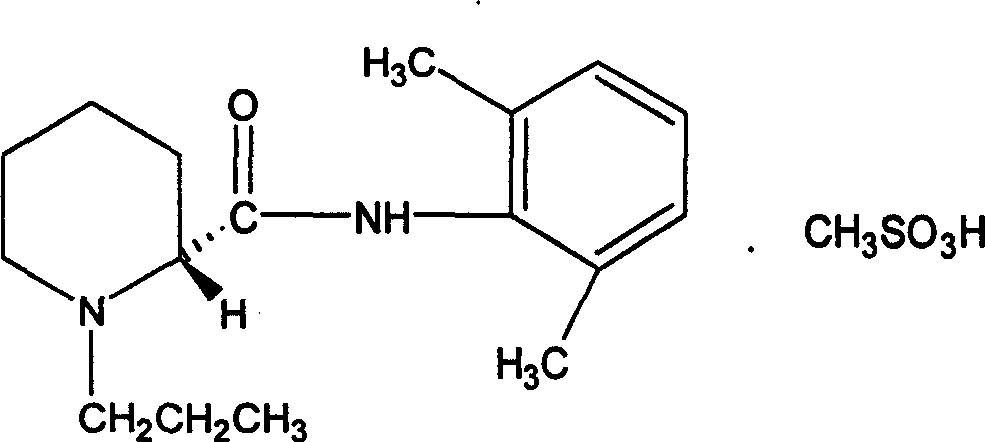
Preparation method of ropivacaine methanesulfonate and its compound and preparation
A technology of ropivacaine mesylate compound and ropivacaine mesylate, which is applied in the fields of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., which can solve the problem of difficult control of drying temperature and stable hygroscopicity Poor performance, high price and other problems, to achieve the effect of good salt stability and water solubility, good stability and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of the ropivacaine mesylate of the present invention comprises the following steps: material preparation—dissolution—adding methanesulfonic acid and a ketone solvent—adding a ketone solvent—filtering and drying—recrystallization. The specific content of described processing step is as follows
[0024] Preparation:
[0025] a.(-)-(S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide.: 200g;
[0026] b. Absolute ethanol: 1000ml;
[0027] c. Methanesulfonic acid: 80ml;
[0028] d. Acetone: 2000ml;
[0029] Dissolution: put ropivacaine single isomer (S) form and absolute ethanol into a container, stir and heat at 20-100°C to reflux, so that ropivacaine is completely dissolved in absolute ethanol;
[0030] Add methanesulfonic acid and ketone solvent: add methanesulfonic acid dropwise to the solution of ropivacaine and absolute ethanol, stir for 20 minutes after the dropwise addition, then add 800ml of acetone, and filter after the solution dr...
Embodiment 2
[0038] Using the ropivacaine mesylate compound of the present invention as a raw material for the preparation of injections comprises the following steps:
[0039] (1) Pretreatment according to the requirements of conventional injections;
[0040] (2) Dissolve 5 g of ropivacaine mesylate compound and 800 mg of sodium chloride in 500 ml of water for injection, add water for injection to 1000 ml after adding activated carbon for decolorization, and adjust the pH to 3.5-6.5 with HCL or NaOH, Finely filter through a 0.45um microporous membrane or ultrafiltration rod, and then treat it according to the conventional method for injection.
Embodiment 3
[0042] The prescription process of ropivacaine mesylate compound boutique configuration 10ml: 23.8mg injection is:
[0043] (1) The ampoule is treated according to the conventional treatment method, and is set aside;
[0044] (2) Dosing: Take 2.38g and 6-10g of sodium chloride, add it to the batching bucket, add injection water to 500ml, stir the solution, add 0.1% g / ml activated carbon for targeting and stir for 30 minutes;
[0045] (3) Filtration: first filter for decarburization, then add water for injection to 1000ml, stir evenly, adjust the pH to 3.9 with HCl or NaOH, and fine filter with a 0.45 micron microporous membrane or ultrafiltration rod;
[0046] (4) Filling: measure the pH value: 3.5-5.5; content: 90-110%, fill according to the requirements of 10ml: 23.8mg injection;
[0047] (5) Fusion sealing: use a wire drawing sealing machine to seal;
[0048](6) Sterilization: 0.07Mpa115°C, heat and then sterilize for 30 minutes;
[0049] (7) Picking up leaks: carry out ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com