Preparation method and application of connected conjugated linoleic acid and gemcitabine prodrug
A technology of conjugated linoleic acid and gemcitabine, which is applied in the field of preparation of the prodrug, can solve the problems of easy drug resistance of tumors, increase of toxic and side effects, and influence of clinical curative effect, and achieve excellent anti-tumor therapeutic effect, reduce Effects of Toxicity and Resistance, Strong Antitumor Efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
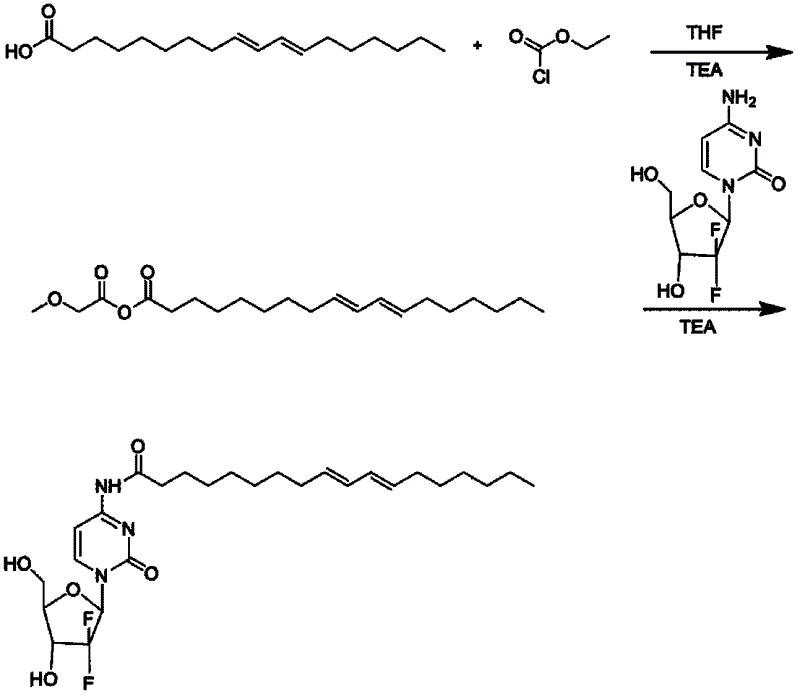
[0035] Example 1 Conjugated linoleic acid and gemcitabine-linked prodrug (CLA-GEM conjugate) Conjugated linoleic acid (0.50g, 1.78mmol) and triethylamine (0.20g, 2.0mmol) were dissolved in 3ml In tetrahydrofuran, keeping the temperature at -10°C, ethyl chloroformate (0.19 g, 1.78 mmol) was added dropwise with stirring, and stirred at -15°C for 30 minutes after the dropwise addition. Gemcitabine hydrochloride (0.53g, 1.78mmol) and triethylamine (0.20g, 2.0mmol) were then dissolved in 5ml of N,N-dimethylformamide, and added dropwise to the above-mentioned conjugated linoleic acid solution at -15°C , the reaction solution was stirred at room temperature for 72 hours under nitrogen protection, and dried in vacuo. Take the material obtained from the above reaction, add 50ml of saturated aqueous sodium bicarbonate solution and ethyl acetate solution to wash with water, collect the ethyl acetate layer, dry it in vacuum, separate it with a silica gel column, and use gradient elution. ...
Embodiment 2
[0036] Example 2 Confirmation of the structure of the prodrug (CLA-GEM conjugate) linked to conjugated linoleic acid and gemcitabine
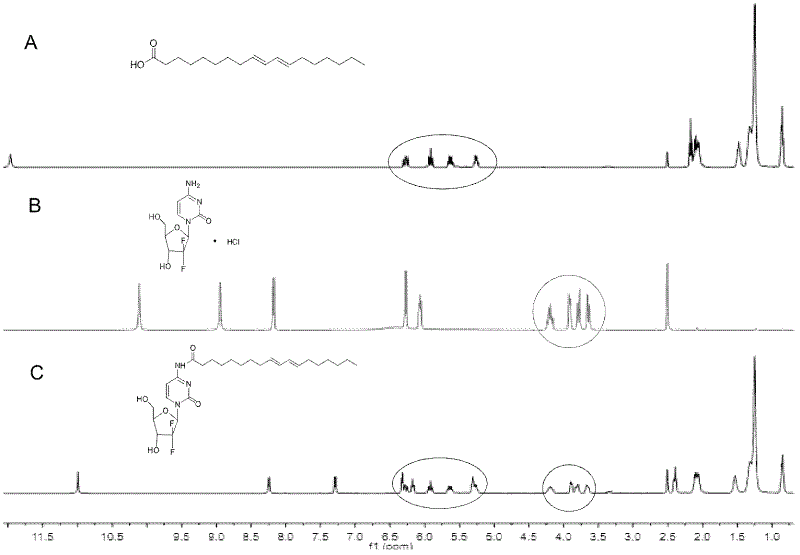
[0037] The structure of the prepared prodrug (CLA-GEM conjugate) was verified by 400MHz 1H-NMR (Bruker AVANCEIII 400, Germany).
[0038] 1H NMR (400MHz, d-DMSO, δppm)
[0039] δ10.97 (1H, s, NHCO), 8.24 (1H, d, J=7.6Hz, 6-CH), 7.29 (1H, d, J=7.6Hz, 5-CH), 6.30-6.31 (1H, m , CH=CH), 6.28 (1H, m, CH=CH), 6.15 (1H, t, J=7.6Hz, 1'-CH), 5.89 (1H, m, CH=CH), 5.65 (1H, m , CH=CH), 4.18 (1H, m, 3'-CH) 3.63-3.90 (3H, m, 4'-CH and 5'-CH2), 2.398 (2H, t, J=7.6, CO-CH2) , 2.1 (4H, m, 2CH2), 1.2-1.5 (18H, m, CH2), 0.88 (3H, t, CH3)
Embodiment 3
[0040] Example 3 Evaluation of prodrug (CLA-GEM conjugate) in vitro cytotoxicity and cell membrane transport mechanism
[0041] Using ammonium thiocyanate B (SulforhodamineB) (SRB) determination method. MCF-7 human breast cancer cells were inoculated in a 96-well plate (2500 cells / well), and after incubation for 24 hours, the gemcitabine hydrochloride aqueous solution and the DMSO solution of the CLA-GEM conjugate were diluted into drug solutions of different concentrations, and added Into the tumor cells, 6 wells per sample, continue to incubate for 24 hours, discard the medium, fix the cells with 10% trichloroacetic acid at 4°C for 1 hour, rinse with purified water, dry and rinse with 0.4% SRB at 4°C After staining for 30 minutes, take it out and wash it with 0.1% acetic acid, add 10uM Tris solution after drying, shake it on a shaker for 30 minutes, and measure the absorbance at 540nm with a microplate reader.
[0042] The research results showed that after 24 hours, 5uM CL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com