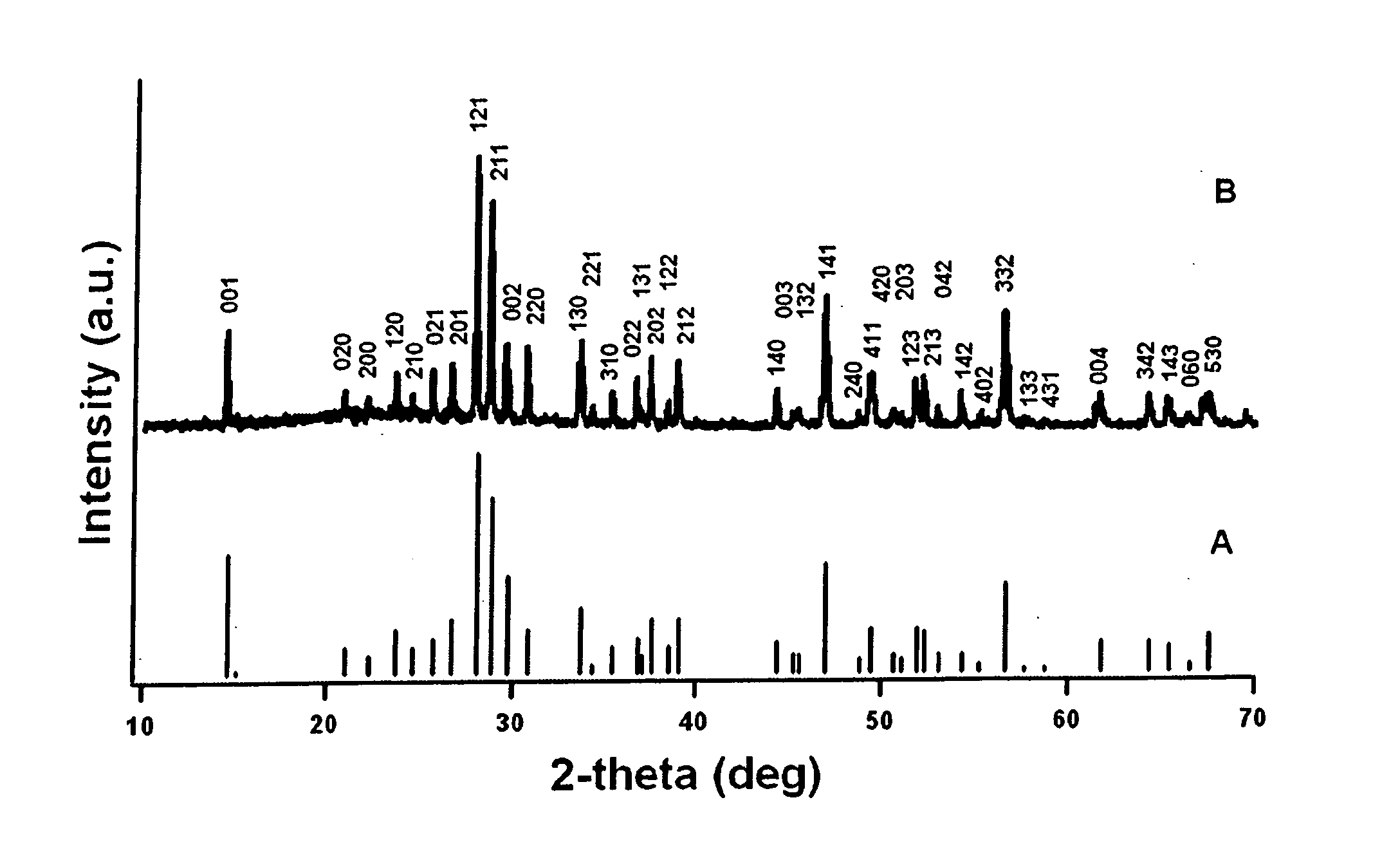
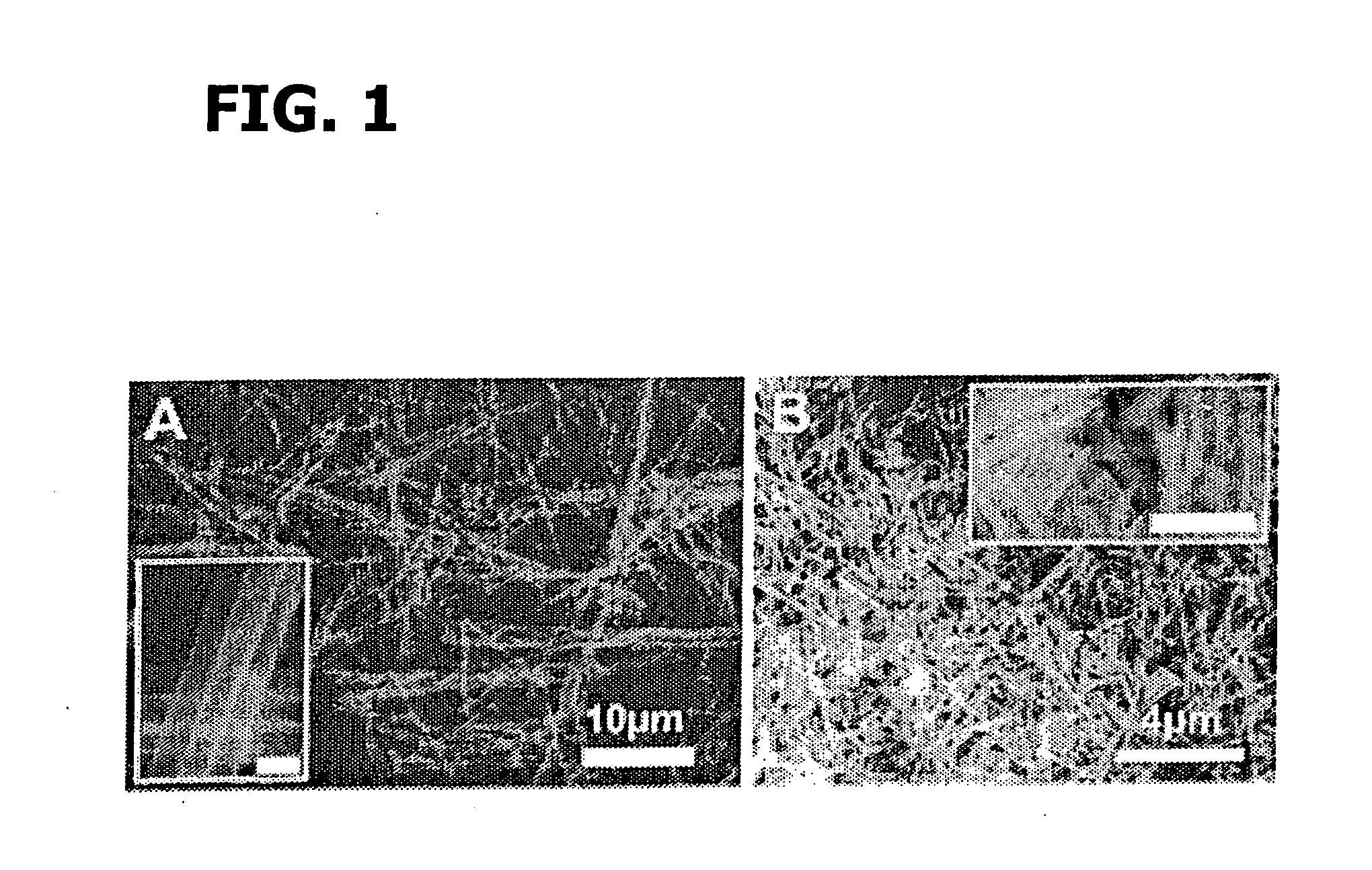
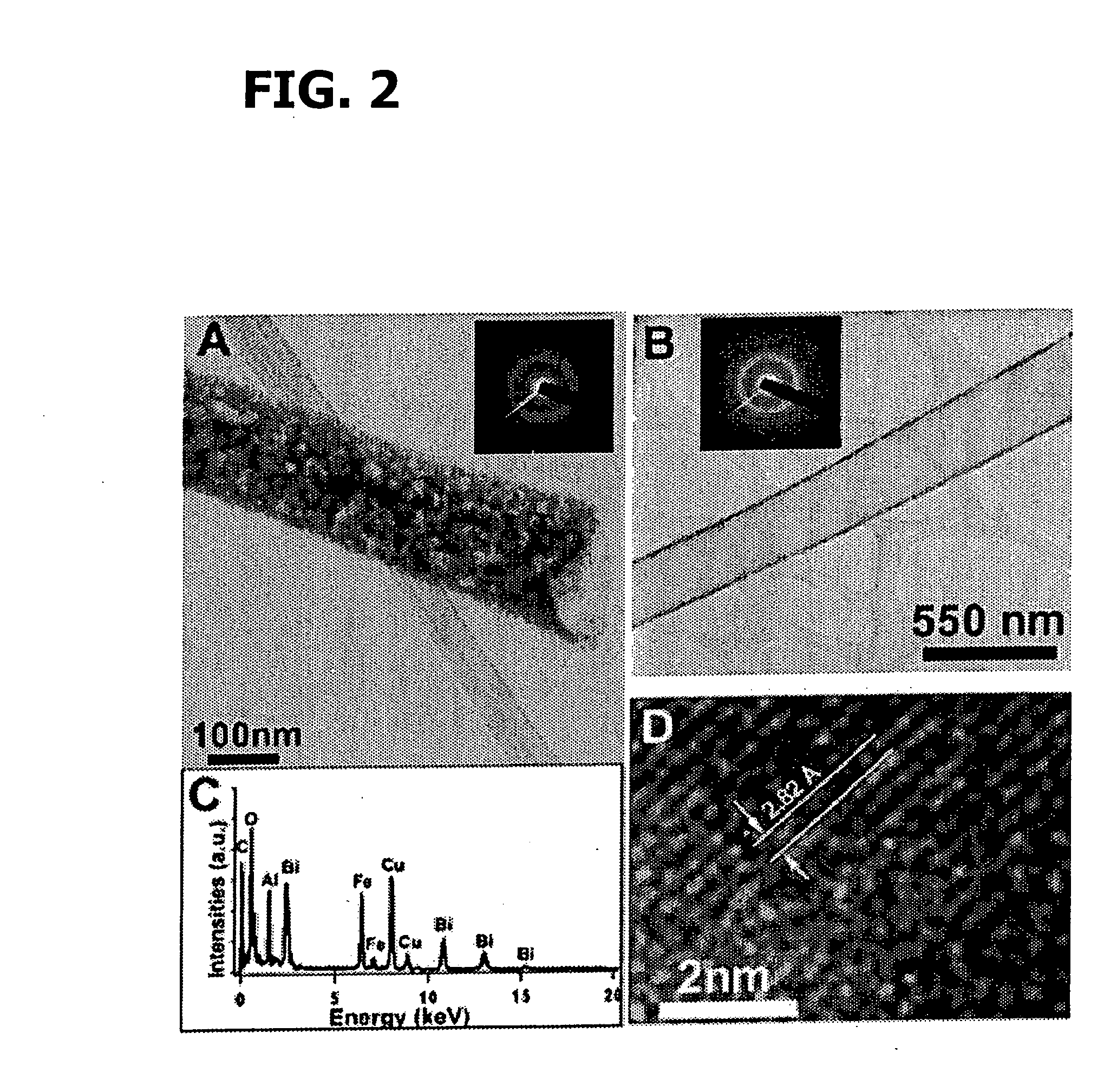
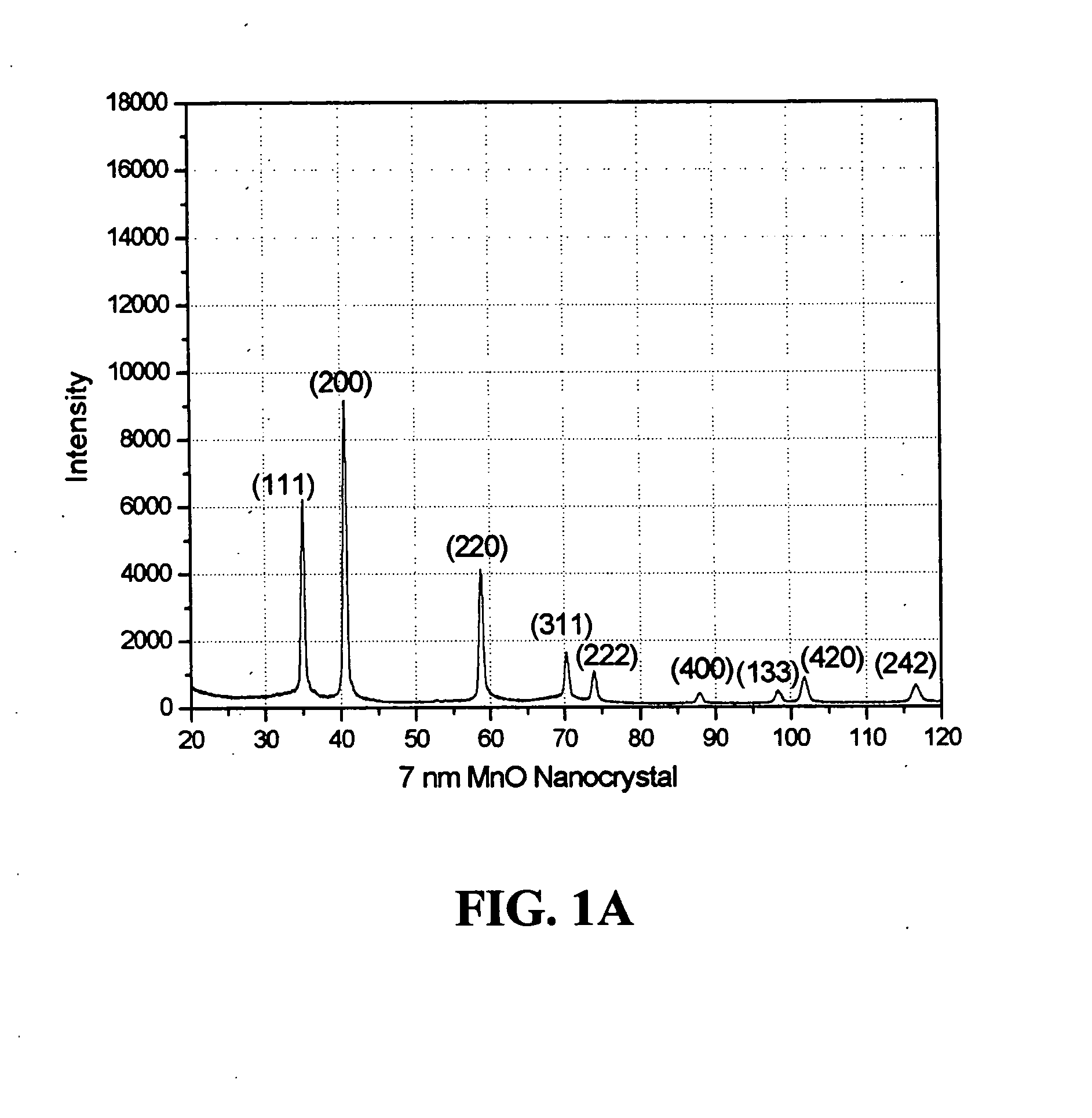
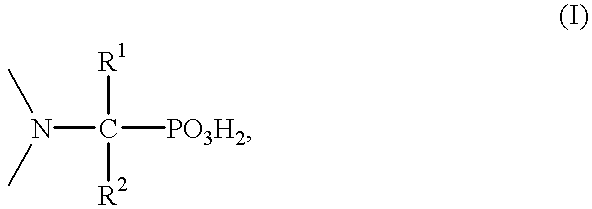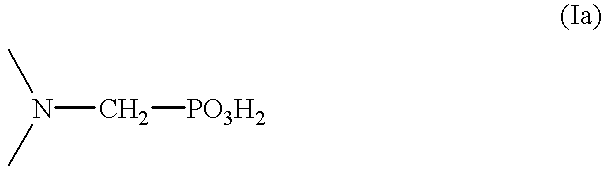Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
319results about "Rhenium compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
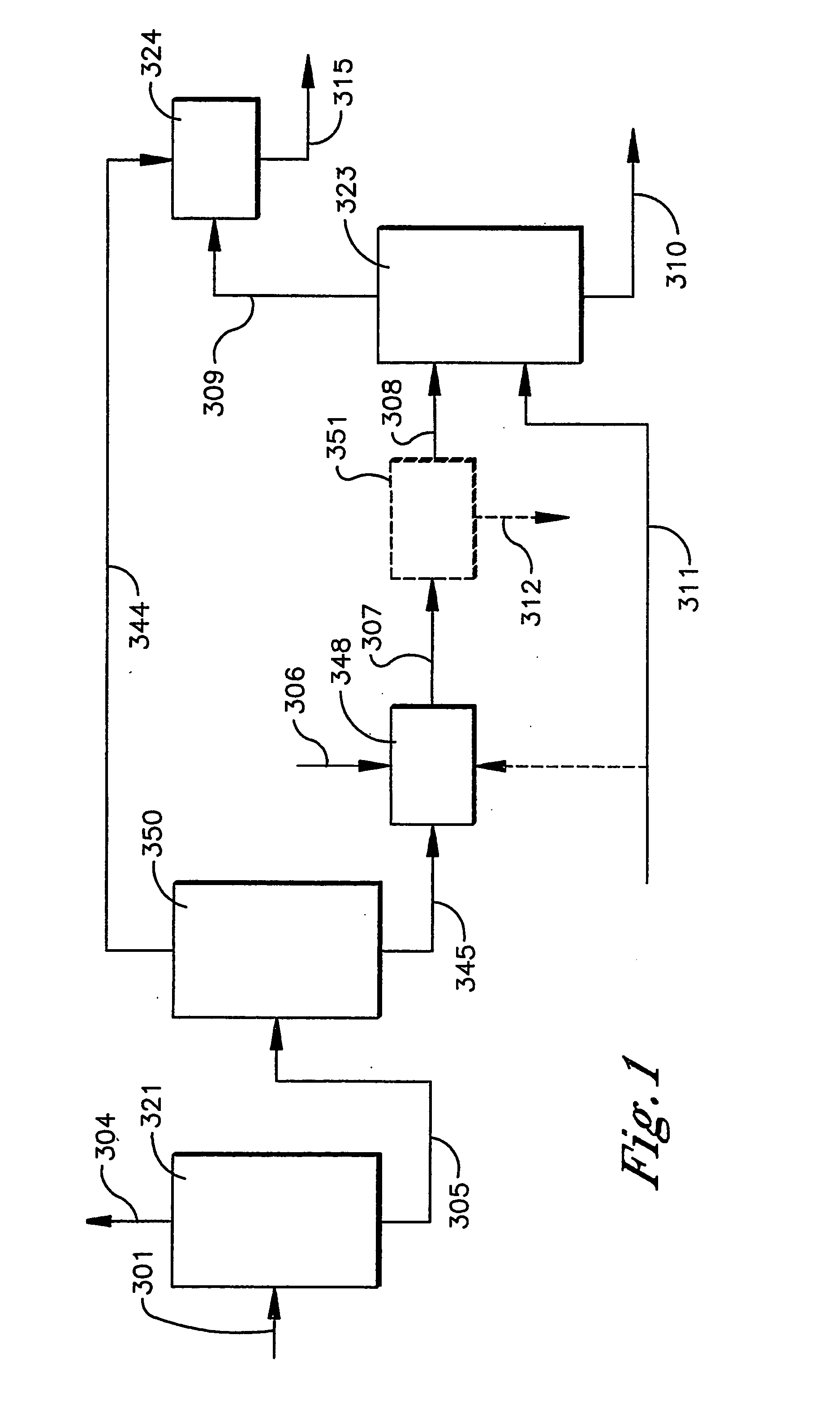
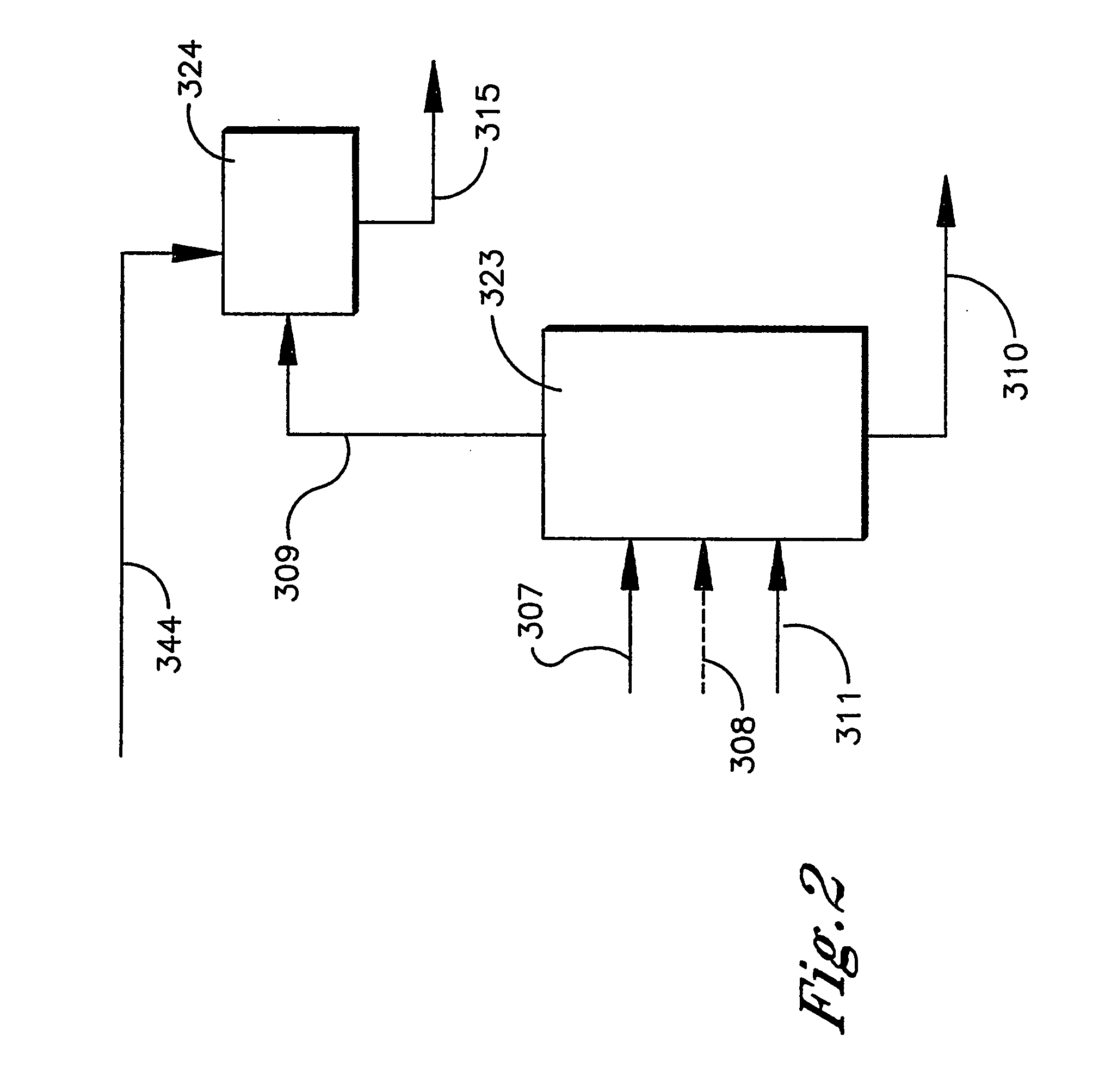
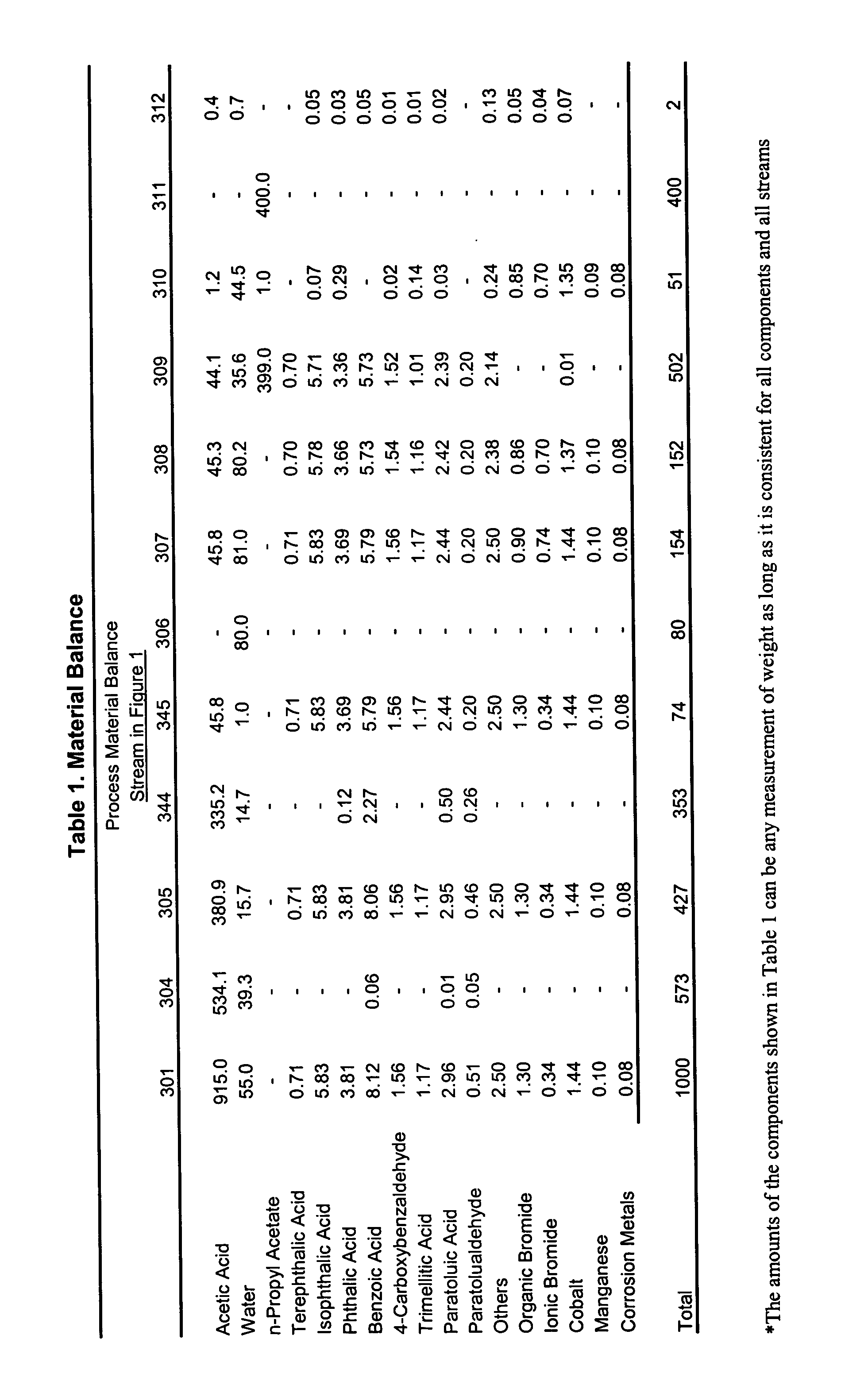
Extraction process for removal of impurities from an oxidizer purge stream in the synthesis of carboxylic acid
InactiveUS20050038288A1Easy to operateImprove reliabilityOrganic compound preparationOrganic chemistry methodsSingle stageMetal catalyst
Disclosed is a process that relates to the recovery of a metal catalyst from an oxidizer purge stream produced in the synthesis of carboxylic acid, typically terephthalic acid. The process involves the addition of a wash solution to a high temperature molten dispersion to recover the metal catalyst and then subjecting an aqueous mixture or purified aqueous mixture so formed to a single stage extraction to remove organic impurities to produce an extract stream and a raffinate stream comprising the metal catalyst.
Owner:GRUPO PETROTEMEX DE C V
Method for preparing precursor for chemical vapor deposition of metallic rhenium
The invention discloses a method for preparing a precursor for chemical vapor deposition of metallic rhenium and belongs to the technical field of material preparation. According to the method, ReCl5 is made to react in an oxidizing atmosphere, efficient solid-liquid-gas separation is conducted on reactants and products with a sand core filter bulb so that reactants, reaction products and waste gas can be effectively separated, the reaction products ReOCl4 and ReO3Cl are gathered in a collection vessel heated by an oil bath pan at the same time, oxygen introduction is stopped after reaction ends, circulation of inert gases is maintained, a tube furnace is cooled, the oil bath pan is heated at the same time to enable ReO3Cl to volatilize to enter a rectification unit to be collected, ReOCl4 is purified, and then the high-purity precursor ReOCl4 for chemical vapor deposition of metallic rhenium is obtained. By the adoption of the method, reaction efficiency is high, ReOCl4 and ReO3Cl are separated through rectification, and the purity of the product ReOCl4 is improved.
Owner:海朴精密材料(苏州)有限责任公司
Ternary oxide nanostructures and methods of making same
InactiveUS20070138459A1Suitable for preparationFrom gel stateAlkaline earth titanatesNanostructureDislocation
A single crystalline ternary nanostructure having the formula AxByOz, wherein x ranges from 0.25 to 24, and y ranges from 1.5 to 40, and wherein A and B are independently selected from the group consisting of Ag, Al, As, Au, B, Ba, Br, Ca, Cd, Ce, Cl, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Ho, I, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, P, Pb, Pd, Pr, Pt, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Ti, Ti, Tm, U, V, W, Y, Yb, and Zn, wherein the nanostructure is at least 95% free of defects and / or dislocations.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Nano-sized particles, processes of making, compositions and uses thereof
InactiveUS20070140951A1Economical and efficientQuality improvementMaterial nanotechnologyToilet preparationsSolventPharmaceutical formulation
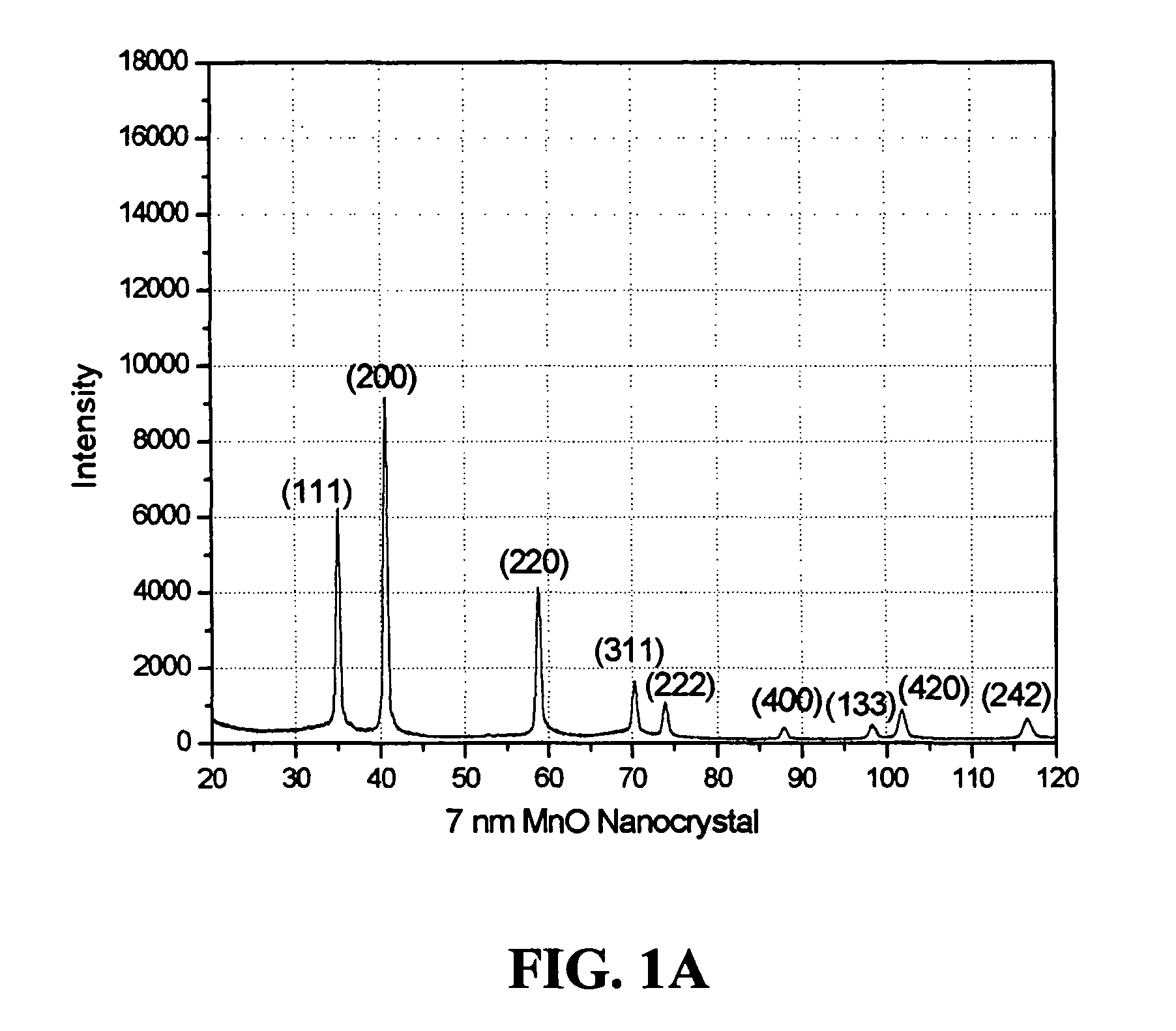
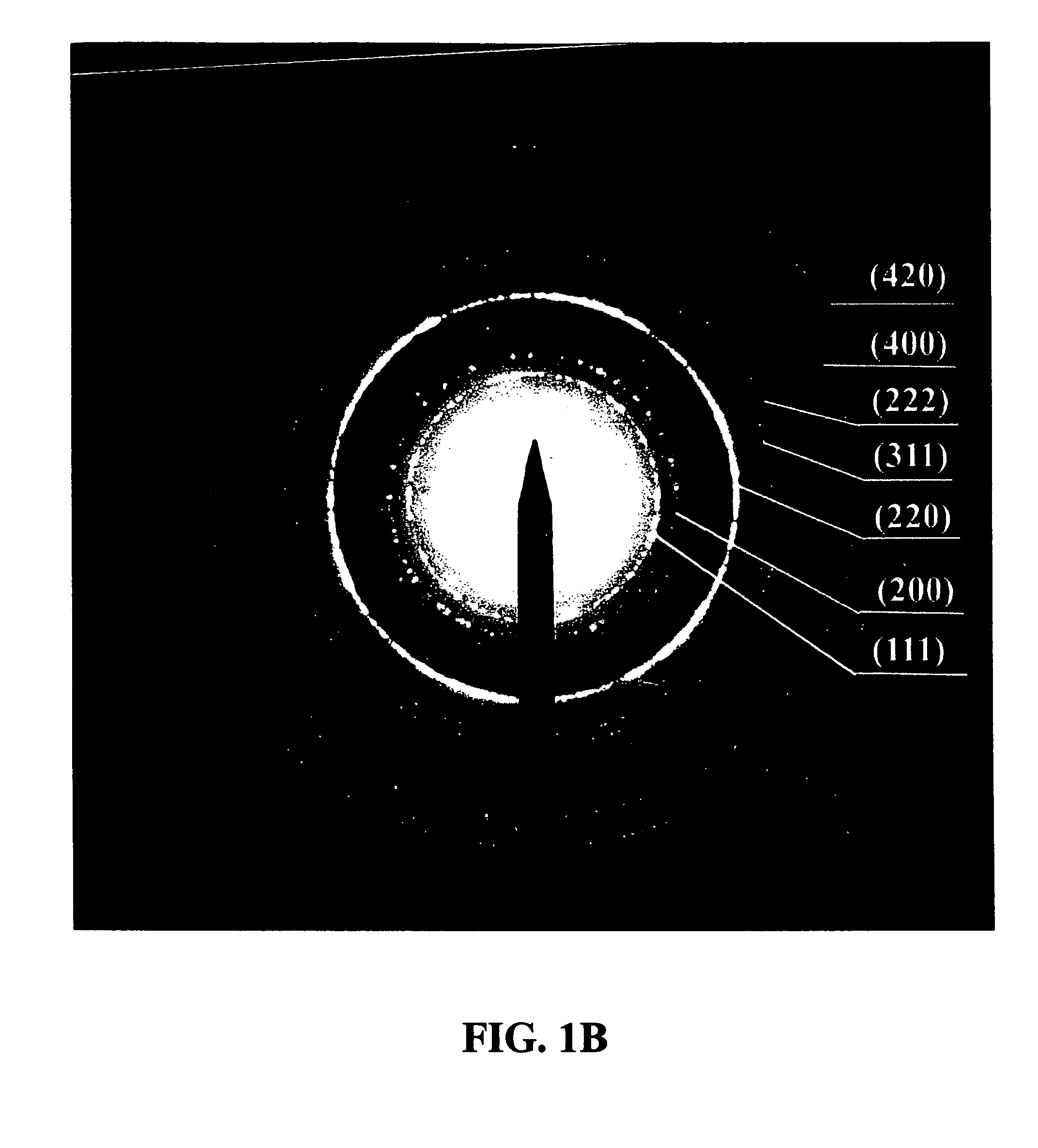
The present invention describes methods for preparing high quality nanoparticles, i.e., metal oxide based nanoparticles of uniform size and monodispersity. The nanoparticles advantageously comprise organic alkyl chain capping groups and are stable in air and in nonpolar solvents. The methods of the invention provide a simple and reproducible procedure for forming transition metal oxide nanocrystals, with yields over 80%. The highly crystalline and monodisperse nanocrystals are obtained directly without further size selection; particle size can be easily and fractionally increased by the methods. The resulting nanoparticles can exhibit magnetic and / or optical properties. These properties result from the methods used to prepare them. Also advantageously, the nanoparticles of this invention are well suited for use in a variety of industrial applications, including cosmetic and pharmaceutical formulations and compositions.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Manganese oxide based materials as ion intercalation hosts in lithium batteries
InactiveUS20050135993A1Enhances electrochemical property and performance of materialImprove stabilityActive material electrodesManganese oxides/hydroxidesRechargeable cellPERMANGANATE ION
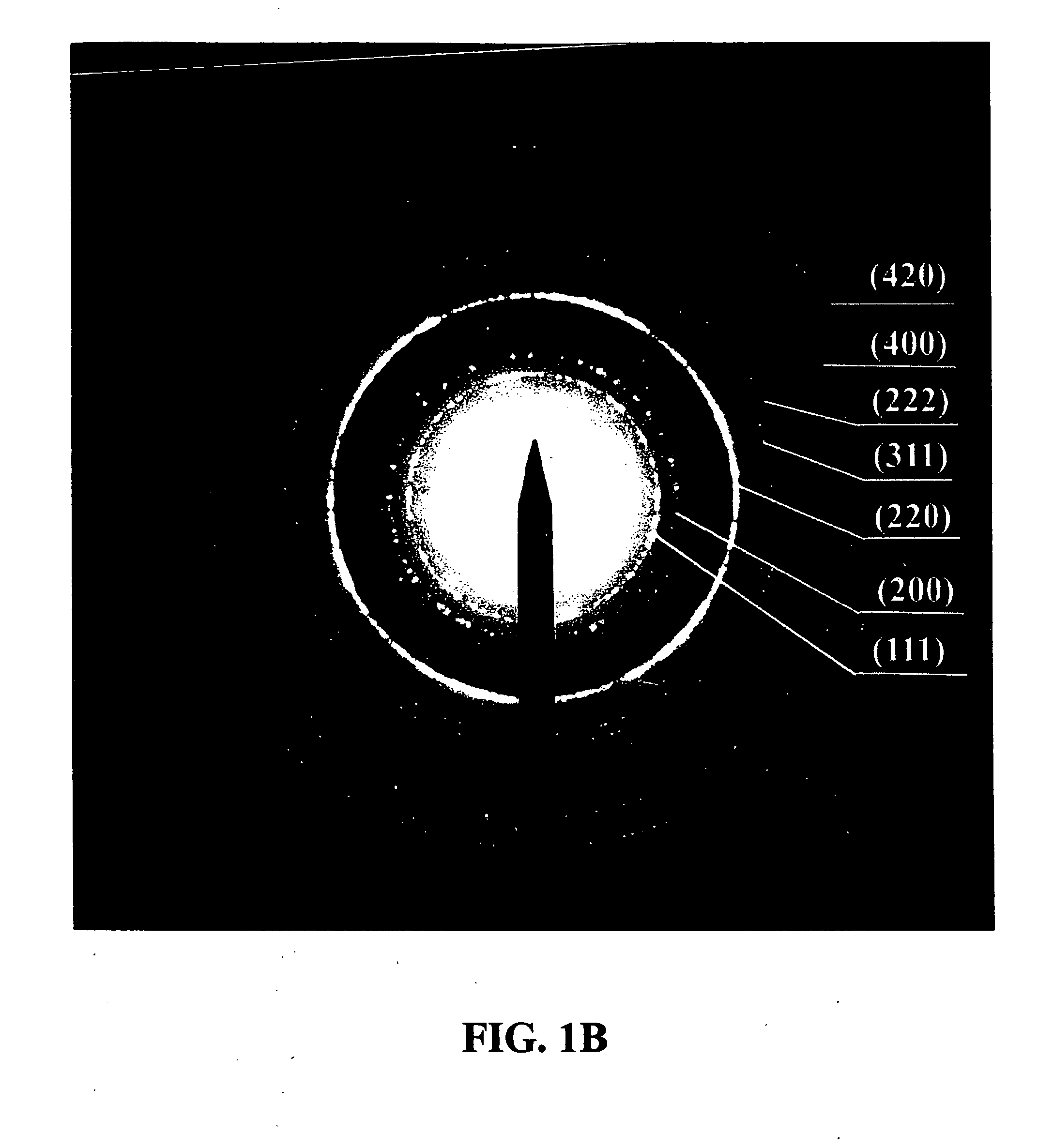
The present invention is directed to a process for making an amorphous nanostructured cation-doped manganese oxide material useful as an ion intercalation host for rechargeable batteries, including the steps of preparing a solution containing cation permanganate combined optionally with a cation donor compound, mixing the solution with a reducing agent to yield a hydrogel comprising a manganese oxide compound, cryogenically freezing the hydrogel, drying the frozen gel to yield a cryogel amorphous nanostructured cation-doped manganese oxide, and heat treating the dried cryogel.
Owner:RUTGERS THE STATE UNIV
Positive active material for rechargeable lithium battery and method of preparing same
InactiveUS20020055042A1Improve thermal stabilityElectrode manufacturing processesZirconium compoundsPhysical chemistryLithium battery
Disclosed is a positive active material for a rechargeable lithium battery. The positive active material includes at least one compound represented by formulas 1 to 4 andl a metal oxide or composite metal oxide layer formed on the compound. <table-cwu id="TABLE-US-00001"> <number>1< / number> <tgroup align="left" colsep="0" rowsep="0" cols="3"> <colspec colname="OFFSET" colwidth="42PT" align="left" / > <colspec colname="1" colwidth="77PT" align="left" / > <colspec colname="2" colwidth="98PT" align="center" / > <row> <entry>< / entry> <entry>< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyF2< / entry> <entry>(1)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-yMnyS2< / entry> <entry>(2)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aFa< / entry> <entry>(3)< / entry> < / row> <row> <entry>< / entry> <entry>LixNi1-y-zMnyMzO2-aSa< / entry> <entry>(4)< / entry> < / row> <row> <entry>< / entry> <entry namest="OFFSET" nameend="2" align="center" rowsep="1">< / entry> < / row> < / tgroup> < / table-cwu> (where M is selected from the group consisting of Co, Mg, Fe, Sr, Ti, B, Si, Ga, Al, Sc, Y, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Ac, Th, Pa, U, Np, IPu, Am, Cm, Bk, Cf, Es, Fm, Md, No and Lr, 0.95<=x<=1.1, 0<=y<=0.99, 0<=,z<=0.5, and 0<=a<=0.5)
Owner:SAMSUNG SDI CO LTD
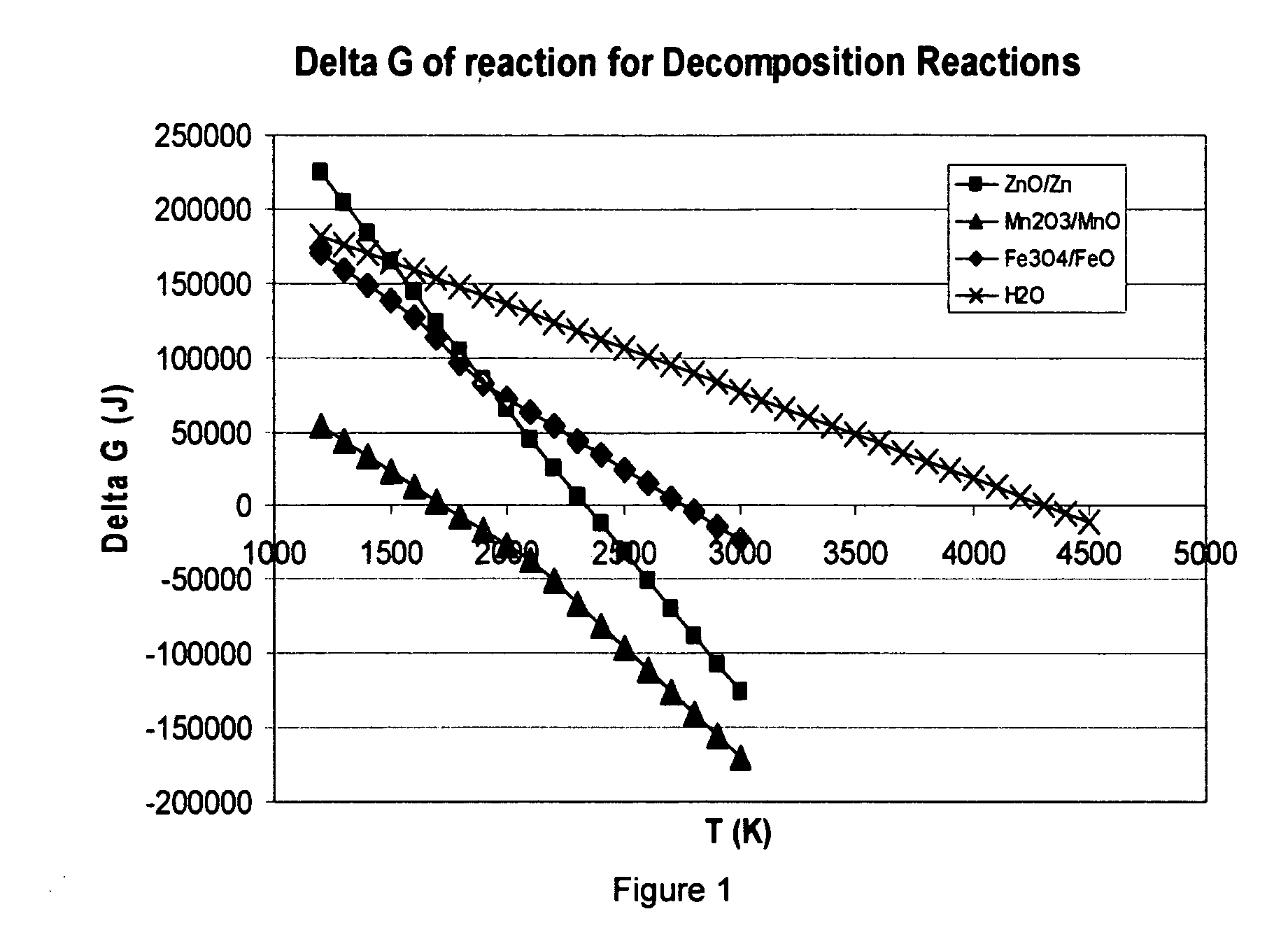
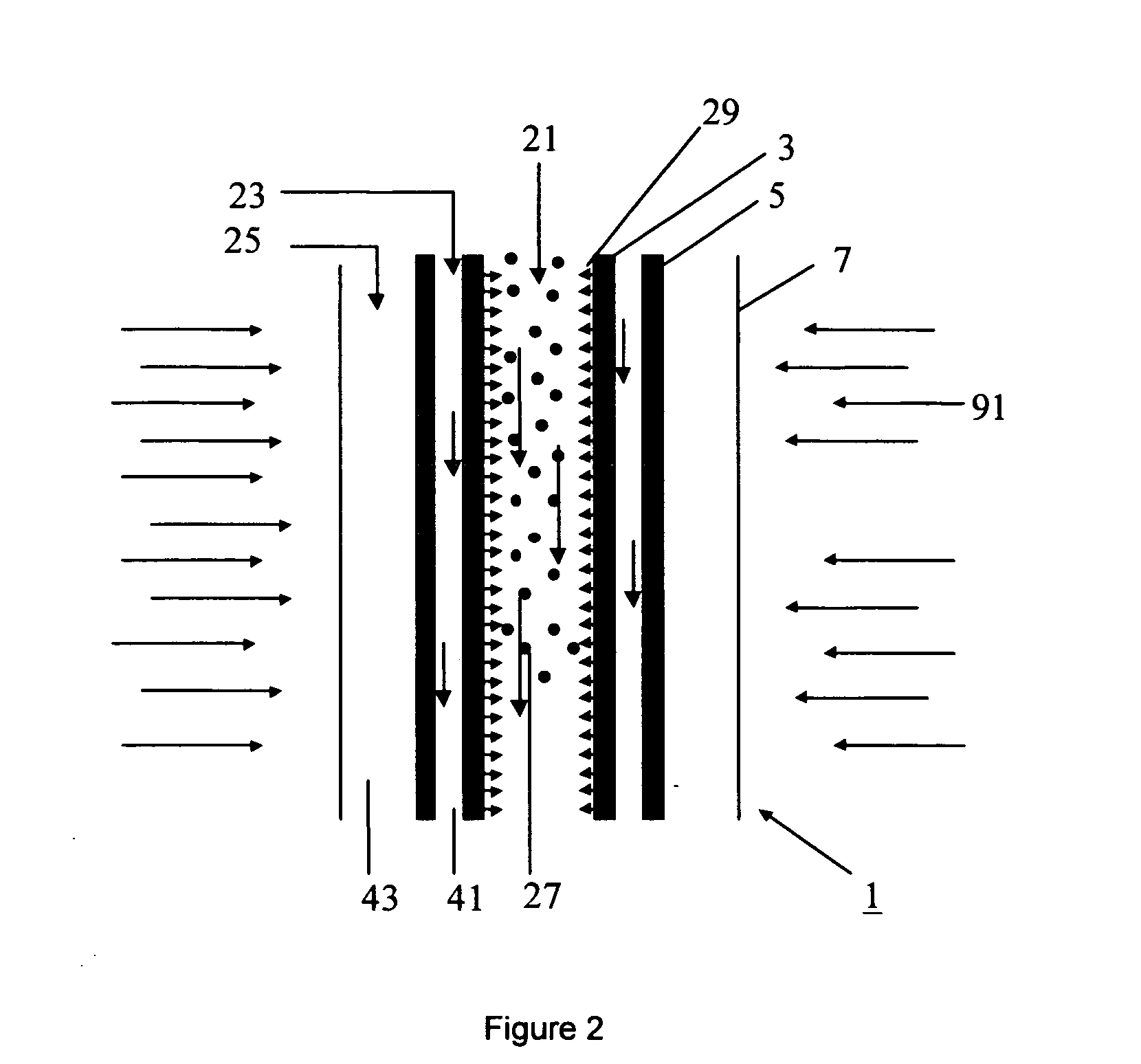
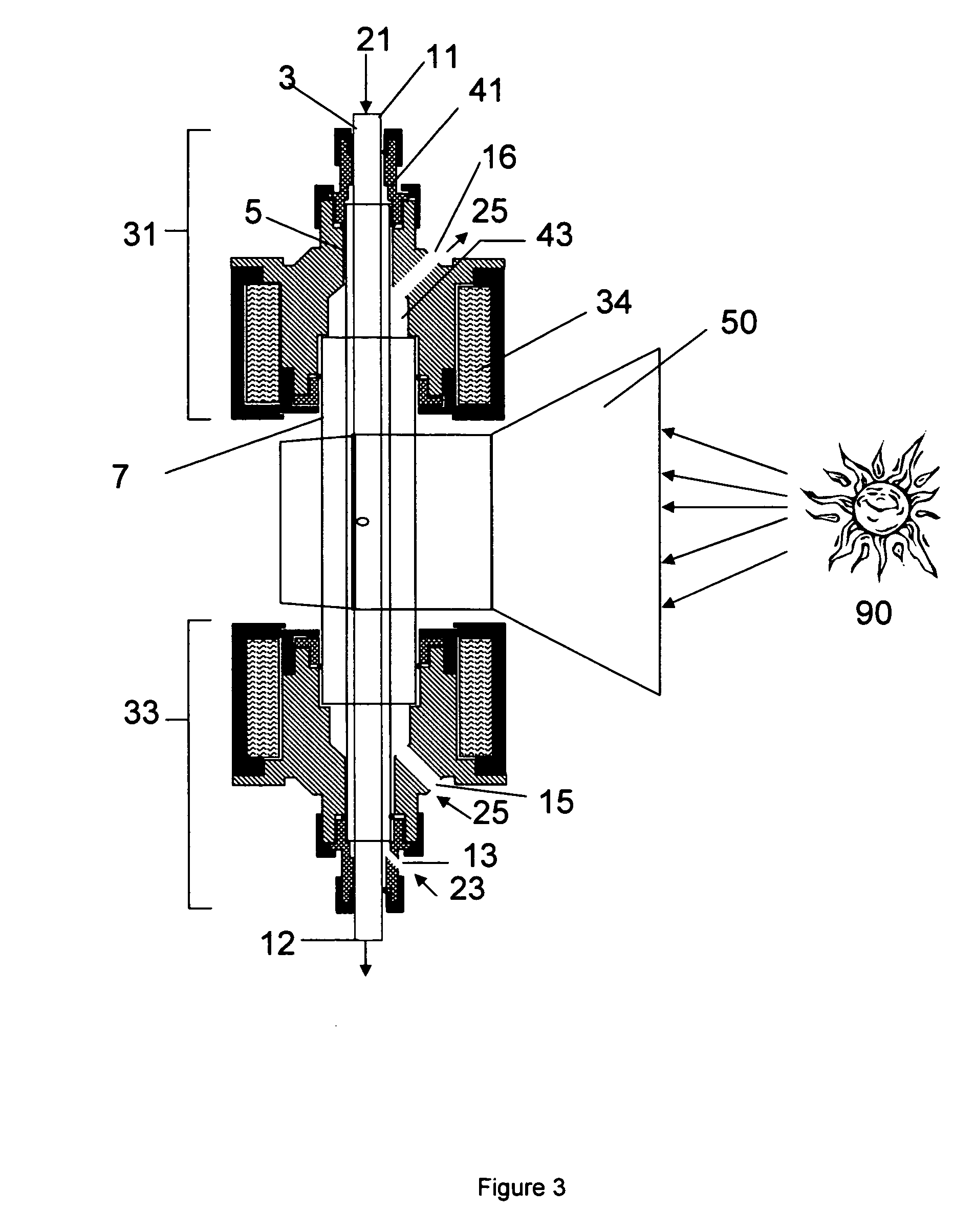
Metal-oxide based process for the generation of hydrogen from water splitting utilizing a high temperature solar aerosol flow reactor
InactiveUS20060188433A1Suppress heterogeneous nucleationReduce recombinationCatalytic gas-gas reactionZinc oxides/hydroxidesHydrogenReactor system
The invention provides methods for reduction of metal oxide particles using a high temperature solar aerosol reactor. The invention also provides metal-oxide based processes for the generation of hydrogen from water splitting using a high temperature solar aerosol reactor. In addition, the invention provides solar thermal reactor systems suitable for use with these processes.
Owner:UNIV OF COLORADO THE REGENTS OF +1
Method for producing positive plate material for lithium secondary cell
InactiveUS20050265909A1Improve battery performanceElectrode manufacturing processesActive material electrodesAlkaline earth metalLithium carbonate
A positive plate material for lithium secondary cells stably exhibiting excellent performance including the cell initial capacity, cycle characteristics, and the safety. The material is produced by dripping an aqueous solution of a salt (e.g., cobalt sulfate) of a doping element (e.g., a transition metal, an alkaline metal, an alkaline-earth metal, B, or Al) into an alkaline solution, a carbonate solution, or a hydrogencarbonate solution in any one of which a compound (e.g., manganese oxide) of a metal (Mn, Co, Ni, or the like) which is the major component of the positive plate material so as to precipitate the compound of the doping element on the major component compound and to cover the major component compound, mixing the major component compound covered with the doping element with a lithium compound (e.g., lithium carbonate), and firing the mixture.
Owner:NIKKO MATERIALS CO LTD
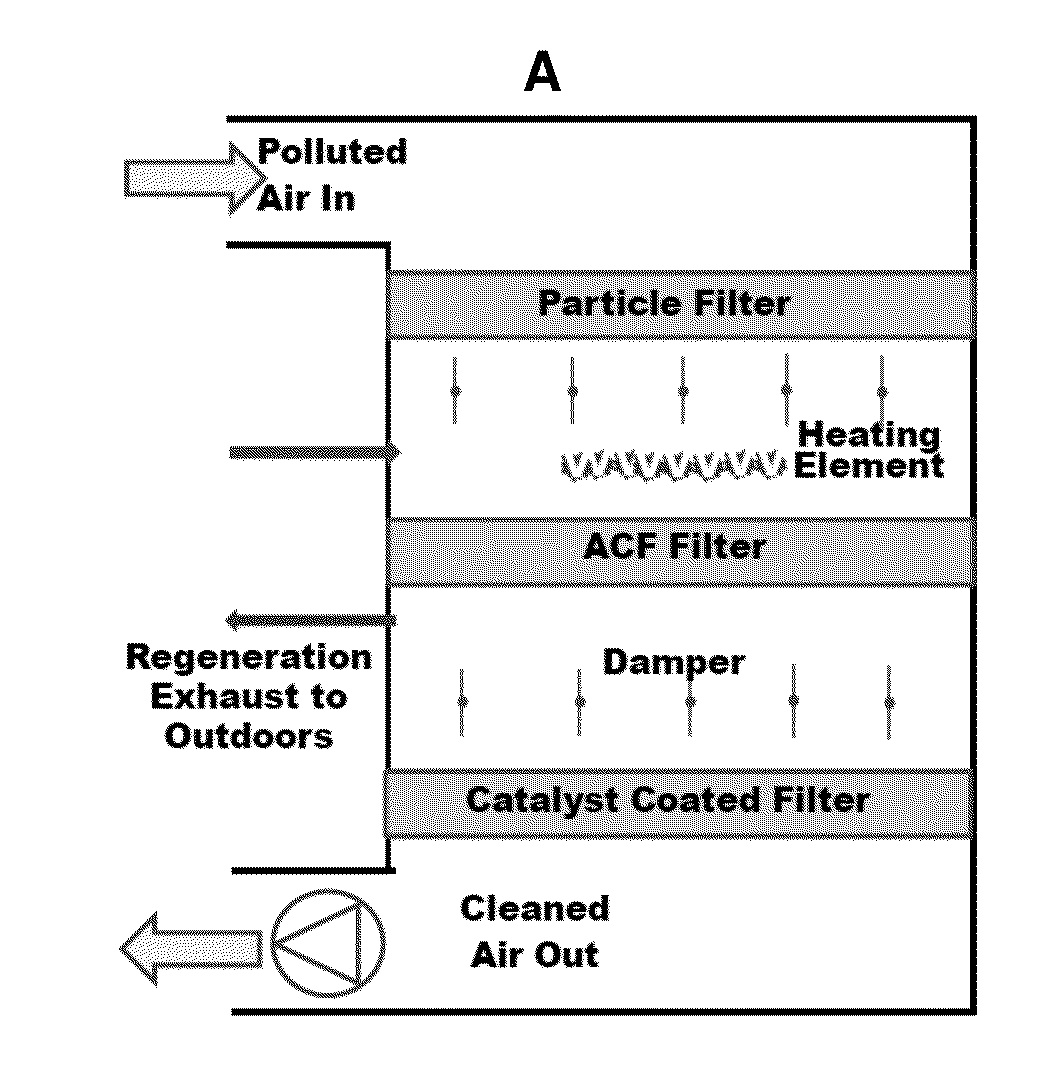
Use of Manganese Oxide and Activated Carbon Fibers for Removing a Particle, Volatile Organic Compound or Ozone from a Gas
InactiveUS20140255283A1Reduce contentLow VOC contentOrganic chemistryManganese oxides/hydroxidesFiberCarbon fibers
The present invention provides for a device for reducing a volatile organic compound (VOC) content of a gas comprising a manganese oxide (MnOx) catalyst. The manganese oxide (MnOx) catalyst is capable of catalyzing formaldehyde at room temperature, with complete conversion, to CO2 and water vapor. The manganese oxide (MnOx) catalyst itself is not consumed by the reaction of formaldehyde into CO2 and water vapor. The present invention also provides for a device for reducing or removing a particle, a VOC and / or ozone from a gas comprising an activated carbon filter (ACF) on a media that is capable of being periodically regenerated.
Owner:RGT UNIV OF CALIFORNIA
Methods of preparing cathode active materials for lithium secondary battery
InactiveUS6071489AEasy to optimizeHigh crystallinityMaterial nanotechnologyNon-aqueous electrolyte accumulatorsManganeseEthyl acetate
The LixMn2O4 powder for cathode active material of a lithium secondary battery of the present invention is prepared by a method of comprising the steps of mixing an acetate aqueous solution using Li acetate and Mn acetate as metal precursors, and a chelating agent aqueous solution using PVB, GA, PAA or GC as a chelating agent; heating the mixed solution at 70 DIFFERENCE 90 DEG C. to form a sol; further heating the sol at 70 DIFFERENCE 90 DEG C. to form a gel precursor; calcining the produced gel precursor at 200 DIFFERENCE 900 DEG C. for 5 DIFFERENCE 30 hours under atmosphere. The cathode active material, LixMn2O4 powder for a lithium secondary battery in accordance with the present invention has a uniform particle size distribution, a high crystallinity and a pure spinel-phase, and a particle size, a specific surface area, a lattice of a cubic structure and the like can be controlled upon the preparing conditions. The present invention also provides a method of preparing LiNi1-xCoxO2 powder, which comprises the steps of providing a gel precursor using PAA as a chelating agent and hydroxide, nitrate or acetate of Li, Co and Ni as metal precursors; heating the gel precursor at 200 DIFFERENCE 900 DEG C. for 5 DIFFERENCE 30 hours to form a powder. The LixMn2O4 and LiNi1-xCoxO2 powder of the present invention can be used for a cathode active material of a lithium secondary battery such as a lithium ion battery or lithium polymer battery.
Owner:SAMSUNG DISPLAY DEVICES CO LTD
Ternary oxide nanostructures and methods of making same
InactiveUS7585474B2Suitable for preparationFrom gel stateAlkaline earth titanatesSingle crystalNanostructure
A single crystalline ternary nanostructure having the formula AxByOz, wherein x ranges from 0.25 to 24, and y ranges from 1.5 to 40, and wherein A and B are independently selected from the group consisting of Ag, Al, As, Au, B, Ba, Br, Ca, Cd, Ce, Cl, Cm, Co, Cr, Cs, Cu, Dy, Er, Eu, F, Fe, Ga, Gd, Ge, Hf, Ho, I, In, Ir, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Nd, Ni, Os, P, Pb, Pd, Pr, Pt, Rb, Re, Rh, Ru, S, Sb, Sc, Se, Si, Sm, Sn, Sr, Ta, Tb, Tc, Te, Ti, Tl, Tm, U, V, W, Y, Yb, and Zn, wherein the nanostructure is at least 95% free of defects and / or dislocations.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Lithium-manganese composite oxides for lithium ion battery and process for preparing same
ActiveUS20090289218A1Improve cycle performanceIncrease battery capacityElectrode manufacturing processesConductive materialLithium-ion batteryComposite oxide
A lithium-manganese composite oxide for a lithium ion battery having a good cycle property at high-temperature and battery property of high capacity is provided.A spinel type lithium-manganese composite oxide for a lithium ion battery represented by a general formula: Li1+xMn2−yMyO4 (wherein M is one or more elements selected from Al, Mg, Si, Ca, Ti, Cu, Ba, W and Pb, and, −0.1≦x≦0.2, and 0.06≦y≦0.3), and when D10, D50 and D90 are defined as a particle size at which point the cumulative frequency of volume reaches 10%, 50% and 90% respectively, d10 is not less than 2 μm and not more than 5 μm, d50 is not less than 6 μm and not more than 9 μm, and d90 is not less than 12 μm and not more than 15 μm, and BET specific surface area thereof is greater than 1.0 m2 / g and not more than 2.0 m2 / g, and the tap density thereof is not less than 0.5 g / cm3 and less than 1.0 g / cm3.
Owner:JX NIPPON MINING& METALS CORP
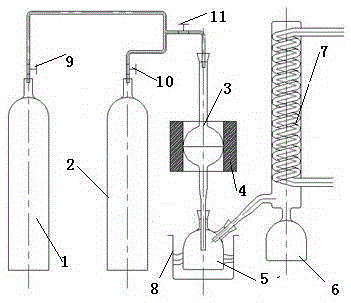
Method for preparing ammonium perrhenate from waste liquid containing molybdenum and rhenium
ActiveCN102173457APromote enrichmentSave resourcesRhenium compoundsCalcium hydroxideAmmonium perrhenate
The invention relates to a method for preparing ammonium perrhenate from waste liquid containing molybdenum and rhenium. In the technical scheme of the invention, the method comprises the following steps of: adding hydrogen peroxide into the waste liquid containing molybdenum and rhenium until the solution turns to yellow, then adding a mixed agent until the pH of the solution is 6 to 7, separating by filter pressing, collecting the filtrate, absorbing the filtrate by a resin exchange column, stopping adsorption until the concentration of rhenium in effluent is constant, eluting with NH3.H2O,collecting the eluate, heating to concentrate the eluate at 98-100 DEG C, cooling, and crystallizing to obtain ammonium perrhenate. The mixed agent is a mixture of calcium hydroxide and calcium oxidein a weight ratio of 5:1. According to the invention, the waste liquid containing molybdenum and rhenium, particularly the absorption liquid of the flue gas during molybdenum roasting, is used as theraw material; the enrichment of rhenium is increased by about 20 times; ammonia water is determined as the eluent of rhenium; the elution rate of rhenium is higher than 98%; the recovery rate of rhenium is higher than 93%, the purity of the ammonium perrhenate product is higher than 99.5%, and the economic, social and environmental benefits are significantly improved.
Owner:爱瑞克(大连)安全技术集团有限公司
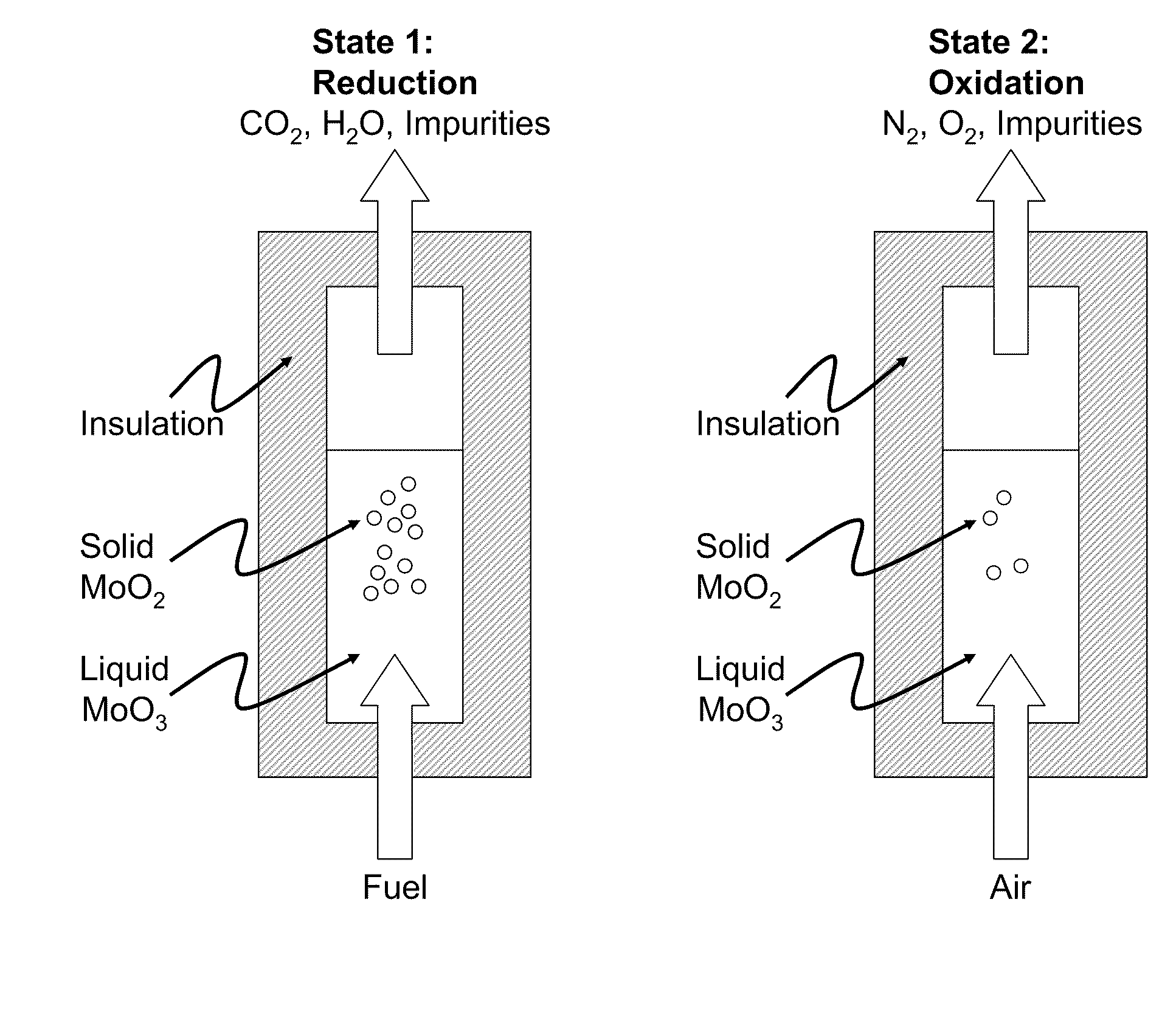
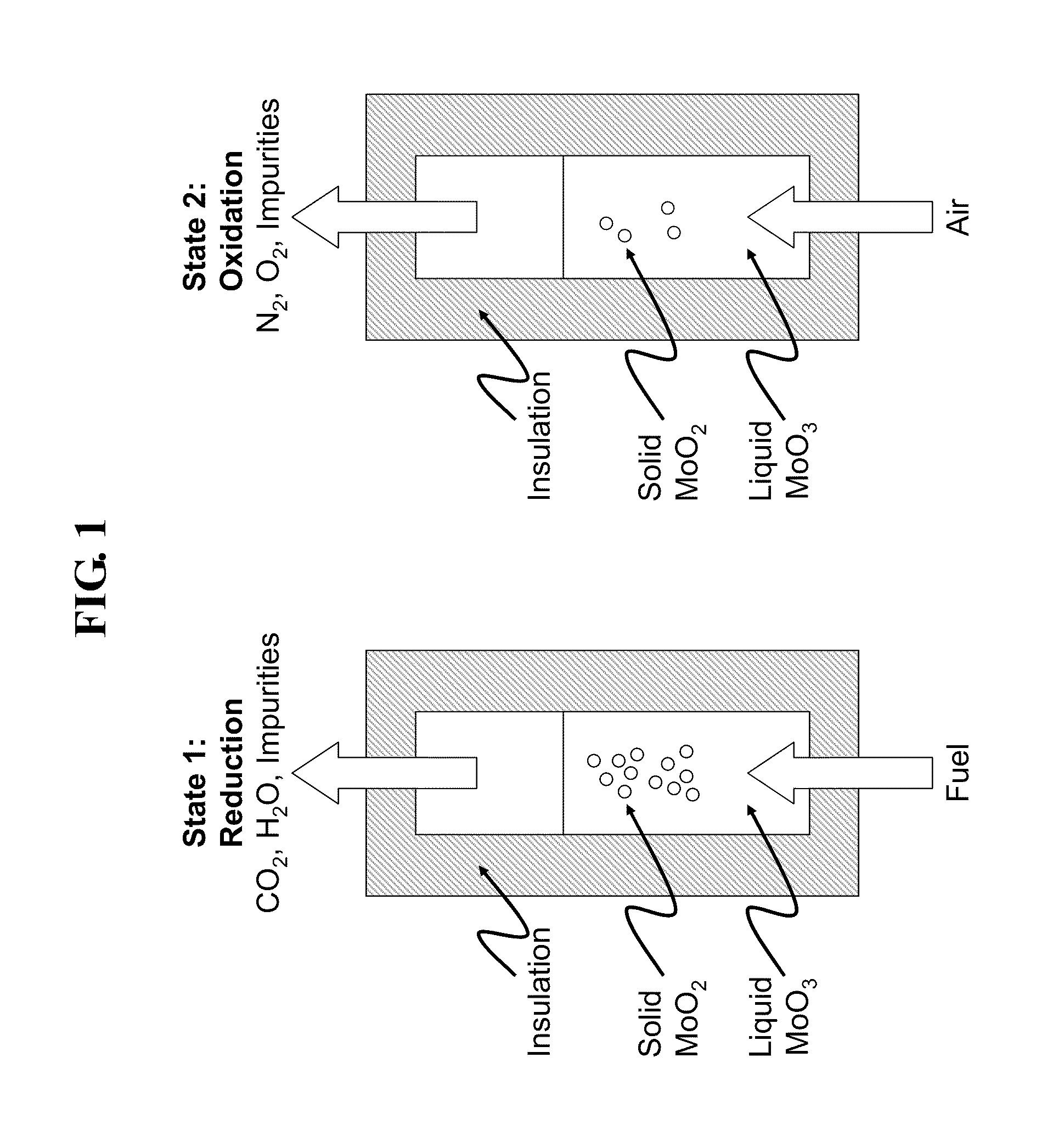
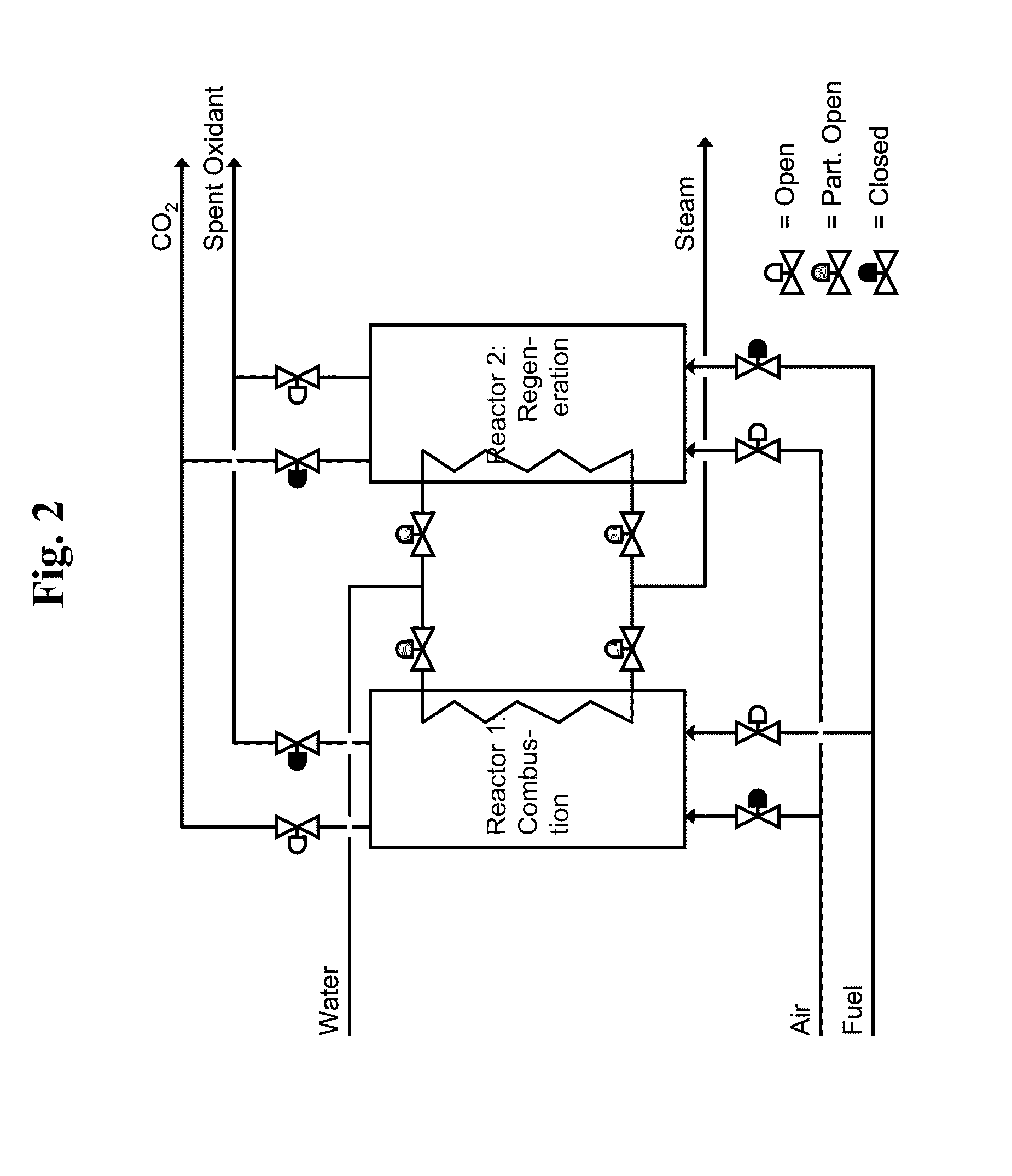
Liquid-phase chemical looping energy generator
Owner:PHILLIPS 66 CO
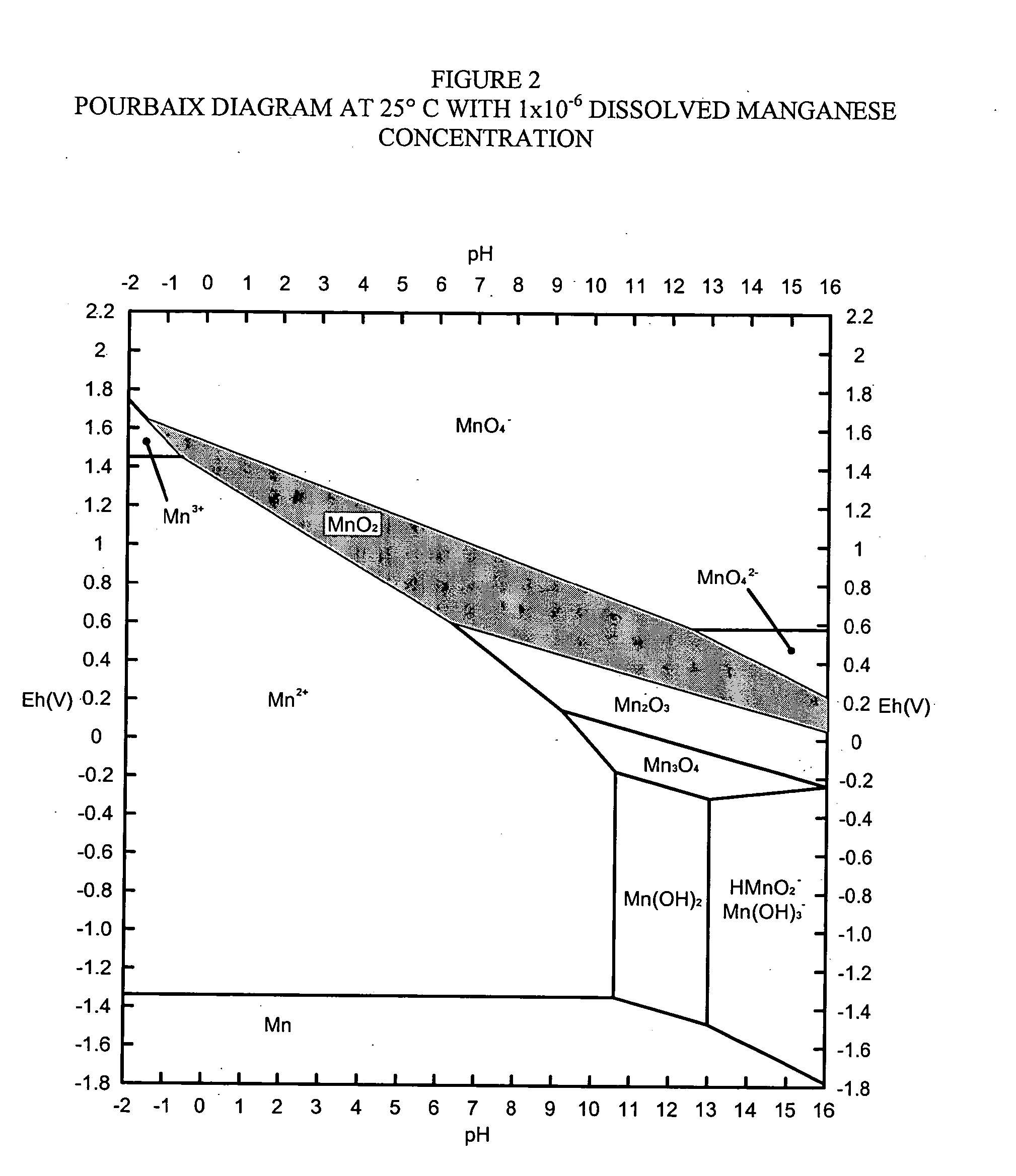
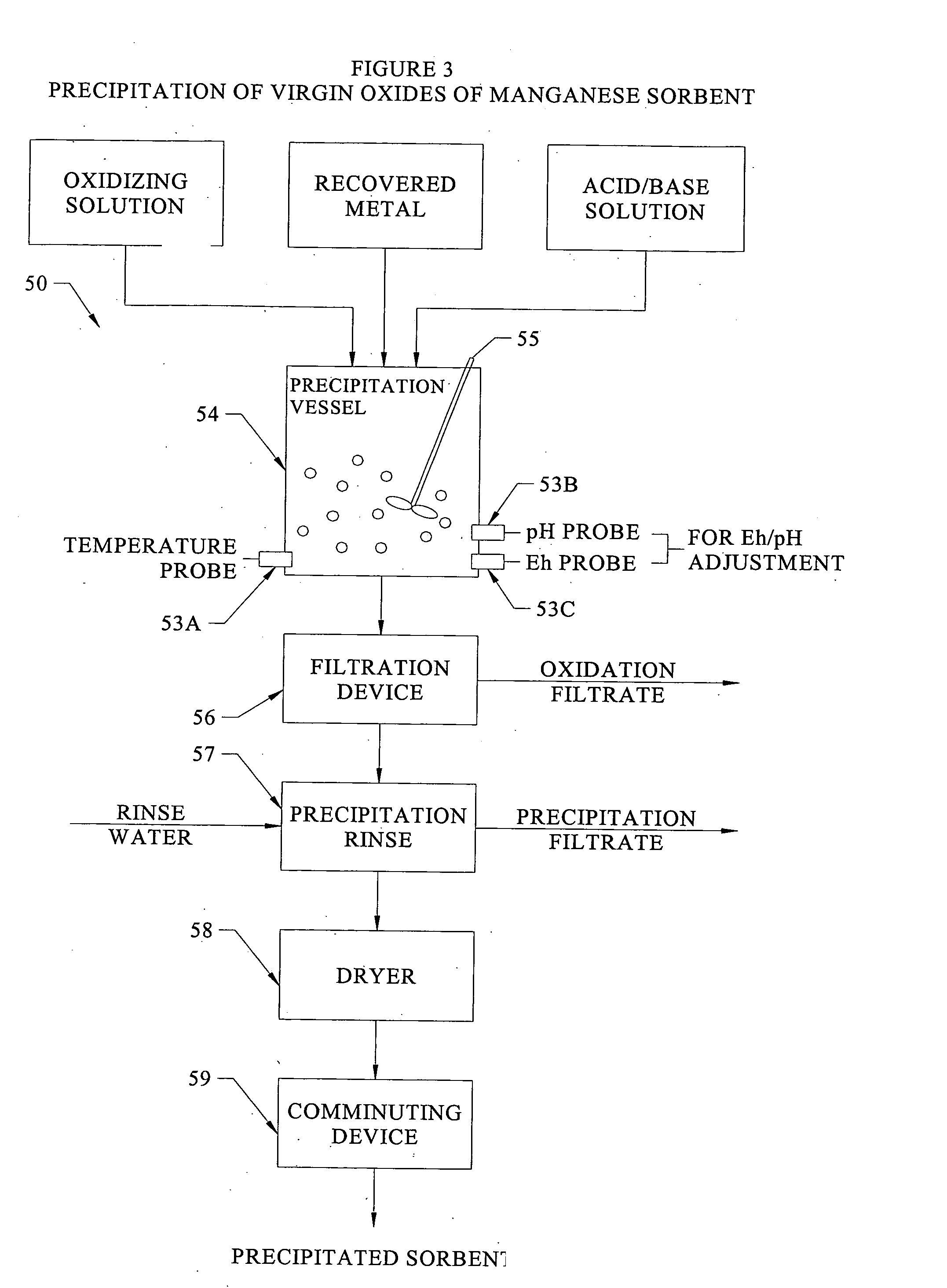
Disassociation processing of metal oxides
InactiveUS20040101457A1Rapid and adaptive recovery of metal valueOther chemical processesCombustible gas purificationSorbentOxidation state
The invention relates to systems and processes for recovery and / or extraction of metal values from ore or other raw material containing oxides of the metal and to precipitation of oxides of metals that have oxidation states and / or pollutant loading capacities equal to or greater than that of the metal oxides in the ore or other raw material which are suitable, amongst other uses, as a sorbent for capture and removal of target pollutants from industrial and other gas streams. Further, the invention relates to oxides of metals so recovered and precipitated.
Owner:ENVIROSCRUB TECH CORP
Chemically bonded phosphate ceramics of trivalent oxides of iron and manganese
A new method for combining elemental iron and other metals to form an inexpensive ceramic to stabilize arsenic, alkaline red mud wastes, swarfs, and other iron or metal-based additives, to create products and waste forms which can be poured or dye cast.
Owner:CHICAGO UNIV OF +1
Recovery of the transition metal component of catalyst used in heavy feed hydroconversion
The invention relates to a process for recovering the transition metal component of catalysts used in the hydroconversion of heavy hydrocarbonaceous materials. In accordance with the invention, a slurry of a transition metal catalyst and hydrocarbon is catalytically desulfurized resulting in a desulfurized product and a solid residue containing the transition metal. The transition metal may be recovered by coking the residue and then dividing the coker residue into two portions are combusted with the flue dust from the first combustion zone being conducted to the second combustion zone. The flue dust from the second combustion zone is treated with ammonia and ammonium carbonate in order to obtain ammonium molybdate.
Owner:EXXON RES & ENG CO
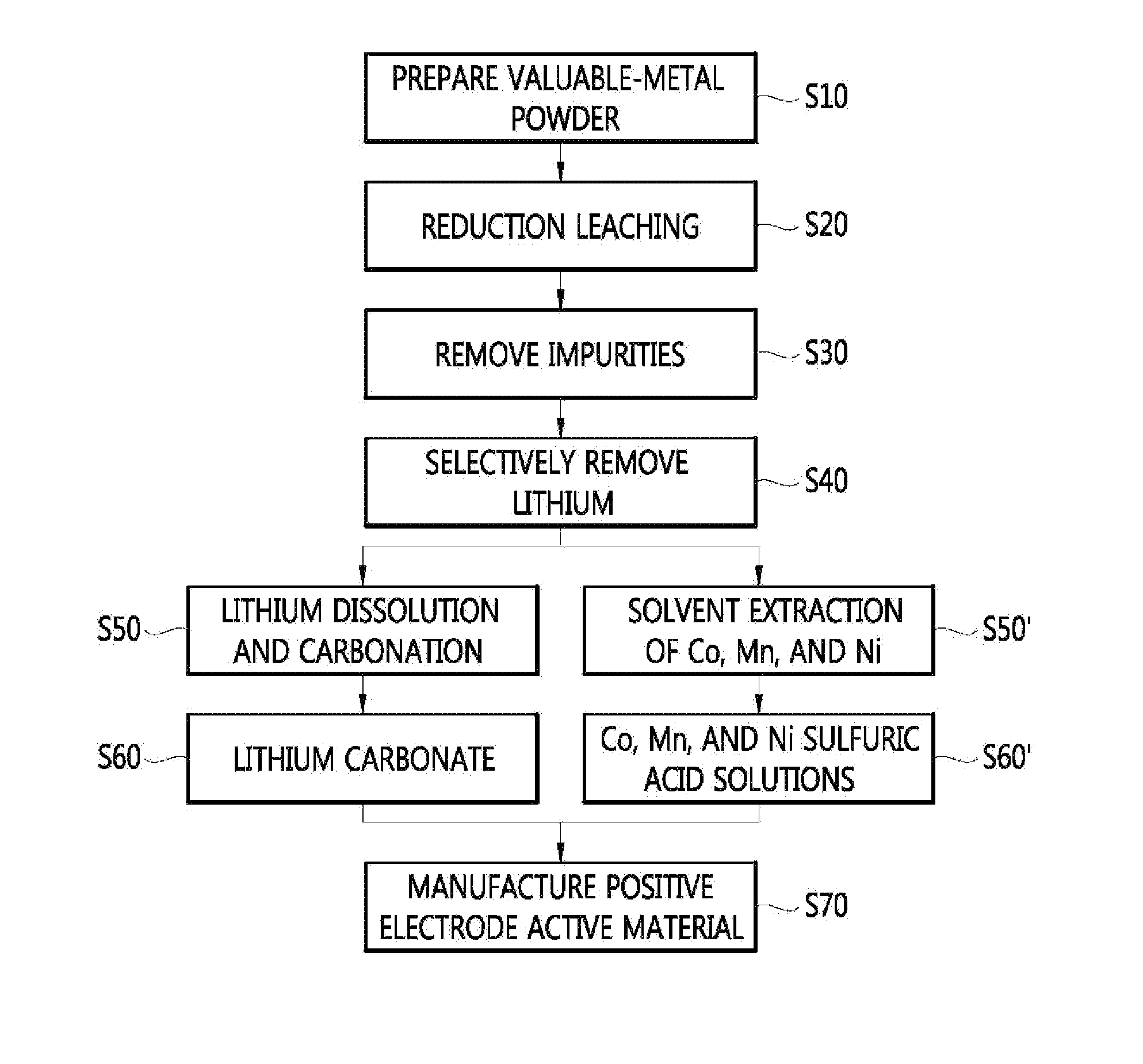
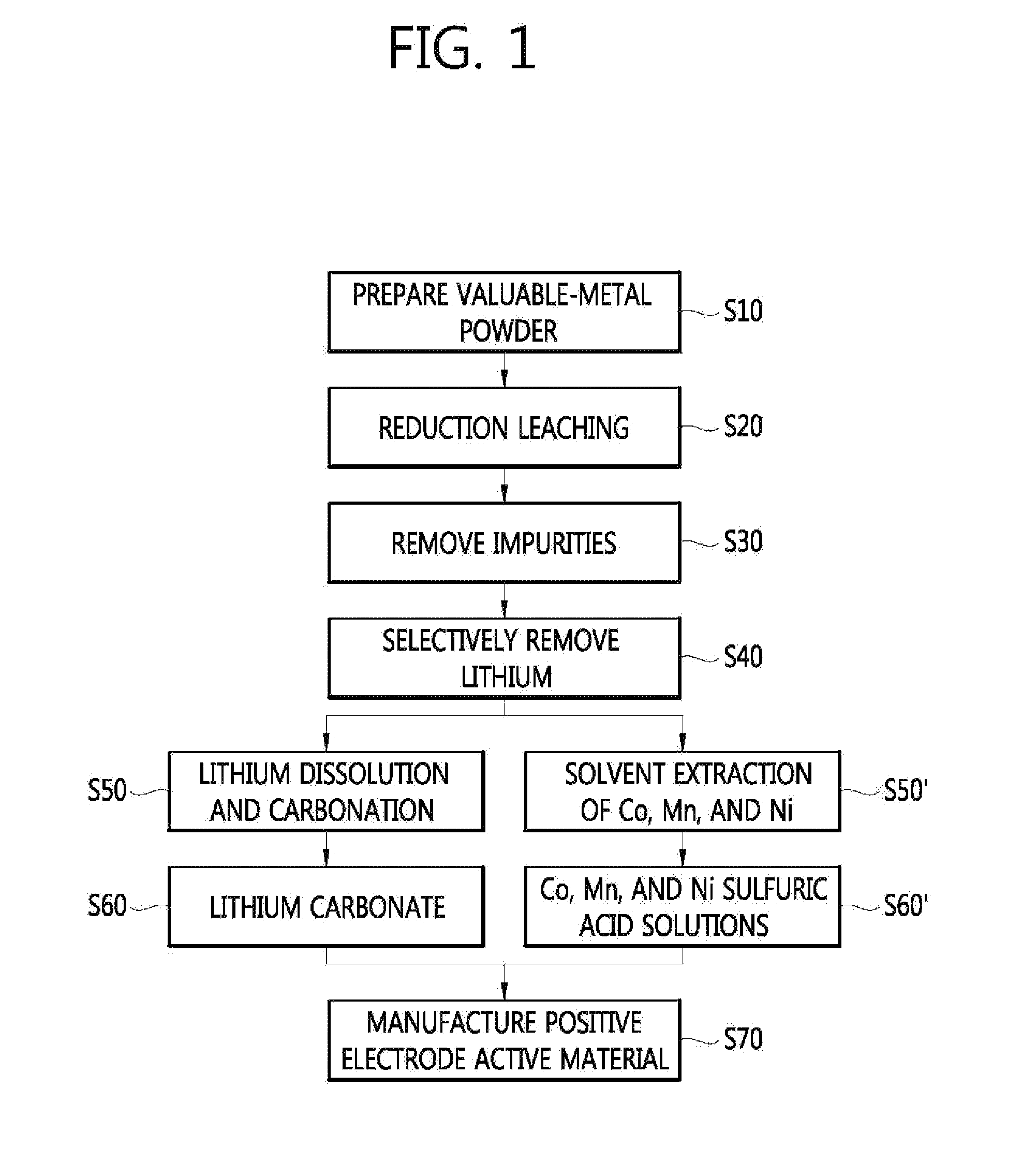
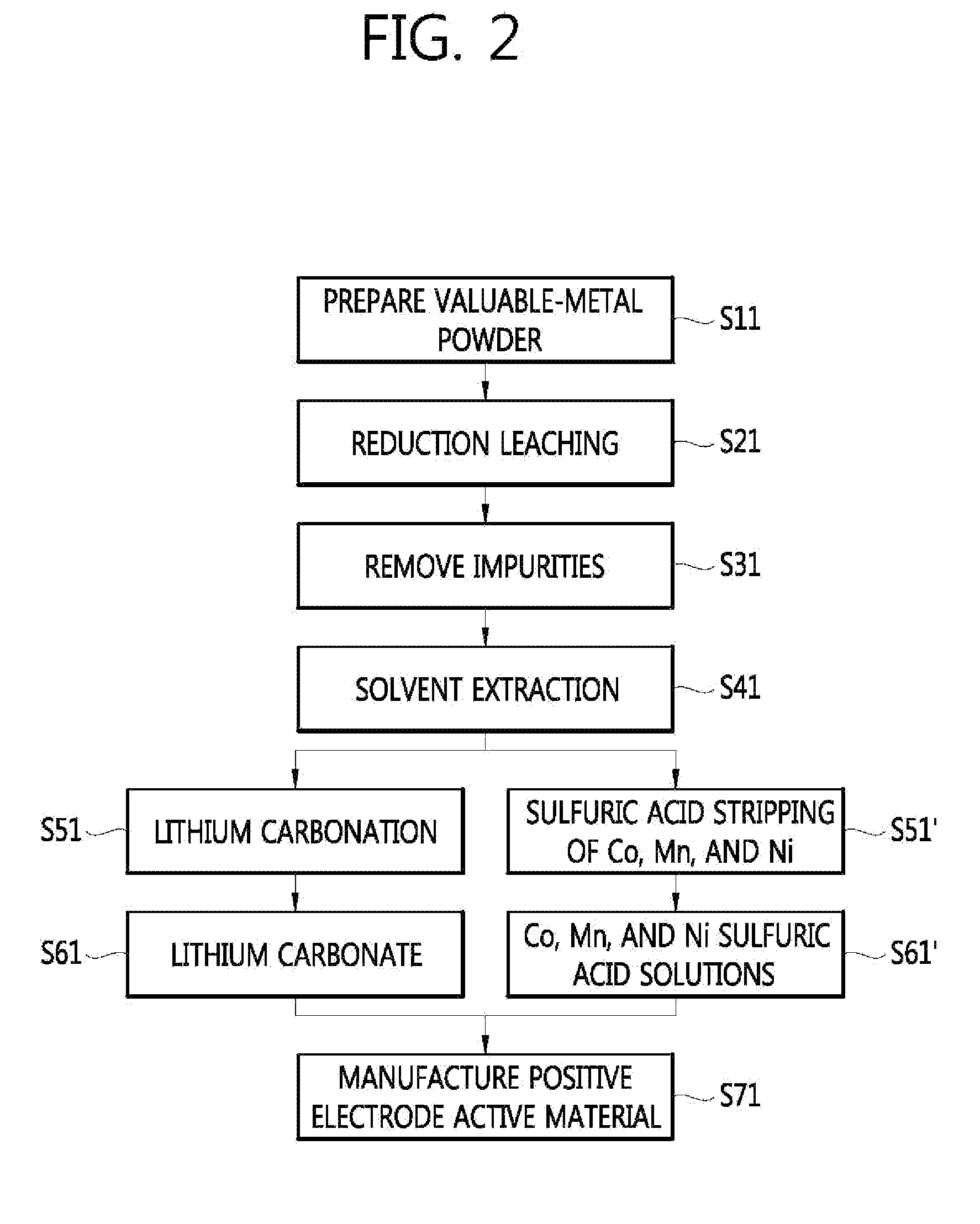
Method for manufacturing a valuable-metal sulfuric-acid solution from a waste battery, and method for manufacturing a positive electrode active material
InactiveUS20130312254A1Efficient preparationPrimary cellsElectrode carriers/collectorsLithiumManganese
The present invention relates to a method for manufacturing a valuable-metal sulfuric-acid solution from a waste battery, and to a method for manufacturing a positive electrode active material. The method for manufacturing the valuable-metal sulfuric-acid solution includes: a step of obtaining valuable-metal powder containing lithium, nickel, cobalt, and manganese from waste batteries; a step of acid-leaching the valuable-metal powder under a reducing atmosphere in order to obtain a leaching solution; and a step of separating the lithium from the leaching solution so as to obtain a sulfuric-acid solution containing the nickel, cobalt, and manganese.
Owner:KOREA INST OF GEOSCI & MINERAL RESOURCES
Pollutant emission control sorbents and methods of manufacture
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing fly ash particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the fly ash particles.
Owner:BASF CATALYSTS LLC
Methods of forming a nanocrystal
InactiveUS20110033368A1Low pour pointMaterial nanotechnologyFrom normal temperature solutionsBoiling pointOxygen donor
Methods of forming a nanocrystal are provided. The nanocrystal may be a binary nanocrystal of general formula M1A or of general formula M1O, a ternary nanocrystal of general formula M1M2A, of general formula M1AB or of general formula M1M2O or a quaternary nanocrystal of general formula M1M2AB. M1 is a metal of Groups II-IV, Group VII or Group VIII of the PSE. A is an element of Group VI or Group V of the PSE. O is oxygen. A homogenous reaction mixture in a non-polar solvent of low boiling point is formed, that includes a metal precursor containing the metal M1 and, where applicable M2. For an oxygen containing nanocrystal the metal precursor contains an oxygen donor. Where applicable, A is also included in the homogenous reaction mixture. The homogenous reaction mixture is under elevated pressure brought to an elevated temperature that is suitable for forming a nanocrystal.
Owner:AGENCY FOR SCI TECH & RES
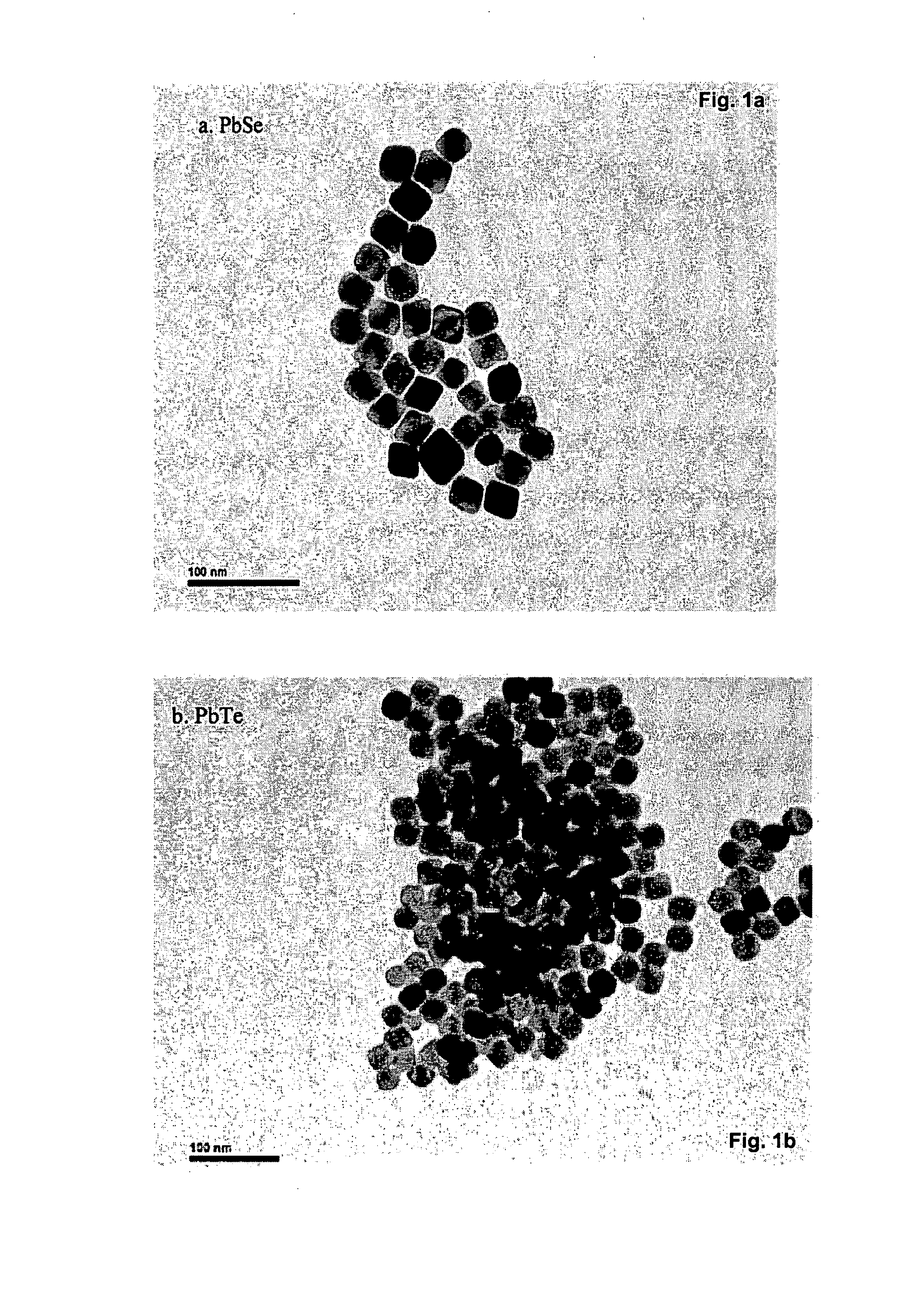
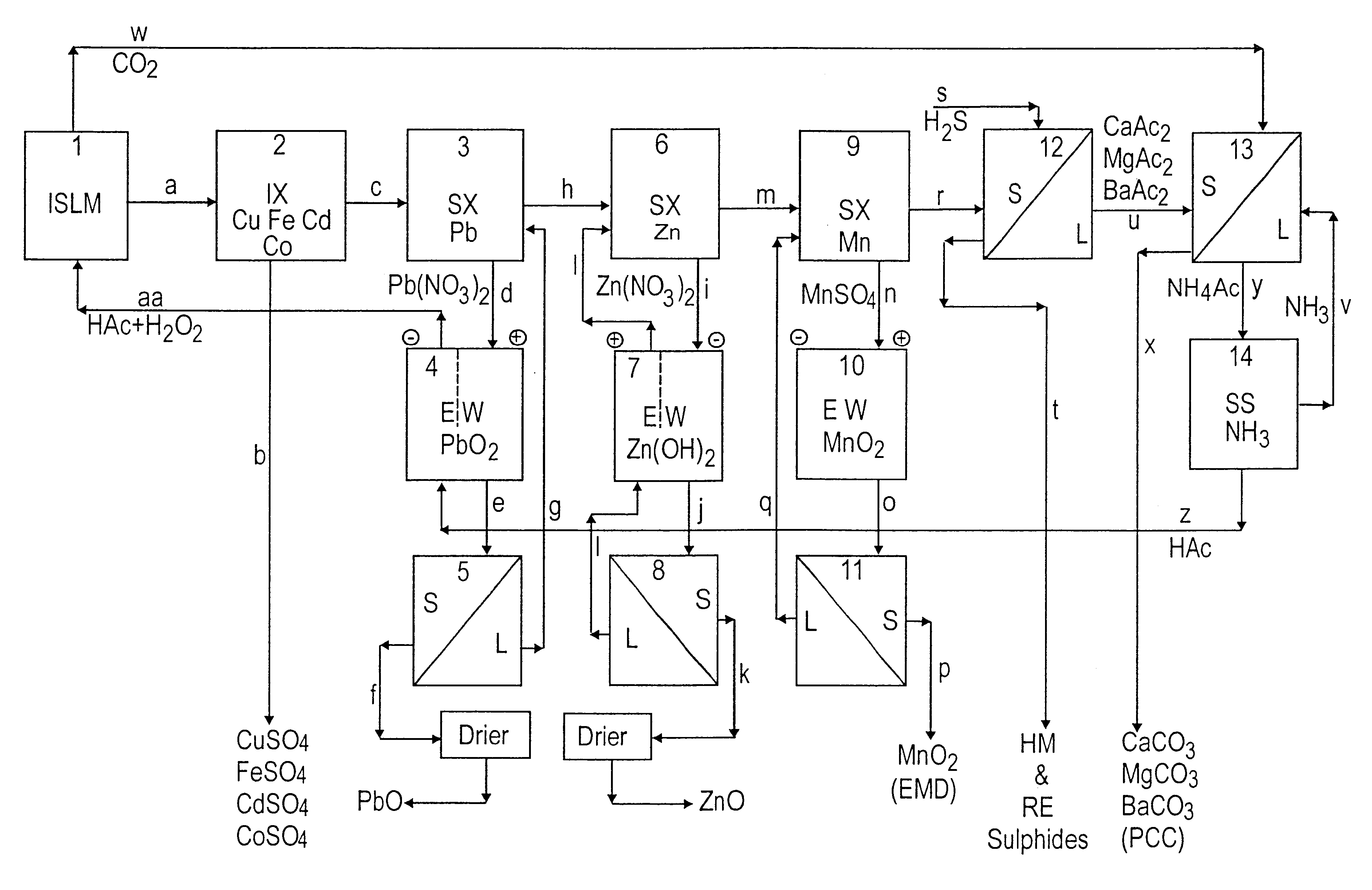
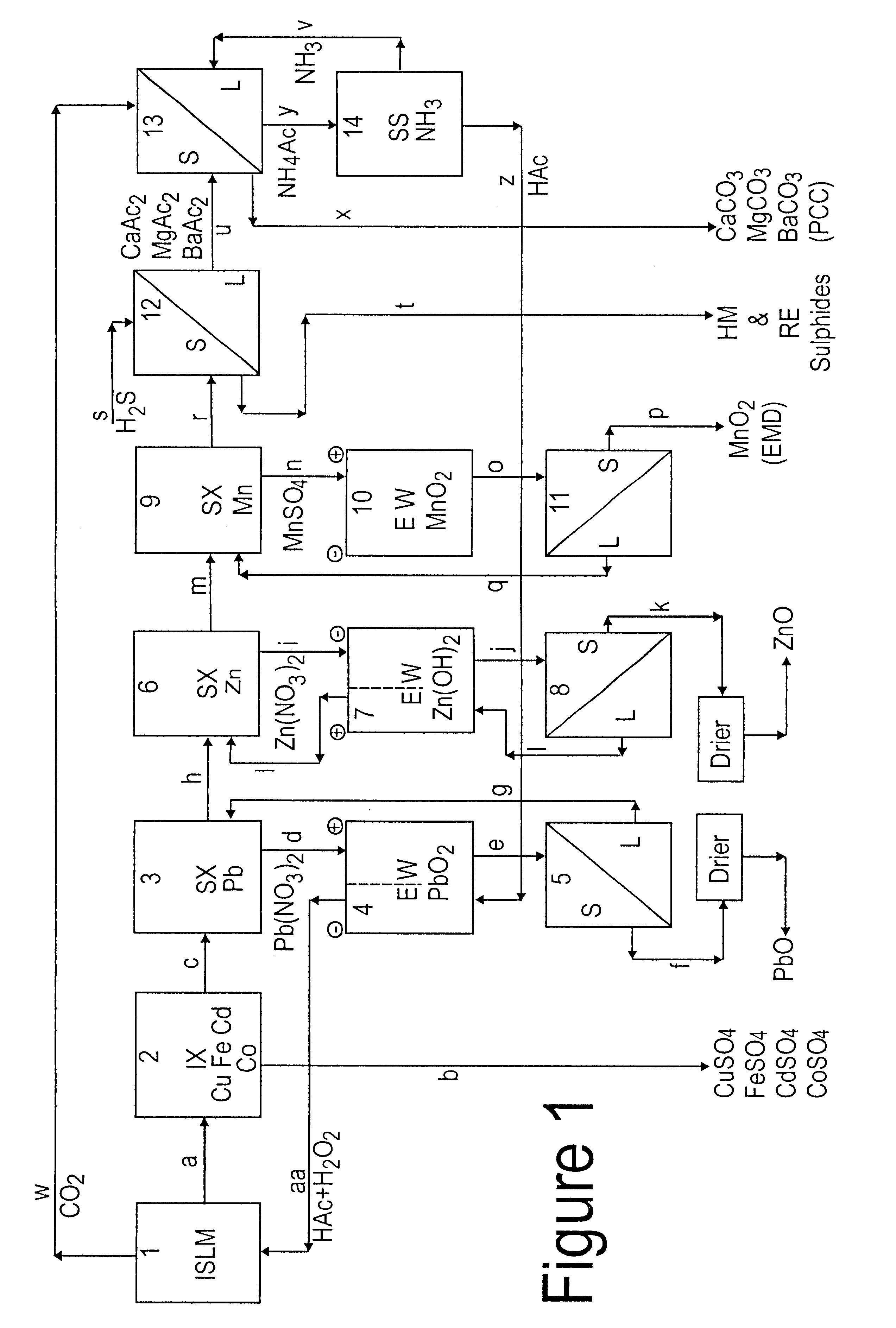
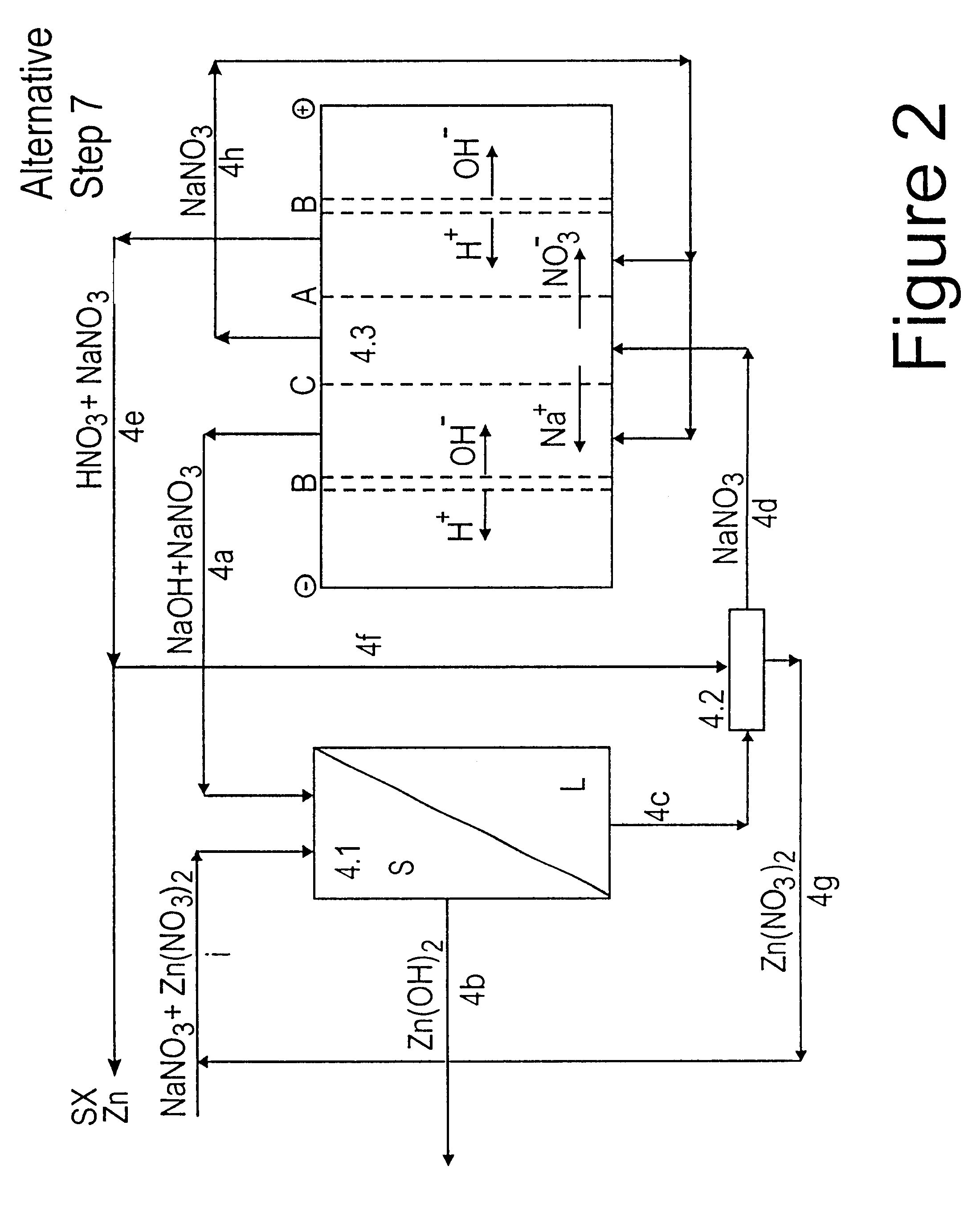
Lead, zinc and manganese recovery from aqueous solutions
InactiveUS6517701B1Low costShorten the timePhotography auxillary processesElectrolysis componentsIon exchangeManganese
Aqueous solutions containing lead, zinc and manganese are treated to recover these metals by sequential solvent extraction steps. Solvent extractants are selected to extract preferentially lead, then zinc and then manganese in that order. Any interfering metals are removed (as by ion exchange) before extraction. The loaded extractant phases are stripped with selected acids and lead, zinc and manganese each recovered from the strip solutions. Optionally calcium can be recovered when present. A preferred type of extractant (for lead especially) is substituted monothiophosphinic acids. A closed loop system is described which is advantageous with leachate from sulphide and carbonate ores.
Owner:CENTAUR MINING EXPLORATION
Nano-sized particles, processes of making, compositions and uses thereof
InactiveUS8182786B2Economical and efficientEfficiently tailoredMaterial nanotechnologyToilet preparationsSolventPharmaceutical formulation
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Method for effecting solvent extraction of metal ions using hydrocarbon soluble aminomethylene phosphonic acid compounds
Solvent extraction of one or more metal ions from an aqueous solution in the presence of hydrocarbon-soluble aminomethylenephosphonic acid derivatives.
Owner:BASF AG +1
Method for recovering active material from waste battery material
ActiveUS20130323142A1Electrode thermal treatmentWaste accumulators reclaimingConductive materialsBiological activation
Method of recovering active material from waste battery materials comprises: (1) an electrode material mixture recovery step of separating an electrode from the waste battery material to recover an electrode material mixture including the active material, a conductive material, and a binder from the electrode; (2) an activation agent mixing step of mixing an activation agent including one or more alkali metal compounds with the recovered electrode material mixture; (3) an activation step of heating the obtained mixture to a retention temperature not less than a melting start temperature of the activation agent to activate the active material included in the mixture; and (4) an active material recovery step of recovering the activated active material from a mixture obtained as a result of cooling after the activation step.
Owner:SUMITOMO CHEM CO LTD
Ultraviolet light screening compositions
InactiveUS6869596B1Minimize migrationReduce productionMaterial nanotechnologyCosmetic preparationsUltraviolet lightsUltraviolet A Radiation
Owner:OXFORD UNIV INNOVATION LTD
Manganese dioxide, method and apparatus for producing the same, and battery active material and battery prepared by using the same
InactiveUS20070020171A1Non-aqueous electrolyte accumulatorsActive material electrodesElectrical batteryCrystal structure
Owner:PANASONIC CORP
Lithium-manganese composite oxide granular secondary particle, method for production thereof and use thereof
ActiveUS20050123832A1Improve output characteristicsMaintain good propertiesAluminium compoundsLithium compoundsMicrometerCompound (substance)
The invention provides granular secondary particles of a lithium-manganese composite oxide which are granular secondary particles made up of aggregated crystalline primary particles of a lithium-manganese composite oxide and have many micrometer-size open voids therein, the open voids having an average diameter in the range of from 0.5 to 3 μm and the total volume of the open voids being in the range of from 3 to 20 vol. % on average based on the total volume of the granules. These particles are suitable for use as a constituent material for non-aqueous electrolyte secondary batteries showing high-output characteristics. The invention further provides a process for producing the granular secondary particles of a lithium-manganese composite oxide which includes spray-drying a slurry prepared by dispersing a fine powder of a manganese oxide, a lithium source, an optional compound containing at least one element selected from Al, Co, Ni, Cr, Fe, and Mg, and an agent for open-void formation to thereby granulate the slurry and then calcining the granules at a temperature of from 700 to 900° C.
Owner:TOSOH CORP
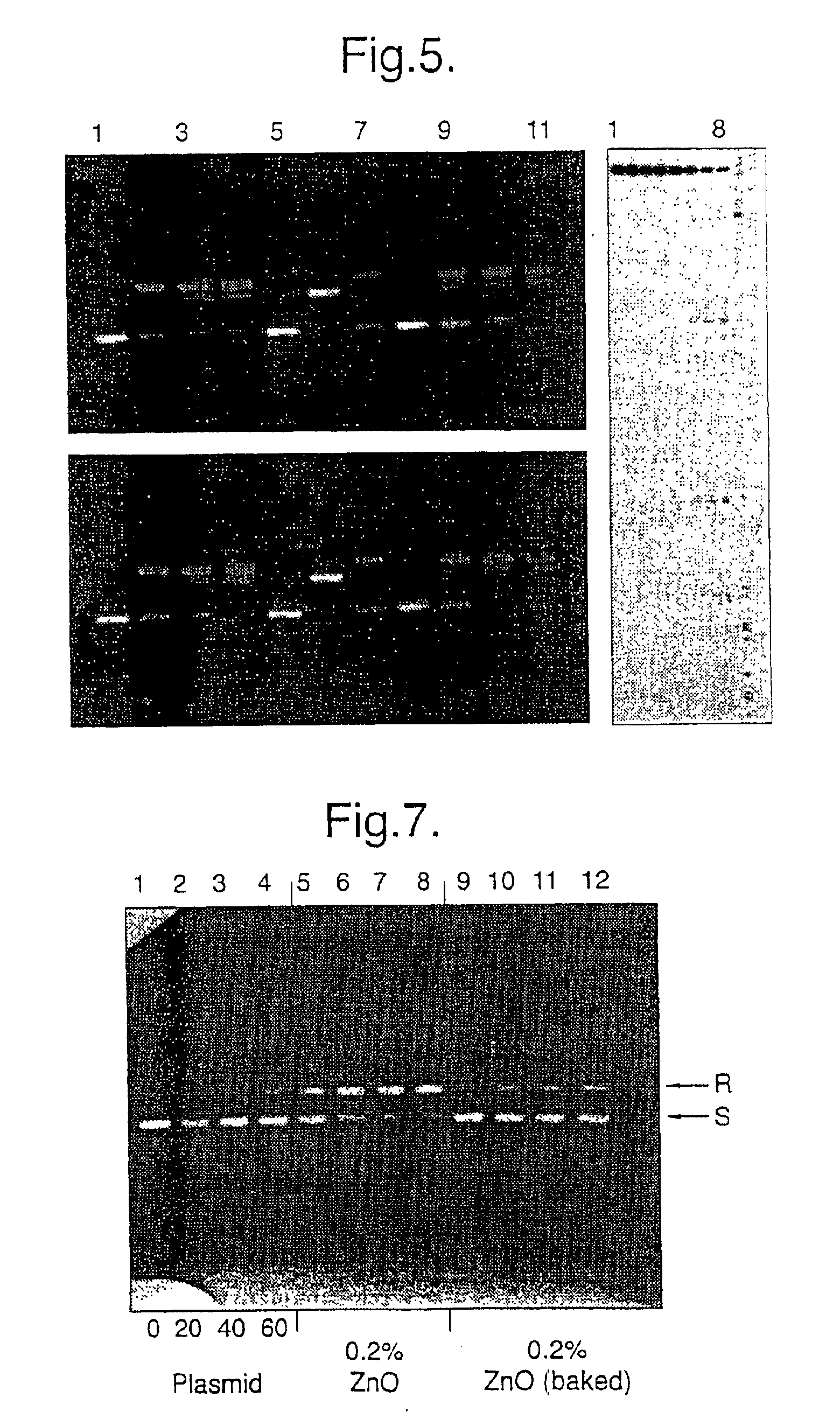
Recycling of used perfluorosulfonic acid membranes
InactiveUS20050211630A1Guaranteed long-term availabilityIncrease consumptionTransuranic element compoundsActive material electrodesSolventMembrane configuration
Owner:ION POWER
Novel metal air cathode: manganese oxide contained in octahedral molecular sieve
ActiveUS20070111095A1High power outputEfficient decompositionFuel and primary cellsAlkaline accumulatorsMolecular sieveManganese oxide
An oxygen reduction electrode, e.g., an air cathode, comprising manganese oxides having octahedral molecular sieve structures as active catalyst materials and use of such an electrode as a component of a metal-air cell.
Owner:ENERGIZER BRANDS +1
Methods for preparation from carbonate precursors the compounds of lithium transition metals oxide
InactiveUS7767189B2Improve cycle lifeSuitable for useOxide/hydroxide preparationElectrode thermal treatmentLithiumCarbonate ester
A method for preparing lithium transitional metal oxides, comprises the steps of: preparing a carbonate precursor using the following substeps: forming a first aqueous solution containing a mixture of at least two of the ions of the following metal elements (“Men+”): cobalt (Co), nickel (Ni), and manganese (Mn); forming a second aqueous solution containing ions of CO32−; and mixing and reacting the first solution and the second solution to produce the carbonate precursor, Ni1-x-yCoxMnyCO3; and preparing the lithium transition metals oxide from the carbonate precursors using the following substeps: evenly mixing Li2CO3 and the carbonate precursor; calcinating the mixed material in high temperature; and cooling and pulverizing the calcinated material to obtain the lithium transition metal oxide, Li Ni1-x-yCoxMnyO2.
Owner:BYD CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com