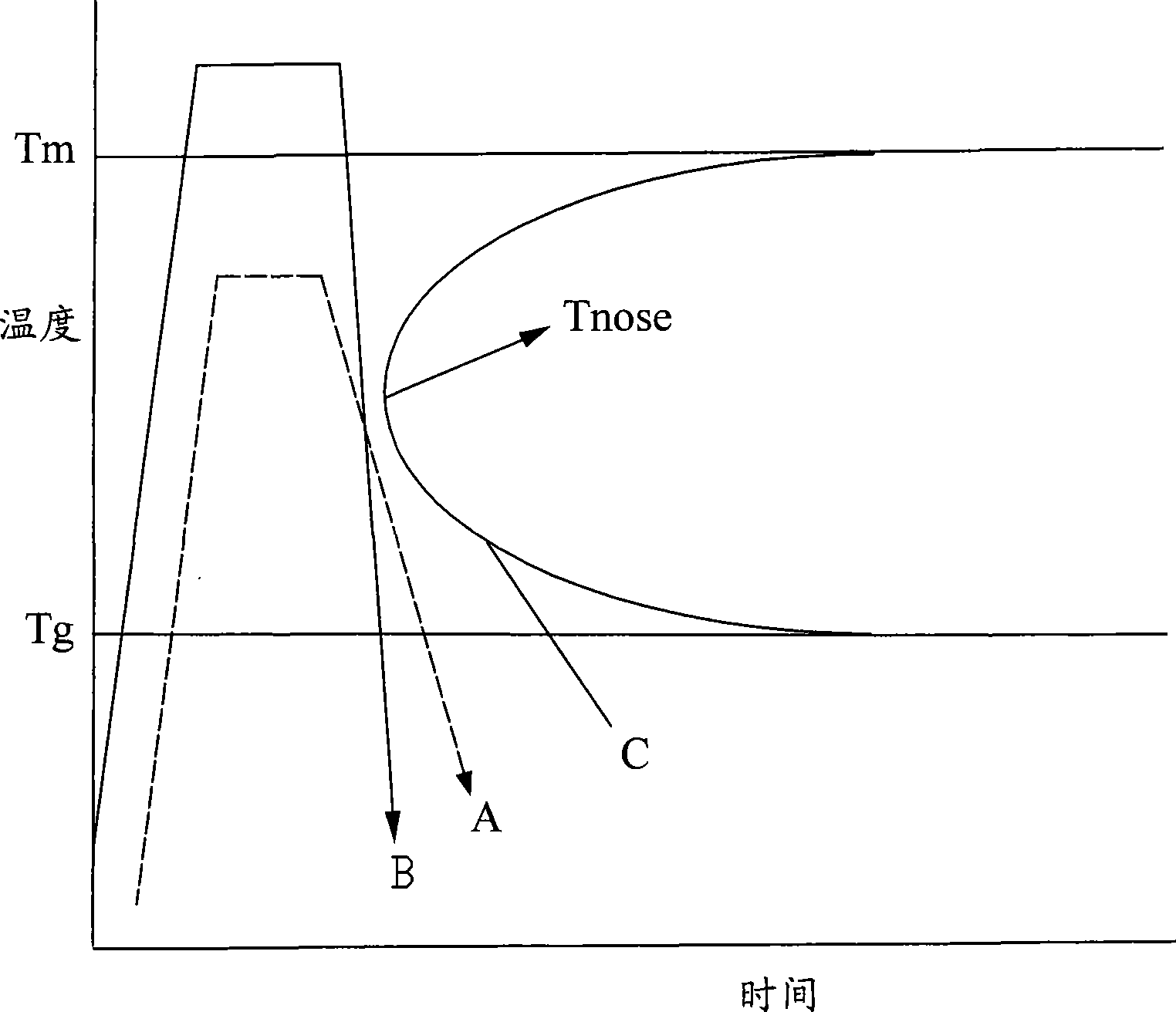
The invention provides a device and a process for the hot pressing and molding of an amorphous alloy. The device is provided with a vacuum, inert gas or nitrogen gas protective environment, and a mould set and an induction heating coil positioned in the environment; the mould set comprises an upper mould, a lower mould and a molding mould; the induction heating coil is at least wound in a peripheral space of the molding mould and is not contacted with the molding mould; and the process sequentially comprises the following steps in the vacuum, inert gas or nitrogen gas protective environment: blank placement, induction heating, hot pressing and molding, cooling, and mould extraction. By adopting an induction heating mode, the process can rapidly concentrate on heating a molded blank to Tg temperature above, and carry out the hot pressing and molding within the temperature range between Tg and Tm or the Tm temperature above; a molding strain rate can reach between 10 and 10S; and the process has high processing efficiency and can effectively prevent crystallization of the amorphous alloy in the molding process within shorter temperature rise time.
Owner:BYD CO LTD
Sulfide-based lithium-ion-conducting solid electrolyte glass, all-solid lithium secondary battery, and method for manufacturing all-solid lithium secondary battery
InactiveCN101494299APrevent precipitationPromote precipitationNon-metal conductorsCell electrodesVitrificationLithium
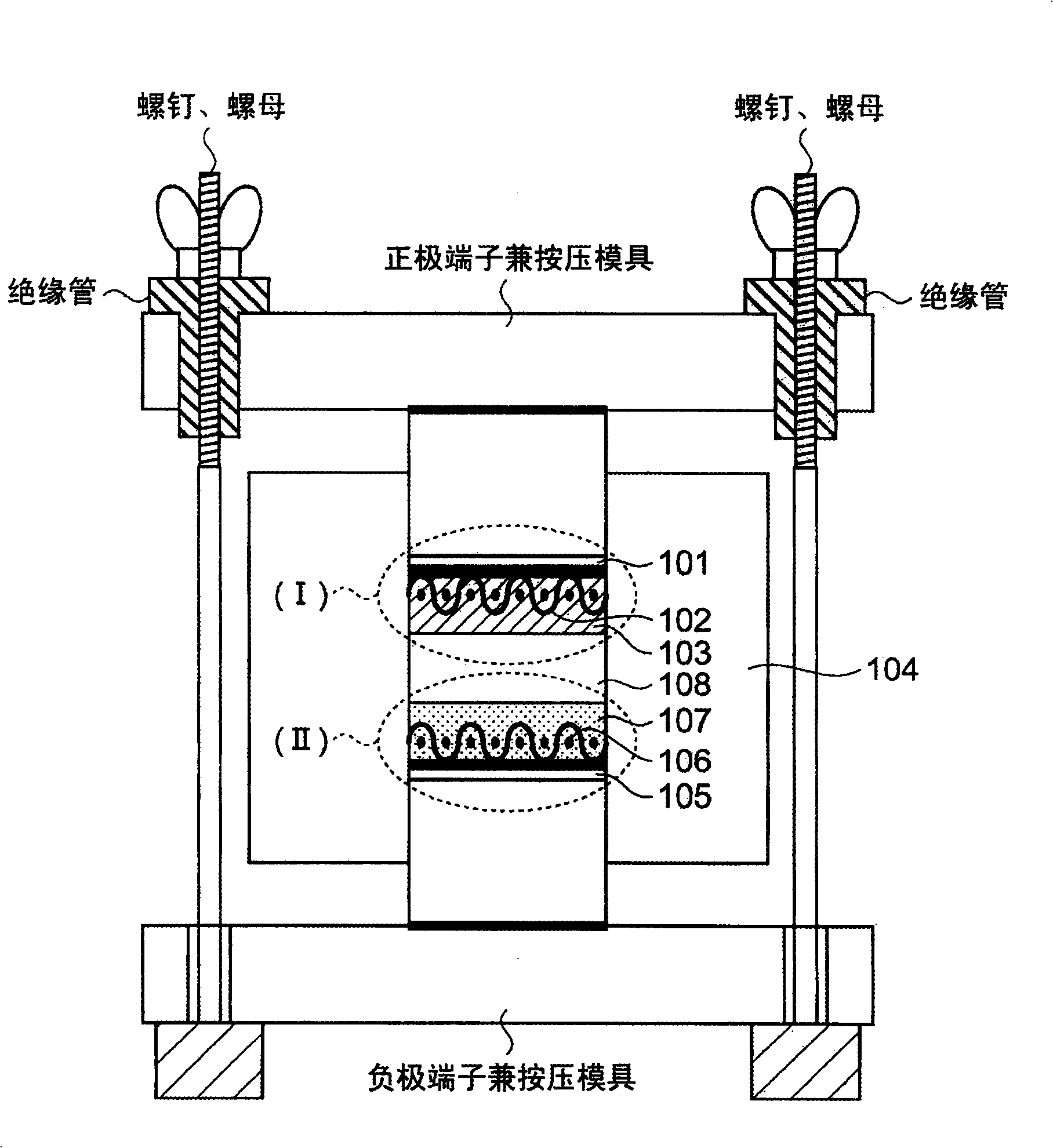
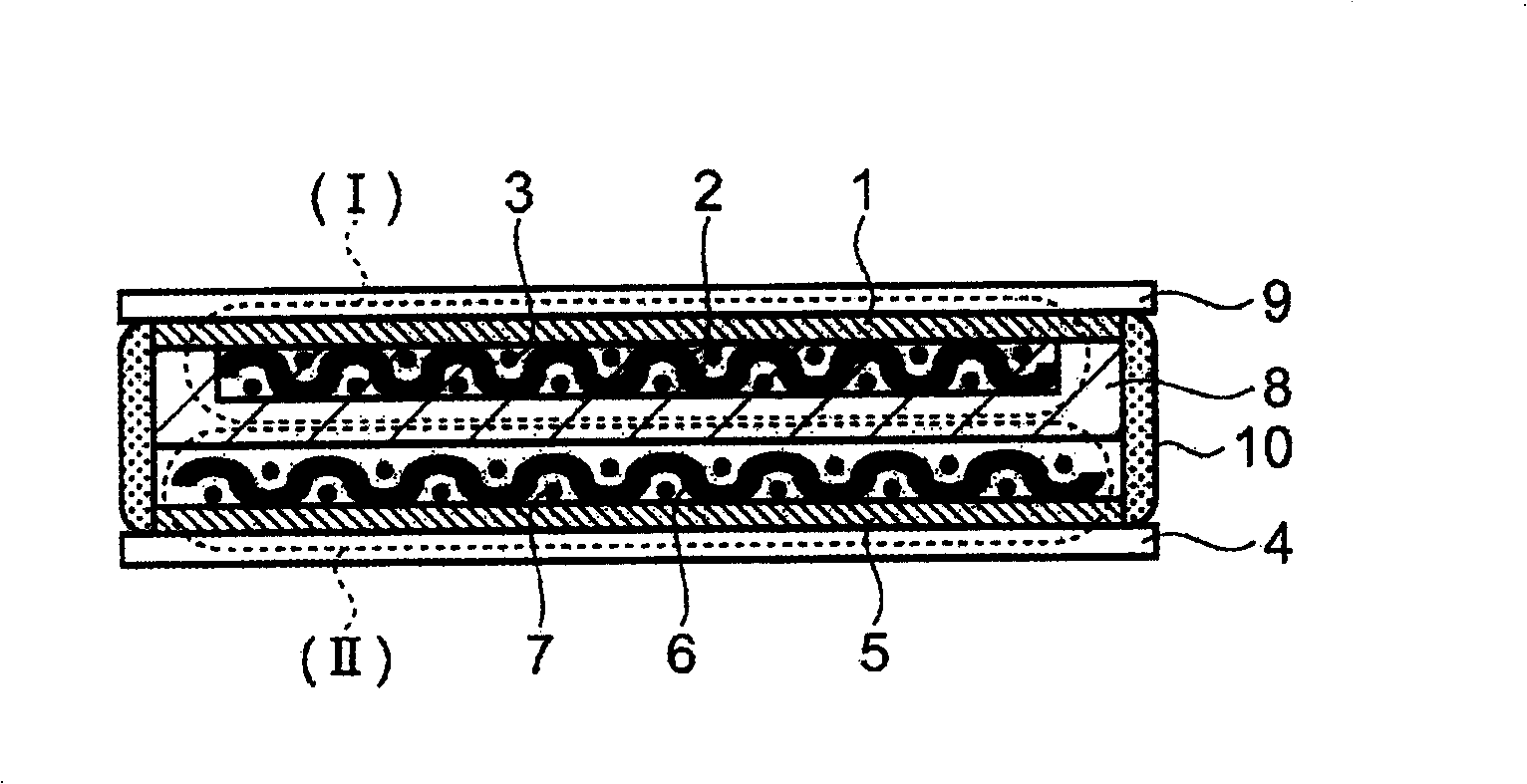
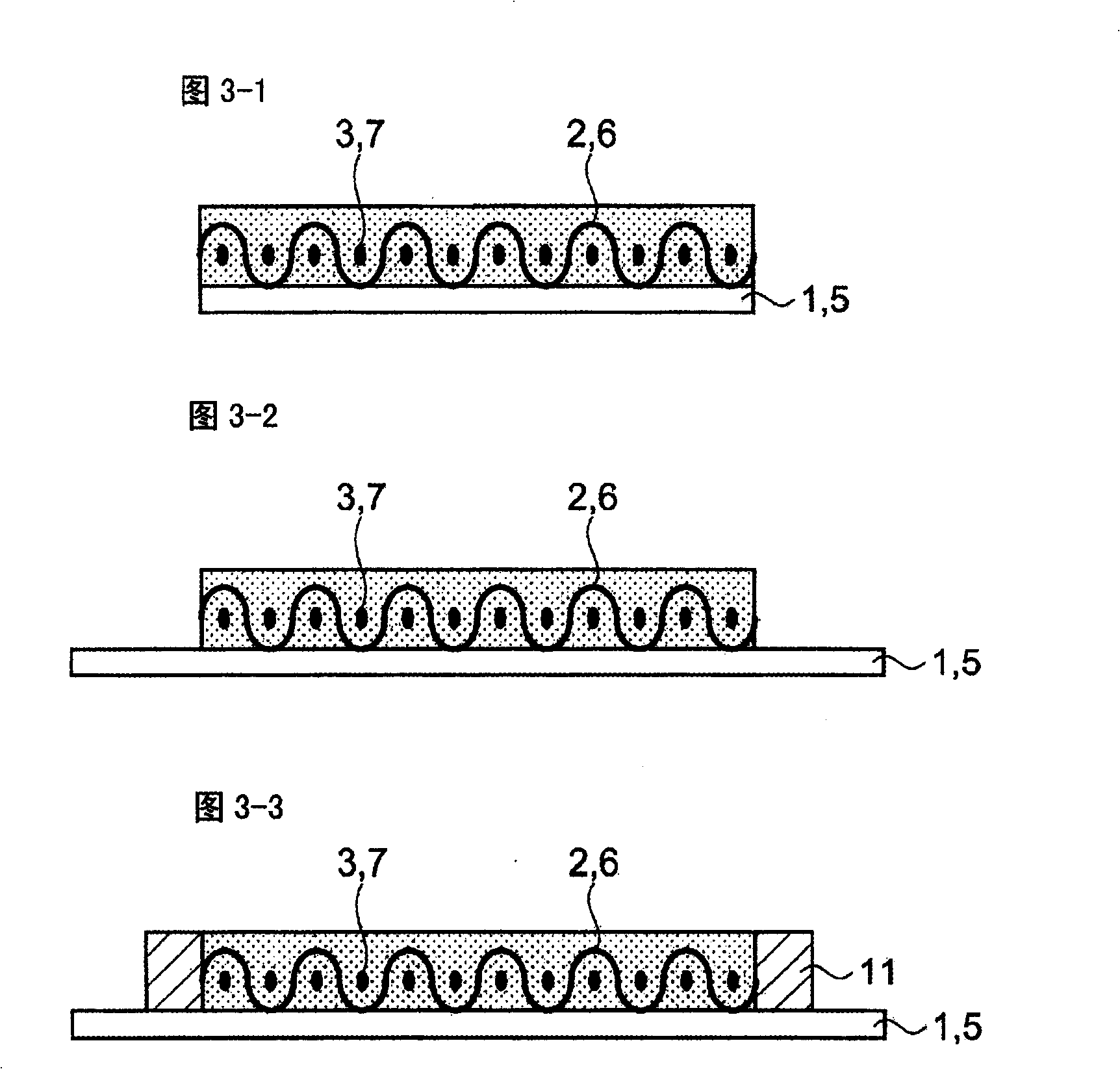
The present invention discloses a all-solid lithium secondary battery that has large chare and discharge output current denseness and excellent charge and discharge cycle life which can be easily manufactured at low cost. In the manufacturing process of the all-solid lithium secondary battery, a new lithium ion conductor with ionic conduction improved can be obtained through vitrification to mixed electrolytes by mixing Alpha-oxide of alumina in various sulfide system lithium ion conductivity solid electrolyte. The electrolytes layer 8 used the lithium ion conductor, and positive and negative electrode (I), (II) are formed by positive and negative electrode material 3, 7 are constituted. Subsequently, cascading at least 1 layer in positive and negative electrode (I), (II) with electrolytes layer, and producing battery while electrolytes not crystallizes by heating and compressing as a whole.
Owner:SEIKO EPSON CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com