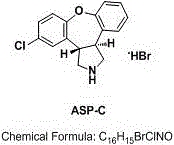
Novel method for removing methyl impurity in preparation of asenapine
A technology of asenapine maleate and demethylation, which is applied in the field of new process synthesis to achieve the effect of simple process, high quality and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
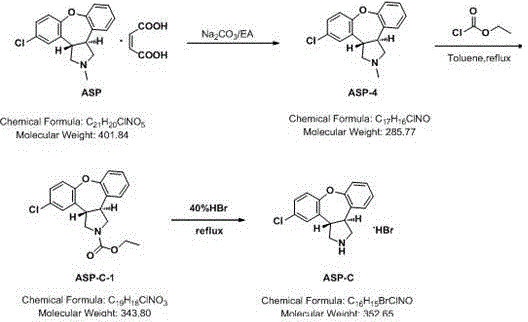
[0020] Add 15.0 g of asenapine maleate (compound ASP) and 15.8 g of sodium carbonate into the reaction flask, then add 250 ml of EA to dissolve, and stir until completely dissolved. The liquid was washed with water for 2 hours, and the filtrate was spin-dried to obtain 11.15 g of a light yellow solid, namely asenapine (compound ASP-1), with a yield of 98%.
Embodiment 2
[0022] Asenapine (Compound ASP-4) 11.15g was dissolved in 350mL of toluene, 25.1g of ethyl chloroformate was added, stirred and heated to reflux, monitored by TLC. After the reaction was complete, the temperature was lowered, washed with water and saturated saline, separated, and the organic phase was spin-dried to obtain an oily substance, stirred with n-hexane to precipitate a solid, filtered with suction, and the filter cake was dried to obtain 10.33g, ASP-C-1, with a yield of 77.1% .
Embodiment 3
[0024] Dissolve 10.33g of ASP-C-1 in 25mL of DMF, add 500mL of 40% hydrobromic acid, heat up to 120°C, and react for 4 hours. TLC monitors that the raw material point disappears. After the reaction was completed, the heating was stopped. During the cooling and stirring process, an off-white solid gradually precipitated. After cooling down to room temperature, it was suction filtered, the filter cake was washed twice with methanol, and dried to obtain 8.52 g of an off-white solid, which was 1-[2-(2,4- Dimethylphenylsulfanyl)-phenyl]methylpiperazine (compound III), yield 80.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com