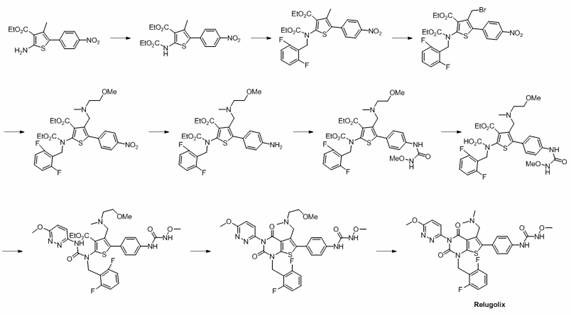
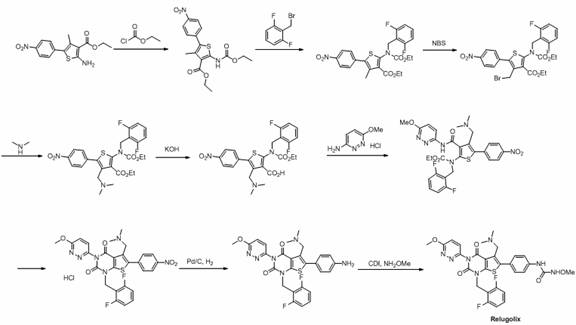
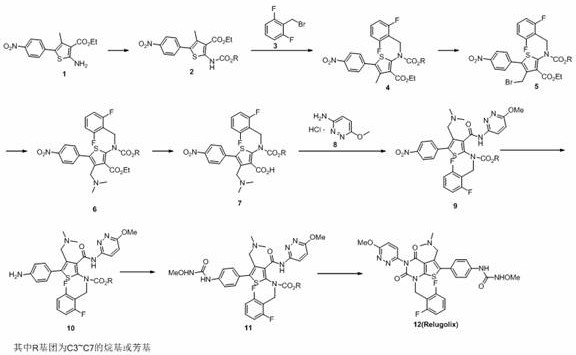
Preparation method of relugolix drug intermediate
A technique of rilugoli and intermediates, applied in the field of drug synthesis, can solve the problems of high flammability, low flash point, high production environment requirements, etc., and achieve the effect of avoiding substitution reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022] Toluene (450 mL) and compound 1 (150 g) were added in sequence to a 2 L reaction flask, stirring was started, propyl chloroformate (120 g) was added dropwise, and the reaction was heated under reflux for 2 h. Cool down to 50-60 °C, control the internal temperature at 50-60 °C and add ethanol (1350 mL) dropwise. After the dropwise addition, slowly lower the temperature to an internal temperature of 0-10 °C, stir for 1 h, filter, and rinse the filter cake with ethanol (300 mL). Drying in vacuo at 45 °C gave a yellow solid with a yield of 84.4% and a purity of 98%.
[0023] The NMR data of compound 2-a are as follows:
[0024] 1 H NMR (400 MHz, CDCl 3 ) δ 10.67 (s, 1H), 8.31-8.22 (m, 2H), 7.59-7.52 (m,2H), 4.39 (q, J = 7.1 Hz, 2H), 4.21 (t, J = 6.7 Hz, 2H) , 2.42 (s, 3H), 1.80-1.67 (m, 2H), 1.42 (t, J = 7.1 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H).
Embodiment 2
[0026]
[0027] Add toluene (1.5 mL) and compound 1 (0.5 g) to the 10 mL reaction flask in sequence, start stirring, add isopropyl chloroformate (0.4 g) dropwise, and heat to reflux for 2 h. Cool down to 20-30 °C, add purified water (4 mL) and dichloromethane (4 mL), separate layers, extract the aqueous phase with dichloromethane (4 mL), combine the organic phases, and concentrate under reduced pressure to obtain compound 2 -b The crude product was purified by column (ethyl acetate: n-heptane = 1:10) to obtain 0.58 g of a bright yellow solid with a yield of 90% and a purity of 98%.
[0028] The NMR data of compound 2-b are as follows:
[0029] 1 H NMR (400 MHz, DMSO-d6) δ 10.45 (s, 1H), 8.29 – 8.23 (m, 2H), 7.69 – 7.63 (m, 2H), 5.02 – 4.91 (m, 1H), 4.32 (m, J = 7.1 Hz, 2H), 2.34 (s, 3H), 1.33 (t, J = 7.1 Hz, 3H), 1.29 (d, J = 6.3 Hz, 6H).
Embodiment 3
[0031]
[0032] Add toluene (1.5 mL), compound 1 (0.10 g) in sequence to the 10 mL reaction bottle, add K 2 CO 3 (90 mg), start stirring, add benzyl chloroformate (0.136 g) dropwise, and heat under reflux for 4 h. Cool down to 20-30 °C, add purified water (4 mL) and toluene (4 mL), separate the layers, extract the aqueous phase with toluene (4 mL), combine the organic phases, and concentrate under reduced pressure to obtain the crude compound 2-c. After column purification (ethyl acetate: n-heptane = 1:6), 0.103 mg of a bright yellow solid was obtained, with a yield of 71.5% and a purity of 98%.
[0033] The NMR data of compound 2-c are as follows:
[0034] 1 H NMR (400 MHz, CDCl 3 ) δ 10.75 (s, 1H), 8.37-8.16 (m, 2H), 7.62-7.50 (m,2H), 7.49-7.32 (m, 5H), 5.27 (s, 2H), 4.37 (q, J = 7.1 Hz, 2H), 2.41 (s, 3H), 1.40 (t, J = 7.1 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com