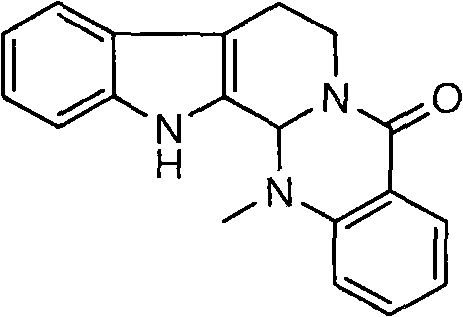
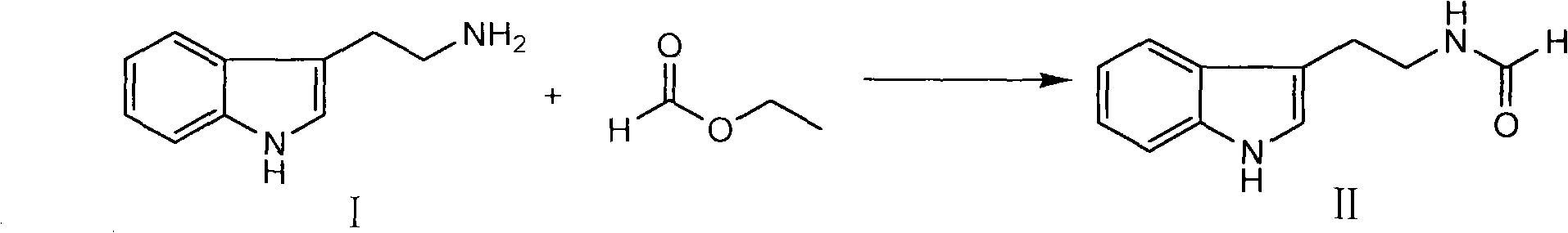
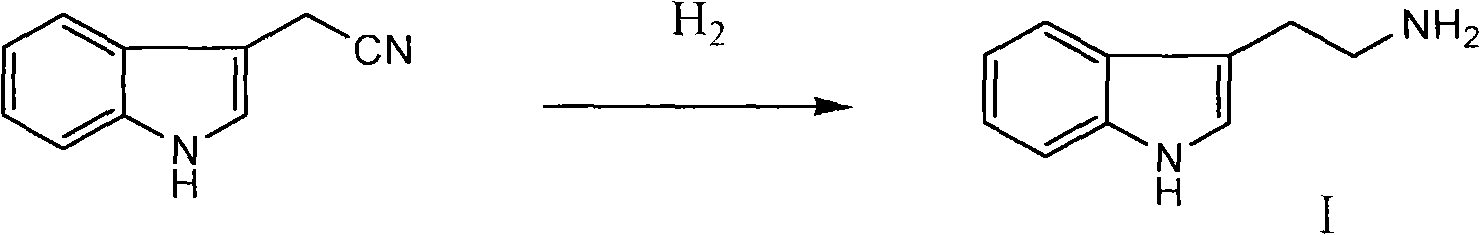Method for synthesizing evodiamine
A technology of evodial alkaloid and a synthesis method, applied in directions such as organic chemistry, can solve problems such as cost cannot be effectively controlled, large environmental pollution, unsuitable for industrialized production, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of tryptamine I
[0037] Indole acetonitrile (15.6g, 0.1mol) was dissolved in 100ml methanol, put into the hydrogenation kettle, add 2.0g Pd / C (5%), seal the kettle, N 2 After the replacement, feed hydrogen gas, react at room temperature for 24 hours, until no hydrogen is absorbed, the reaction solution is suction filtered to the concentration tank to concentrate methanol, and 100ml of ethyl acetate is added, cooled, separated, and centrifuged to obtain tryptamine I (quantitative yield) .
Embodiment 2
[0038] Example 2 Preparation of tryptamine I
[0039] Indole acetonitrile (15.6g, 0.1mol) was dissolved in 100ml ethanol, put into the hydrogenation kettle, add 2.0g Pd / C (5%), seal the kettle, N 2 After the replacement, pass in hydrogen, raise the temperature to 50°C and react for 12 hours until no hydrogen is absorbed. After cooling, filter the reaction solution to the concentration kettle to concentrate ethanol, add 100ml of ethyl acetate, cool, separate the material, and centrifuge to obtain tryptamine I (quantitative yield).
Embodiment 3
[0040] Example 3 Preparation of Tryptamine I
[0041] Indole acetonitrile (15.6g, 0.1mol) was dissolved in 100ml DMF, put into the hydrogenation kettle, add 2.0g Pd / C (5%), seal the kettle, N 2 After the replacement, pass in hydrogen, raise the temperature to 100°C and react for 2 hours until no hydrogen is absorbed. After cooling, filter the reaction solution to the concentration kettle to concentrate DMF, add 100ml of ethyl acetate, cool, precipitate, and centrifuge to obtain tryptamine I 13g, yield 85%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com