Intermediate compound, carbamazepine and derivative thereof as well as preparation method of oxcarbazepine and derivative thereof
A carbamazepine and compound technology, applied in the field of intermediate compounds, can solve the problems of high raw material price, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
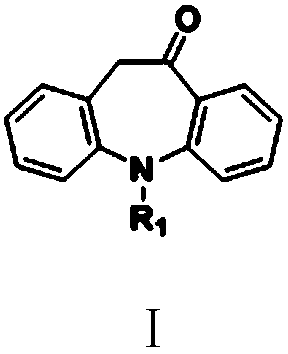
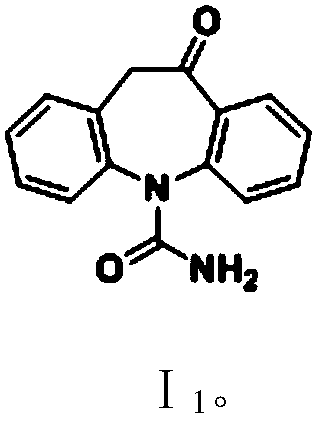
[0104] Example 1: Oxcarbazepine (5-carbamoyl-10-oxa-10,11-dihydro-5H-dibenzo[b,f]azepine I 1 ) preparation
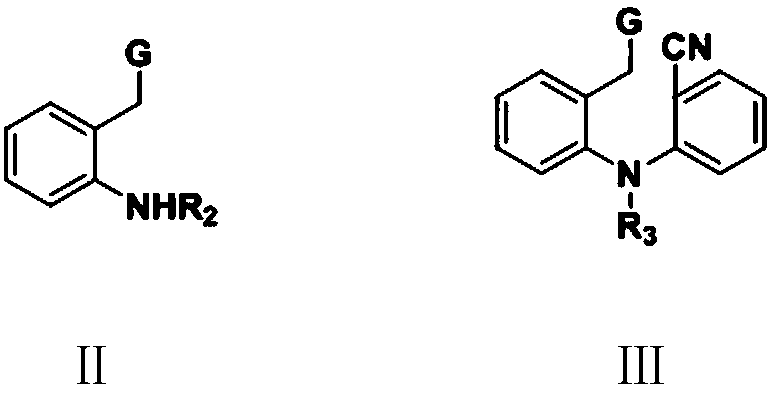
[0105] Step (1): N-cyano-N-2'-cyanophenyl-2-aminophenylacetic acid methyl ester (Ⅲ 1 ) preparation
[0106] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser, add 250 grams of N,N-dimethylformamide, 38.0 grams (0.2 moles) of methyl 2-cyanoaminophenylacetic acid, 38.0 grams ( 0.21 mol) of 2-bromobenzonitrile, 35.0 g of potassium carbonate, and reacted with stirring at 95 to 100° C. for 5 hours. Cool to 20 to 25°C, filter, wash the filter cake with 30 g of N,N-dimethylformamide, combine the filtrates, distill under reduced pressure to recover the solvent, add 200 g of isopropyl ether to the residue for recrystallization, and obtain 52.2 g N-cyano-N-2'-cyanophenyl-2-aminophenylacetic acid methyl ester (Ⅲ 1 ), the yield is 89.7%, and the liquid phase purity is 99.3%.
[0107] Step (2): Oxcarbazepine (5-carbamoyl-10...
Embodiment 2
[0109] Embodiment 2: Oxcarbazepine (Ⅰ 1 ) preparation
[0110] Step (1): Ethyl N-cyano-N-2'-cyanophenyl-2-aminophenylacetate (Ⅲ 4 ) preparation
[0111] In a 500 ml four-neck flask connected with stirring, a thermometer and a reflux condenser, add 250 grams of N,N-dimethylformamide, 40.8 grams (0.2 moles) of ethyl 2-cyanoaminophenylacetate, 38.0 grams ( 0.21 mol) of 2-bromoxynil, 35.0 g of potassium carbonate, and reacted with stirring at 100 to 105° C. for 4 hours. Cool to 20 to 25°C, filter, wash the filter cake with 30 g of N,N-dimethylformamide, combine the filtrates, distill under reduced pressure to recover the solvent, add 200 g of isopropyl ether to the residue for recrystallization, and obtain 55.1 g N-cyano-N-2'-cyanophenyl-2-aminophenylacetic acid ethyl ester (Ⅲ 4 ), the yield is 90.3%, and the liquid phase purity is 99.2%.
[0112] Step (2): Oxcarbazepine (Ⅰ 1 ) preparation
[0113] To a 500 ml four-necked flask connected with stirring, a thermometer, a refl...
Embodiment 3
[0116] Embodiment 3: Carbamazepine (5-carbamoyliminostilbene, IV 1 ) preparation
[0117] In the 250 milliliters of four-neck flasks that are connected with stirring, thermometer, reflux condenser, add 20 grams of methanol, 2.52 grams (10 mmol) of oxcarbazepine (I) prepared in embodiment 1 1 ), 0.31 g (8 mmol) of sodium borohydride, and stirred at 45 to 50° C. for 3 hours. Cool to 20 to 25°C, use 35wt% hydrochloric acid acidification system pH value 2.0-2.5, 25 to 30°C stirring reaction for 2 hours, add 20 grams of water, filter, dry to obtain 2.16 grams of carbamazepine (IV 1 ), the yield is 91.5%, and the liquid phase purity is 99.7%.
[0118] The NMR data of the resulting product are as follows:
[0119] 1 H-NMR (CDCl 3 , 400MHz) δ: 7.28–7.47 (8H, m), 6.92 (2H, s), 4.81 (2H, br).
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com