Slowly released tablet of compound atenolol, and preparation method
A sustained-release tablet and compound technology, which is applied in the field of medicine, can solve problems such as difficult erosion release synchronous coordination, low bioavailability, prolonging the period of medication, etc., achieve important economic value and social significance, reduce drug costs, reduce The effect of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
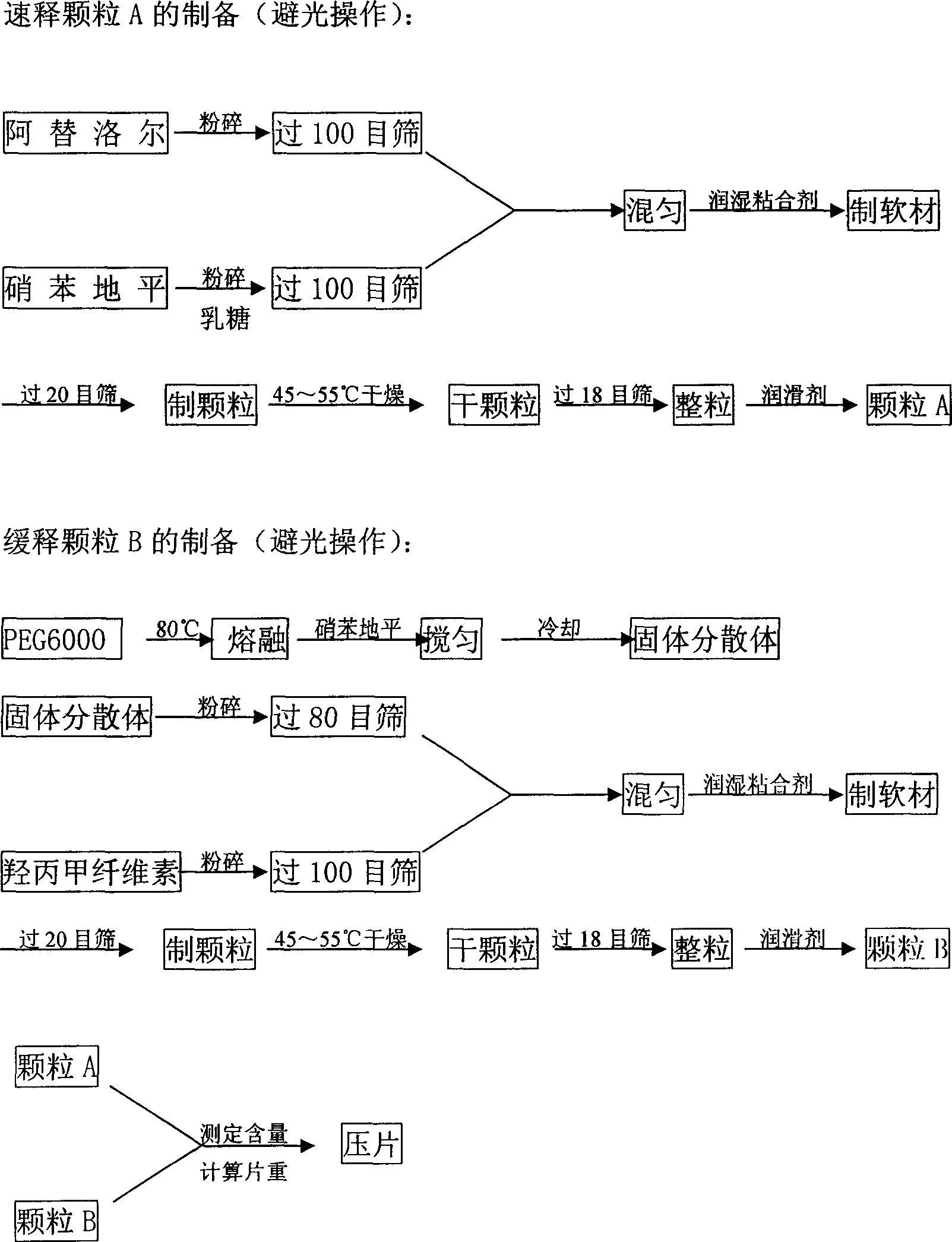
[0061] Unit prescription of compound atenolol sustained-release tablets (1000 tablets, specification: atenolol 50mg, nifedipine 20mg / tablet), in which:
[0062] Immediate-release granules A contain:
[0063] Atenolol 50g
[0064] Nifedipine 5g
[0065] Lactose 10g
[0066] Talc 1.0%
[0067] 5% PVPk 30 80% ethanol solution in appropriate amount
[0068] Sustained-release granules B contain:
[0069] Nifedipine 15g
[0070] PEG6000 60g
[0071] Hypromellose (K 15M ) 70g
[0072] Talc 1.0%
[0073] 5% PVPk 30 An appropriate amount of 80% ethanol solution.
[0074] The preparation method of the above-mentioned compound atenolol sustained-release tablet is to mix atenolol with a small amount of nifedipine to make quick-release granules A, as the quick-release layer, use the hydrophilic gel skeleton material PEG6000 to make the remaining nifedipine Dipine is made into a solid dispersion and then mixed with a slow-release material to make a slow-release granule B as a s...
Embodiment 2
[0077] Unit prescription of compound atenolol sustained-release tablets (1000 tablets, specification: atenolol 50mg, nifedipine 20mg / tablet), in which:
[0078] Immediate-release granules A contain:
[0079] Atenolol 50g
[0080] Nifedipine 5g
[0081] Lactose 3g
[0082] Talc 0.5%
[0083] 5% PVPk 30 80% ethanol solution in appropriate amount
[0084] Sustained-release granules B contain:
[0085] Nifedipine 15g
[0086] PEG6000 60g
[0087] Hypromellose (K 15M ) 15g
[0088] Talc 0.5%
[0089] 5% PVPk 30 An appropriate amount of 80% ethanol solution.
[0090] The preparation method of the above-mentioned compound atenolol sustained-release tablet is to mix atenolol with a small amount of nifedipine to make quick-release granules A, as the quick-release layer, use the hydrophilic gel skeleton material PEG6000 to make the remaining nifedipine Dipine is made into a solid dispersion and then mixed with a slow-release material to make a slow-release granule B as a slow...
Embodiment 3
[0093] Unit prescription of compound atenolol sustained-release tablets (1000 tablets, specification: atenolol 50mg, nifedipine 20mg / tablet), in which:
[0094] Immediate-release granules A contain:
[0095] Atenolol 50g
[0096] Nifedipine 5g
[0097] Lactose 24g
[0098] Talc 2.0%
[0099] 5% PVPk 30 80% ethanol solution in appropriate amount
[0100] Sustained-release granules B contain:
[0101] Nifedipine 15g
[0102] PEG6000 60g
[0103] Hypromellose (K 15M ) 140g
[0104] Talc 2.0%
[0105] 5% PVPk 30 An appropriate amount of 80% ethanol solution.
[0106] The preparation method of the above-mentioned compound atenolol sustained-release tablet is to mix atenolol with a small amount of nifedipine to make quick-release granules A, as the quick-release layer, use the hydrophilic gel skeleton material PEG6000 to make the remaining nifedipine Dipine is made into a solid dispersion and then mixed with a slow-release material to make a slow-release granule B as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com