Long acting drug delivery system for treating breast cancer and preparation method and applications thereof
A drug delivery system and breast cancer technology, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problems of large local irritation, large volume, and low solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
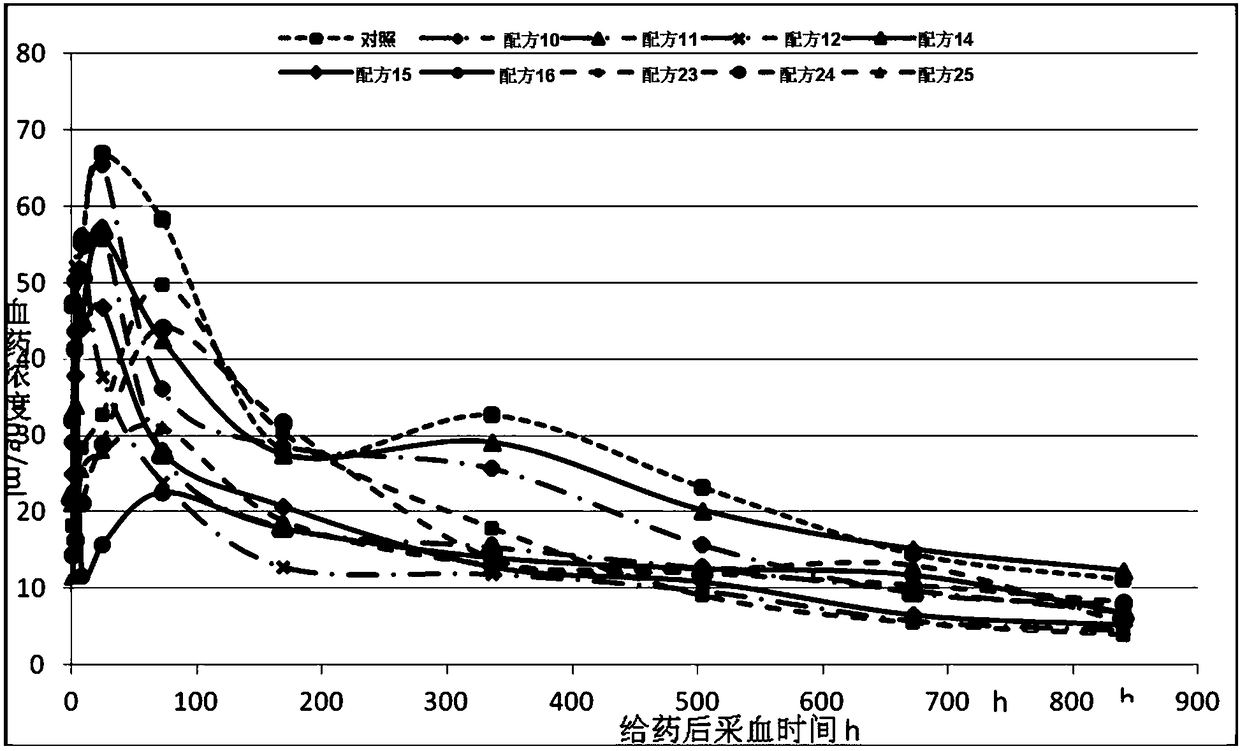
Examples
Embodiment 1
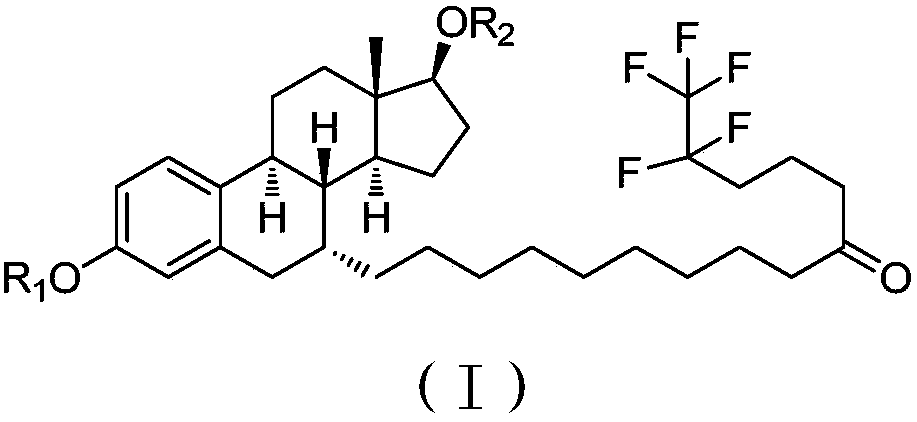
[0134] formula:
[0135]
[0136] Preparation process:
[0137] A liquid preparation: Accurately weigh a certain amount of fulvestrant and dissolve it in the required final amount of ethyl lactate and ethanol. Stir until the drug is completely dissolved, then add the formulated amount of analgesic and antioxidant, stir until completely dissolved, then add the formulated amount of slow-release material, ultrasonically or stir to mix to prepare the required medicinal solution; when all the components are dissolved, Back weigh the total weight to confirm that the volatilization of components is within an acceptable range (less than 0.5%). The above operations were all carried out at room temperature.
[0138] B Aseptic subpackaging: Under aseptic conditions, pass the prepared medicinal solution through a 0.2um microporous membrane to sterilize, then subpackage into prefilled syringes, exhaust the air, and compress the PTFE-coated plunger.
Embodiment 2
[0140] formula:
[0141]
[0142] The preparation process is the same as in Example 1.
Embodiment 3
[0144] formula:
[0145]
[0146] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com