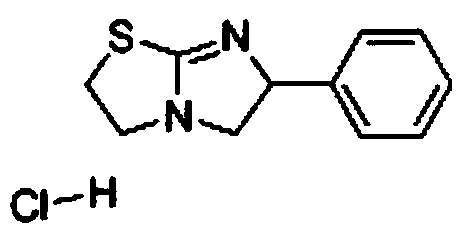
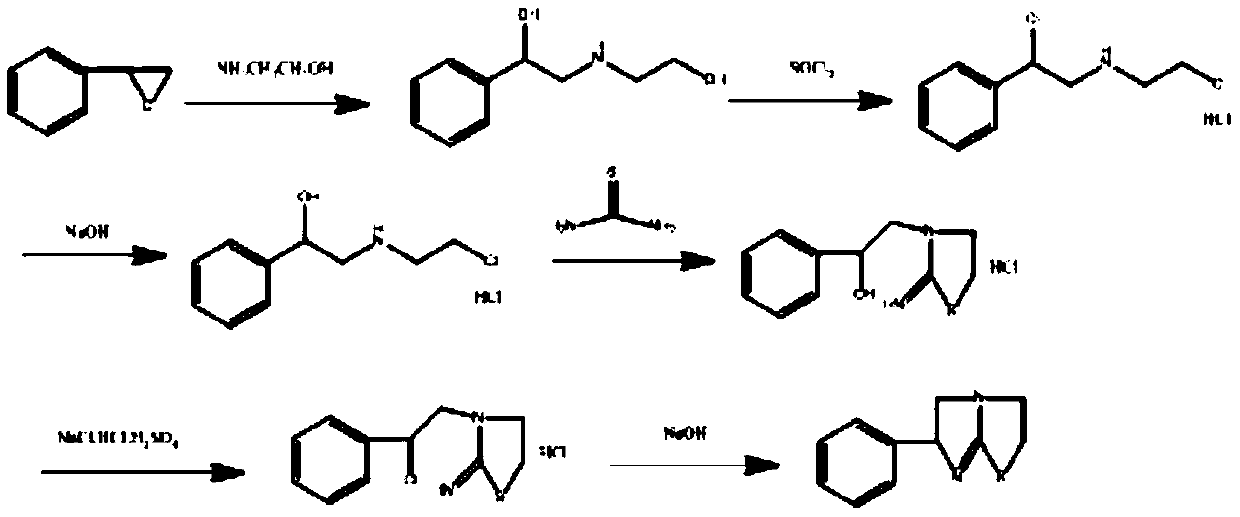
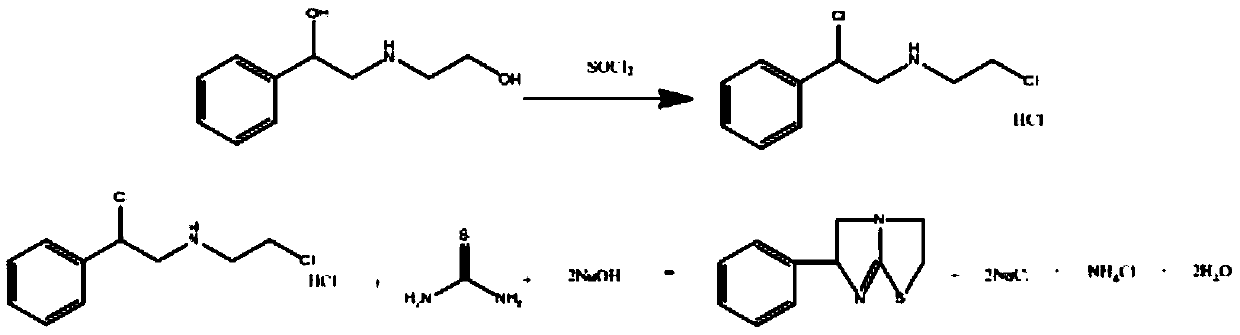
Preparation method of tetraimidazole free alkali
A technology of free base and tetramisole, which is applied in the field of preparation of tetramisole free base, can solve the problems of long reaction steps, uneconomical, cost increase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Using a 2L four-neck flask, add 1000 g of a buffer solution of ammonium chloride and ammonia water, and raise the temperature to 60°C. At a speed of 500-600rpm, using a micro-quantitative feeder, a total of 200g of N-(2-chloroethyl)-α-(chloromethyl)-benzylamine hydrochloride solid and a total of 71.67g of Thiourea solid (molar ratio 1:1.2), where the feeding rate of N-(2-chloroethyl)-α-(chloromethyl)-benzylamine hydrochloride is 1.11g / min, the feeding speed of thiourea It is 0.4g / min, and the dosing is completed in 3h. During the reaction, the pH of the solution will decrease continuously. Use 30% sodium hydroxide solution to control the pH value, so that the pH value of the reaction solution is controlled at pH=9. After feeding is complete, heat up to 80~85°C, keep warm for 2.5h, cool down to 25°C, obtain 148.75g product after filtration, detect purity 98.56%, calculate and obtain the yield of tetramisole free base 91.45%.
Embodiment 2
[0036] Similar to Example 1, wherein the initial reaction temperature was adjusted to 55°C. Finally, 144.64 g of the product was obtained by filtration, the detection purity was 98.83%, and the calculated yield was 89.17%.
Embodiment 3
[0038] Similar to Example 1, wherein the initial reaction temperature was adjusted to 65°C. Finally, 141.08 g of the product was obtained by filtration, the detection purity was 96.87%, and the calculated yield was 85.25%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com