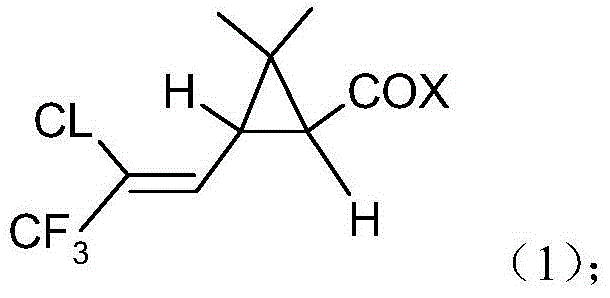
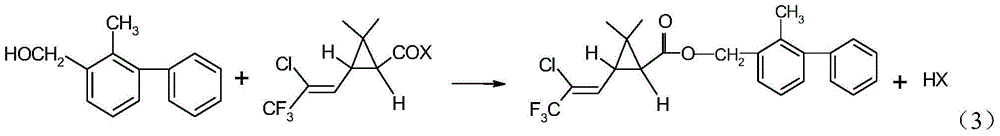
Method for producing bifenthrin with clean synthesizing process
A production method and bifenthrin technology are applied in the field of bifenthrin production with a clean synthesis process, which can solve the problems of long reaction time, excessive waste water, and low yield, and achieve improved synthesis conversion rate, simple process, and The effect of small amount of three wastes produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 194Kg of 2-methyl-3-phenylbenzyl alcohol and 700Kg of n-hexane were put into a 2000L synthesis kettle equipped with stirring, condenser and tail gas absorption device, and the temperature was raised to 45°C. Tail gas is absorbed by falling film water in three stages in series, 80L of water is added to each stage, and the absorption temperature is 10-55°C.
[0024] The temperature of the reaction kettle was controlled at 45-55°C, and 261Kg of trifluorochrysanthemum acid chloride was started to be added dropwise into the synthesis kettle for 3 hours. After the dropwise addition was completed, the reaction was incubated for 6 hours. At the end of the heat preservation reaction, the conversion rate of 2-methyl-3-phenylbenzyl alcohol in Zhongkong was 99.5%. 114.5Kg of hydrochloric acid, with a content of 30.08%, was recovered in the first stage of tail gas absorption.
[0025] The materials in the synthesis kettle were discharged into the crystallization kettle to cool dow...
Embodiment 2
[0027] 198Kg of 2-methyl-3-phenylbenzyl alcohol and 990Kg of n-hexane were put into a 2000L synthesis kettle equipped with stirring, condenser and tail gas absorption device, and the temperature was raised to 60°C. Tail gas is absorbed by falling film water in three stages in series, 80L of water is added to each stage, and the absorption temperature is 10-55°C.
[0028] The temperature of the reaction kettle was controlled at 60-65°C, and 261Kg of trifluorochrysanthemum acid chloride was started to be added dropwise into the synthesis kettle for 3 hours. After the dropwise addition was completed, the reaction was incubated for 6 hours. At the end of the heat preservation reaction, the conversion rate of 2-methyl-3-phenylbenzyl alcohol in Zhongkong was 99.7%. 115.0Kg of hydrochloric acid with a content of 30.23% is recovered in the first stage of tail gas absorption.
[0029] The materials in the synthesis kettle were discharged into the crystallization kettle to cool down a...
Embodiment 3
[0031] 198Kg of 2-methyl-3-phenylbenzyl alcohol and 790Kg of cyclohexane were put into a 2000L synthesis kettle equipped with stirring, condenser and tail gas absorption device, and the temperature was raised to 45°C. Tail gas is absorbed by falling film water in three stages in series, adding 80L of water to each stage, and the absorption temperature is 10-60°C.
[0032] The temperature of the reaction kettle was controlled at 45-55°C, and 261Kg of trifluorochrysanthemum acid chloride was started to be added dropwise into the synthesis kettle for 3 hours. After the dropwise addition was completed, the reaction was incubated for 6 hours. At the end of the heat preservation reaction, the conversion rate of 2-methyl-3-phenylbenzyl alcohol in Zhongkong was 99.2%. 115.2Kg of hydrochloric acid, with a content of 30.16%, was recovered in the first stage of tail gas absorption.
[0033] The material in the synthesis kettle was discharged into the crystallization kettle to cool down...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com