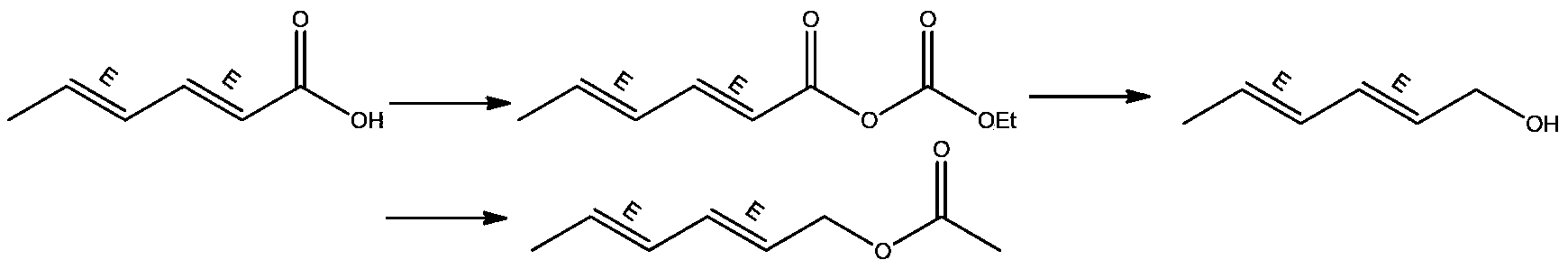
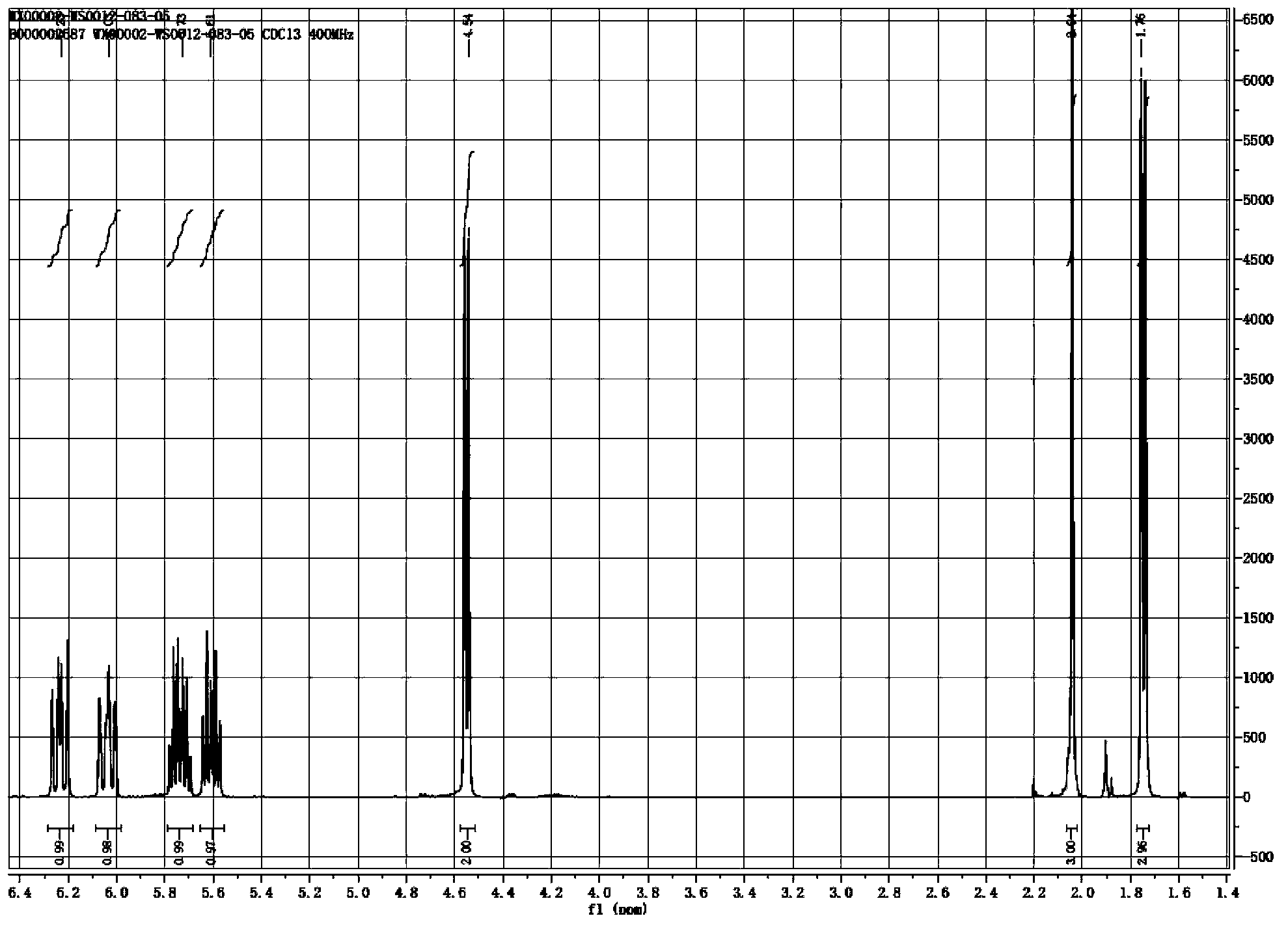
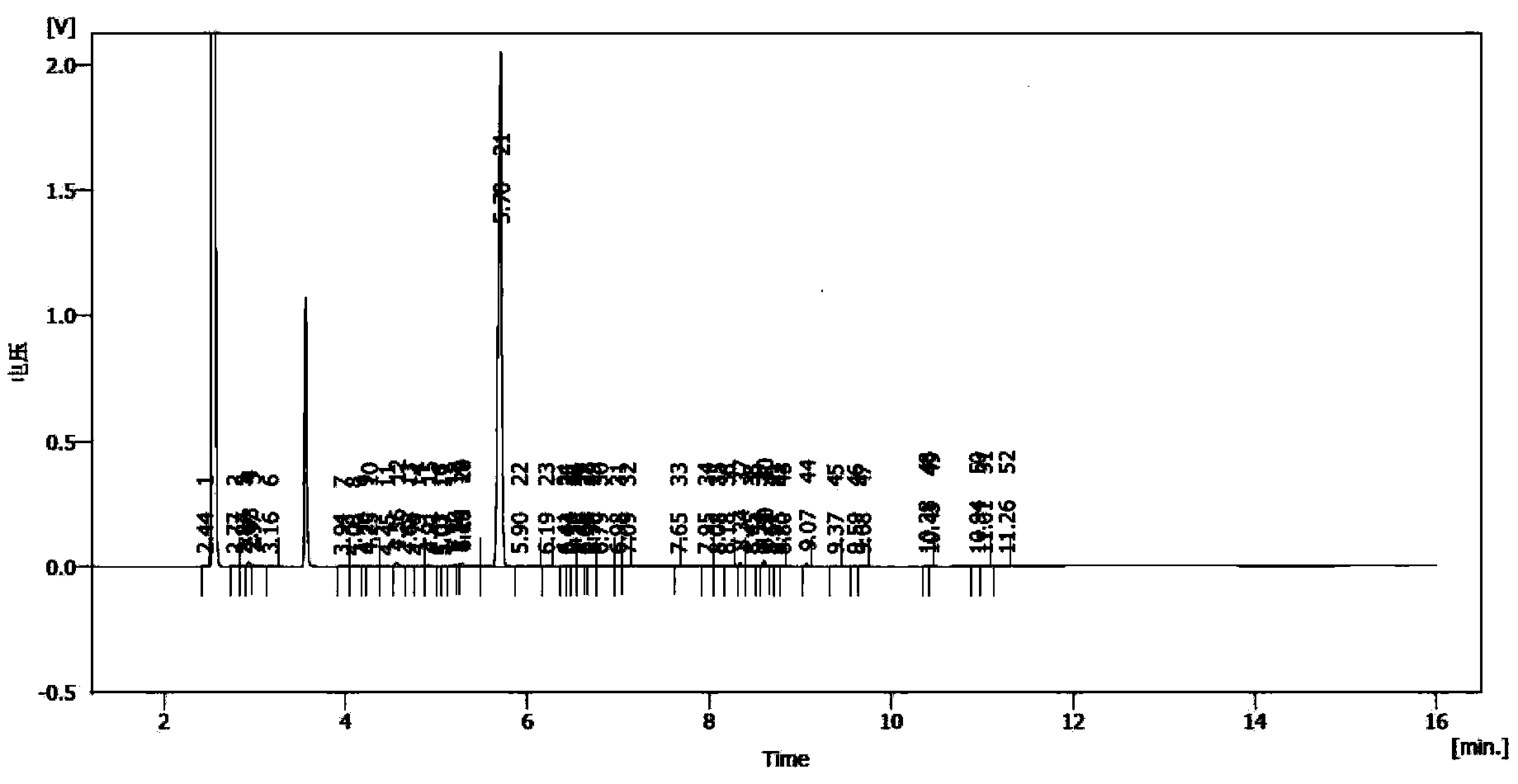
Synthesis method of laspeyresia pomonella sex pheromone intermediate (2E, 4E)-2,4-hexadienol acetate
A technology of hexadienol acetate and codling moth is applied in the field of chemical synthesis of insect sex pheromone intermediates, which can solve the problems of inconvenient industrial production and operation, difficulty in filtering aluminum salts, harsh requirements and the like, and achieve good technical results. , easy handling, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step (1) Synthesis of active acid anhydride:
[0039] Under nitrogen protection, 10 g (0.089 mol) of sorbic acid, 60 mL of tetrahydrofuran and 9 g (0.089 mol) of triethylamine were added to a 250 mL reaction flask, and the temperature was lowered to 20°C. Add 9.7g (0.089mol) of ethyl chloroformate dropwise at a rate of 0.01mL / s. After the feeding is completed, the reaction temperature is 35°C, and the reaction is continued for 1.5 hours. The reaction progress is tracked by HPLC, and the reaction is stopped after the reaction is complete. The white solid was removed by filtration to finally obtain 70 g of filtrate, which was directly put into the next reaction without treatment.
[0040] Step (2) reduction reaction:
[0041] Put the above 70g filtrate into a 500mL reaction flask, cool down to -20°C, add sodium borohydride / sodium hydroxide aqueous solution dropwise, wherein the preparation method of sodium borohydride / sodium hydroxide aqueous solution is: hydroboration o...
Embodiment 2
[0046] Step (1) Synthesis of active acid anhydride:
[0047] Under nitrogen protection, add 60 g (0.54 mol) of sorbic acid, 360 mL of tetrahydrofuran and 65.6 g (0.648 mol) of triethylamine into a 1 L reaction flask, and cool down to 5 °C. Add 64g (0.59mol) ethyl chloroformate dropwise at a rate of 2.0mL / s. After feeding, the reaction temperature is 35°C. Continue to react for 2 hours. HPLC tracks the reaction process, and stops the reaction after the reaction is complete. The white solid was removed by filtration to finally obtain 430 g of filtrate, which was directly put into the next step reaction without treatment.
[0048] Step (2) reduction reaction:
[0049] Put the above 430g filtrate into a 1L reaction bottle, cool down to -10°C, add potassium borohydride / potassium hydroxide aqueous solution dropwise, wherein the preparation method of potassium borohydride / potassium hydroxide aqueous solution is: hydroboration of 43.7g (0.81mol) Potassium solids were added to 180g o...
Embodiment 3
[0053] Step (1) Synthesis of active acid anhydride:
[0054] Under nitrogen protection, 120g (1.07mol) of sorbic acid, 720mL of tetrahydrofuran and 129.5g (1.28mol) of triethylamine were added to a 2L reaction flask, and the temperature was lowered to 20°C. Add 138.9g (1.28mol) ethyl chloroformate dropwise at a rate of 0.05mL / s. After the feeding is completed, the reaction temperature is 50°C, and the reaction is continued for 0.5 hours. The reaction process is tracked by HPLC, and the reaction is stopped after the reaction is complete. The white solid was removed by filtration to finally obtain 920 g of filtrate, which was directly put into the next step reaction without treatment.
[0055] Step (2) reduction reaction:
[0056] Put the above-mentioned 920g filtrate into a 2L reaction flask, cool down to -15°C, add sodium borohydride / potassium hydroxide aqueous solution dropwise, and the preparation method of sodium borohydride / potassium hydroxide aqueous solution is as follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com