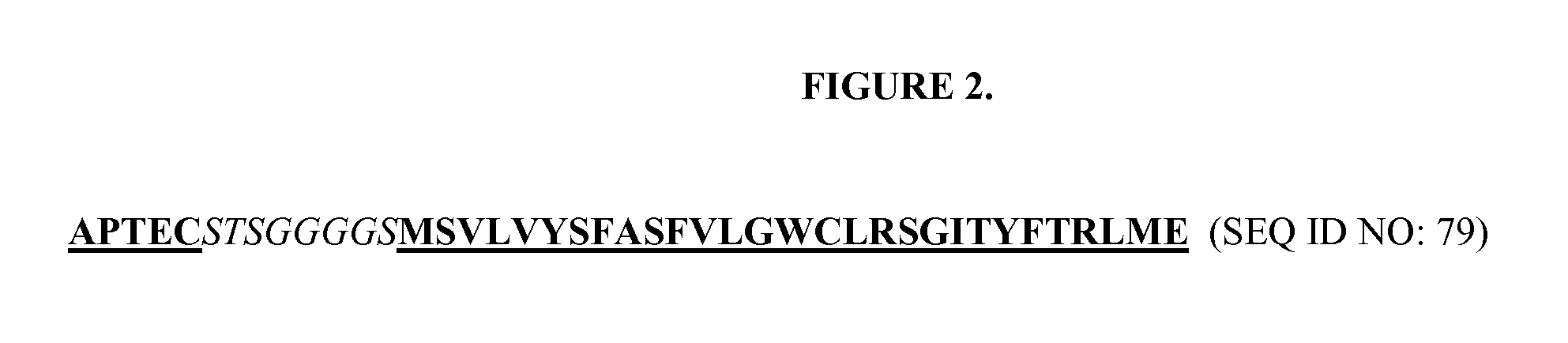Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
413results about How to "Minimize contamination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
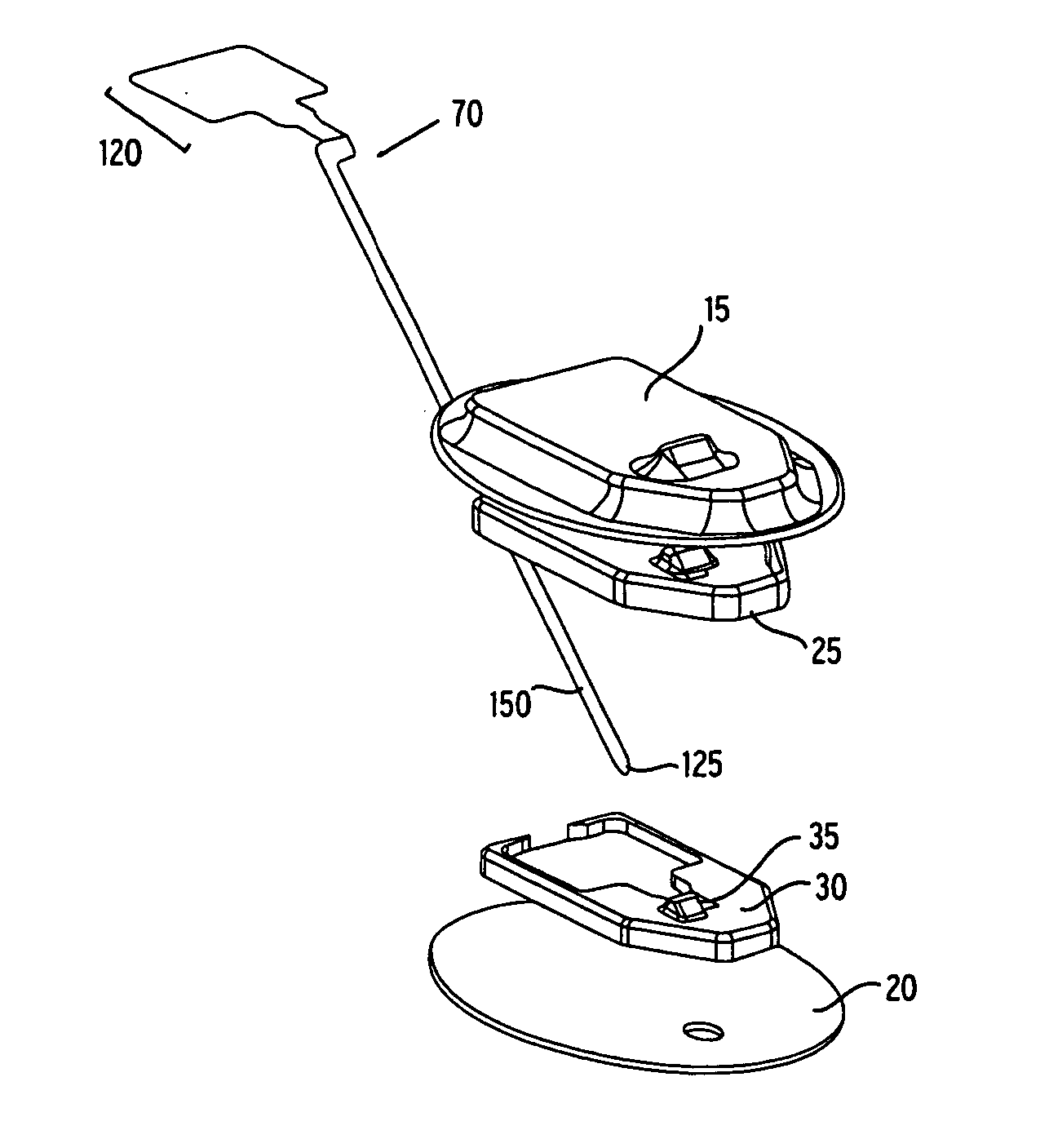
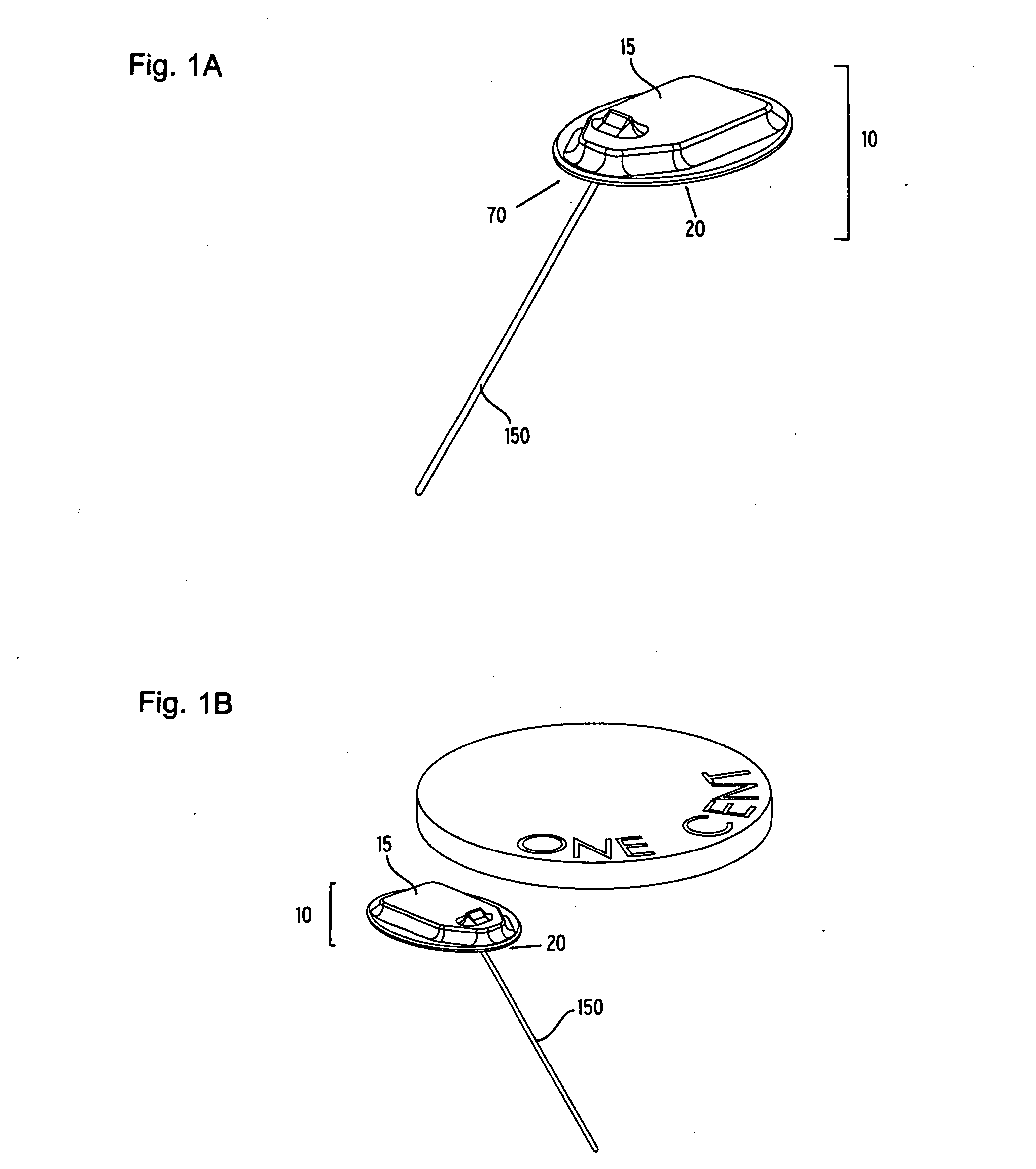
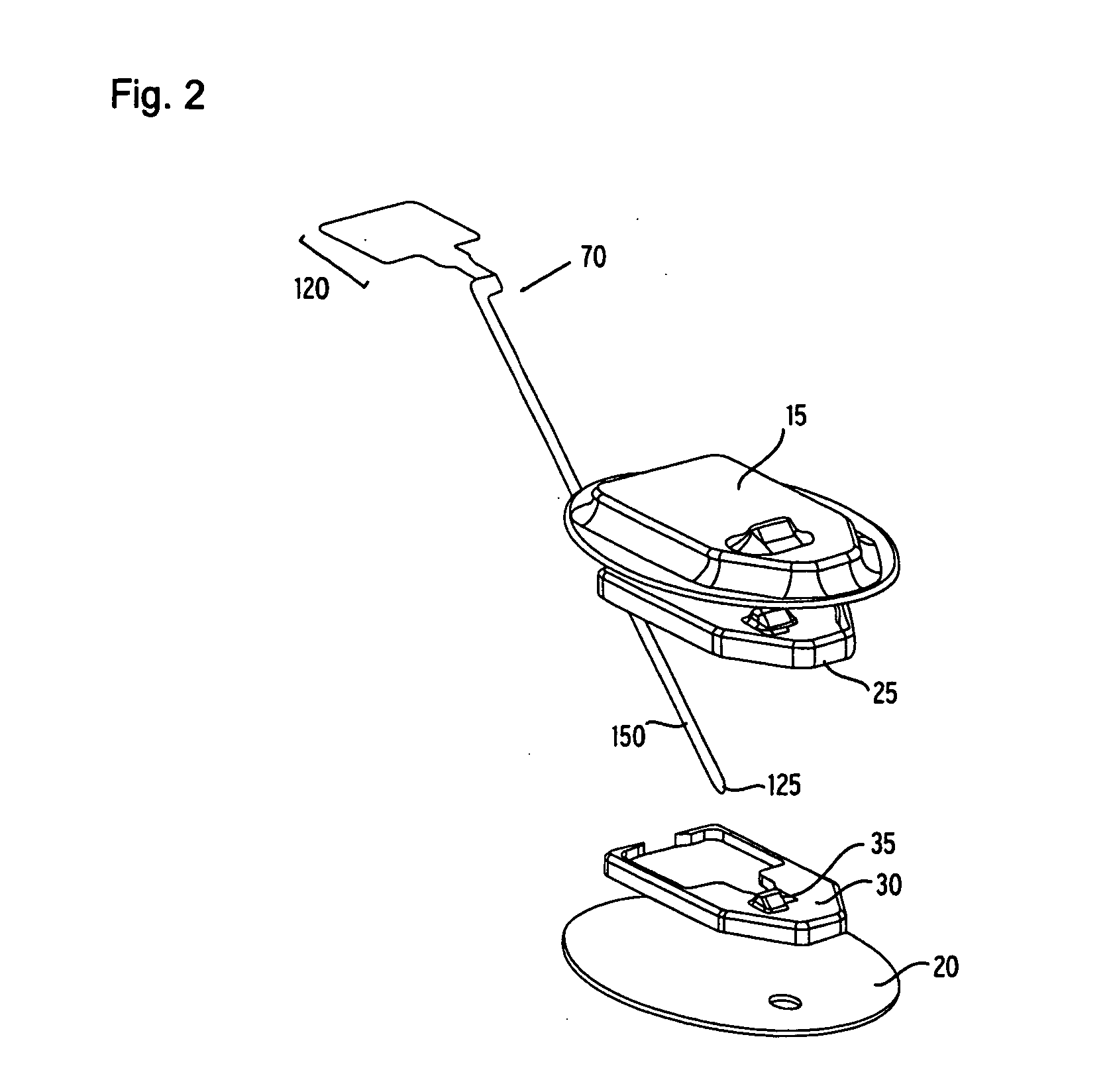
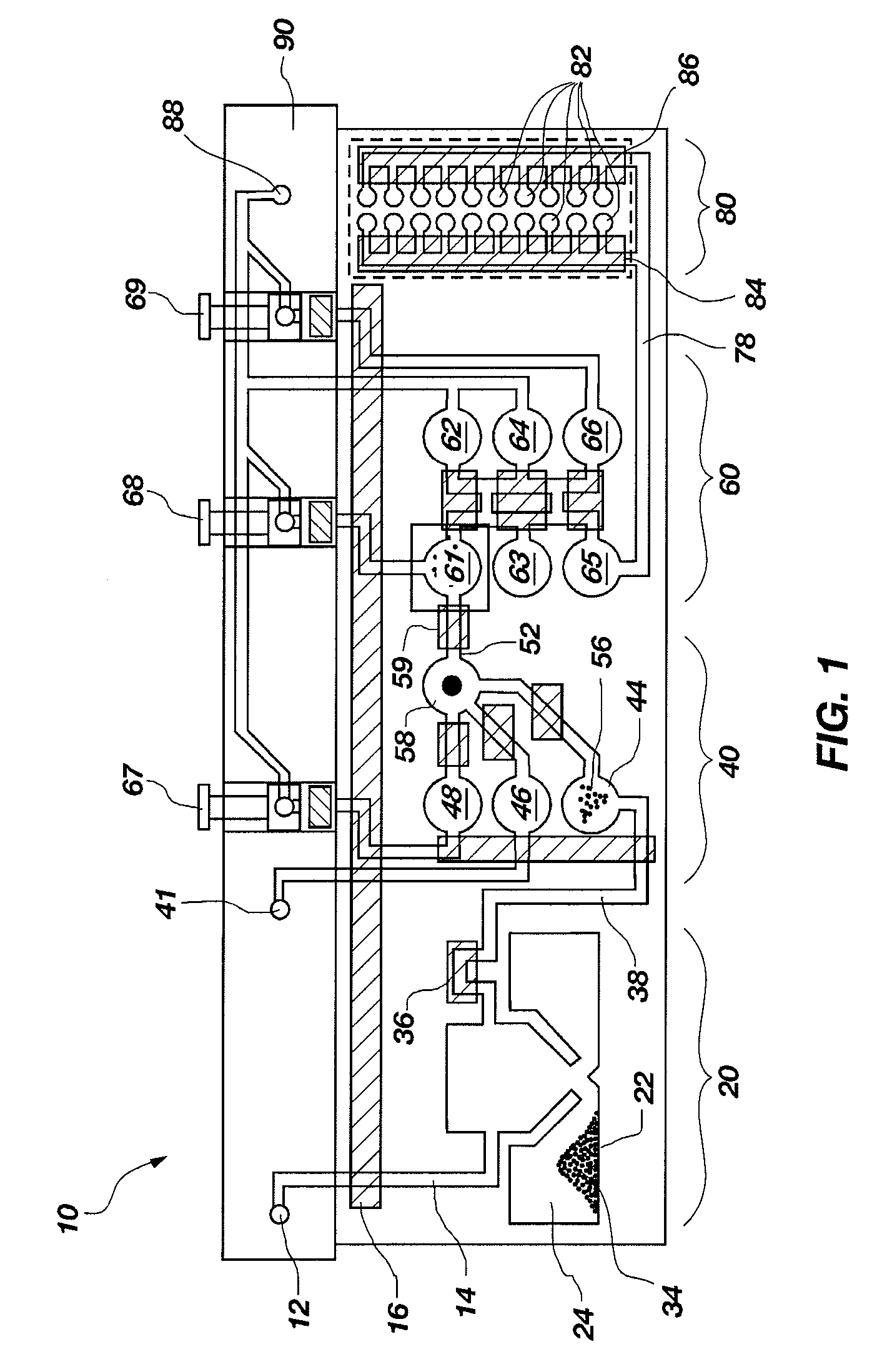
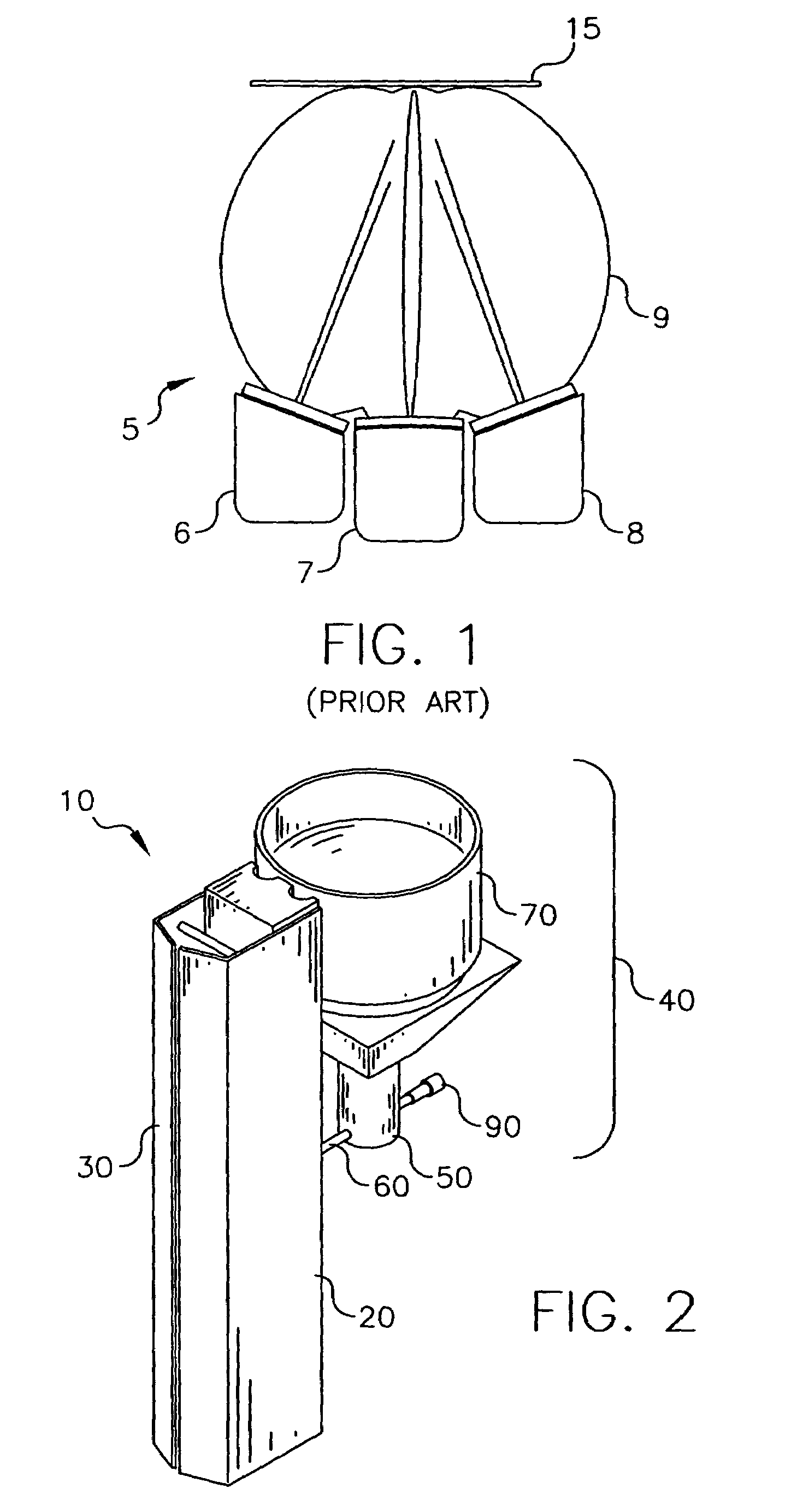
Flexible sensor apparatus
ActiveUS20070073129A1Quick and easy transcutaneous placementImprove stabilitySurgical needlesCatheterAnalyteAdhesive
A flexible mounting base to hold a sensor at an infusion site, the sensor being a removable in vivo sensor for monitoring analyte concentration level in a patient, such as blood glucose (BG) level. The mounting base comprises a flexible adhesive that anchors the flexible sensor set at an infusion site to provide stability for the sensor set in a convenient and comfortable manner. Placement of the mounting base onto the patient's skin causes the insertion needle to pierce the skin for transcutaneous placement of the cannula with the sensor therein. The insertion needle can then be withdrawn to leave the cannula and sensor at the selected insertion position, with the distal segment of the sensor being exposed to patient extracellular fluid via apertures formed in the cannula.
Owner:MEDTRONIC MIMIMED INC
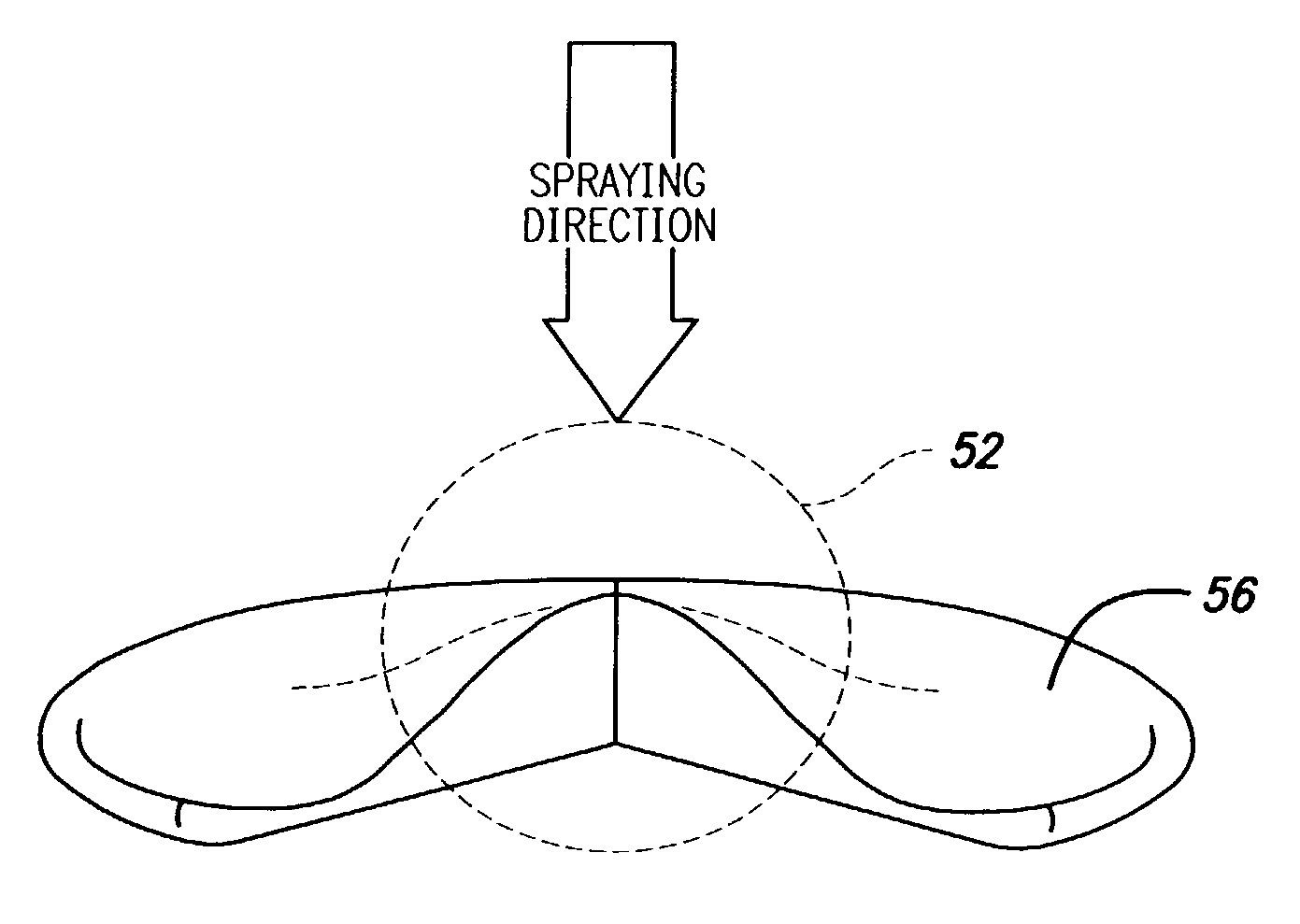
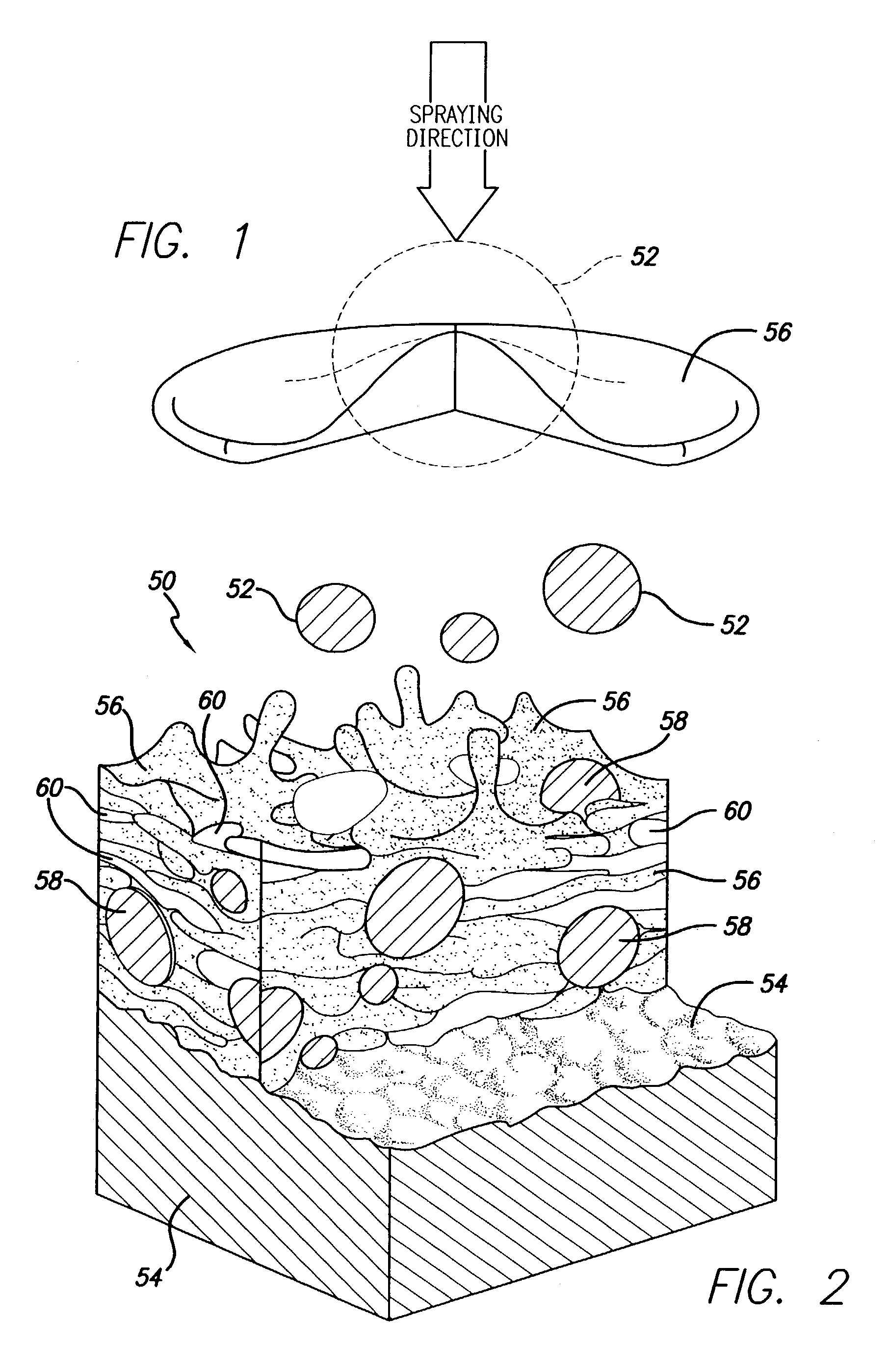
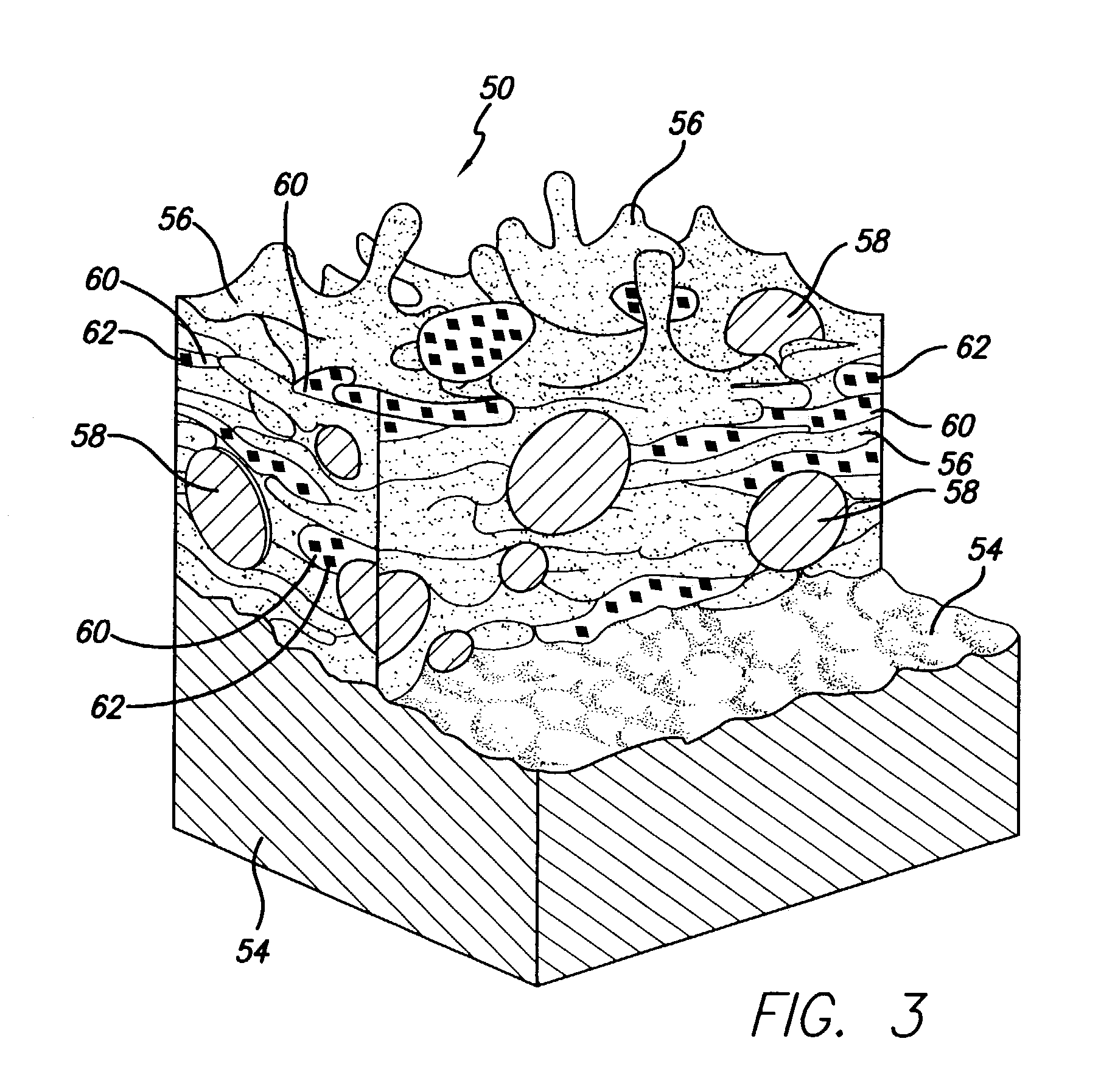
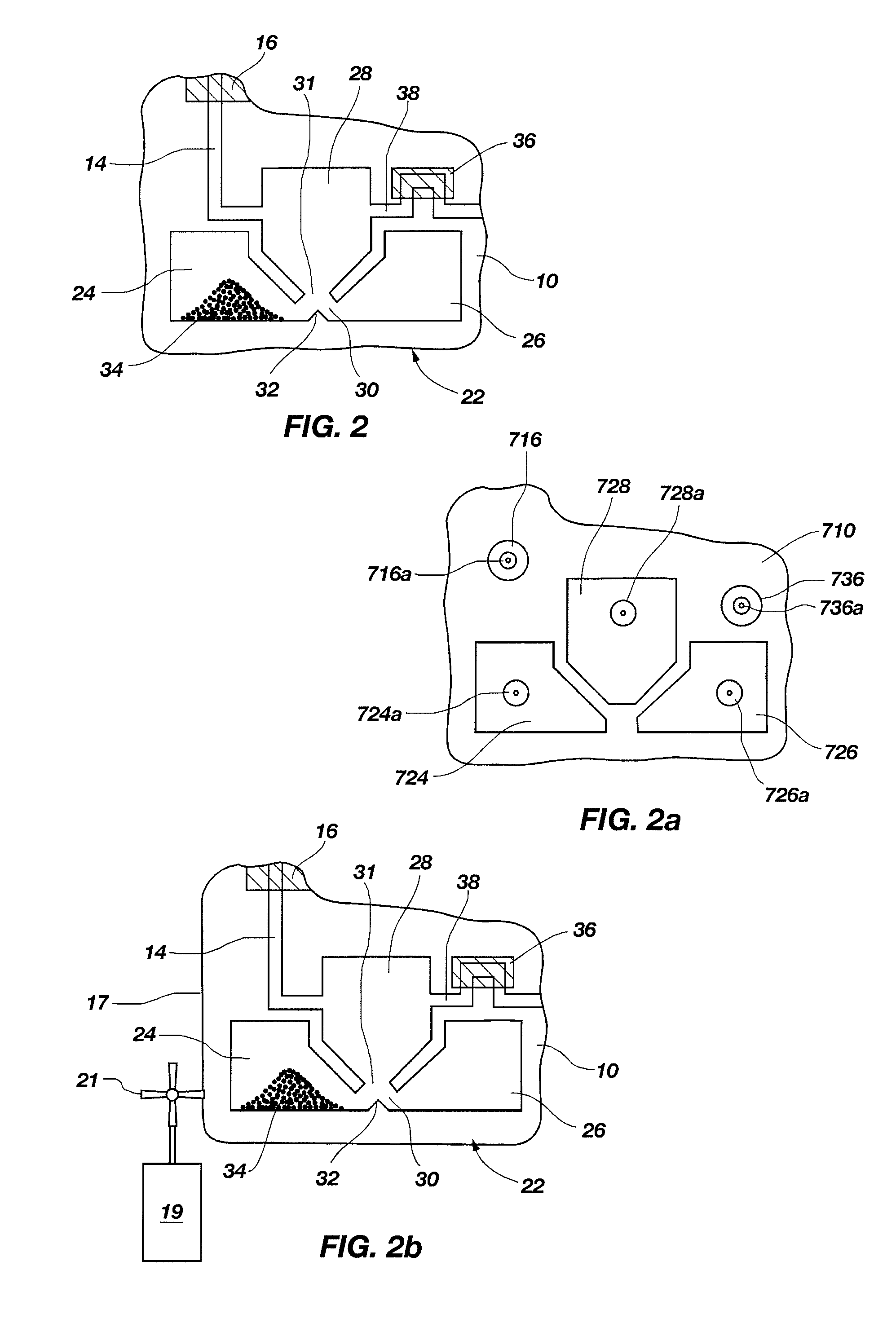
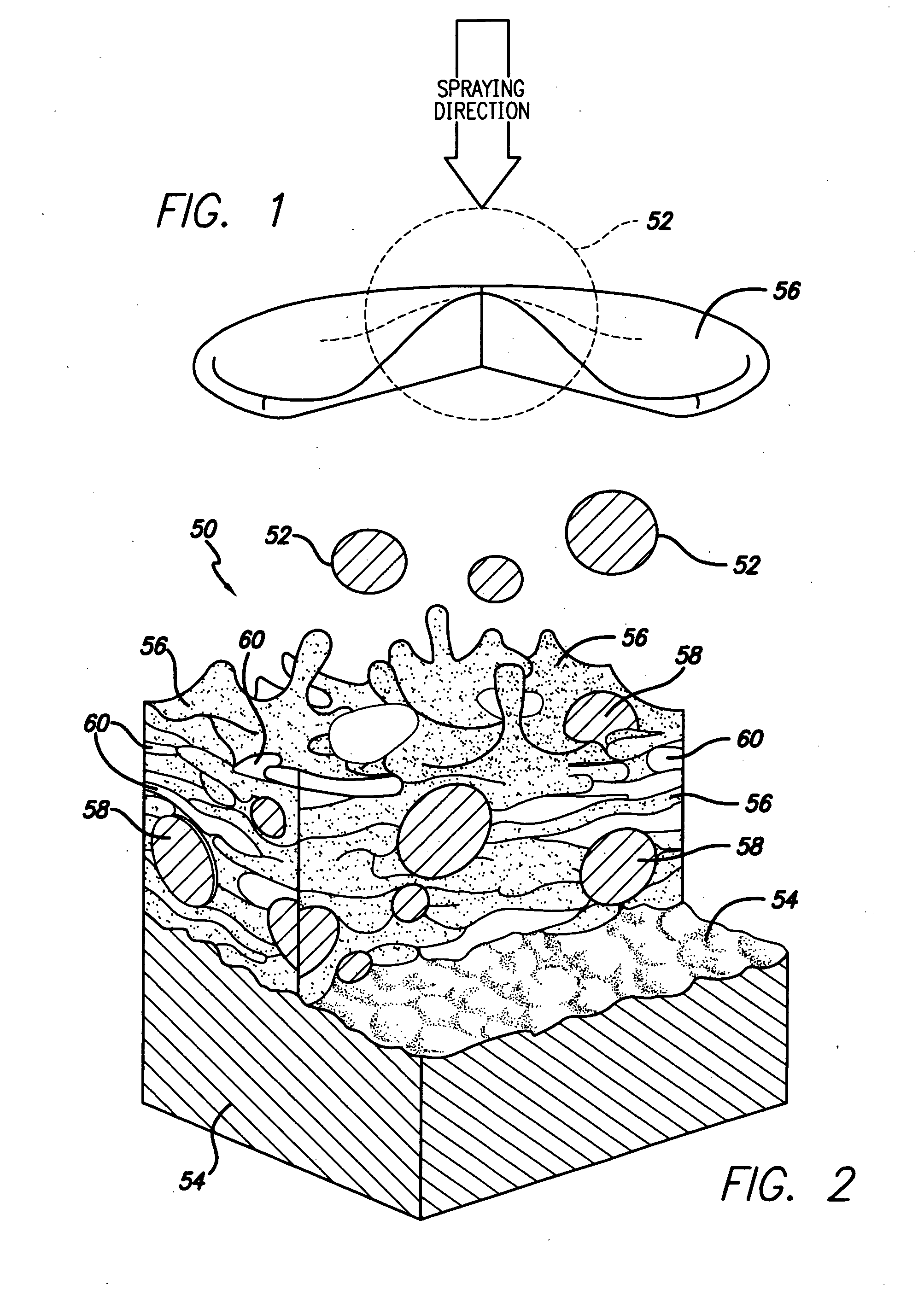
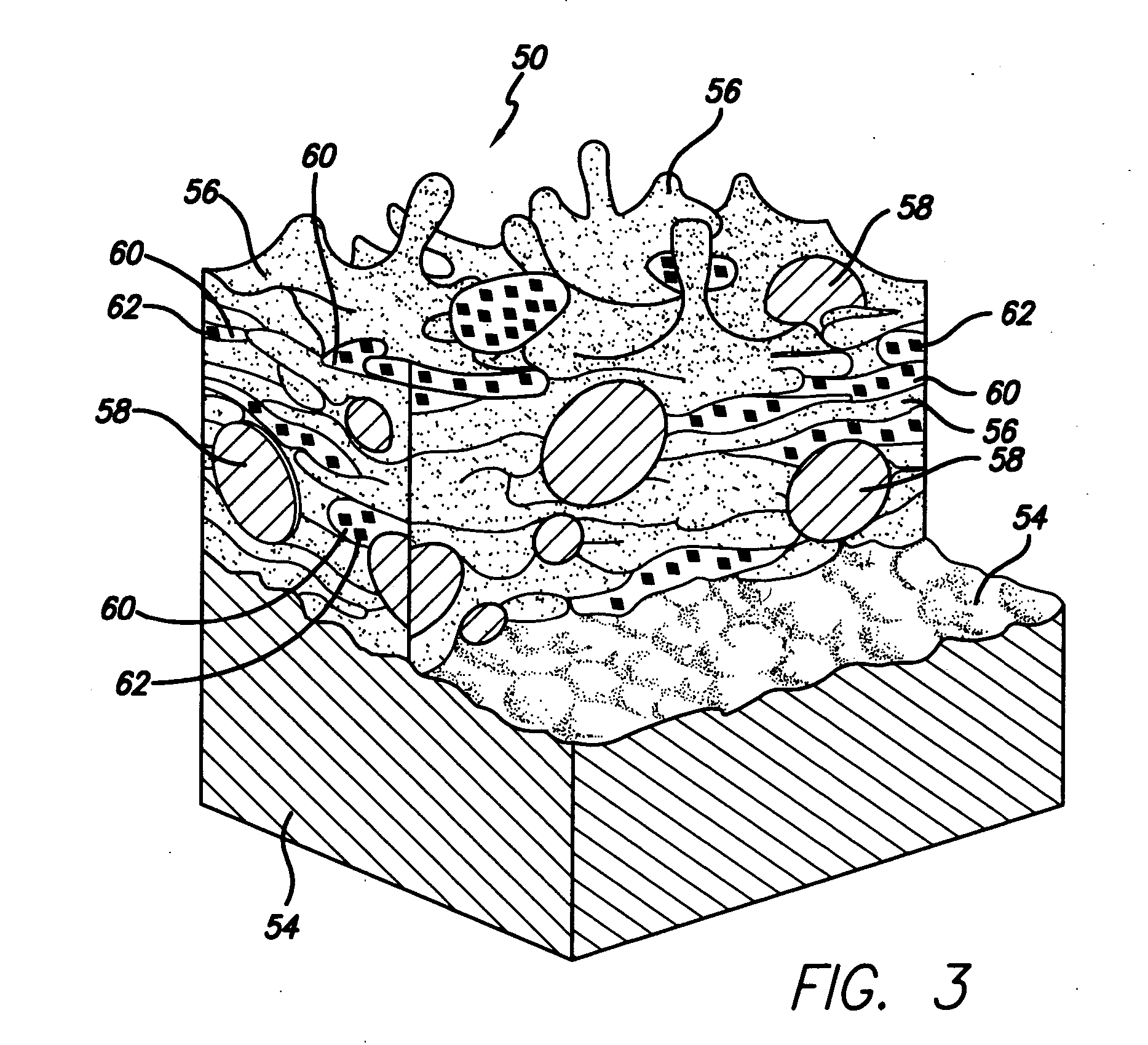
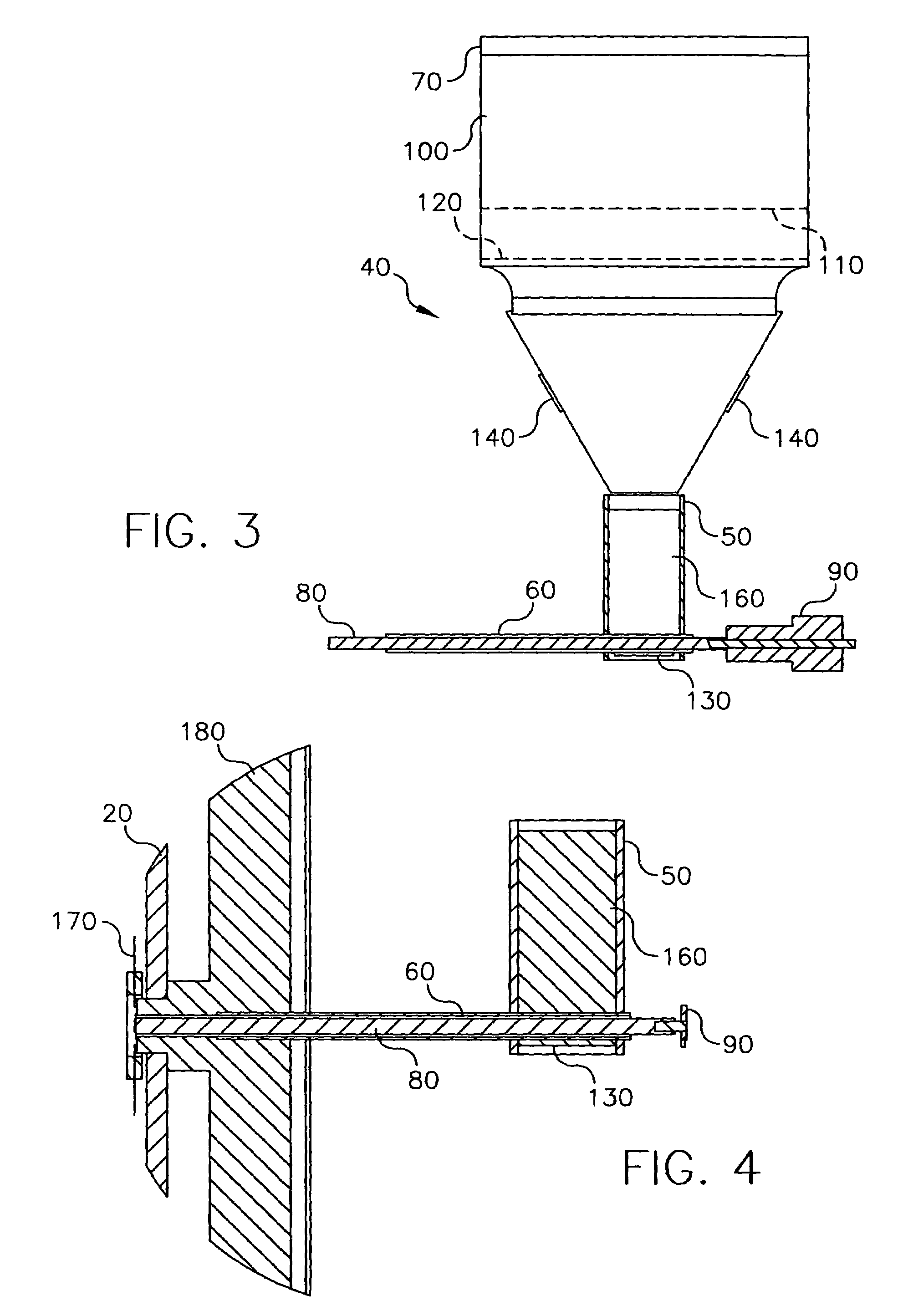
Spray processing of porous medical devices
InactiveUS7163715B1Minimize drug releaseMinimize contaminationMolten spray coatingSurgeryPolymerPorous substrate
Thermal spray processing and cold spray processing are utilized to manufacture porous starting materials (such as tube stock, wire and substrate sheets) from biocompatible metals, metal alloys, ceramics and polymers that may be further processed into porous medical devices, such as stents. The spray processes are also used to form porous coatings on consolidated biocompatible medical devices. The porous substrates and coatings may be used as a reservoir to hold a drug or therapeutic agent for elution in the body. The spray-formed porous substrates and coatings may be functionally graded to allow direct control of drug elution without an additional polymer topcoat. The spray processes are also used to apply the drug or agent to the porous substrate or coating when drug or agent is robust enough to withstand the temperatures and velocities of the spray process with minimal degradation.
Owner:ABBOTT CARDIOVASCULAR
Microfluidic sequencing systems
InactiveUS7238323B2Minimize contaminationIncrease speedBioreactor/fermenter combinationsBiological substance pretreatmentsGenomic screeningSoftware
Owner:CAPLIPER LIFE SCI INC
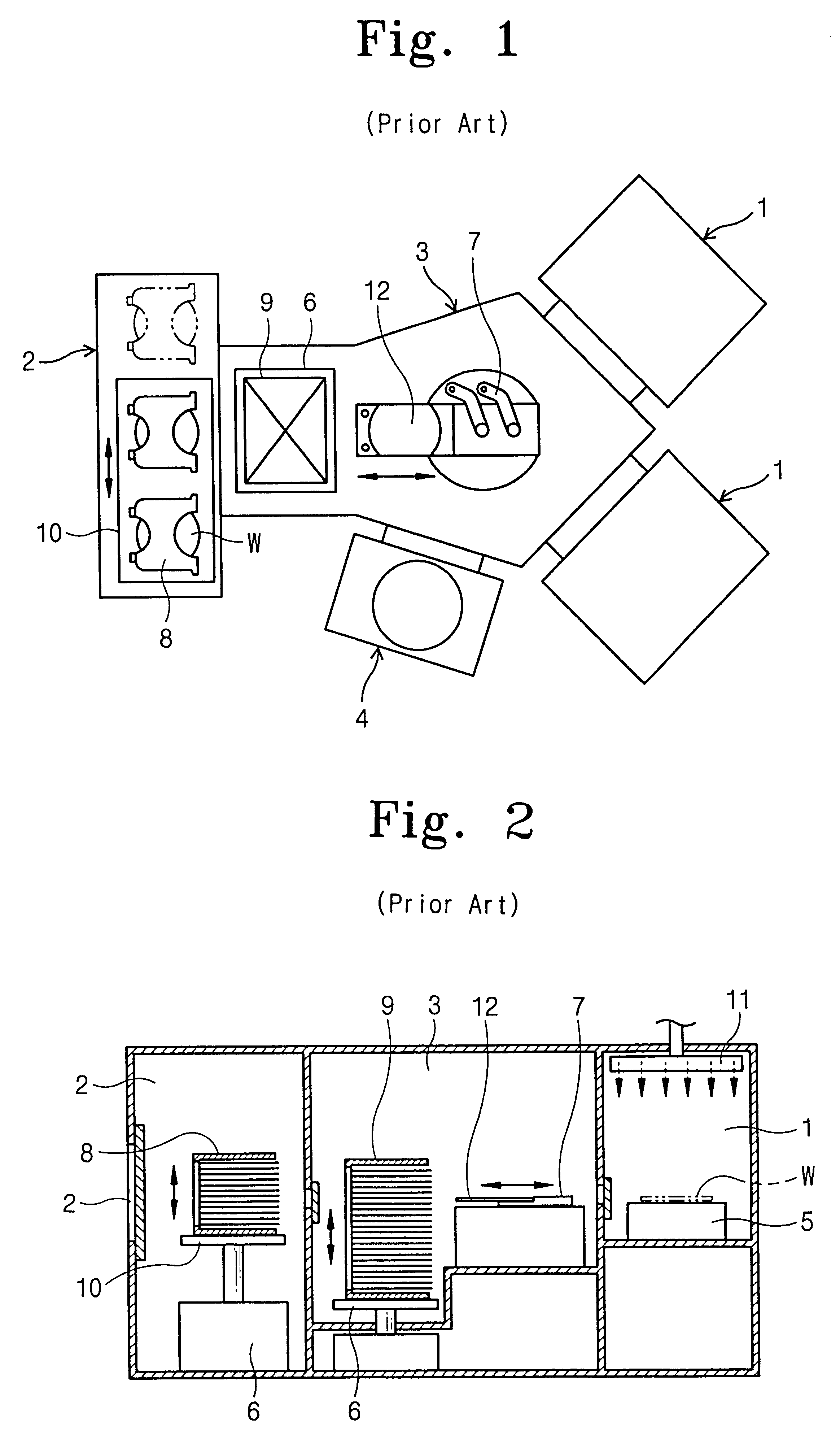
Etching apparatus for manufacturing semiconductor devices
InactiveUS20010037856A1Operation efficiency can be improvedReduce processing timeSemiconductor/solid-state device manufacturingCharge manipulationMagnetic tapeWafer stacking
An etching apparatus for manufacturing semiconductor devices which reduces contamination of the processing surface of a wafer by transporting a plurality of wafers stacked in a cassette with their processing surfaces facing down from the cassette supply chamber to one or more process chambers where the etching operation is performed on each wafer, one at a time. The apparatus has a load lock chamber for transferring the wafers stacked in the cassette from the cassette supply chamber, which is maintained under atmospheric conditions, to the process chamber, which is maintained under a strong vacuum. The process chamber has a cathode to which a wafer is clamped by a wafer holder with its processing surface facing down; the process chamber may also have a removable lower cover for easy repair and cleaning. The apparatus may also have a wafer aligning chamber installed between the cassette supply chamber and the load lock chamber for simultaneously aligning all of the wafers n the cassette before they are transported to the load lock chamber. The wafer aligning chamber also has a cassette transport mechanism for transferring the cassette from a cassette supply table in the cassette supply chamber to an elevator installed in the load lock chamber.
Owner:SAMSUNG ELECTRONICS CO LTD
System for Conducting the Identification of Bacteria in Urine
ActiveUS20110008825A1Rapid diagnosisImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsCuvettePipette
A system for conducting the identification and quantification of micro-organisms, e.g., bacteria in urine samples which includes: 1) several disposable cartridges for holding four disposable components including a centrifuge tube, a pipette tip having a 1 ml volume, a second pipette tip having a 0.5 ml volume, and an optical cup or cuvette; 2) a sample processor for receiving the disposable cartridges and processing the urine samples including transferring the processed urine sample to the optical cups; and 3) an optical analyzer for receiving the disposable cartridges and configured to analyze the type and quantity of micro-organisms in the urine sample. The disposable cartridges with their components including the optical cups or cuvettes are used in the sample processor, and the optical cups or cuvettes containing the processed urine samples are used in the optical analyzer for identifying and quantifying the type of micro-organism existing in the processed urine samples.
Owner:POCARED DIAGNOSTICS
Self-contained biological analysis
ActiveUS8394608B2Rapid and sensitiveMinimize contaminationHeating or cooling apparatusMicrobiological testing/measurementImmuno pcrBiological organism
Owner:BIOFIRE DIAGNOSTICS LLC
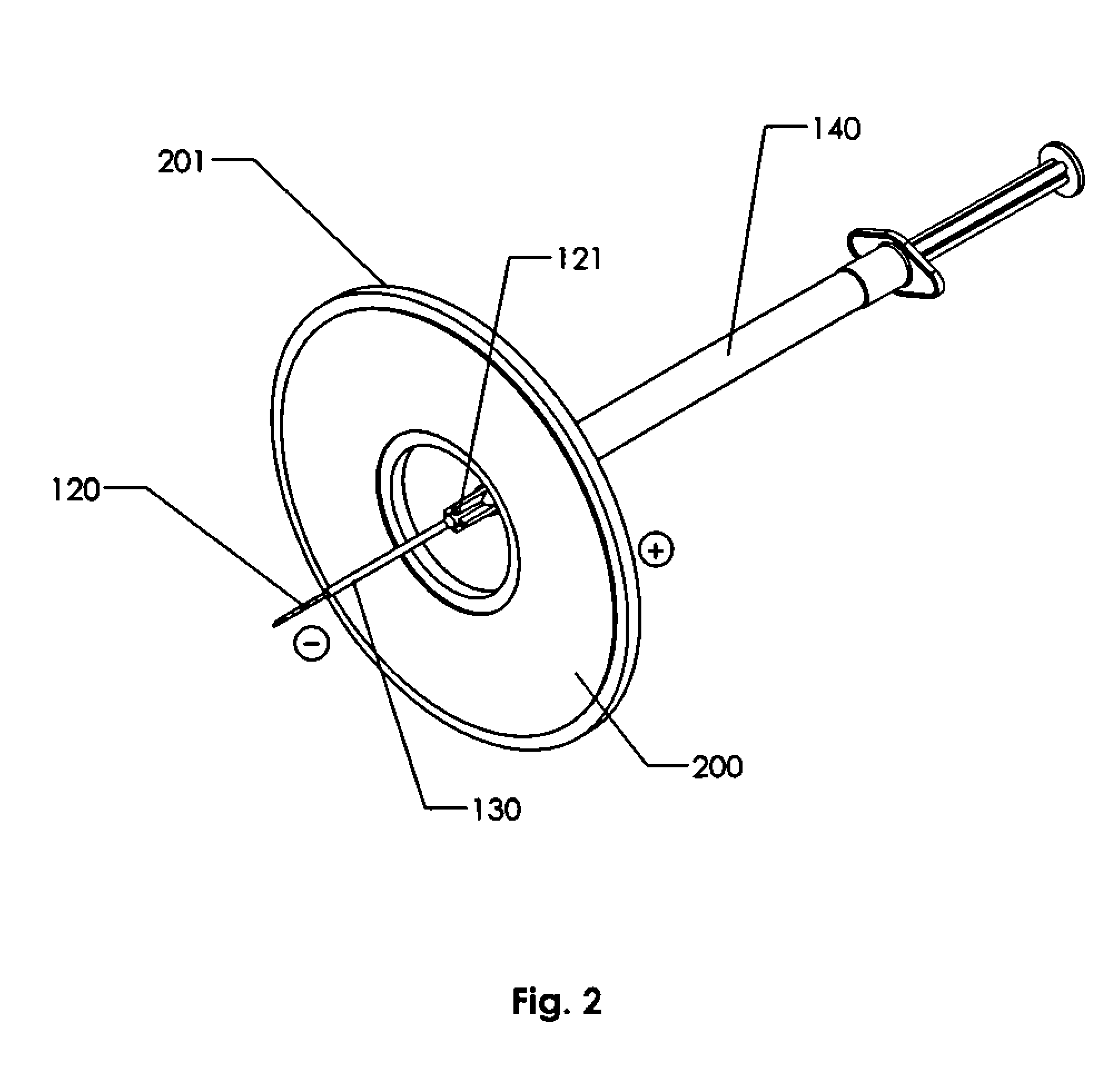
Variable current density single needle electroporation system and method
ActiveUS20110009807A1Efficient use ofSignificant differenceElectrotherapyMedical devicesPower flowElectroporation
This invention comprises an improved electroporation electrode system comprising a single needle and a ring or donut shaped electrode wherein the difference in surface area of the electrodes provide for a substantial reduction of current density near the surface of the treated tissue and a more concentrated current density sufficient for electroporation only in tissues adjacent to the terminal portion of the single needle electrode. Thus, this invention provides for targeting specific tissue for electroporation and also should provide for lessening the sensation of electric current in the treated tissue.
Owner:INOVIO PHARMA
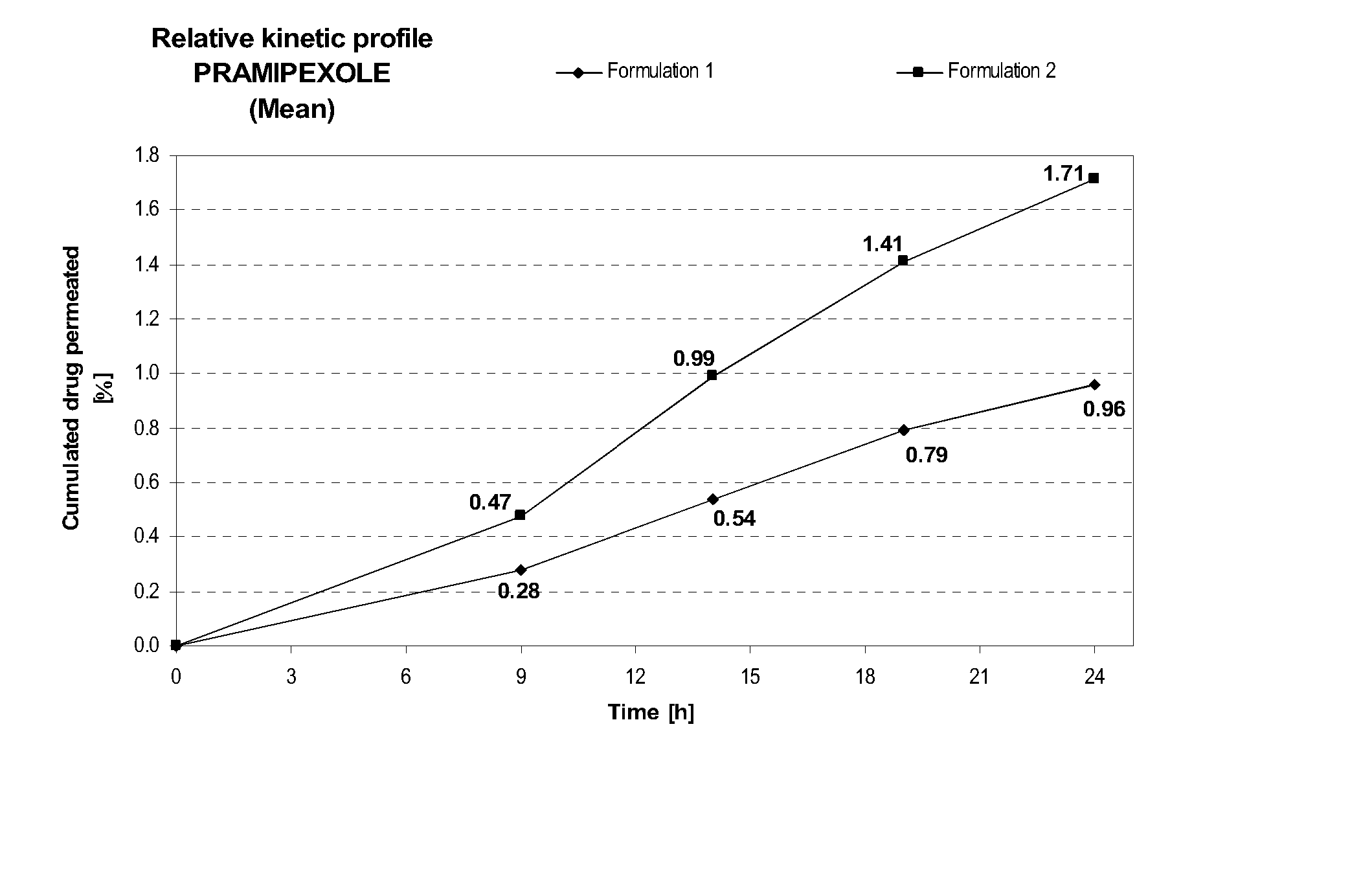
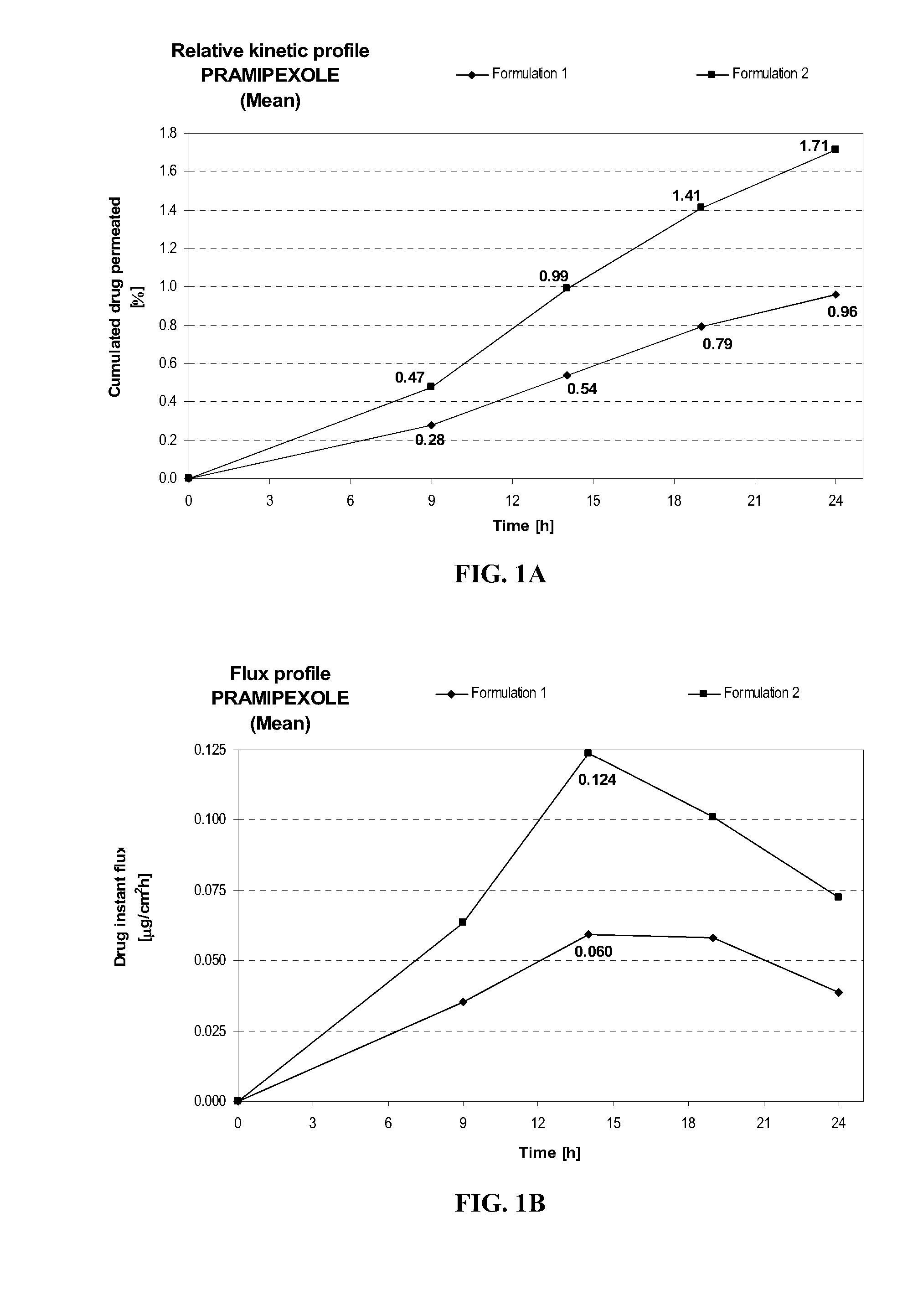
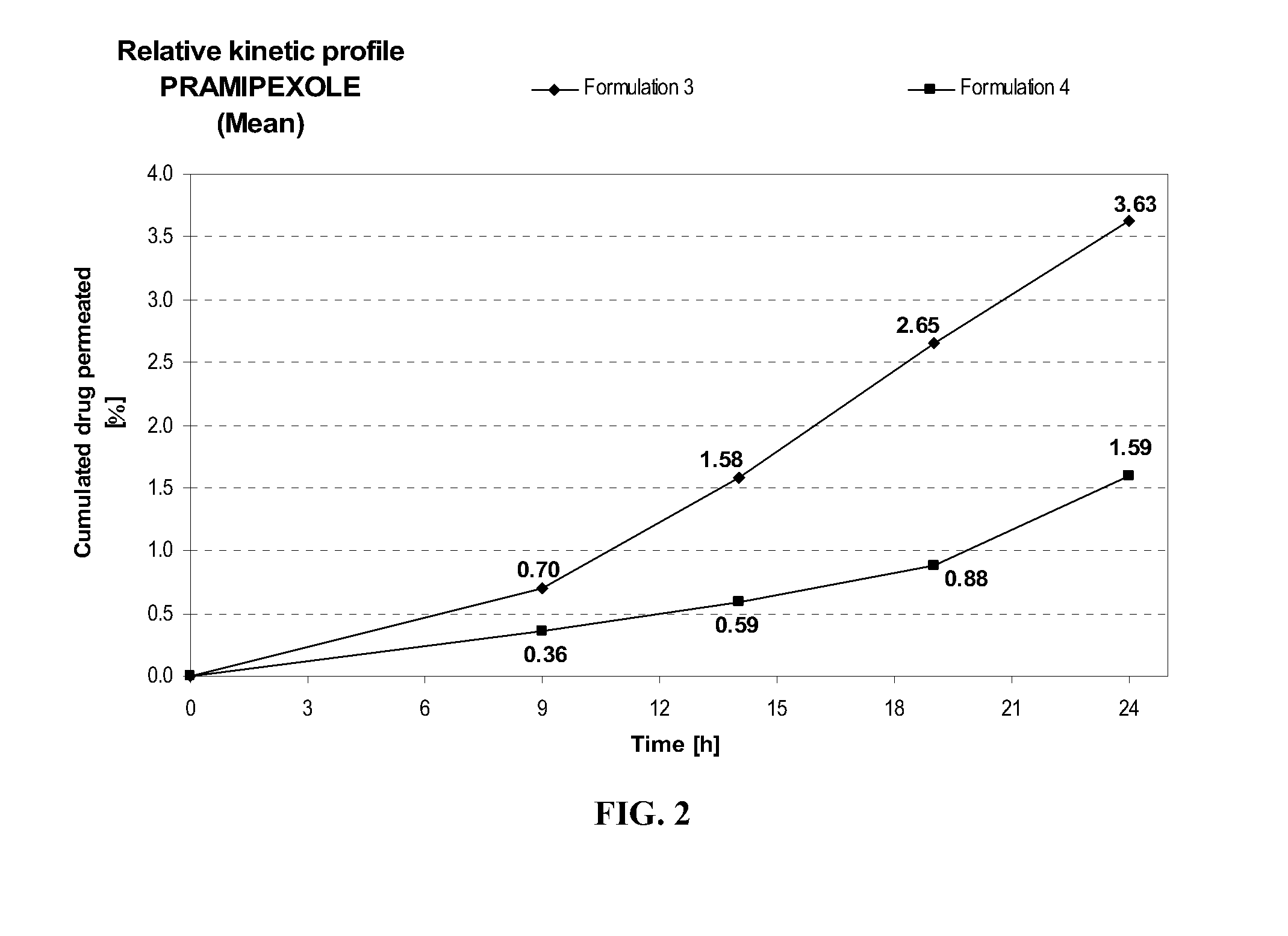
Transdermal delivery of systemically active central nervous system drugs
InactiveUS20070225379A1Reduce transferLoss of therapyBiocideOrganic non-active ingredientsNervous systemActive agent
The invention relates to a transdermal or transmucosal non-occlusive, semi-solid pharmaceutical formulation that includes at least one systemically active agent that acts on the Central Nervous System (CNS) of a mammal; and a permeation enhancing solvent system present in an amount sufficient to solubilize the at least one active ingredient. The permeation enhancing solvent system includes a pharmaceutically acceptable monoalkyl ether of diethylene glycol; a pharmaceutically acceptable glycol; preferably also a fatty alcohol and or a fatty acid; and a mixture of a C2 to C4 alcohol and water so that the permeation enhancing solvent system (a) inhibits crystallization of the at least one active ingredient on a skin or mucosal surface of a mammal, (b) reduces or prevents transfer of the formulation to clothing or to another being, (c) modulates biodistribution of the at least one active agent within different layers of skin, (d) facilitates absorption of the at least one active agent by a skin or a mucosal surface of a mammal, or (e) provides a combination of one or more of (a) through (d).
Owner:ANTARES PHARMA IPL
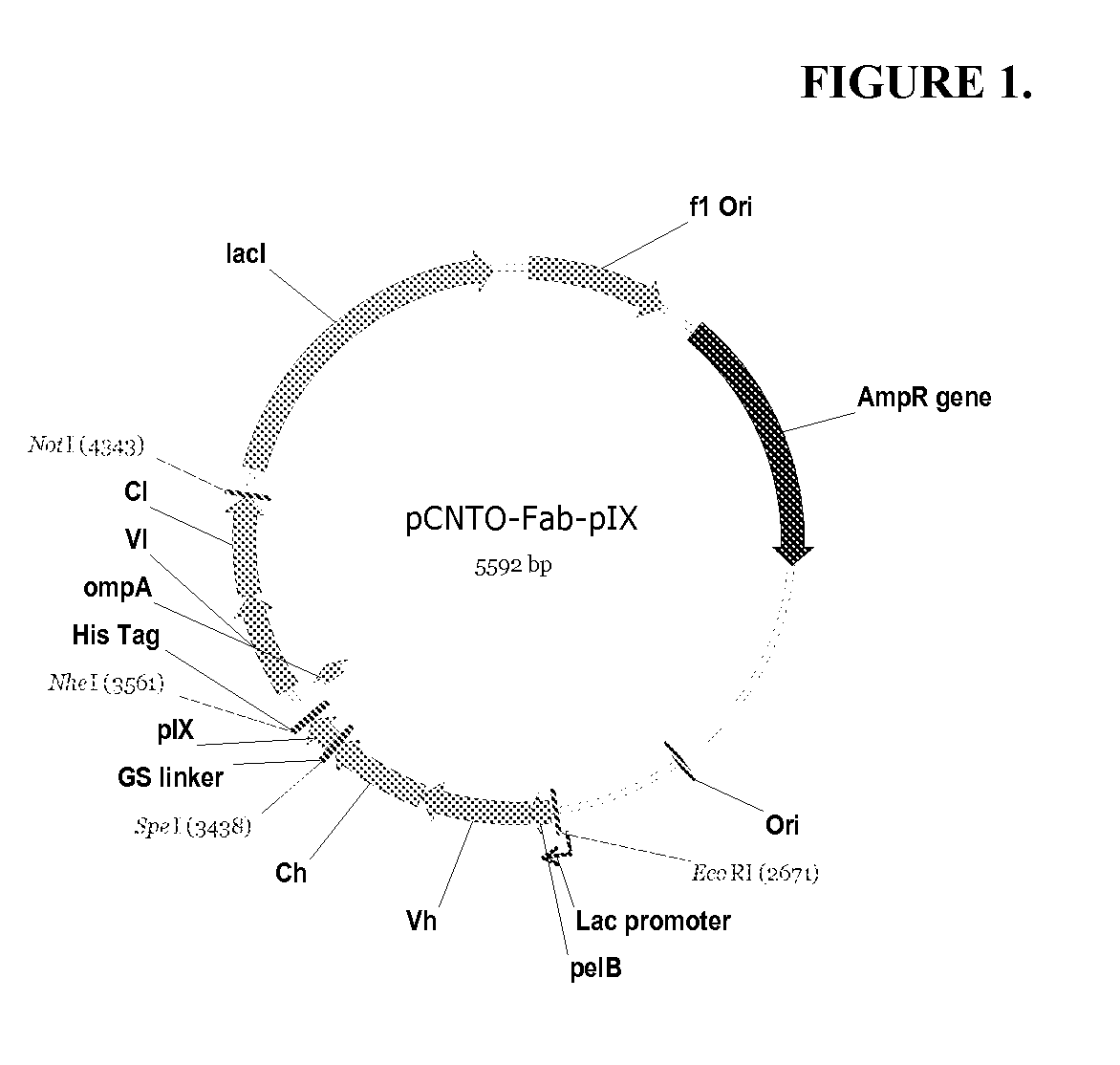
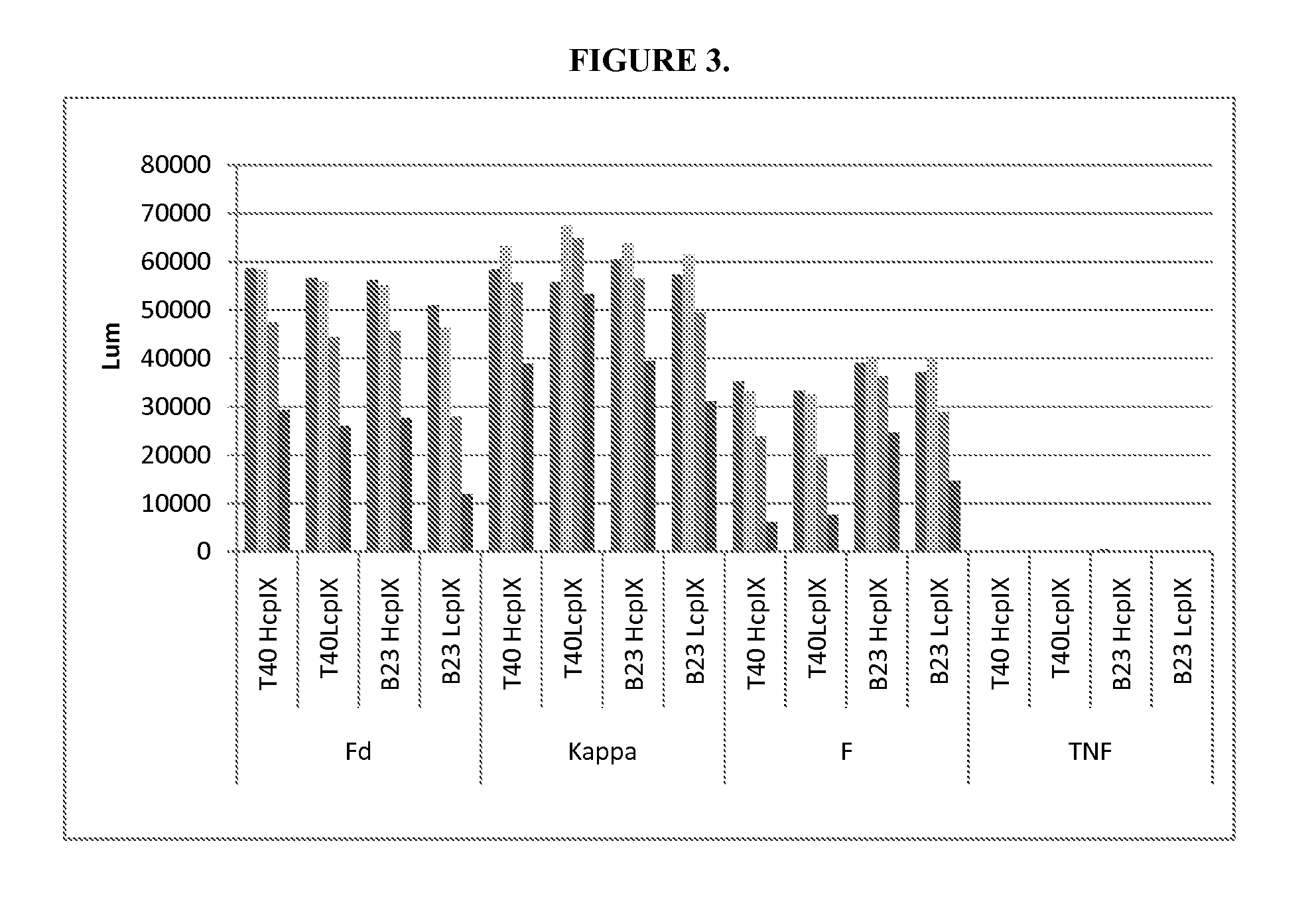
DESIGN AND GENERATION OF HUMAN DE NOVO pIX PHAGE DISPLAY LIBRARIES
InactiveUS20100021477A1Easy to understandIncrease diversityBacteriaLibrary screeningNatural antibodyStructure function
Described and claimed herein are combinatorial synthetic Fab libraries displayed on a phage pIX protein. The libraries were built on scaffolds representing the most frequently used genes in human antibodies, which were diversified to mirror the variability of natural antibodies. After selection using a diverse panel of proteins, numerous specific and high-affinity Fabs were isolated. By a process called in-line maturation the affinity of some antibodies was improved up to one hundred-fold yielding low pM binders suitable for in vivo use. This work thus demonstrates the feasibility of displaying complex Fab libraries as pIX-fusion proteins for antibody discovery and lays the foundations for studies on the structure-function relationship of antibodies.
Owner:JANSSEN BIOTECH INC
Microfluidic devices and methods for optimizing reactions
InactiveUS6440722B1Increase speedMinimize contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsGenomic screeningSoftware
Integrated systems, apparatus, software, and methods for performing biochemical analysis, including DNA sequencing, genomic screening, purification of nucleic acids and other biological components and drug screening are provided. Microfluidic devices, systems and methods for using these devices and systems for performing a wide variety of fluid operations are provided. The devices and systems of are used in performing fluid operations which require a large number of iterative, successive or parallel fluid manipulations, in a microscale, or sealed and readily automated format.
Owner:CAPLIPER LIFE SCI INC
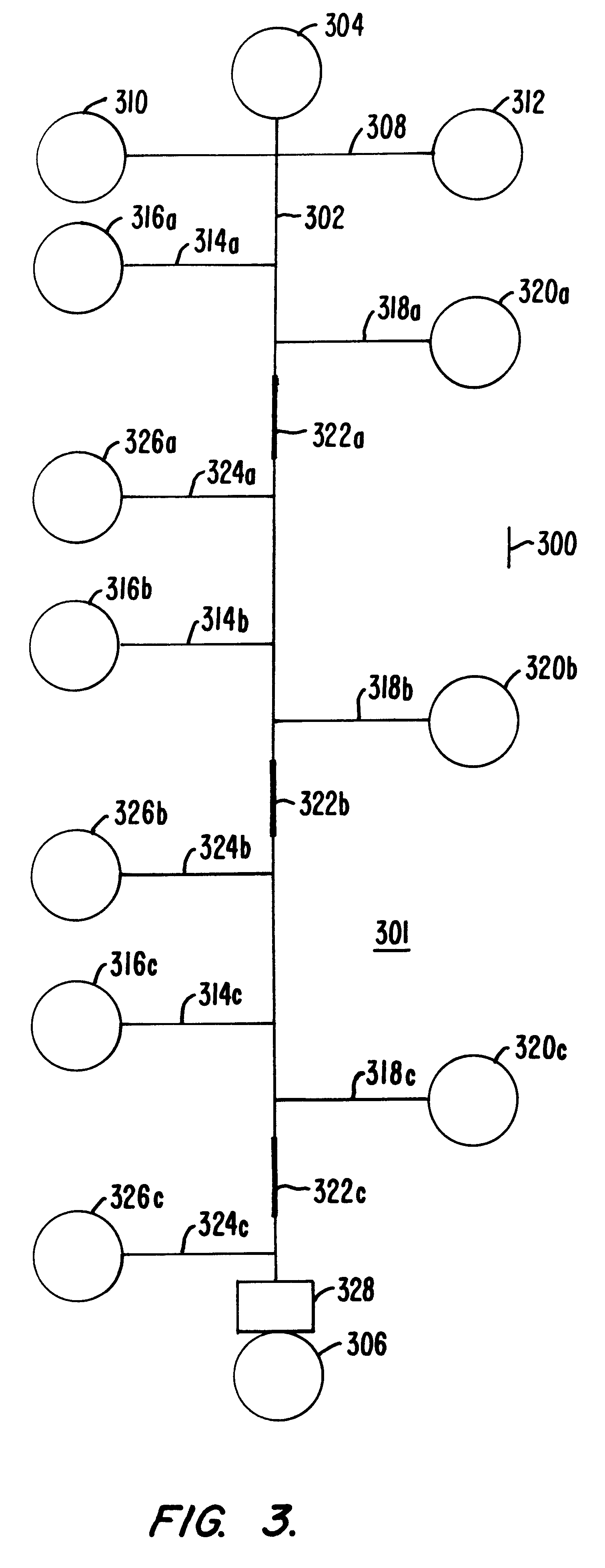
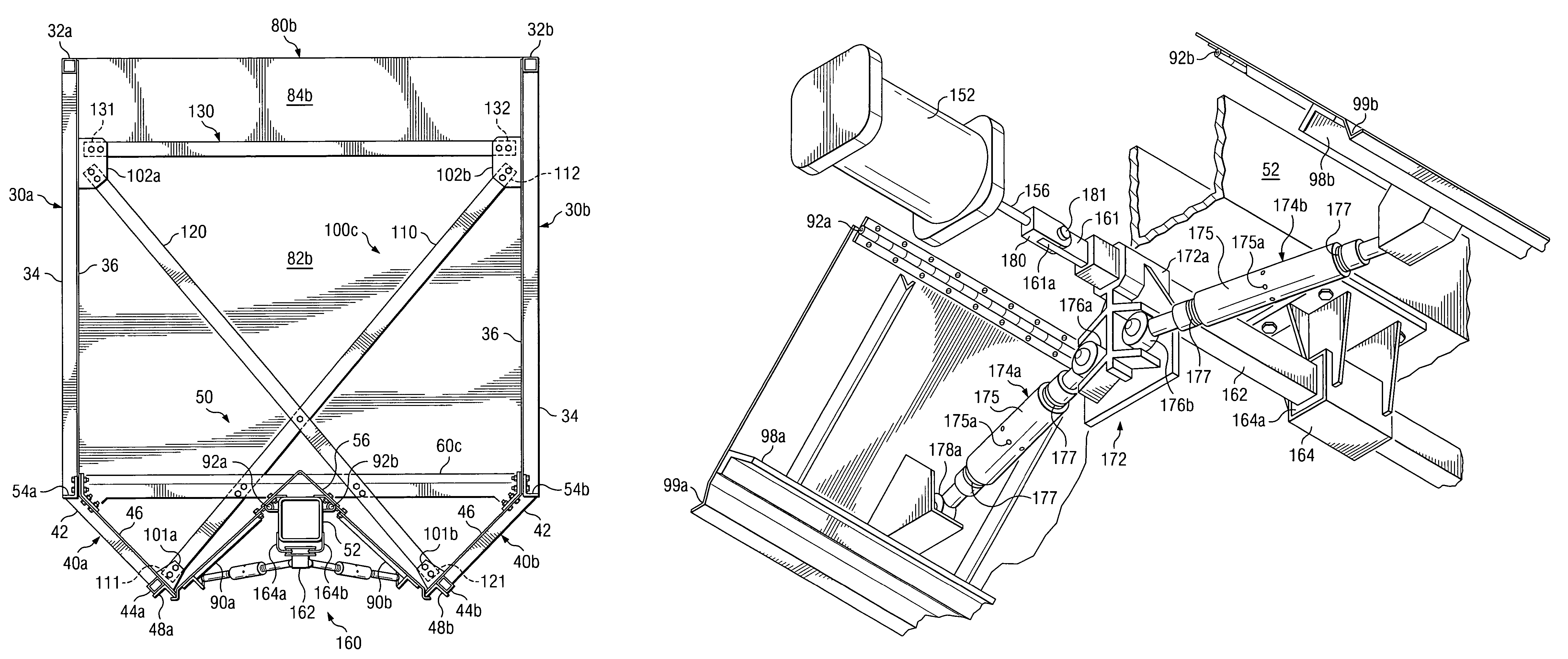
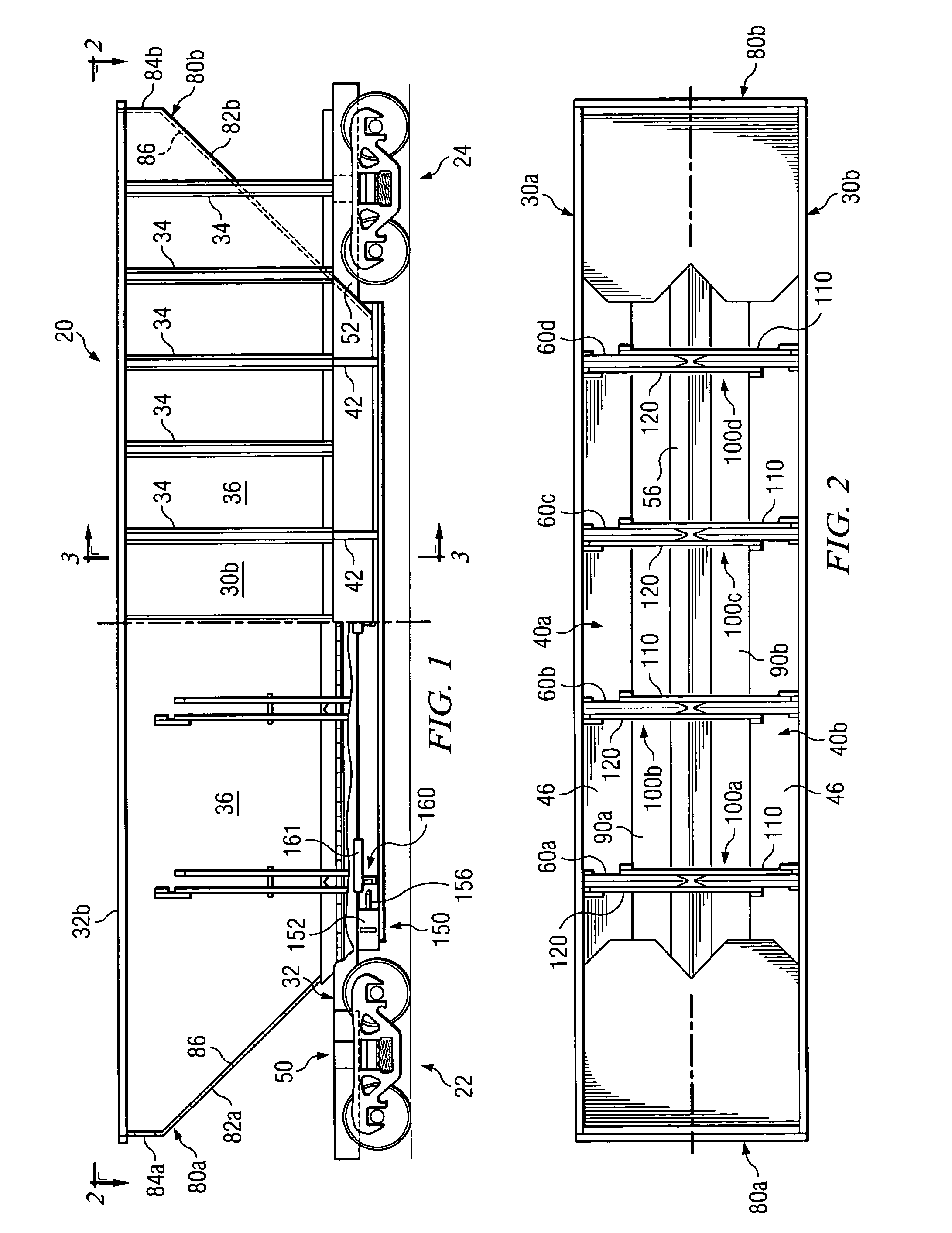
Railcar with discharge control system
ActiveUS7051661B2Improve carrying capacityReduce weightHopper carsWagons/vansControl systemOpen form
A railcar with discharge control system is disclosed. In one embodiment, a railway car includes an underframe and at least one hopper for transporting lading. The railway car further including the underframe including a center sill which defines in part a longitudinal axis of the railway car. A discharge opening formed proximate to a lower portion of the hopper. A respective door assembly pivotally mounted adjacent to the discharge opening to control the flow of lading from the hopper. The door assembly operable for movement between a first, closed position and a second, open position relative to the discharge opening. A discharge control system operable to move the door assembly between the first position and the second position. The discharge control system operably moves generally longitudinally along the axis of the railway car to move the door assemblies between the first, closed position and the second, open position.
Owner:TRINITY IND INC
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS20060153905A1Reduce transferLoss of therapyOrganic active ingredientsNervous disorderActive agentBULK ACTIVE INGREDIENT
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Pre-filled syringe or autoinjector
ActiveUS20120130318A1Minimize contaminationEasy piercingAmpoule syringesAutomatic syringesDrugAutoinjector
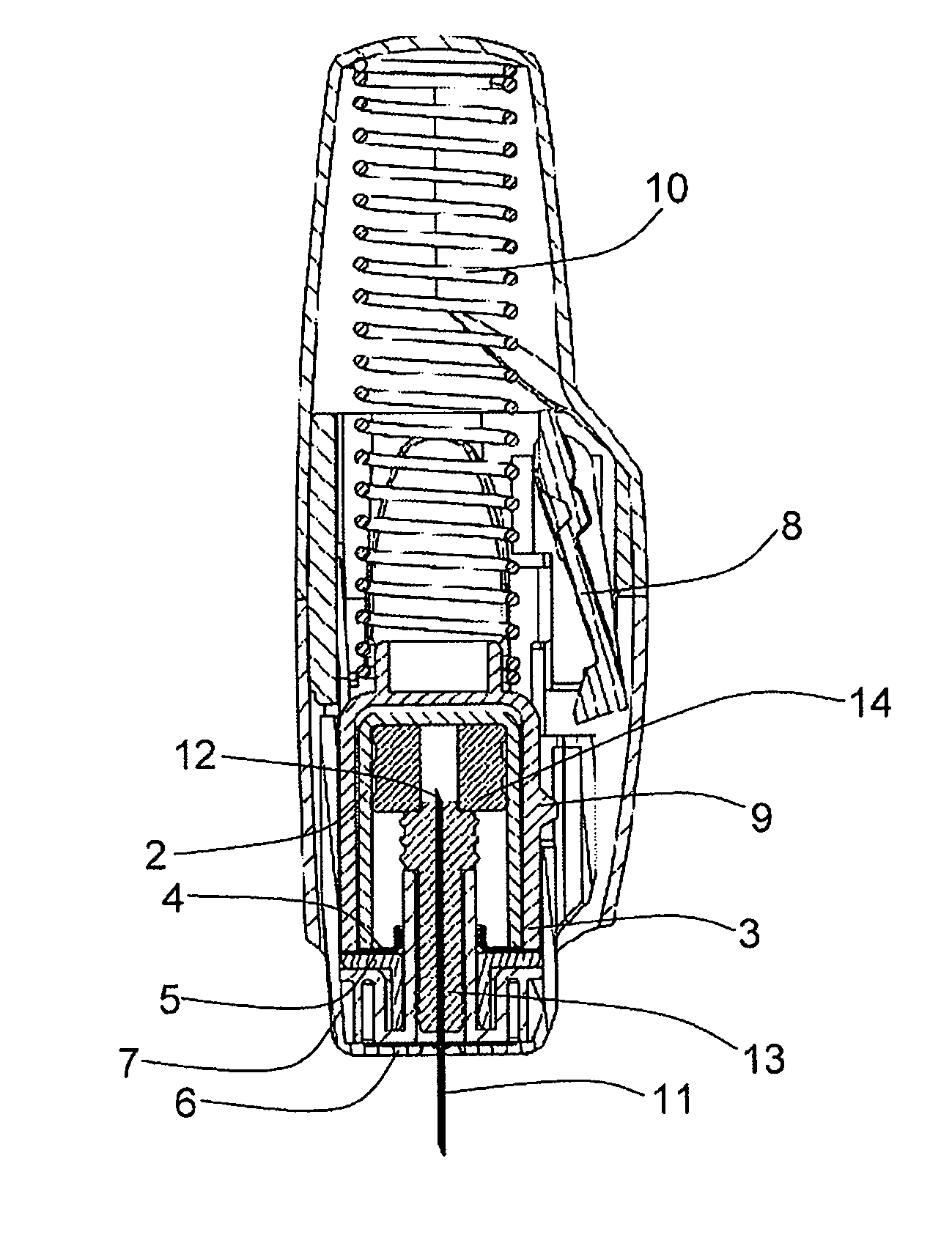
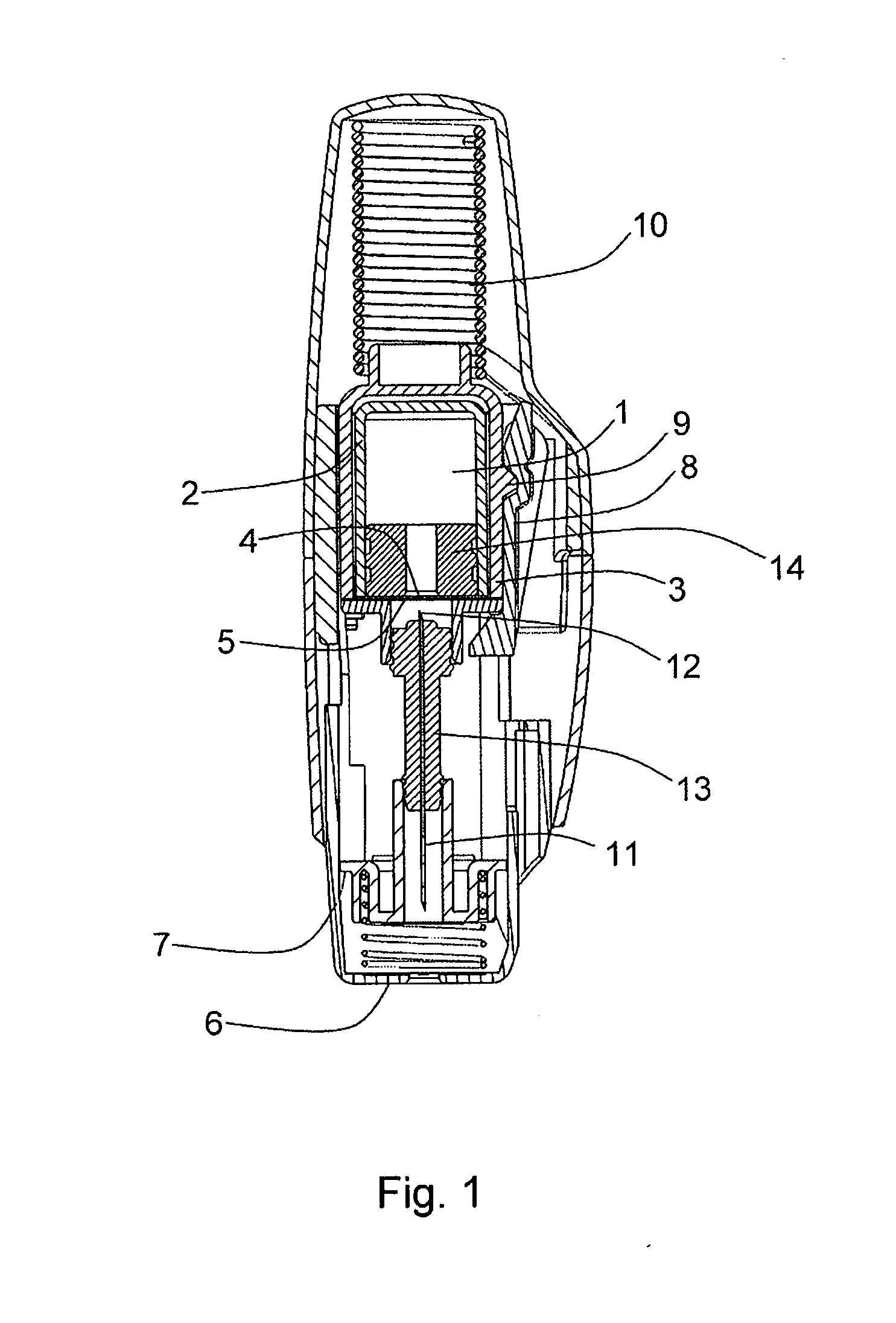
The invention provides a syringe for dispensing a drug (1), comprising: a rigid syringe body,—a first container (2), in contact with and enclosing the drug; and a second container (3), enclosing the first container, the second container being less gas permeable than the first container, wherein the second container partially or fully forms the rigid syringe body or is held within the rigid syringe body. The invention preserves the drug but allows the drug to be easily accessed without the need for a user to remove a gas barrier structure as a separate action in addition to the other actions needed to deliver the drug.
Owner:OVAL MEDICAL TECH
Method and apparatus for spray processing of porous medical devices
InactiveUS20070036905A1Minimize contaminationMinimize oxidationMolten spray coatingPretreated surfacesPorous substrateThermal spraying
Owner:ABBOTT CARDIOVASCULAR
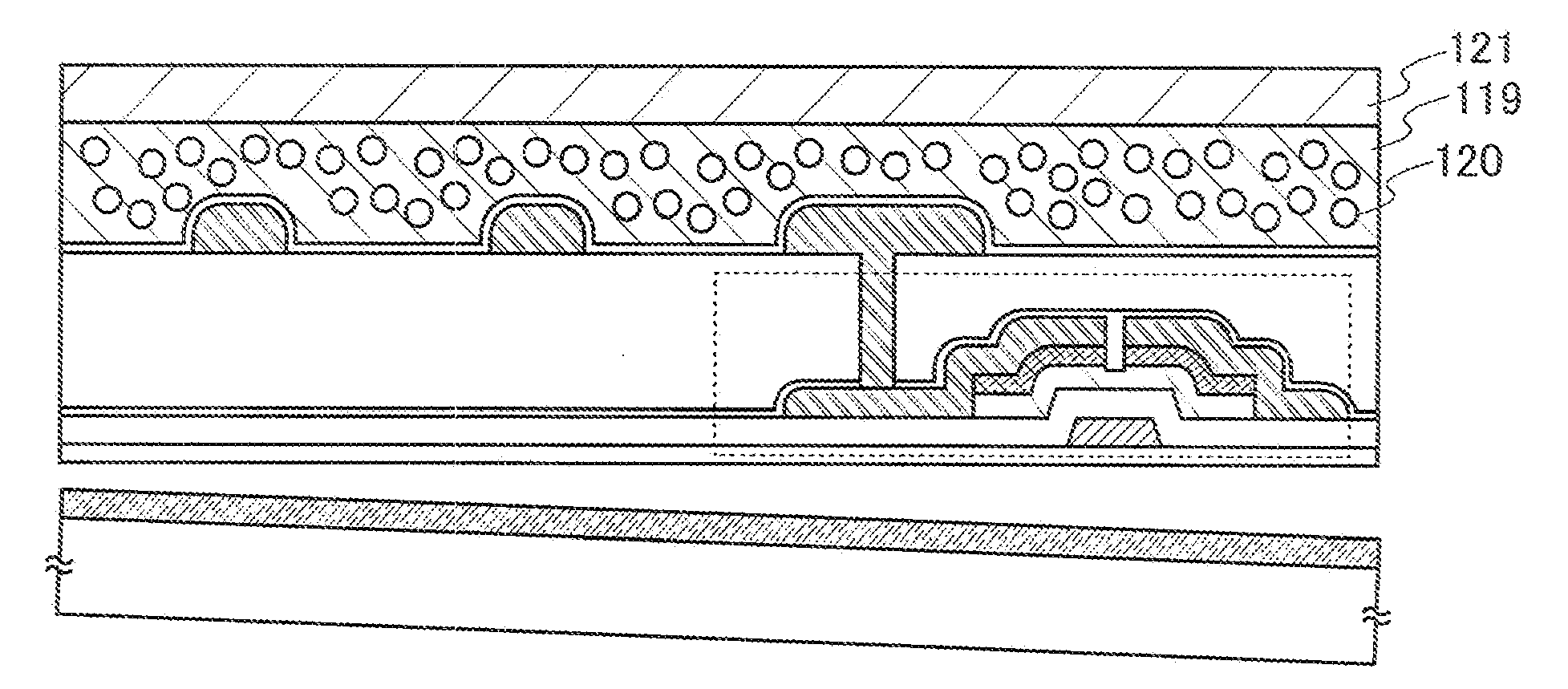
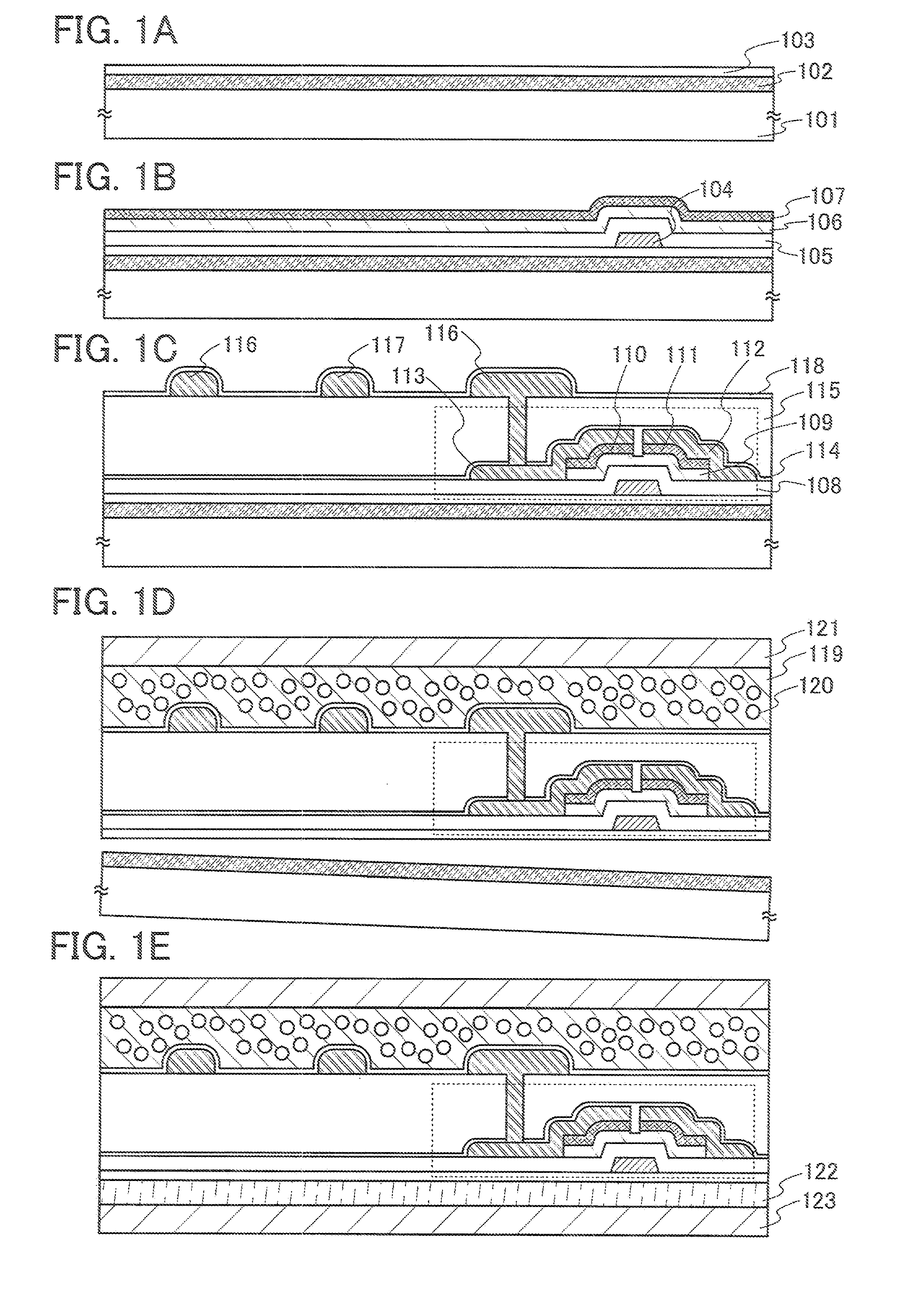
Method for manufacturing semiconductor device
ActiveUS20070254456A1Process stabilityPoor heat resistanceFinal product manufactureSemiconductor/solid-state device detailsEngineeringPlastic film
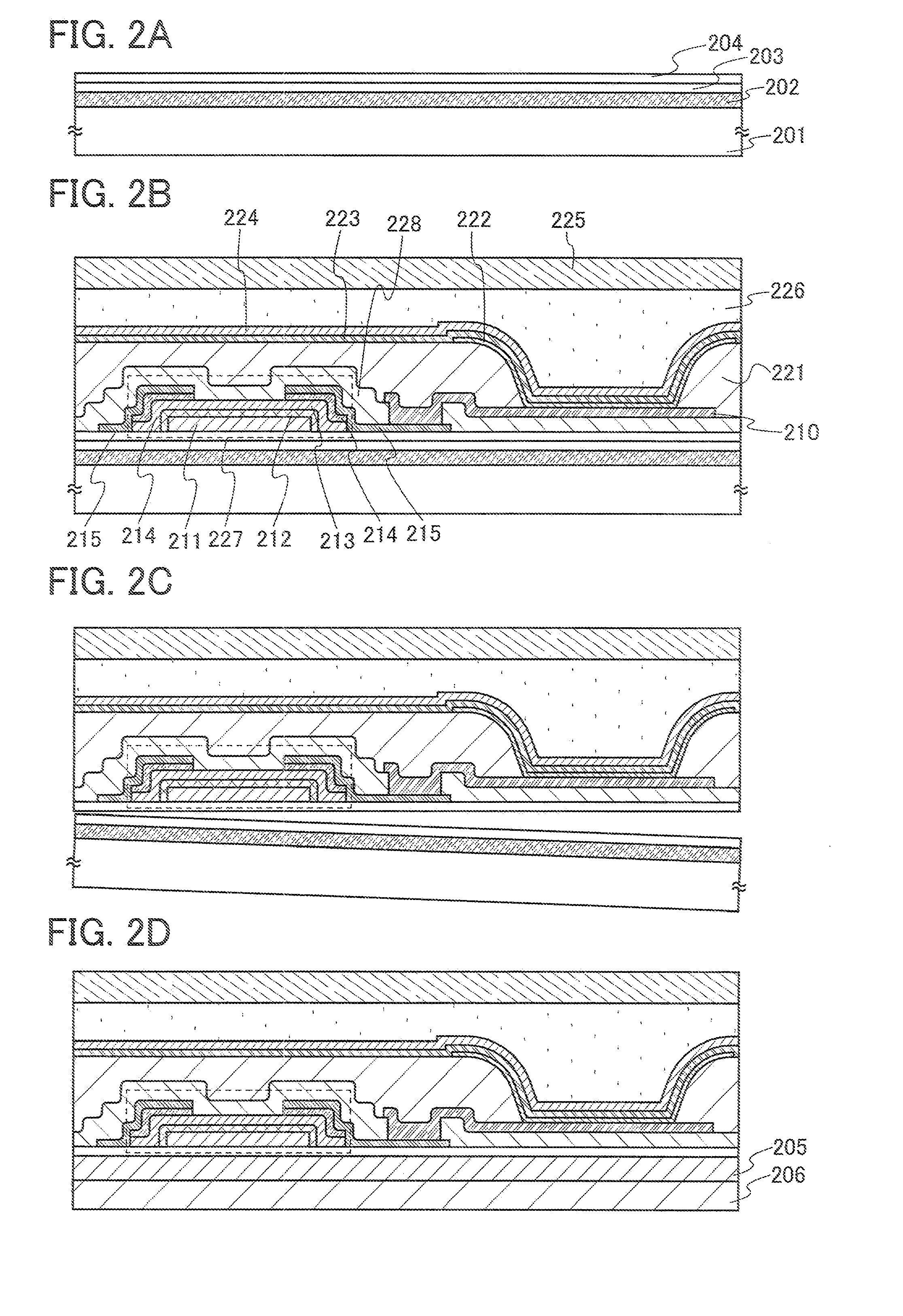
A technique for peeling an element manufactured through a process at relatively low temperature (lower than 500° C.) from a substrate and transferring the element to a flexible substrate (typically, a plastic film). With the use of an existing manufacturing device for a large glass substrate, a molybdenum film (Mo film) is formed over a glass substrate, an oxide film is formed over the molybdenum film, and an element is formed over the oxide film through a process at relatively low temperature (lower than 500° C.). Then, the element is peeled from the glass substrate and transferred to a flexible substrate.
Owner:SEMICON ENERGY LAB CO LTD
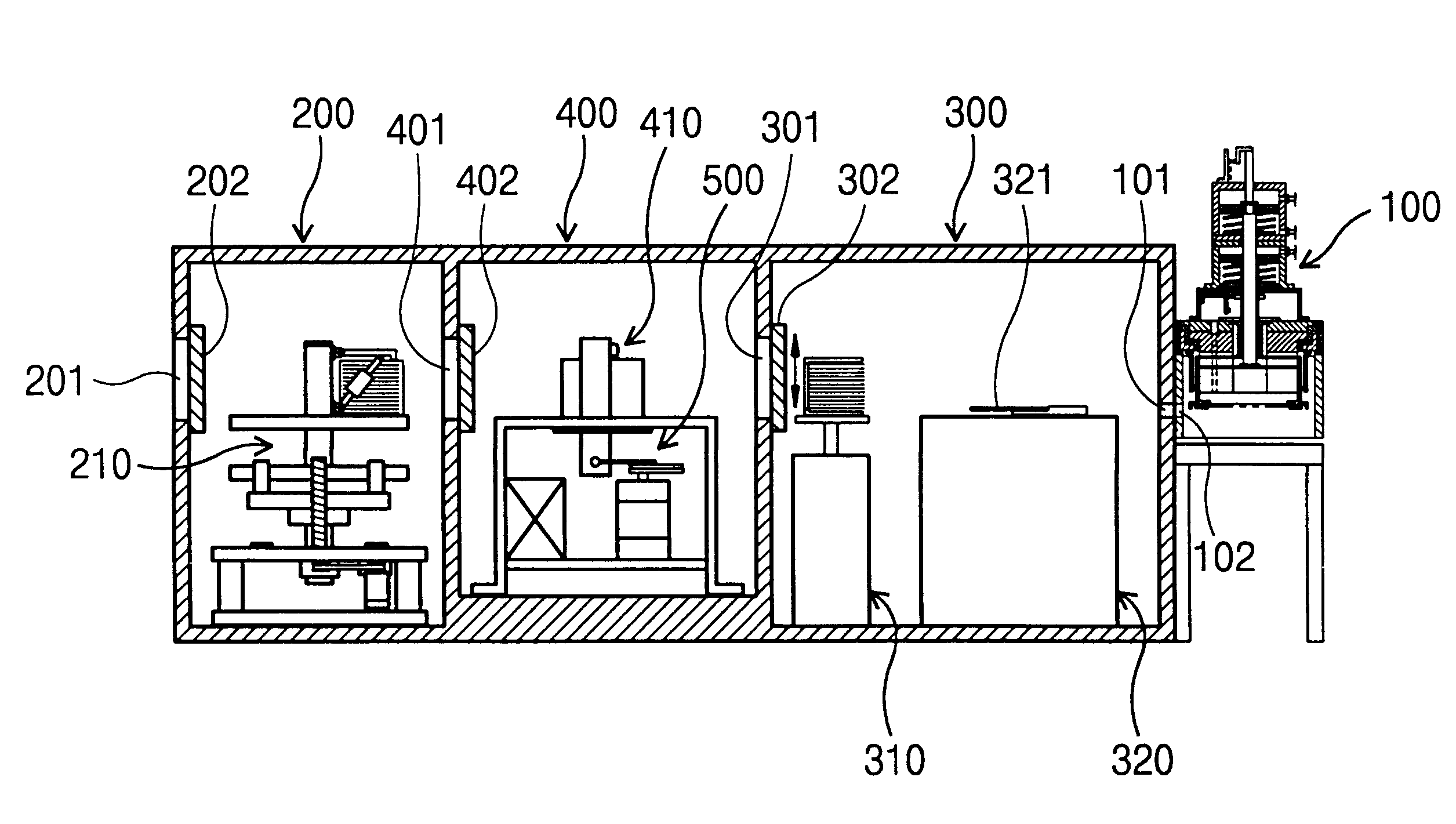
Methods and apparatus for selective pre-coating of a plasma processing chamber
ActiveUS20070204797A1Minimize contaminationSimple manufacturing processElectric discharge tubesSemiconductor/solid-state device manufacturingEngineeringDelivery system
An apparatus for selectively pre-coating a plasma processing chamber, including a chamber wall is disclosed. The apparatus includes a first set of RF electrodes, the first set of RF electrodes configured to strike a first pre-coat plasma, the first set of RF electrodes defining a first plasma chamber zone. The apparatus also includes a first set of confinement rings disposed around the first set of RF electrodes; and a second set of confinement rings disposed between the first set of confinement rings and the chamber wall. The apparatus further includes a gas delivery system configured to apply a first pre-coat layer to the first plasma zone when a first pre-coat gas is delivered and the first set of RF electrodes is energized. The apparatus also includes the gas delivery system configured to apply a second pre-coat layer to the second plasma zone when a second pre-coat gas is delivered and the second set of RF electrodes is energized.
Owner:LAM RES CORP
Transdermal pharmaceutical formulation for minimizing skin residues
InactiveUS7335379B2Reducing and preventing transferMinimize contaminationOrganic active ingredientsNervous disorderAlcoholActive agent
This invention relates to novel transdermal or transmucosal pharmaceutical formulation which reduces the occurrences of contamination of other individuals and the transference to clothing of the user. The novel formulation includes at least one pharmacologically active ingredient, and a solvent system having a monoalkylether of diethylene glycol and a glycol present in specified ratios, and a mixture of water and alcohol. The invention also relates to a method for inhibiting or delaying crystallization of an active agent in a pharmaceutical formulation.
Owner:ANTARES PHARMA IPL
Reduced contaminant gas injection system and method of using
InactiveUS20070235136A1Minimize contaminationElectric discharge tubesSemiconductor/solid-state device manufacturingProduct gasProcess engineering
A gas injection system includes a diffuser to distribute a process gas in a processing chamber. The gas injection system may be utilized in a polysilicon etching system involving corrosive process gases.
Owner:TOKYO ELECTRON LTD
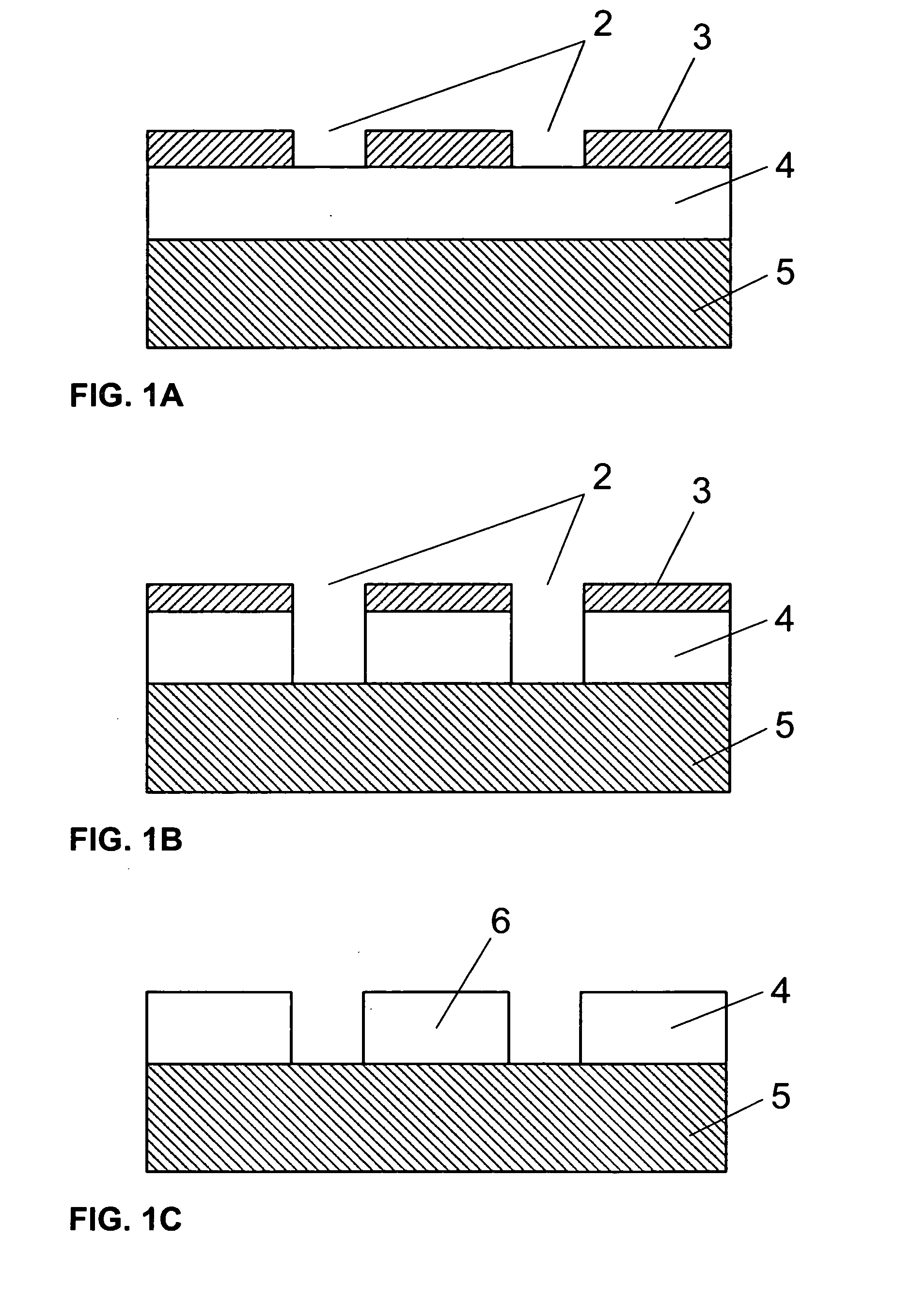
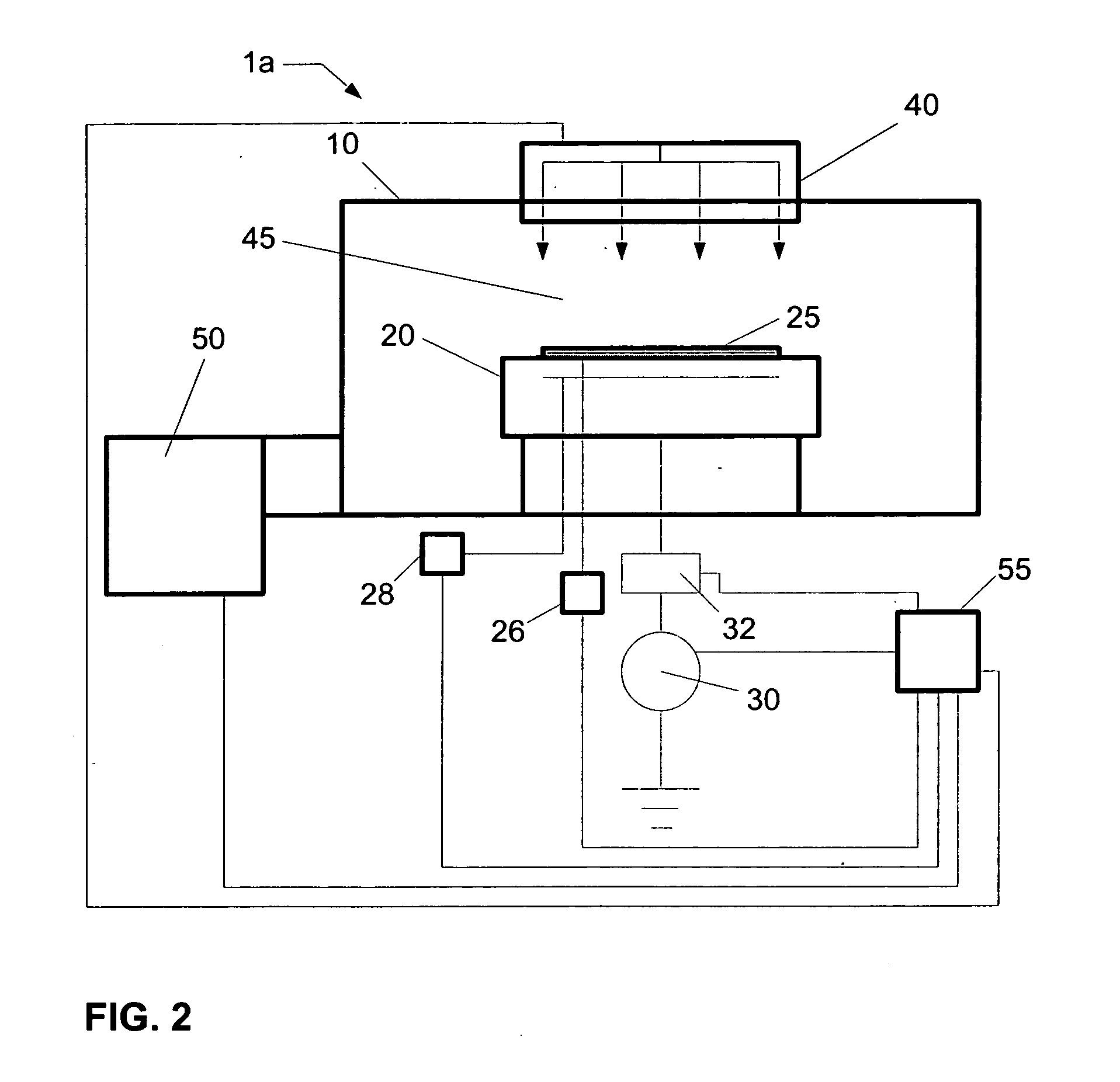
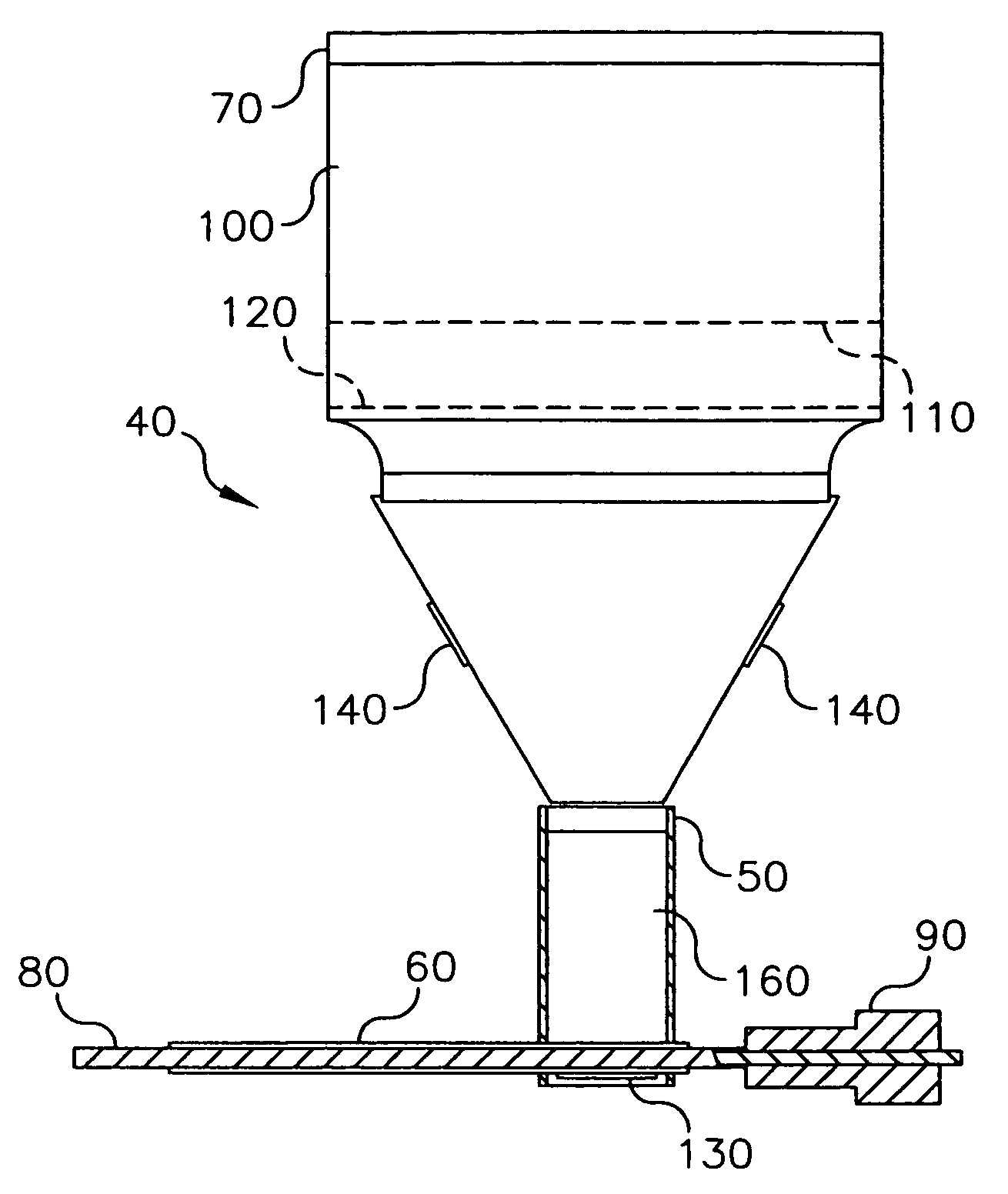
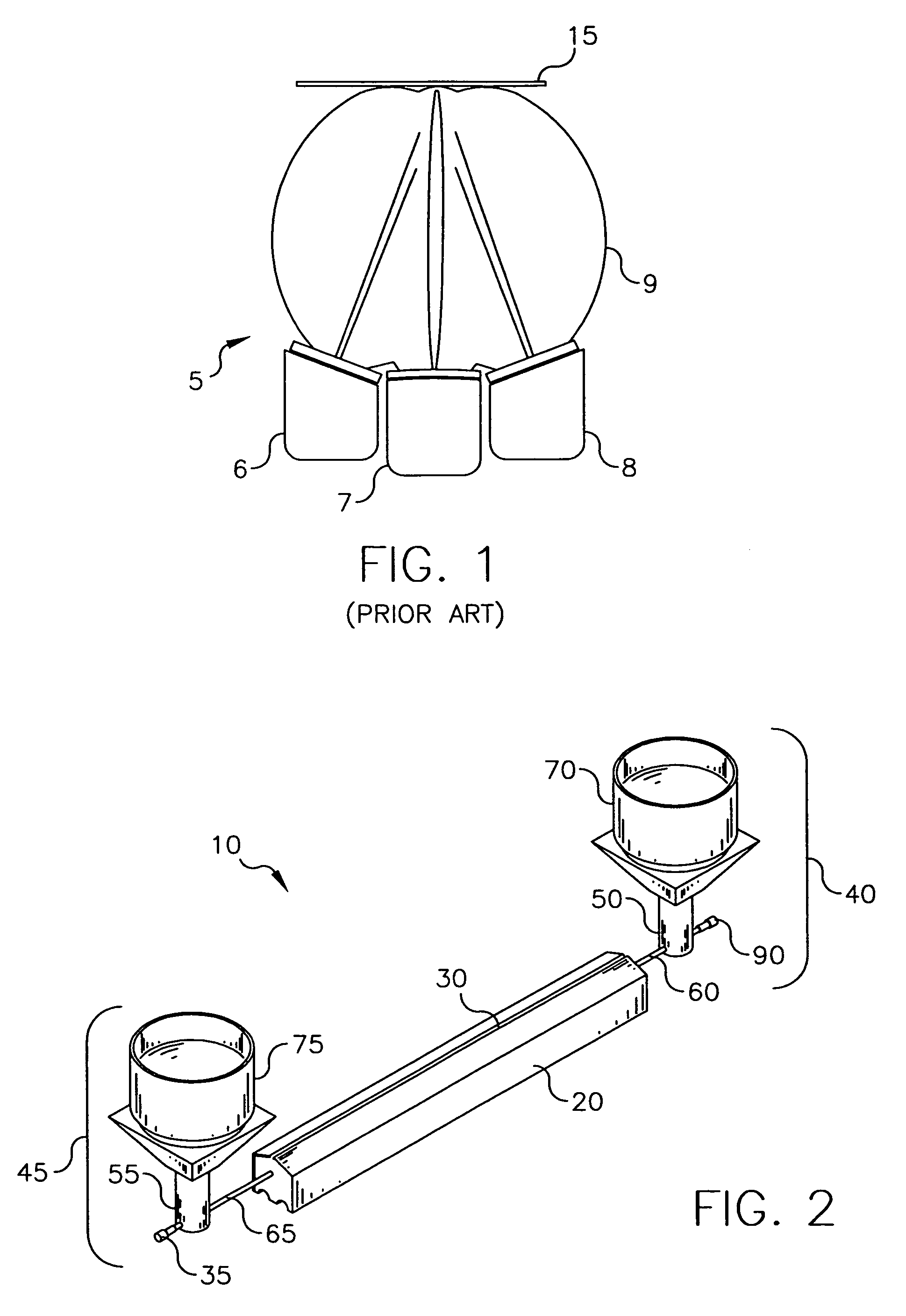
Delivering organic powder to a vaporization zone
ActiveUS7288286B2Reduce riskStable rateLiquid surface applicatorsVacuum evaporation coatingVaporizationSubstrate surface
A method for vaporizing organic material and condensing it onto a surface to form a layer, comprising: providing a quantity of first organic material in a powdered form in a first container and a quantity of second organic material in a second container spaced from the first container; fluidizing the first organic material in the first container, transferring the fluidized first organic material from the first container into a manifold, and vaporizing the first organic material in the manifold; fluidizing the second organic material in the second container, transferring the fluidized second organic material from the second container into the manifold, and vaporizing the second organic material in the manifold where the first and second vaporized organic materials are mixed; and delivering from the manifold the mixed vaporized materials to the substrate surface to form the layer.
Owner:GLOBAL OLED TECH
Vaporizing fluidized organic materials
A method for vaporizing organic materials onto a surface, to form a film includes providing a quantity of organic material in a fluidized powdered form; metering the powdered organic material and directing a stream of such fluidized powder onto a first member; heating the first member so that as the stream of fluidized powder is vaporized; collecting the vaporized organic material in a manifold; and providing a second member formed with at least one aperture in communication with the manifold that permits the vaporized organic material to be directed onto the surface to form a film.
Owner:GLOBAL OLED TECH
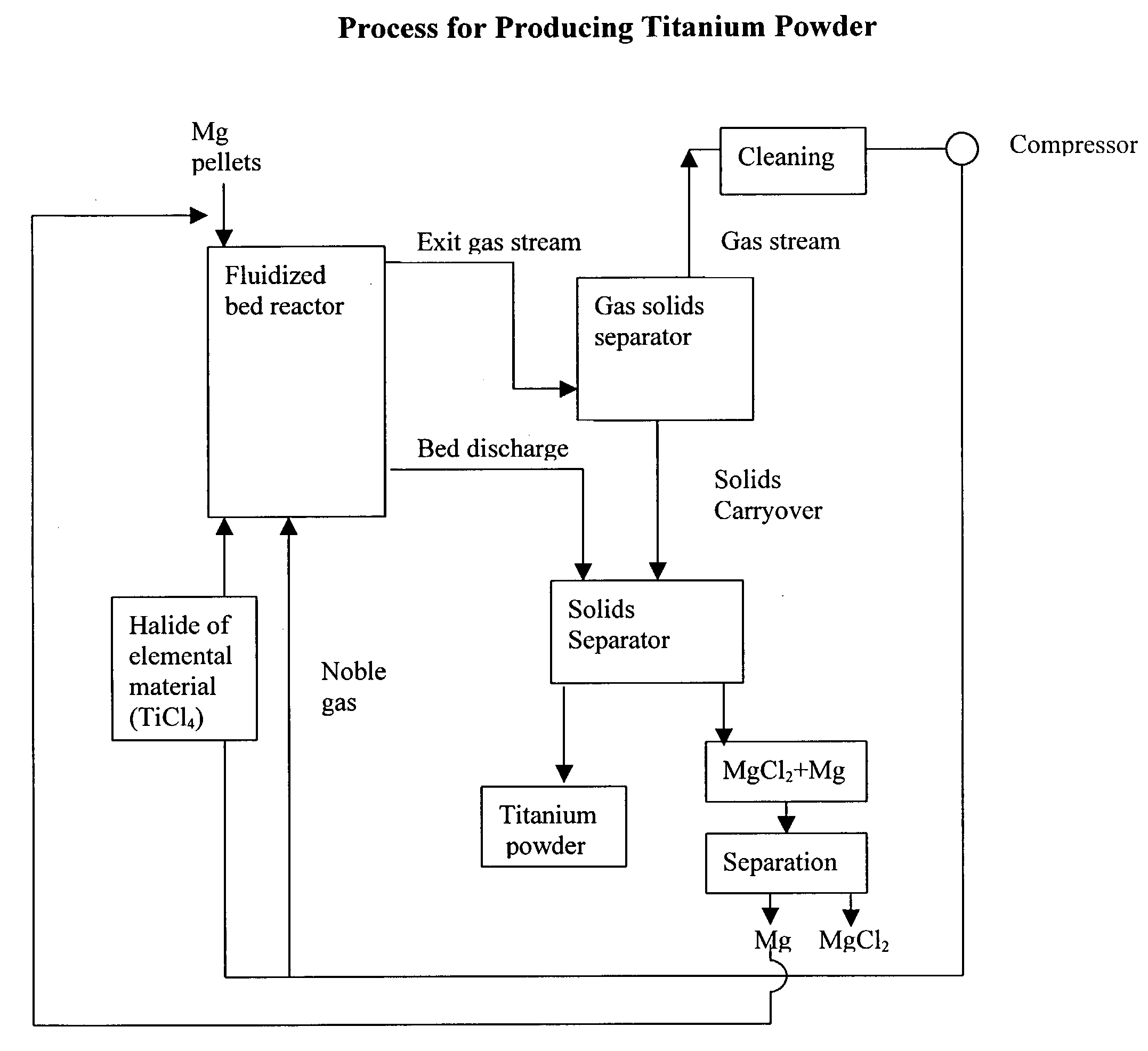
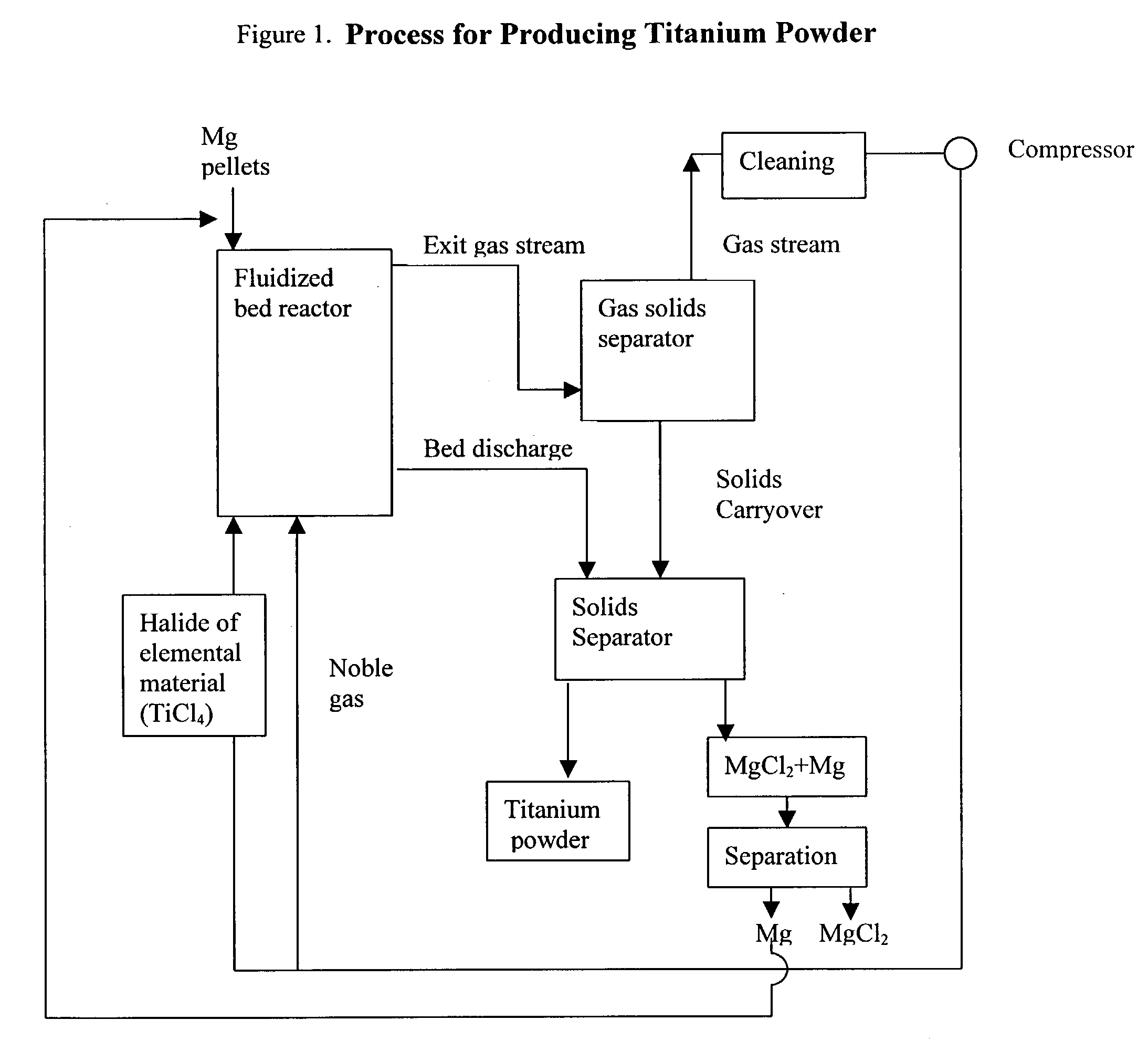
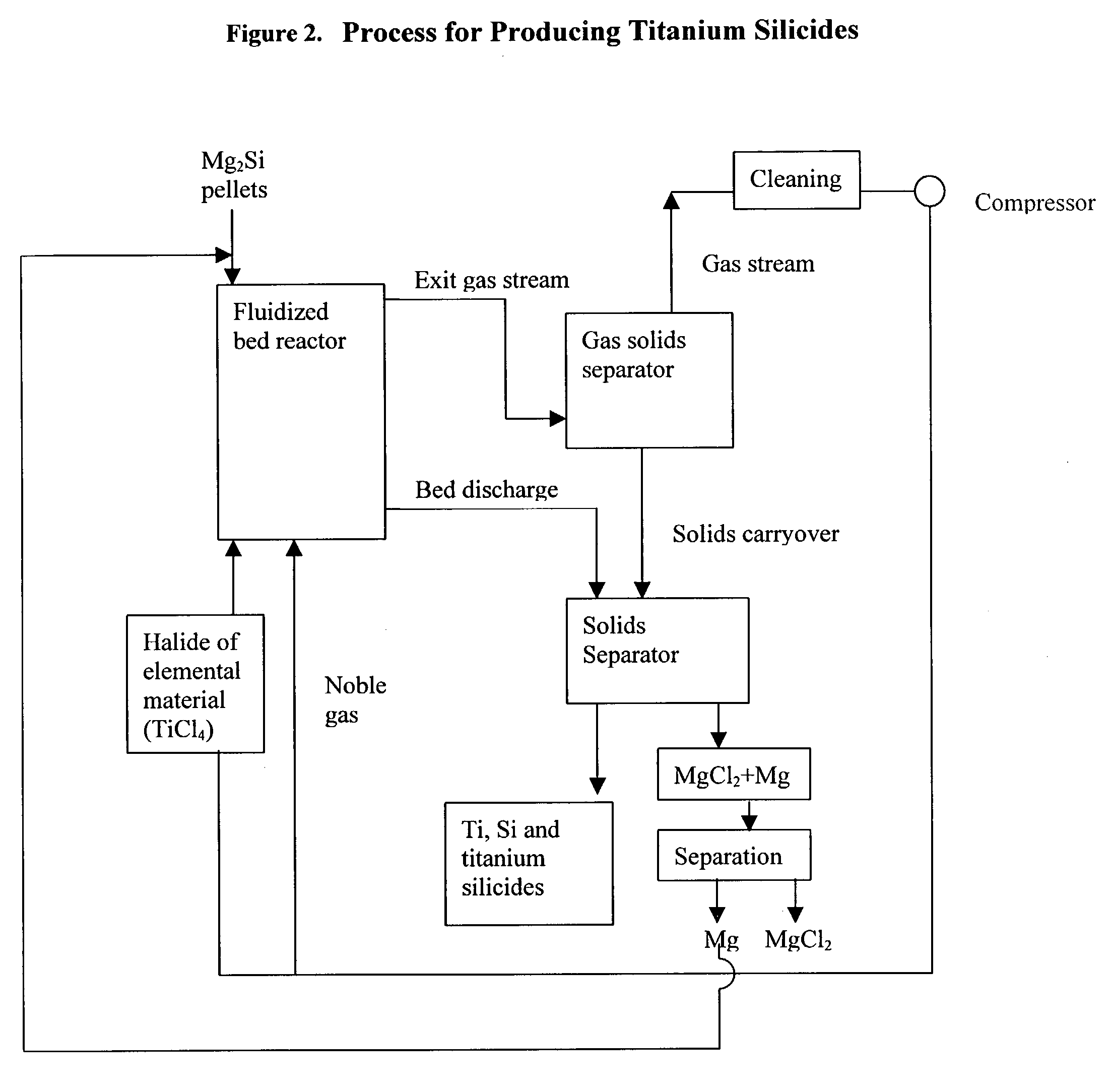
Process for the production of elemental material and alloys
The present invention relates to a process for the production of an elemental material, comprising the step of reacting a halide of the elemental material with a reducing agent in solid form in a fluidized bed reactor at a reaction temperature which is below the melting temperature of the reducing agent. In a preferred embodiment of the present invention, the elemental material is titanium and the titanium is produced in powder form. The invention also relates to the production of alloys or intermetallics of the elemental materials.
Owner:MILLENNIUM INORGANIC CHEM
Delivering organic powder to a vaporization zone
ActiveUS7288285B2Reduce riskStable rateVacuum evaporation coatingSputtering coatingVaporizationMaterial transfer
A method for vaporizing organic materials and condensing them onto a surface to form a layer, comprising: providing a quantity of organic material in a powdered form in a first container; fluidizing the organic material in the first container and transferring such fluidized material to an auger structure; and rotating at least a portion of the auger structure to transfer fluidized powder from the first container along a feeding path to a vaporization zone where such powder is vaporized and delivered to the substrate to form the layer.
Owner:GLOBAL OLED TECH
Etching apparatus for manufacturing semiconductor devices
InactiveUS6340405B2Operation efficiency can be improvedReduce processing timeDecorative surface effectsSemiconductor/solid-state device manufacturingMagnetic tapeWafer stacking
An etching apparatus for manufacturing semiconductor devices which reduces contamination of the processing surface of a wafer by transporting a plurality of wafers stacked in a cassette with their processing surfaces facing down from the cassette supply chamber to one or more process chambers where the etching operation is performed on each wafer, one at a time. The apparatus has a load lock chamber for transferring the wafers stacked in the cassette from the cassette supply chamber, which is maintained under atmospheric conditions, to the process chamber, which is maintained under a strong vacuum. The process chamber has a cathode to which a wafer is clamped by a wafer holder with its processing surface facing down; the process chamber may also have a removable lower cover for easy repair and cleaning. The apparatus may also have a wafer aligning chamber installed between the cassette supply chamber and the load lock chamber for simultaneously aligning all of the wafers n the cassette before they are transported to the load lock chamber. The wafer aligning chamber also has a cassette transport mechanism for transferring the cassette from a cassette supply table in the cassette supply chamber to an elevator installed in the load lock chamber.
Owner:SAMSUNG ELECTRONICS CO LTD
Isolation of inner cell mass for the establishment of human embryonic stem cell (hESC) lines
InactiveUS7294508B2Easy to useAvoid possibilityMammal material medical ingredientsDead animal preservationGerm layerHuman embryonic stem cell line
A method for isolating an inner cell mass comprising the steps of immobilizing a blastocyst stage embryo having a zona pellucida, trophectoderm, and inner cell mass, creating an aperture in the blastocyst stage embryo by laser ablation, and removing the inner cell mass from the blastocyst stage embryo through the aperture. The aperture is through the zona pellucida and the trophectoderm. The laser ablation is acheived using a non-contact diode laser. The inner cell mass removed from the blastocyst stage embryo is used to establish human Embryonic Stem Cell lines.
Owner:RELIANCE LIFE SCI PVT
Pelvic balloon tamponade
An implantable device is provided for controlling hemorrhage in a body cavity, comprising an expandable balloon and a conduit for supplying a physiologically compatible fluid to inflate the balloon. When the balloon tamponade device is implanted or inserted into the body cavity, it is inflated with a physiologically suitable fluid, so that the balloon generally conforms to the body cavity and exerts compressive force against the walls, tissues or structures of the body cavity to control hemorrhage. The balloon may have a deforming means to limit expansion of the balloon in a direction to facilitate expansion of the balloon in another direction. The device may have additional tubes within the conduit, or a plurality of separate lumens within the conduit or tubes to allow drainage and irrigation to the body cavity. There is also provided a cuff for attachment of an external traction to the balloon tamponade, to facilitate the compressive effect of the device. In a preferred embodiment, there is provided a dual balloon tamponade in which two balloons are axially spaced along the conduit, providing a means to control hemorrhage from two distinct body cavities, such as a uterus and a vagina. There is also provided a method to control hemorrhage in a body cavity by implantation or insertion of the balloon tamponade, inflating the balloon with a fluid to a sufficient pressure and retaining the fluid pressure within the balloon for a sufficient period of time to determine whether hemorrhage has been controlled. A kit comprising the balloon tamponade apparatus is also provided.
Owner:B & D MEDICAL DEV
High density self-contained biological analysis
ActiveUS8895295B2Rapid and sensitiveMinimize contaminationBioreactor/fermenter combinationsBiological substance pretreatmentsImmuno pcrHigh density
Devices, containers, and methods are provided for performing biological analysis in a closed environment. Illustrative biological analyses include high density nucleic acid amplification and detection and immuno-PCR.
Owner:BIOFIRE DIAGNOSTICS LLC
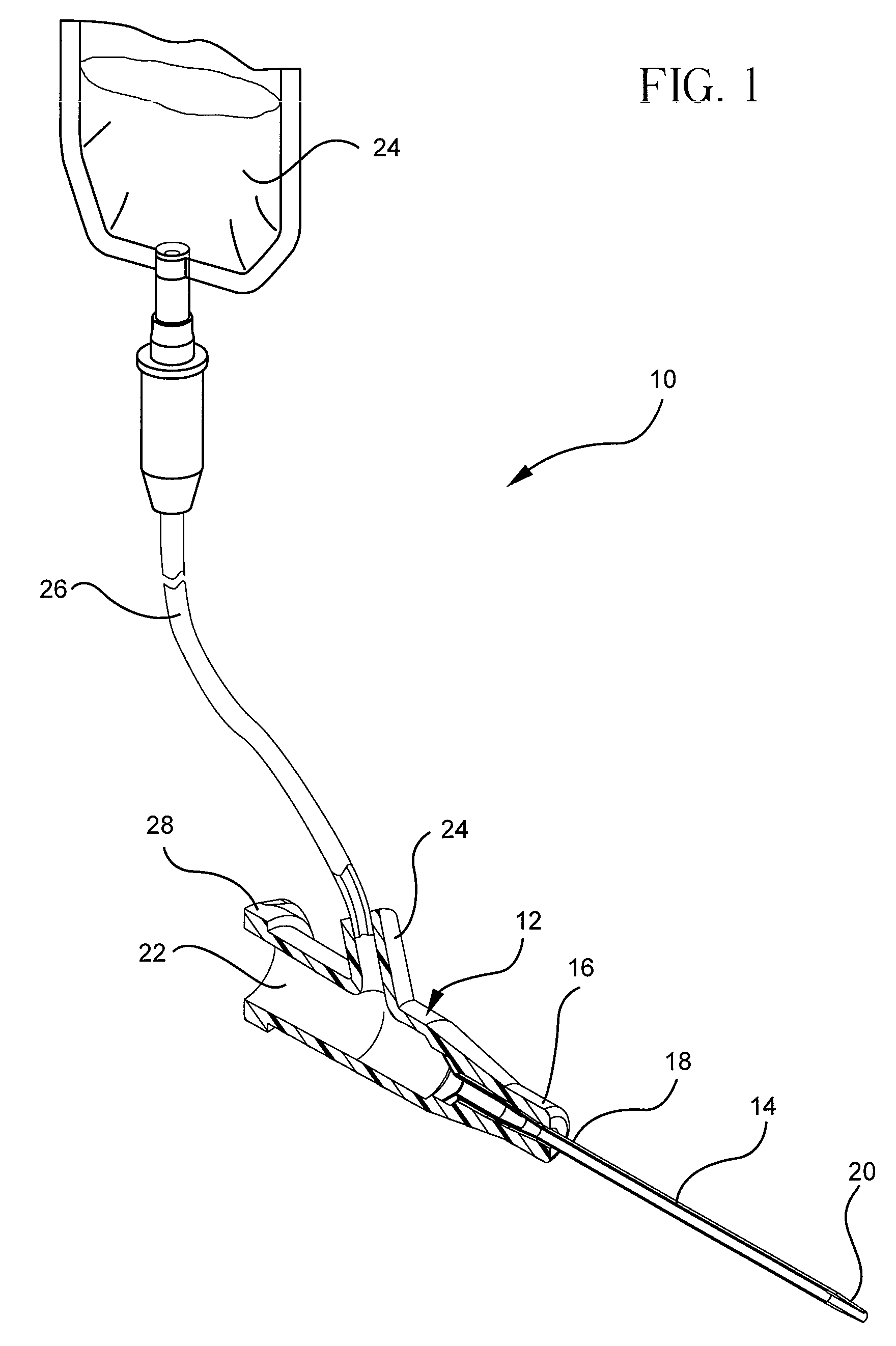
Lay flat tubing
ActiveUS20060229586A1Facilitates fluid deliveryEasy to removeLayered productsHollow filament manufactureElectrical conductorEntry site
Lay flat tubing is used as a fluid conductor for transferring fluid to and from the body of a patient. The lay flat tubing lies flat or collapsed when not in use, utilizing minimal space during transportation and storage, but which expands to form a passage when used to conduct fluid. A plurality of lumens may be formed in the lay flat tubing. At least one lumen transports infused fluid end-to-end from a source to an entry site on a patient, or in the alternative from an entry site on or in a patient. All lumens are isolated with respect to each other in order to prevent crossover of fluid from one lumen to the other.
Owner:MEDICAL SOLUTIONS
Gas ring and method of processing substrates
InactiveUS20070087533A1Improve thickness uniformityReduce turbulenceGaseous chemical processesLiquid-gas reaction of thin-film typeSusceptorProcess engineering
A process gas to a reactor volume of a semiconductor processing reactor is provided through gas injector ports of a gas ring. The process gas flows horizontally from the gas injector ports across a principal surface of a rotating susceptor to exhaust ports of the gas ring. The spent process gas is removed from the reactor volume through the exhaust ports.
Owner:MOORE EPITAXIAL
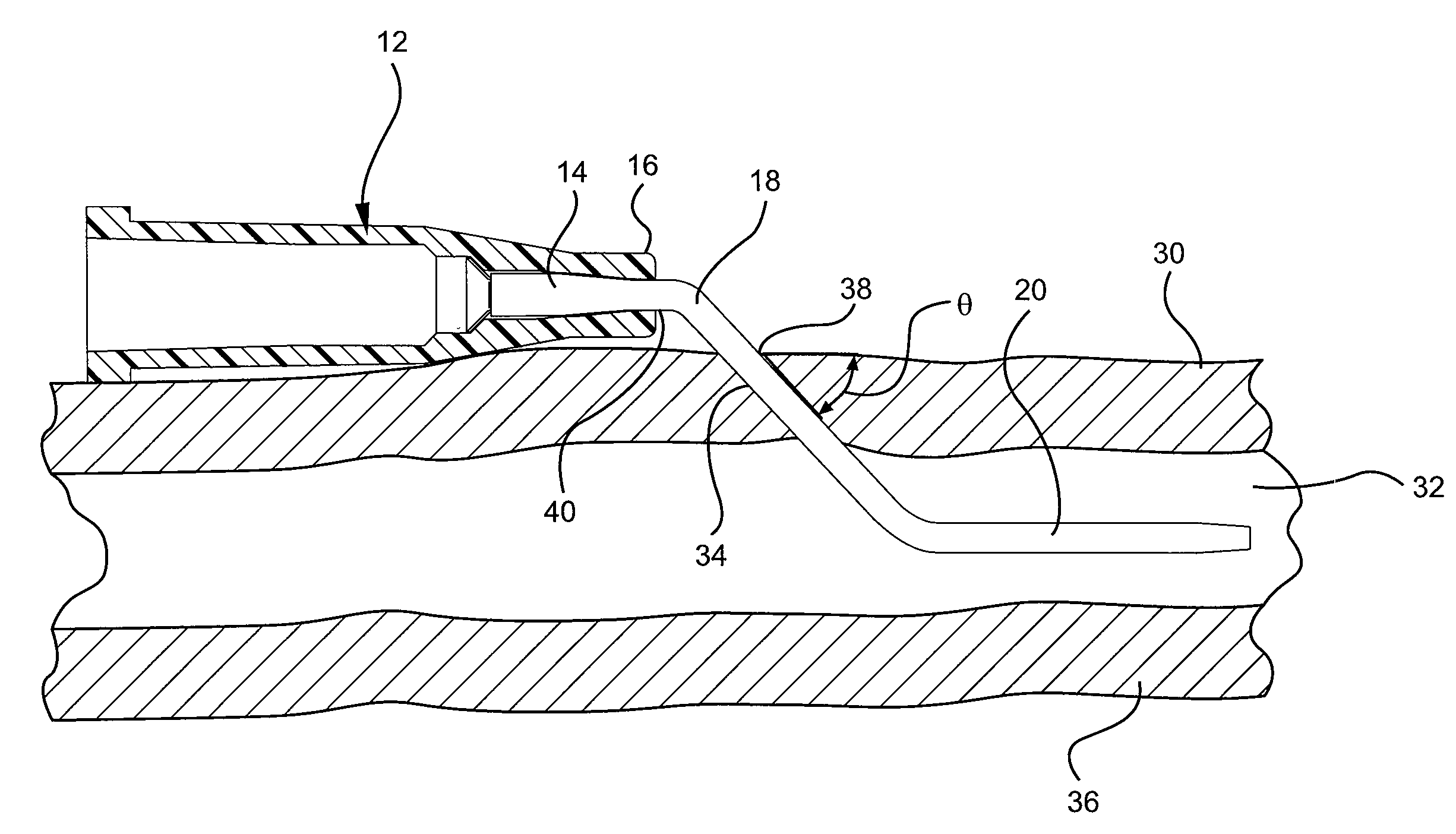
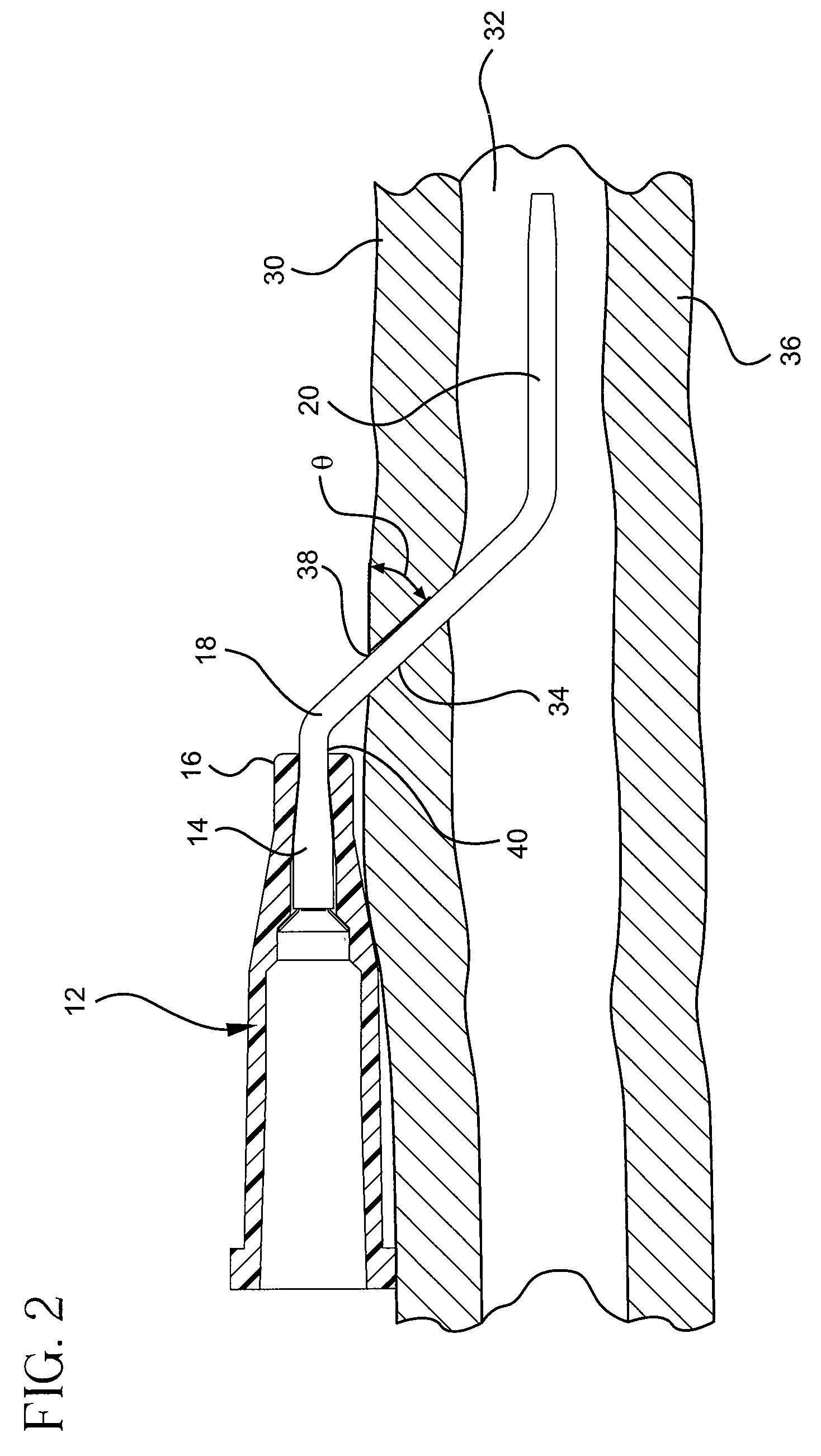
Occlusion resistant catheters
ActiveUS20090192496A1Avoid occlusionPrevent over-insertionMedical devicesCatheterCATHETER ADAPTEROcclusion catheter
A device and method for preventing an occlusion of a catheter including a catheter adapter and a catheter where a flexured portion of the catheter is supported by a bending surface of the catheter adapter. The flexured portion of the catheter may also include a maximum insertion length mark and / or a flexible support member to support and strengthen the flexured portion of the catheter against occlusions. A bending surface is provided over which a flexured portion of the catheter may gently bend to accommodate the transition of the catheter from the catheter adapter to the insertion site without occluding the catheter.
Owner:BECTON DICKINSON & CO
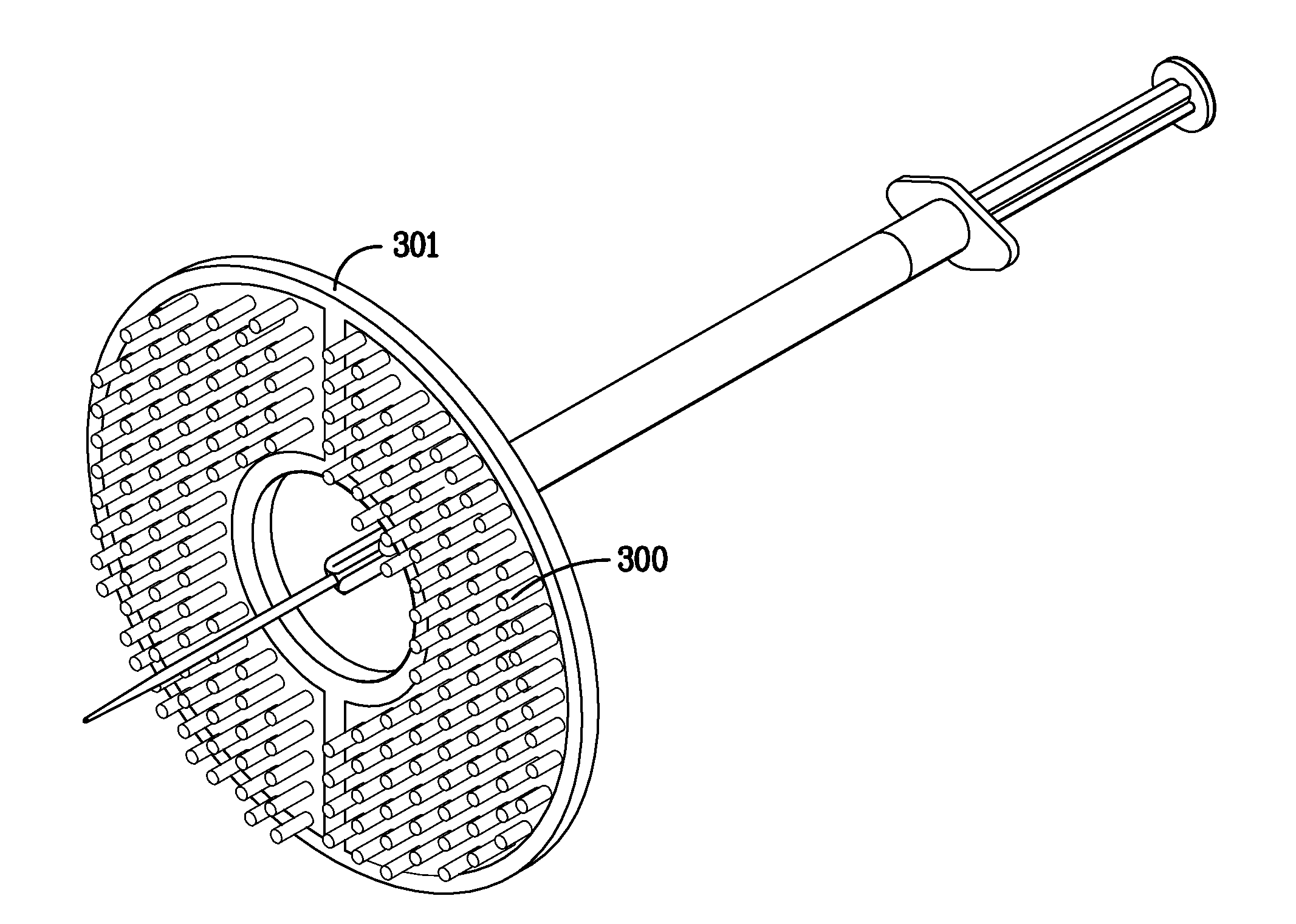
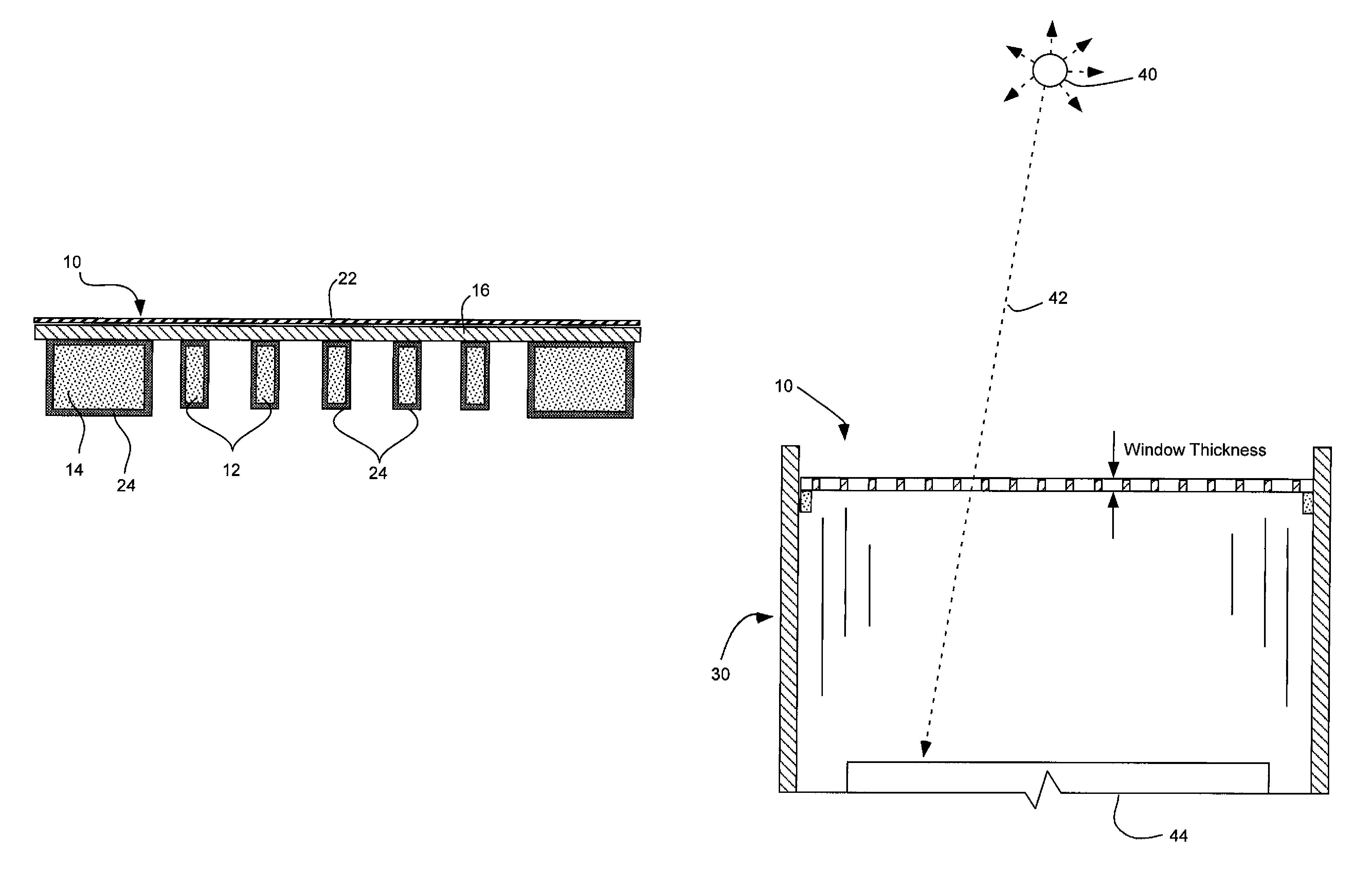
Radiation window with coated silicon support structure
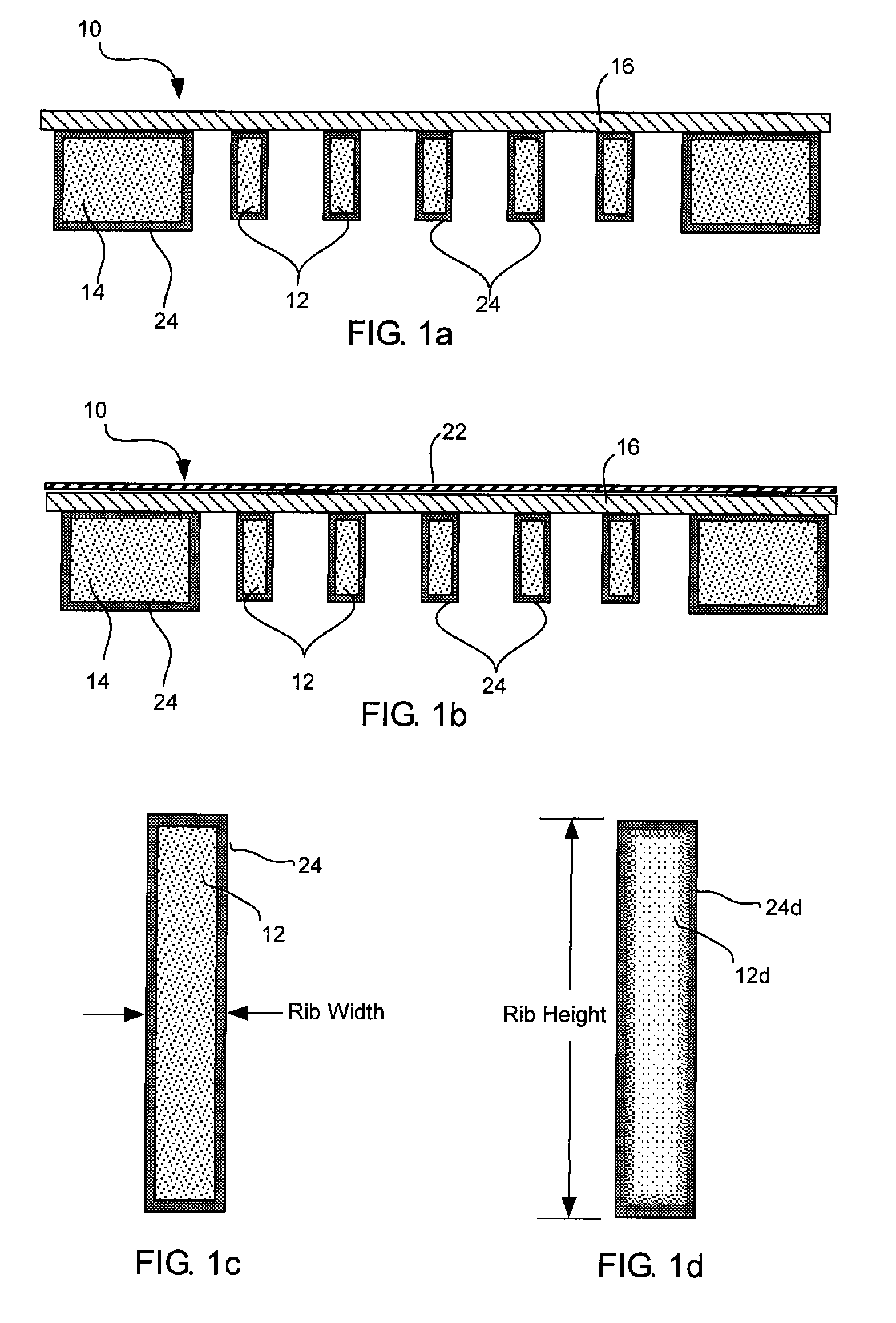
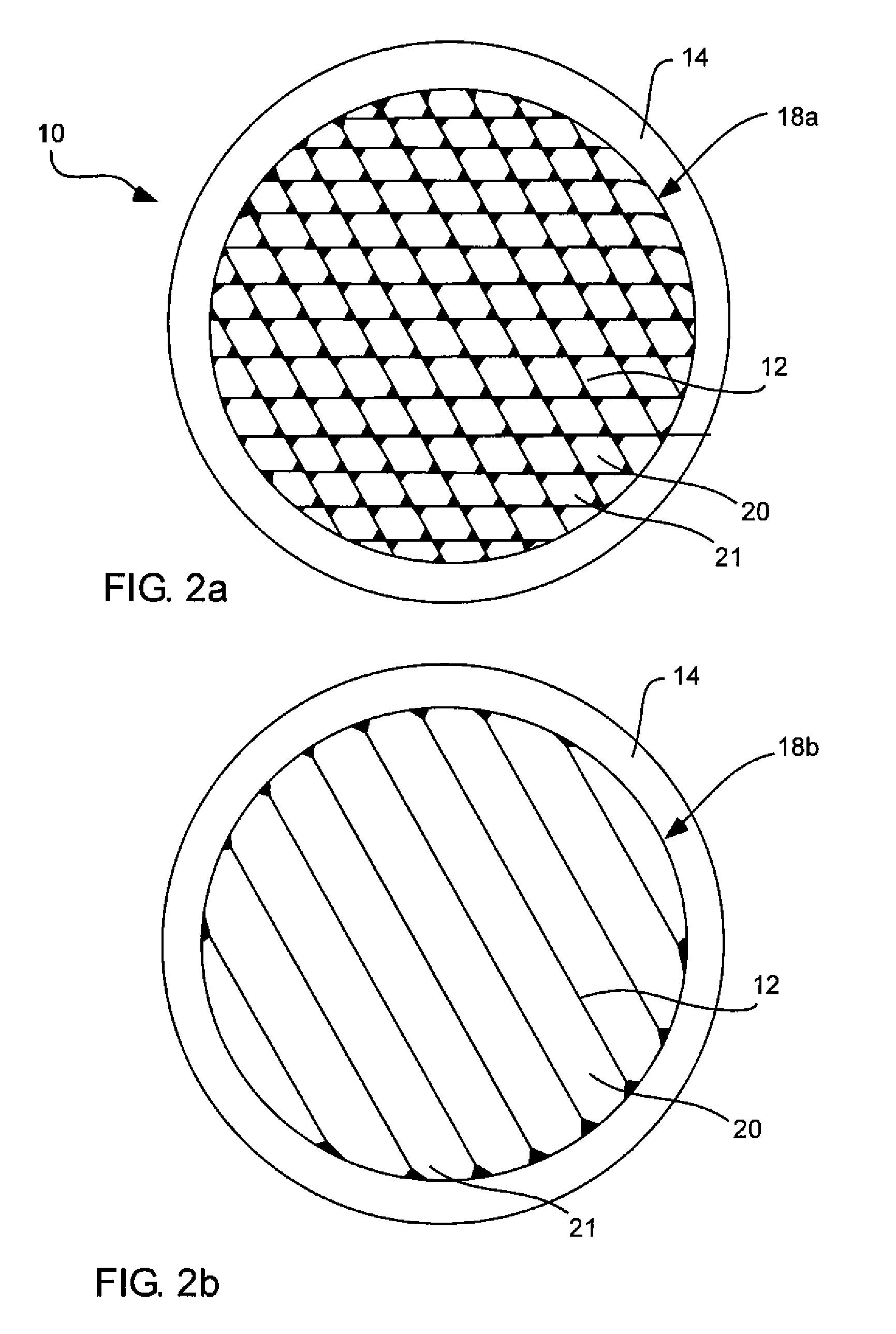
InactiveUS7709820B2Maximize transmissionMinimize contaminationMaterial analysis using wave/particle radiationRadiation/particle handlingSiliconPhysics
A window for a radiation detection system includes a frame with an aperture therein configured to receive radiation therethrough. A plurality of silicon ribs span the aperture and are carried by the frame. A coating substantially envelopes each of the plurality of silicon ribs. A thin film covers the aperture and is carried by the plurality of silicon ribs and is configured to pass radiation therethrough.
Owner:MOXTEK INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com