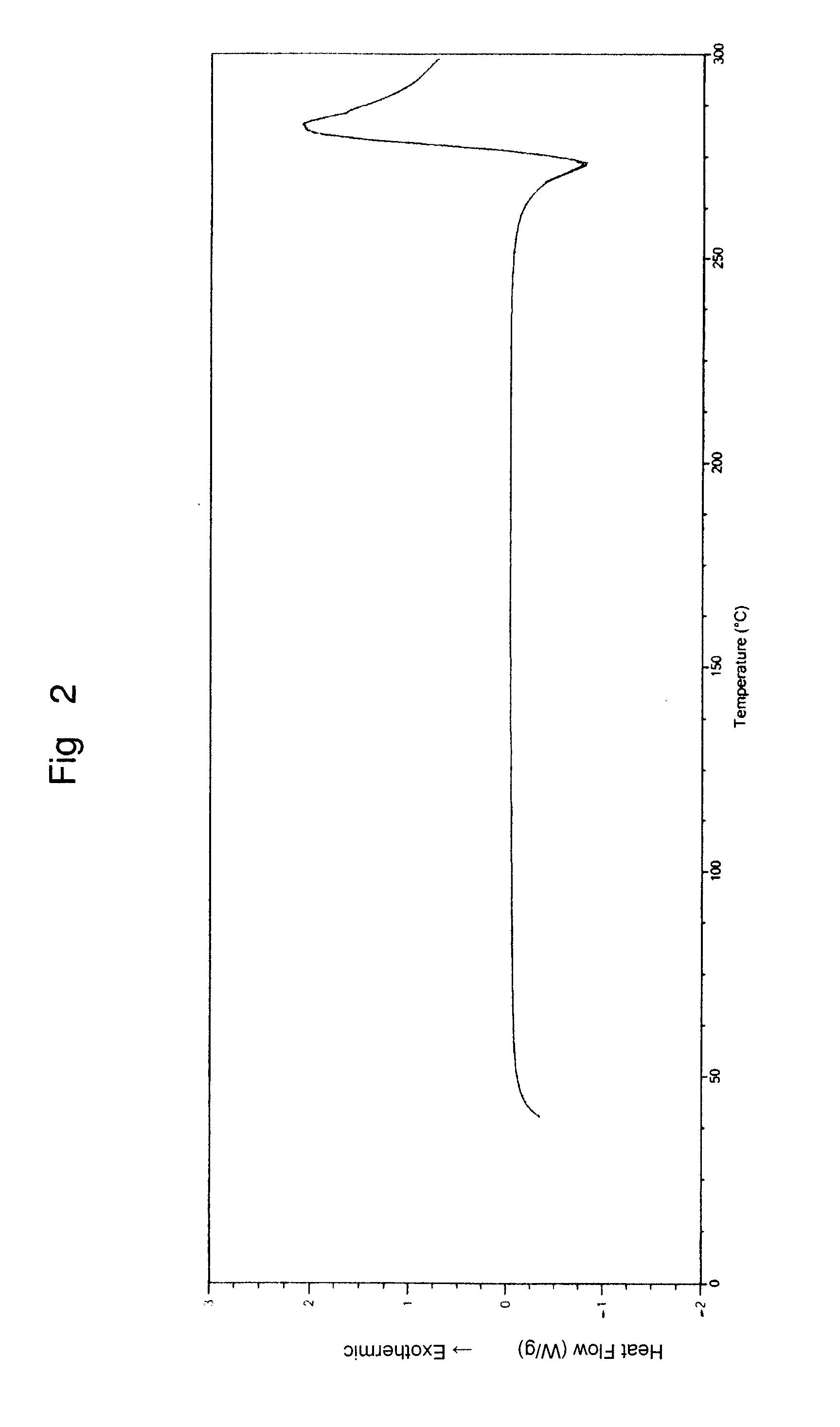
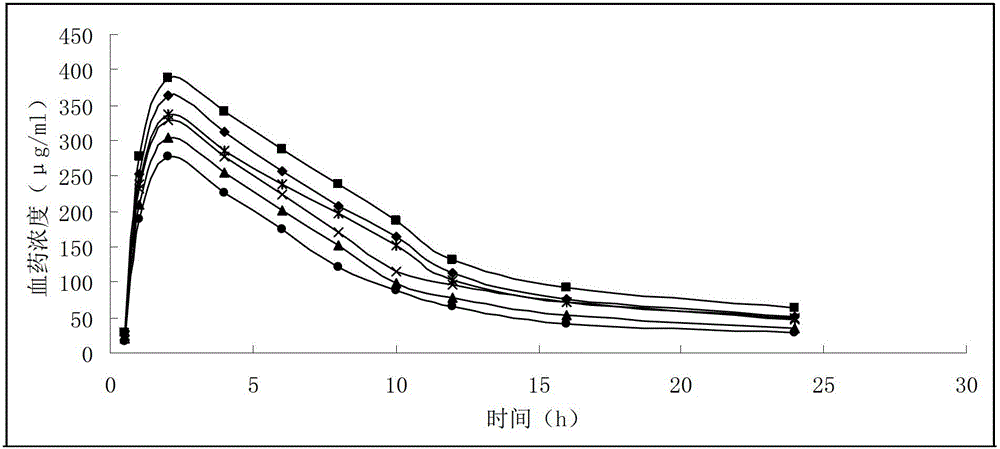
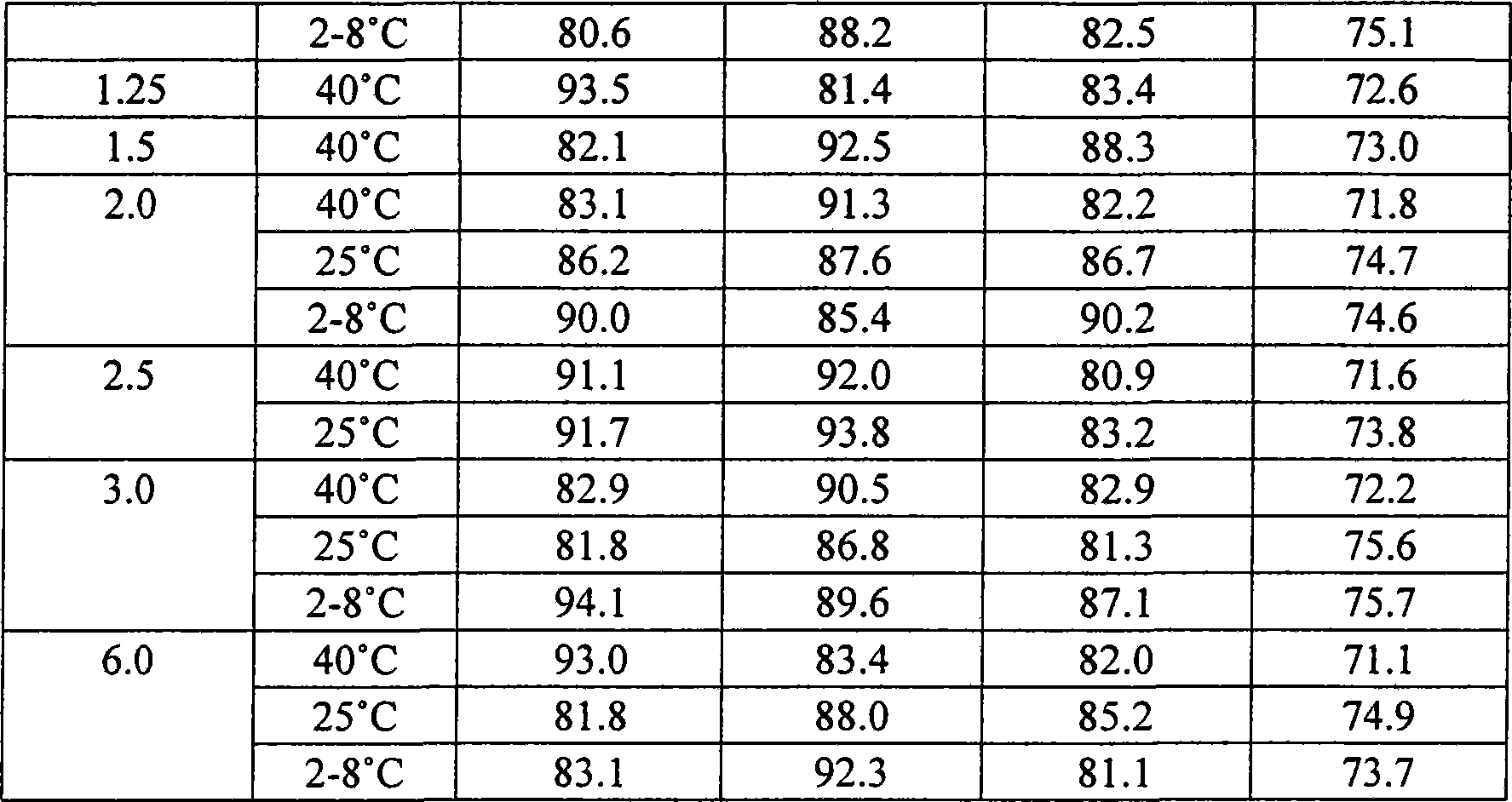
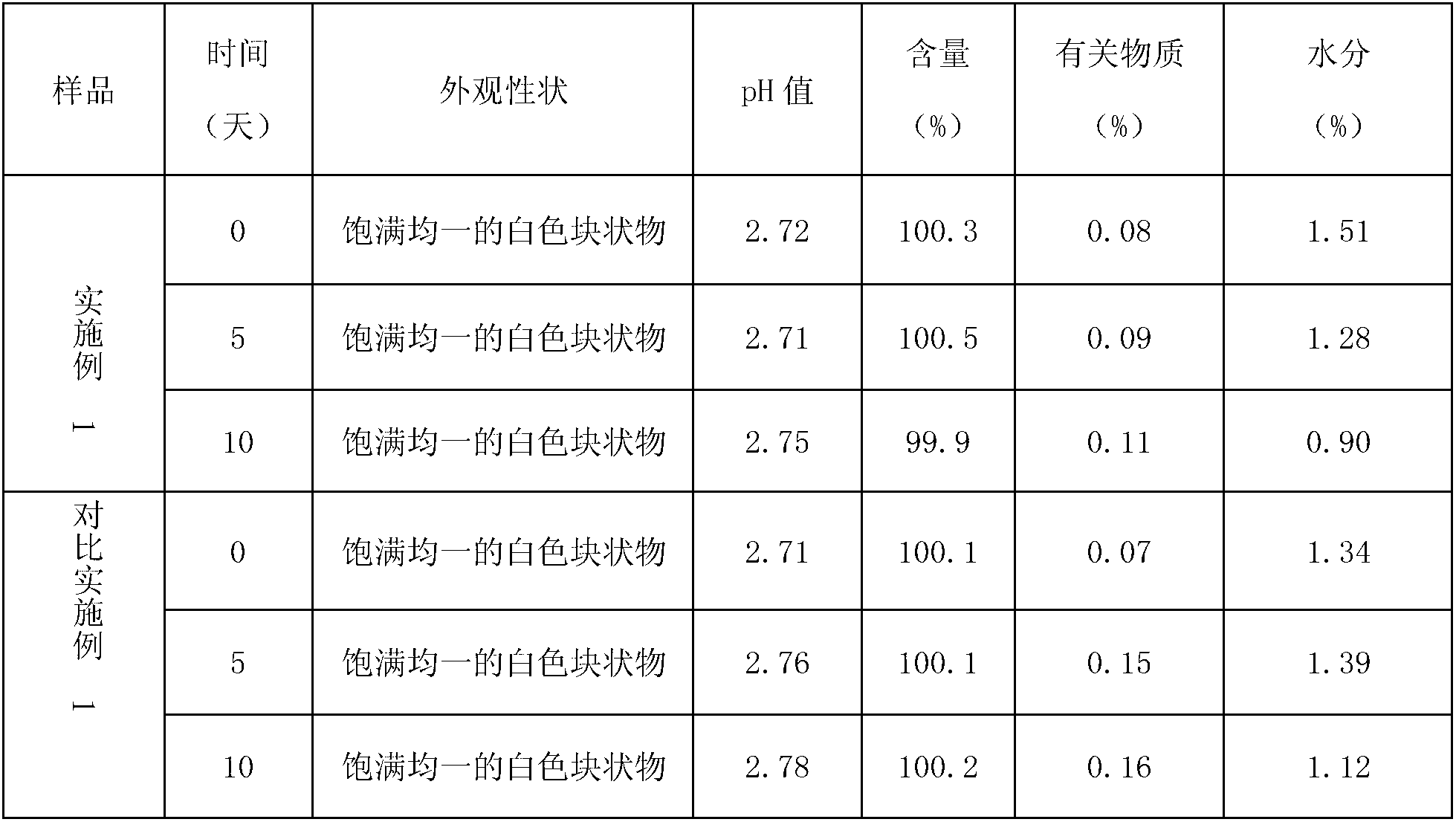
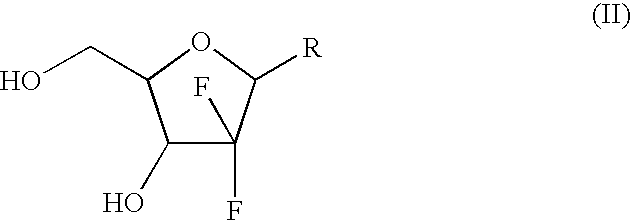
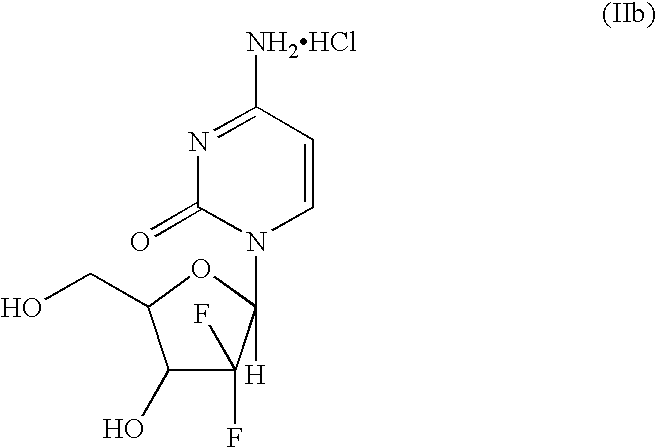
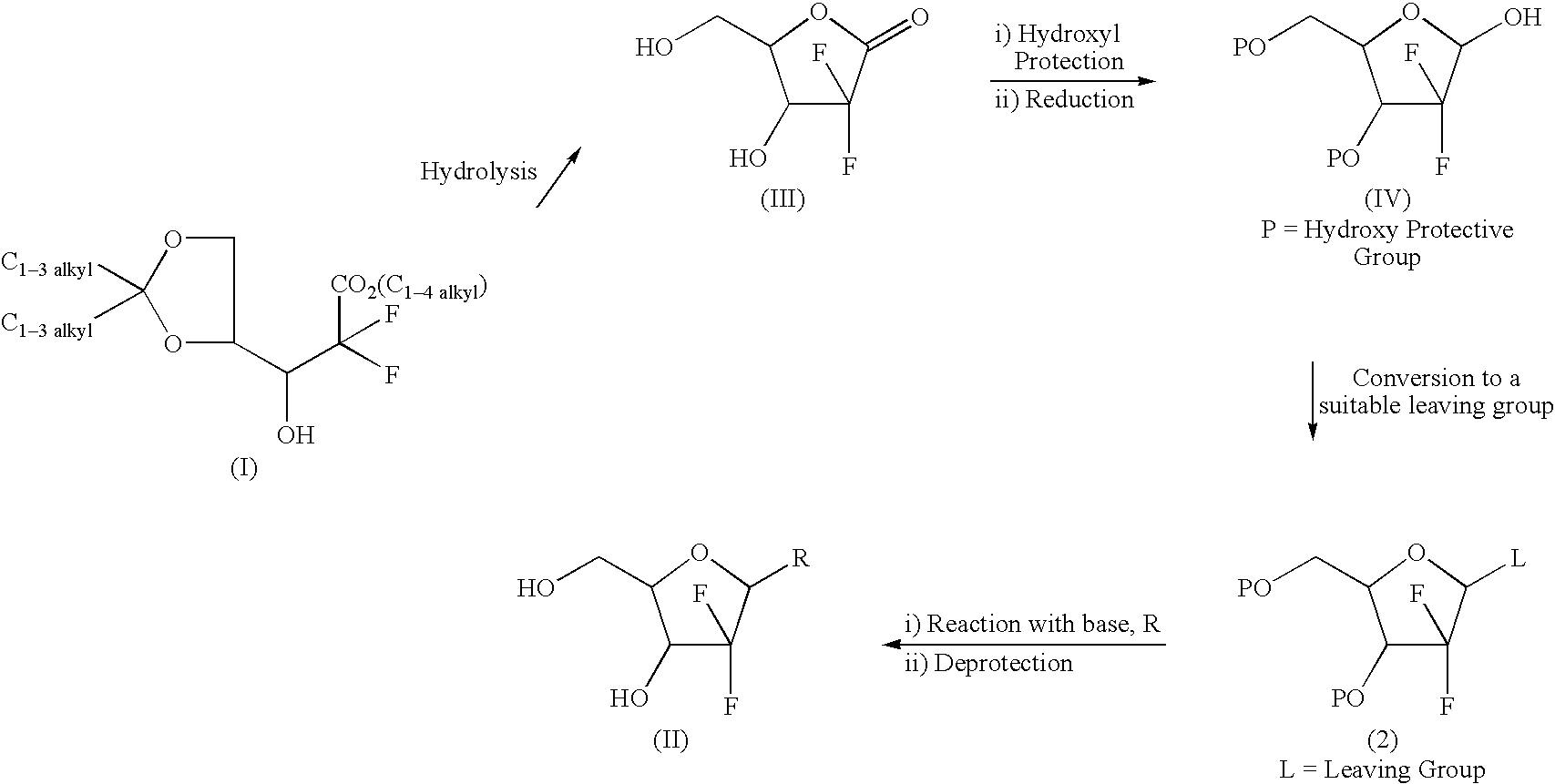
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
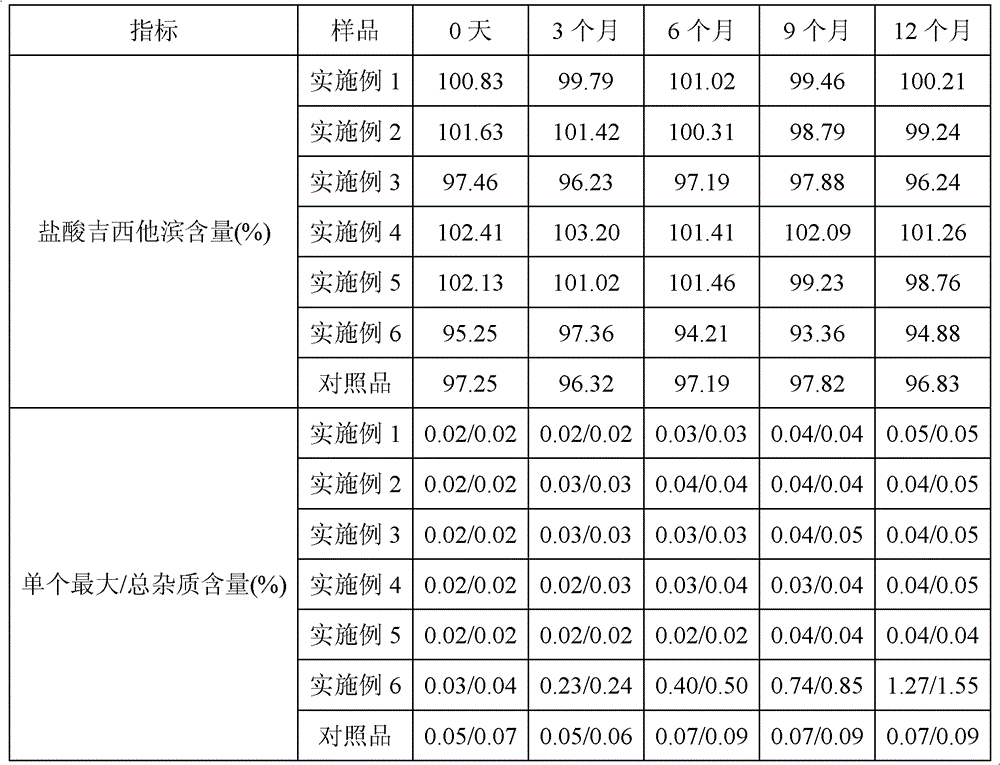
104 results about "Gemcitabine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
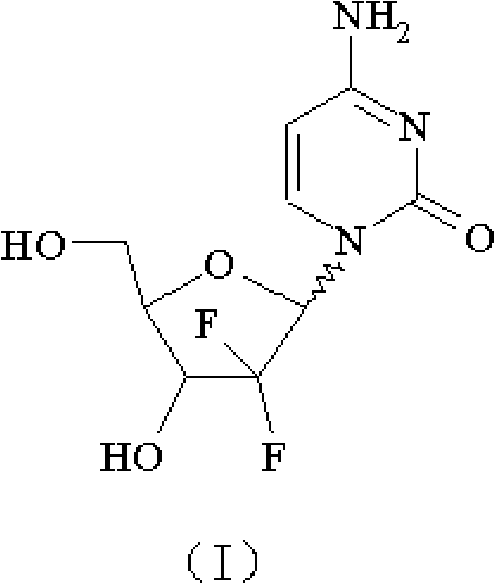
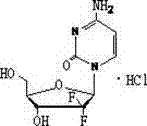
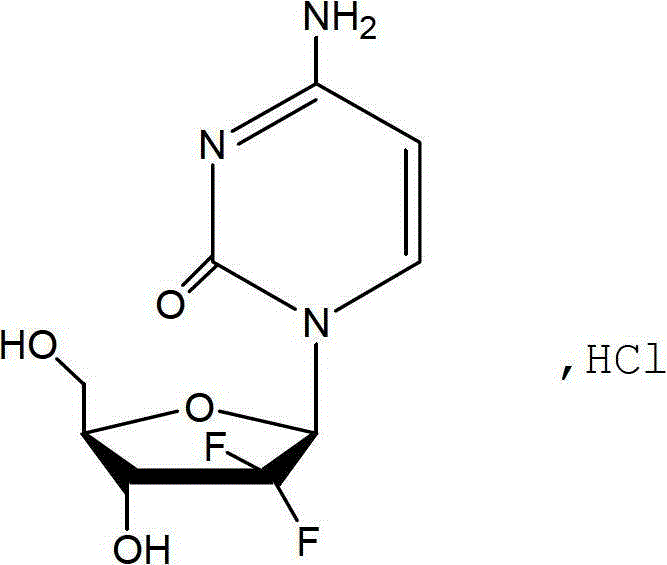
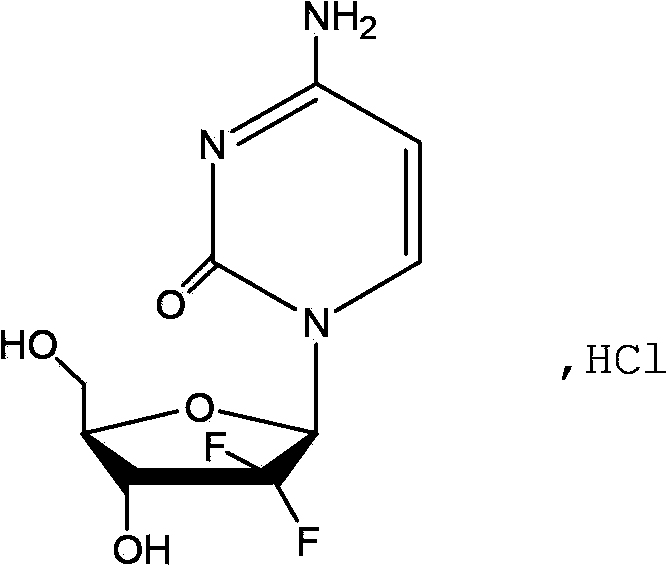
The hydrochloride salt of an analogue of the antimetabolite nucleoside deoxycytidine with antineoplastic activity. Gemcitabine is converted intracellularly to the active metabolites difluorodeoxycytidine di- and triphosphate (dFdCDP, dFdCTP). dFdCDP inhibits ribonucleotide reductase, thereby decreasing the deoxynucleotide pool available for DNA synthesis; dFdCTP is incorporated into DNA, resulting in DNA strand termination and apoptosis.
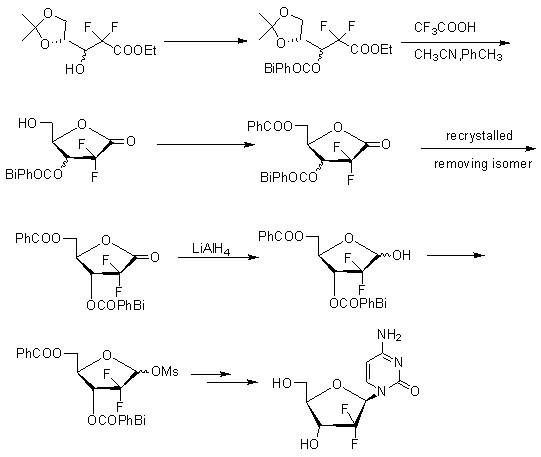
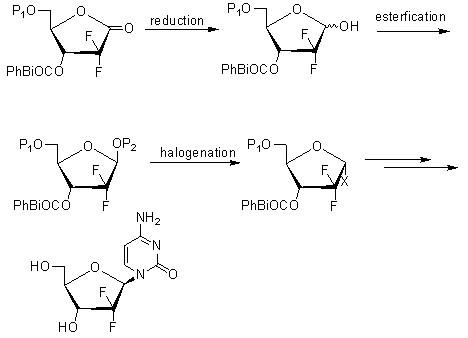
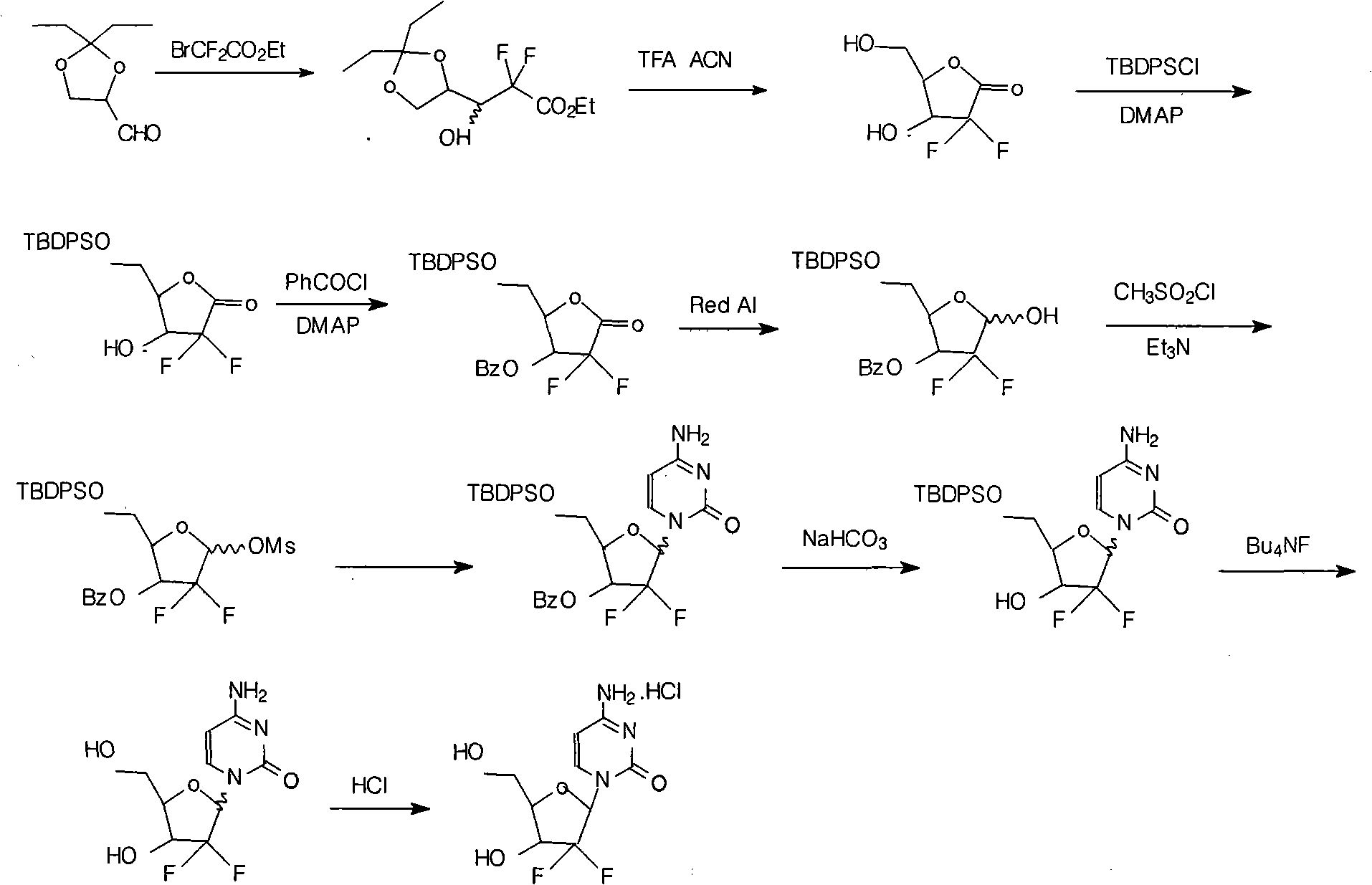
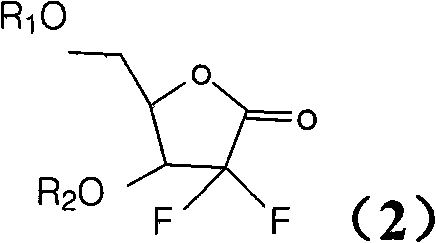
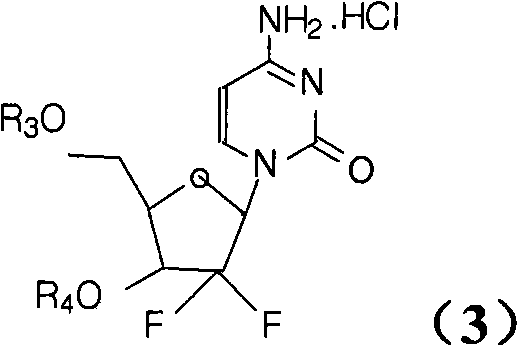
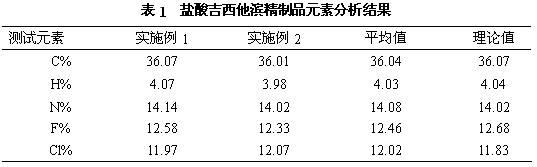
Method for preparing gemcitabine hydrochloride
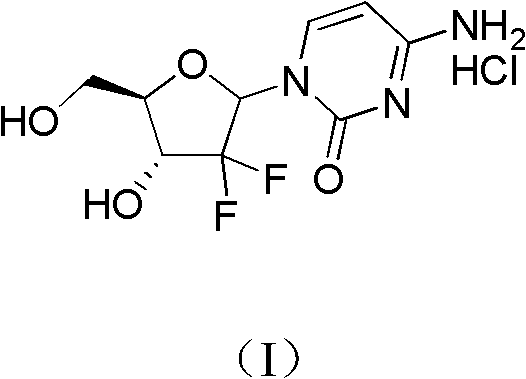
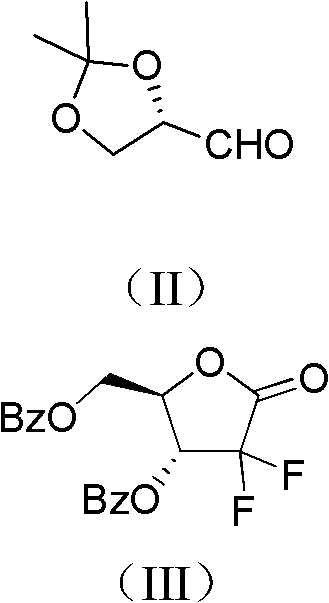
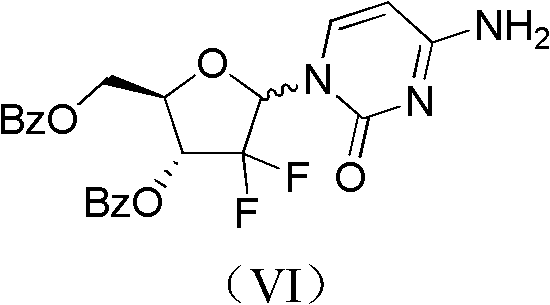
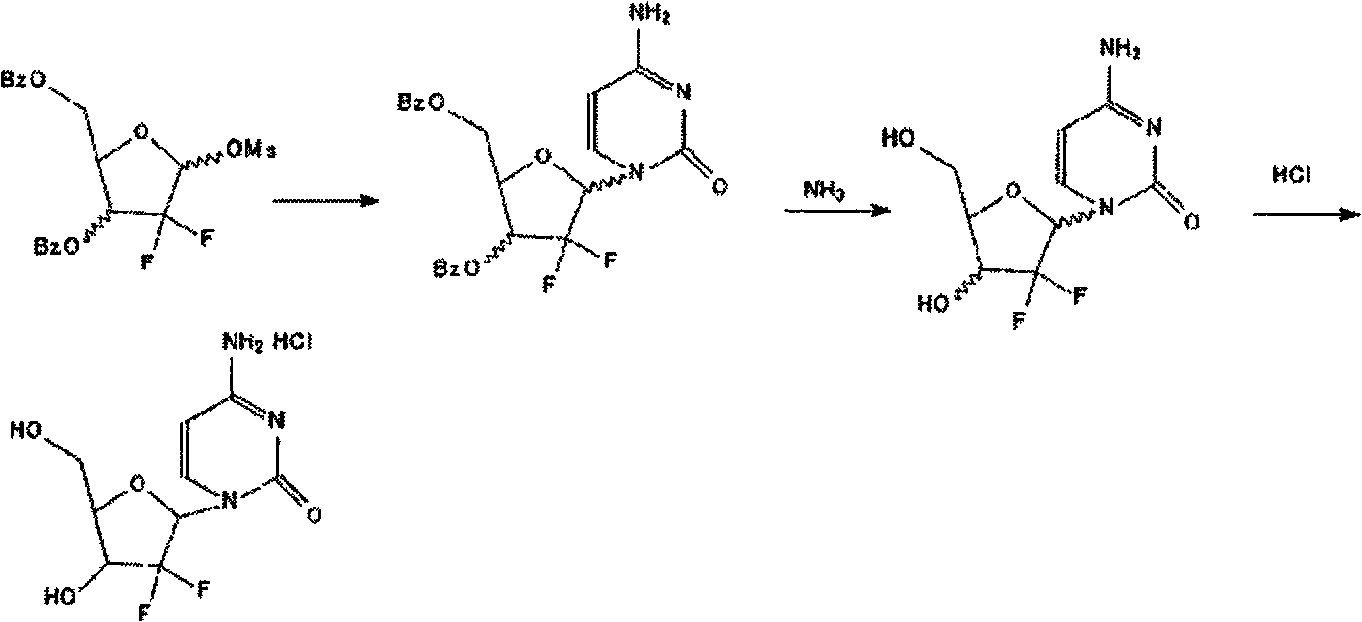
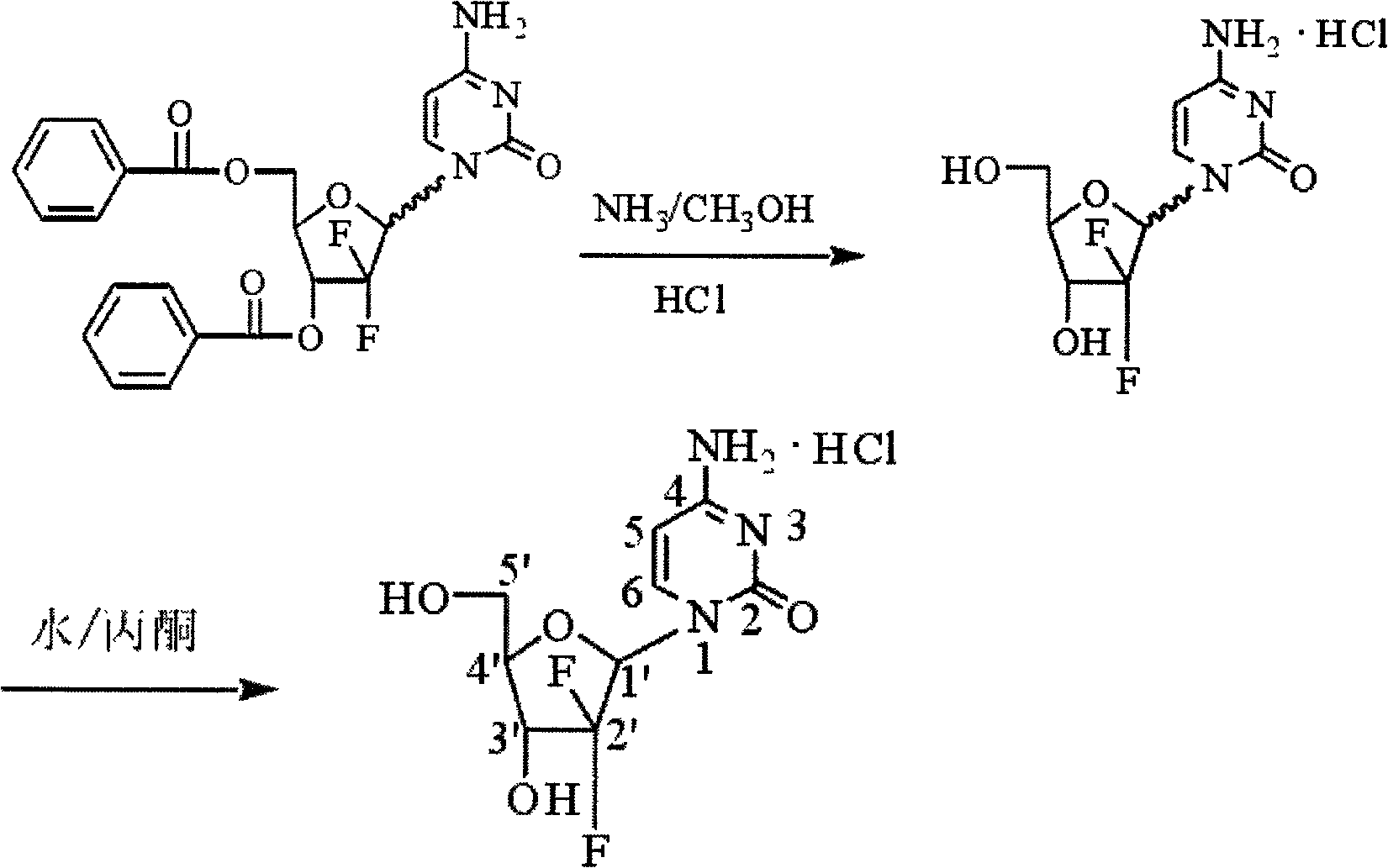
ActiveCN102617678AFew stepsSimple and fast operationSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideProtecting group
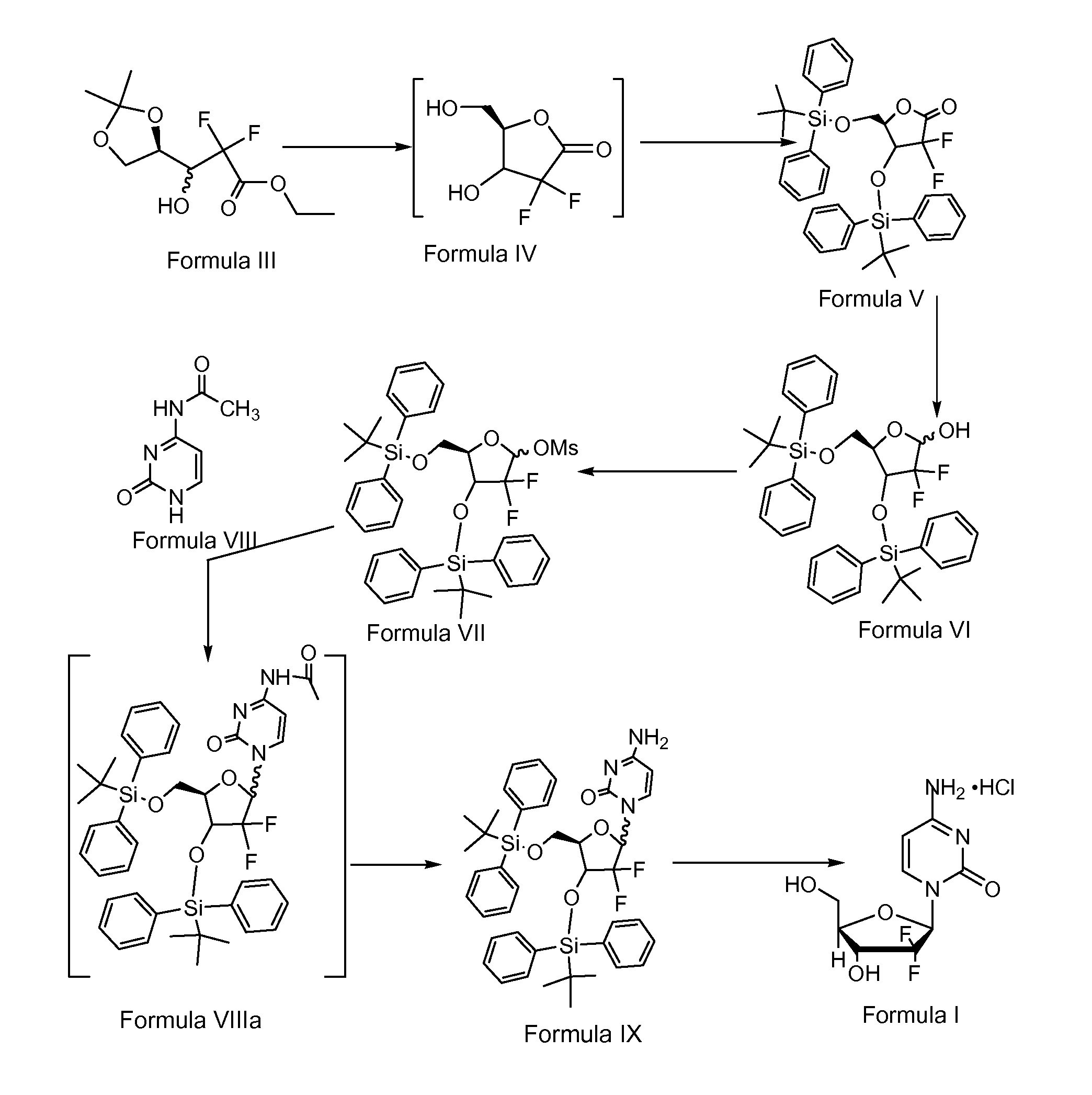
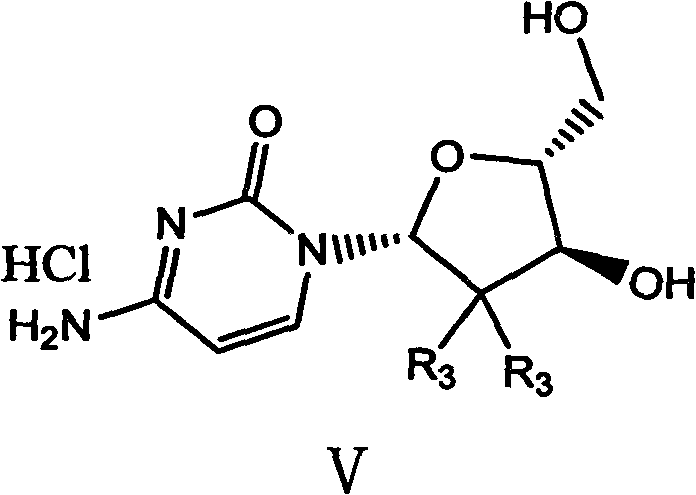
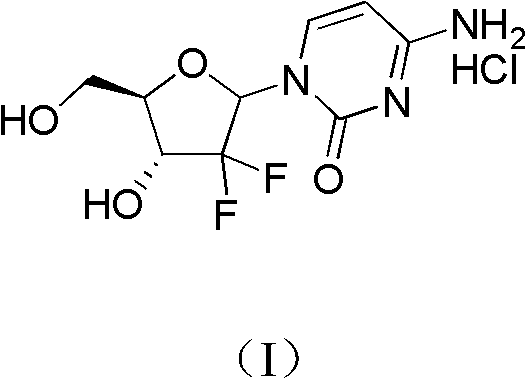
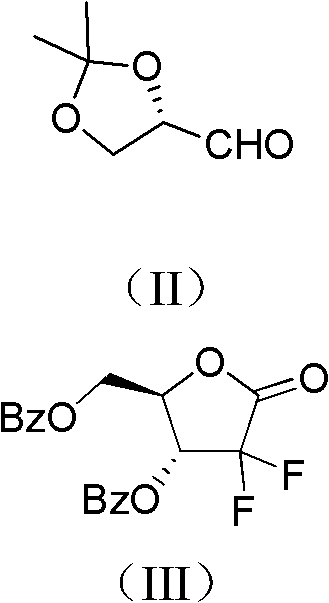
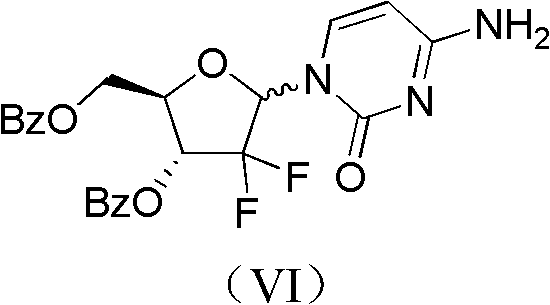
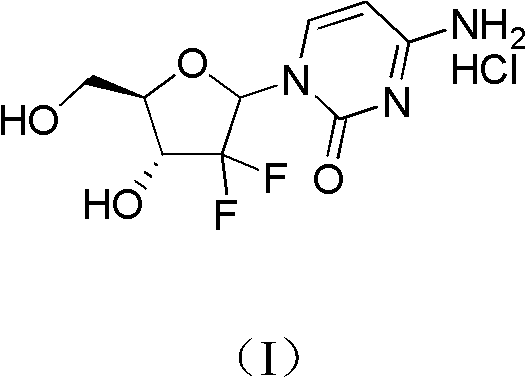
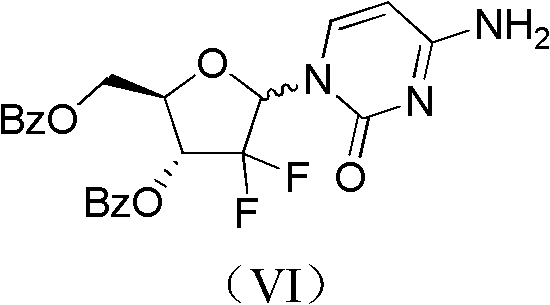
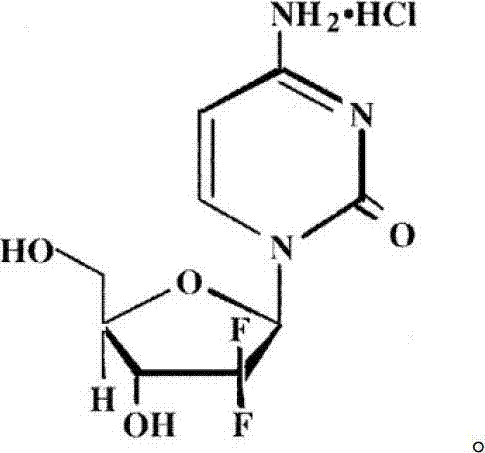
The invention relates to a compound in a formula (I), and discloses a method for preparing gemcitabine hydrochloride. The method includes preparing an intermediate type (III) compound by means of realizing Reformatsky reaction, removing a protecting group and realizing lactonization and double benzoylation; obtaining a compound in a formula (VI) via reduction, methyl sulfonylation and condensation; and finally removing a protecting group, saltifying and realizing crystallization to obtain a final product. The method is simple in process, high in yield and quite suitable for industrial production, the purity of the product is fine, and harsh reaction conditions are omitted.
Owner:JIANGSU HANSOH PHARMA CO LTD
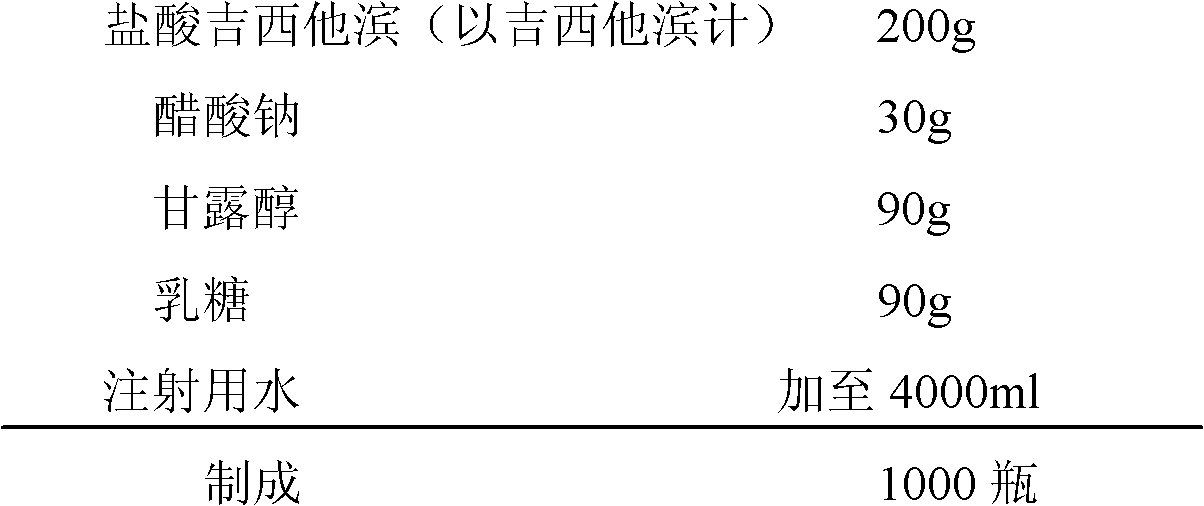
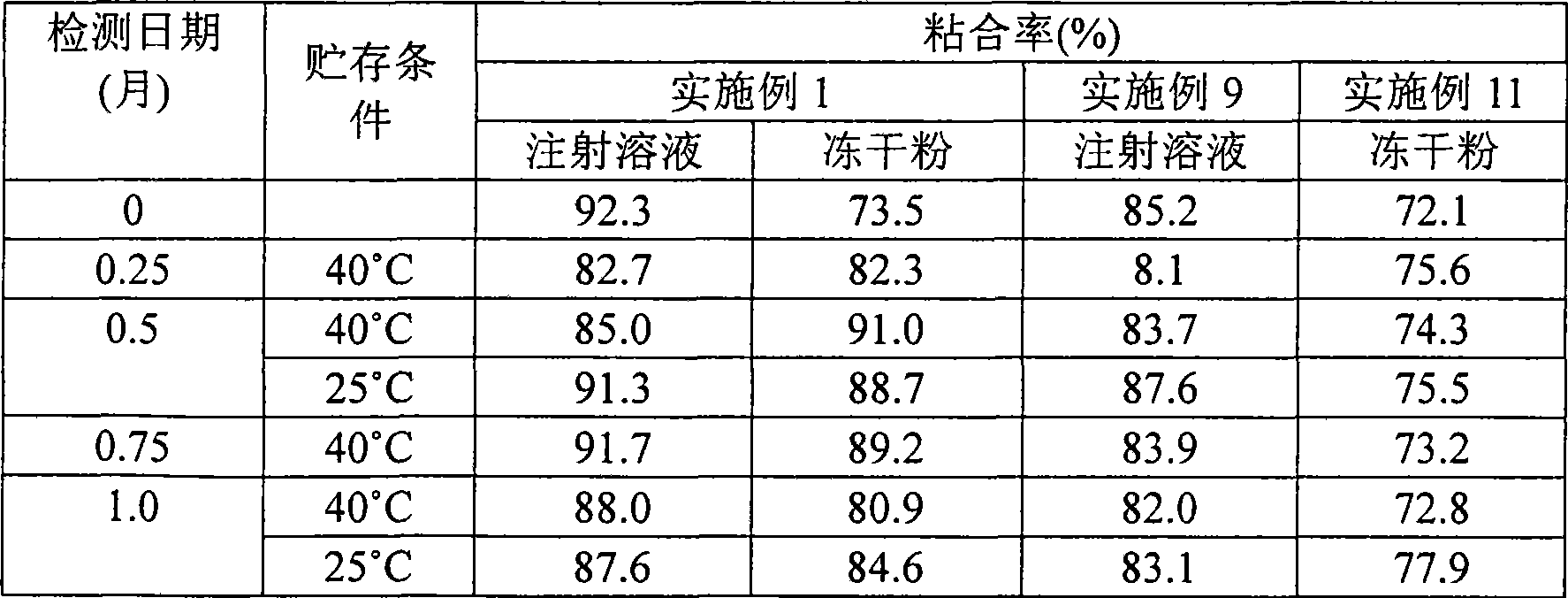
Gemcitabine hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN102144981AReduce dosageImprove stabilityPowder deliveryOrganic active ingredientsSodium acetateAdjuvant
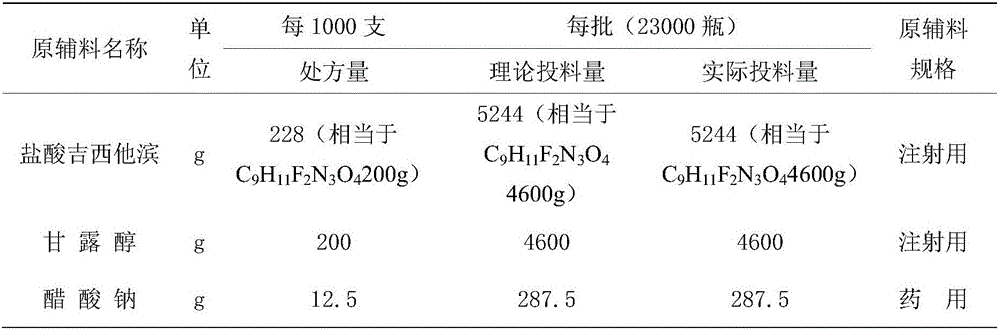
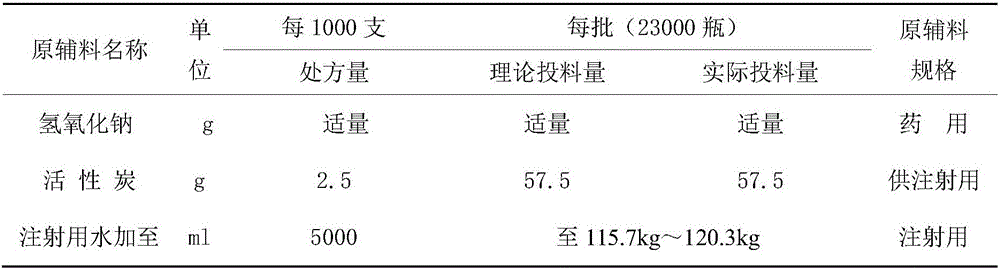
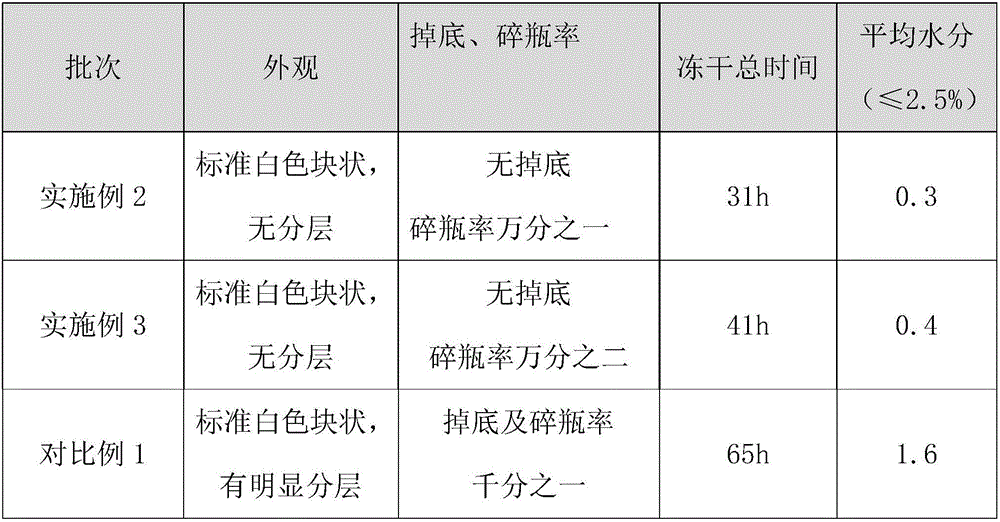
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The lyophilized powder injection comprises the following components in parts by weight: 20-30 parts of gemcitabine hydrochloride, 5-9 parts of mannitol, and 3-10 parts of sodium acetate. The freeze-drying step includes the following three stages: a pre-freezing stage, a primary drying stage and a secondary drying stage, and the entire freeze-drying time is lower than 20 hours. The gemcitabine hydrochloride lyophilized powder injection provided by the invention has the advantages of less types and amounts of adjuvants, easily-controlled technological parameters, simple process route, short freeze-drying time, convenience in operation, good repeatability, low contents of related substances, and controllable quality; and the redissolved lyophilized powder injection has good clarity and forming performance. The lyophilized powder injection has stable and controllable quality, is easy to realize industrial production, and can generate considerable economic and social benefits.
Owner:HAINAN JINRUI PHARMA CO LTD
Gemcitabine hydrochloride composition and preparation method thereof
ActiveCN101606947AImprove appearance qualityIncrease contentOrganic active ingredientsPowder deliverySodium acetateGemcitabine Hydrochloride
The invention provides a gemcitabine hydrochloride composition which comprises the following components by weight portions: 57 portions of gemcitabine hydrochloride, 20 to 50 portions of mannitol and proper amount of sodium acetate. The gemcitabine hydrochloride is firstly recrystallized to improve the purity of the product after synthesizing gemcitabine, and the gemcitabine hydrochloride is prepared. The method has high yield and the obtained gemcitabine hydrochloride has high purity. Freeze-drying powder prepared by the gemcitabine hydrochloride synthesized by the method has good stability. The invention also provides a preparation of the gemcitabine hydrochloride composition, and the adopted freeze-drying method comprises the following steps of: reducing the temperature of a freeze-drying box to -42 to -38 DEG C, putting encapsulated medicines, keeping the temperature for 3 hours, starting a vacuum pump, keeping the vacuum degree in the drying box to 3 to 9Pa, raising the temperature slowly to minus 22 to minus 18 DEG C with the speed of raising the temperature of 0.3 DEG C / min, keeping the temperature for 2 hours, raising the temperature to 0 DEG C with the speed of 0.5 DEG C / min, keeping the temperature for 15 hours, raising the temperature of the drying box to 8 to 12 DEG C with the speed of 1 DEG C / min, keeping the temperature for 3 to 5 hours, raising the temperature to 34 to 36 DEG C with the speed of 2 DEG C / min and keeping the temperature for 10 hours.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
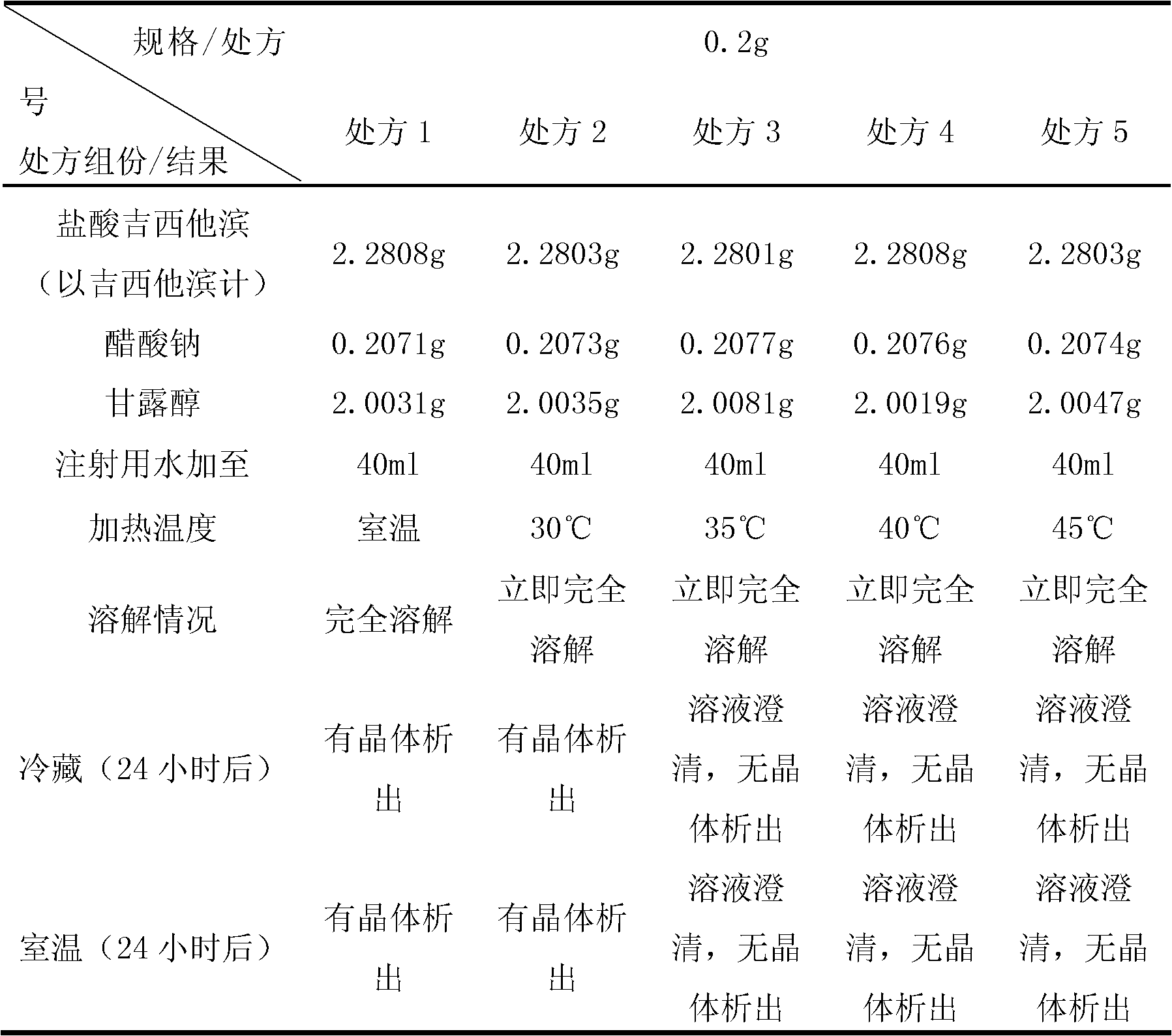
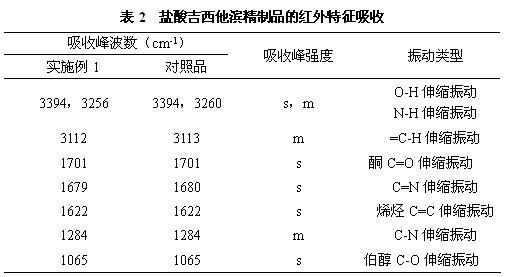
Jixitabing hydrochloride solution type injection agent
InactiveCN1650883AOrganic active ingredientsPharmaceutical delivery mechanismGemcitabine HydrochlorideHydrochloride
A solution-type injection of Jixitabin hydrochloride features that it contains alkaline substance or weak acid's salt as stabilizer.
Owner:华润医药商业集团国际贸易有限公司
Combination therapy for hyperproliferative disease
InactiveUS20070197517A1Ease of detectabilityEasy to prepareBiocideHeavy metal active ingredientsCarboplatinDisease
This invention relates a method of treating hyperproliferative diseases. More particularly, the present invention relates to a method of treating hyperproliferative diseases, such as cancer, comprising the step of administering to a mammal in need of such treatment, either simultaneously or sequentially, (i) a therapeutically effective amount of a taxane derivative, a platinium coordination complex selected from the group consisting of carboplatin, tetraplatin, and topotecan, a nucleoside analog selected from the group consisting of gemcitabine hydrochloride and 5-FU, an anthracycline, a topoisomerase selected from the group consisting of etoposide, teniposide, amsacrine, topotecan, and Camptosar®, an aromatase inhibitor; and (ii) a therapeutically effective amount of an isothiazole derivative. The combinations of the present invention may optionally include an anti-hypertensive agent. This invention also relates to pharmaceutical compositions useful in the treatment of hyperproliferative diseases in mammals, containing such combinations. The present invention also relates to kits having a first compartment with a compound of formula 1 and a second compartment containing a taxane derivative, a platinum coordination complex, a nucleoside analog, an anthracycline, a topoisomerase inhibitor, or an aromatase inhibitor and a third compartment containing an anti-hypertensive agent.
Owner:PFIZER INC
Synthesis method of gemcitabine hydrochloride
InactiveCN102417533AHigh yieldSimple methodSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideMedicinal chemistry
Owner:JIANGSU QINGJIANG PHARMA
Gemcitabine hydrochloride lyophilized preparation
ActiveCN102302462AFix stability issuesGuarantee product qualityOrganic active ingredientsPowder deliveryGemcitabine HydrochlorideAntioxidant
The present invention discloses a gemcitabine hydrochloride lyophilized preparation. The lyophilized preparation comprises, by weight, 20-70 parts of gemcitabine hydrochloride, 15-60 parts of a lyoprotectant and 0.005-0.5 parts of an antioxidant. Compared to the prior art, with the gemcitabine hydrochloride lyophilized preparation provided by the present invention, the problem of the stability ofthe gemcitabine hydrochloride is solved; the product quality is ensured; the risk of drug using is reduced; the preparation process is simple, the cost is low; and the preparation is suitable for theindustrial production, and has practical value.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Liposome Preparation
InactiveUS20090169610A1Improve targetingReduce drug doseOrganic active ingredientsAntibody ingredientsGnRH AntagonistLiposome
The present invention provides cancer treatment preparations of excellent targetability. The sugar chain-modified liposomes of the present invention, which contain an aromatase inhibitor, anti-androgenic agent, lyase inhibitor, GnRH agonist, GnRH antagonist, anti-angiogenic agent, tyrosine kinase inhibitor, serine-threonine kinase inhibitor, antibody having an anticancer activity, ansamitocin, capecitabine, celmoleukin, docetaxel hydrate, gemcitabine hydrochloride, oxaliplatin, prednisolone, tegafur-uracil mixtures, zinostatin stimalamer or arsenic trioxide may be used as cancer treatment preparations having an excellent targetability.
Owner:SIEMENS AG +1
Preparation of gemcitabine
InactiveUS20090069557A1Simple, ecofriendly, cost-effectiveSuitable for useSugar derivativesSugar derivatives preparationGemcitabine HydrochloridePurification methods
Owner:DR REDDYS LAB LTD +1
Gemcitabine hydrochloride liposome injection
InactiveCN102716089AWon't breakHigh encapsulation efficiencyOrganic active ingredientsPowder deliverySolubilitySide effect
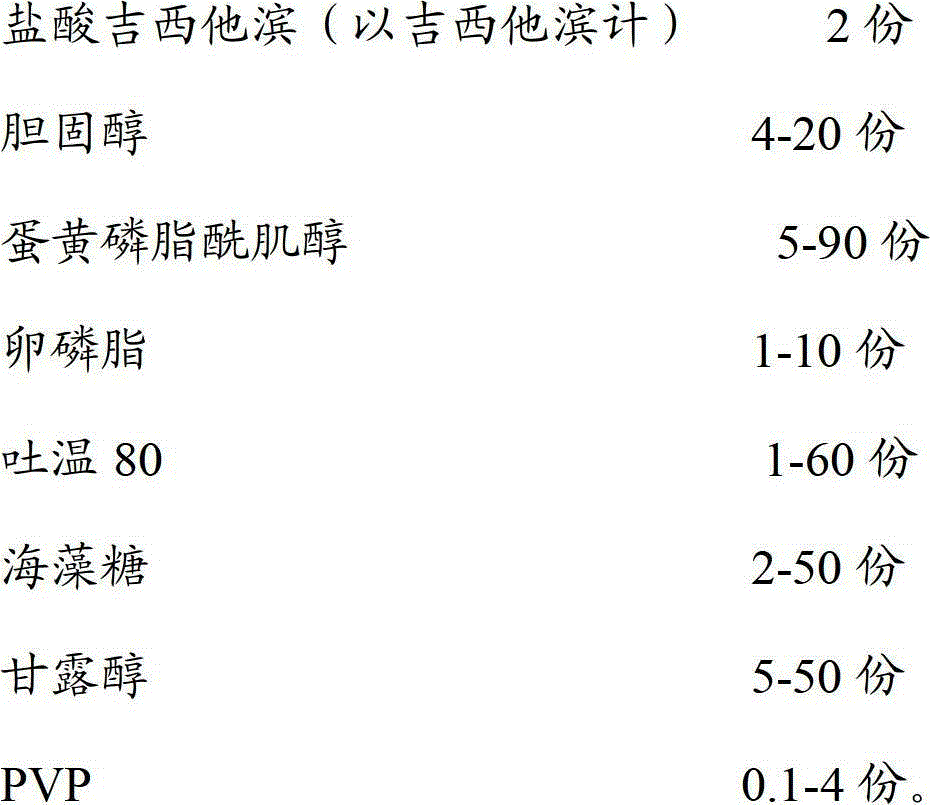
The invention discloses a gemcitabine hydrochloride liposome injection and a preparation method thereof. The gemcitabine hydrochloride liposome injection is prepared from the following components in specific percentage by weight: gemcitabine hydrochloride, cholesterol, egg yolk phosphatidylinositol, phosphatidylcholine, tween 80, trehalose, mannitol and polyvinylpyrrolidone. The liposome injection is high in stability and cannot be cracked due to fusion, ice crystal and the like during freezing; and after long-term storage, liposome is still high in package rate. According to the gemcitabine hydrochloride liposome injection, the solubility of gemcitabine hydrochloride is improved; the quality of a product is improved; the toxic and side effects are reduced; the retention time of medicines during general circulation is prolonged; the biological utilization rate of the medicine is increased; the treating effect is obviously enhanced; the preparation method is simple; and the gemcitabine hydrochloride liposome injection is suitable for industrial large-scale production.
Owner:灵康药业集团股份有限公司
Method for preparing gemcitabine hydrochloride
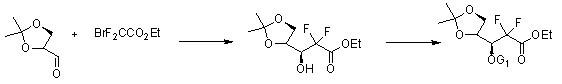
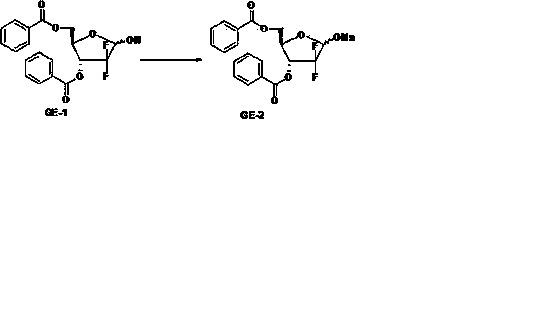
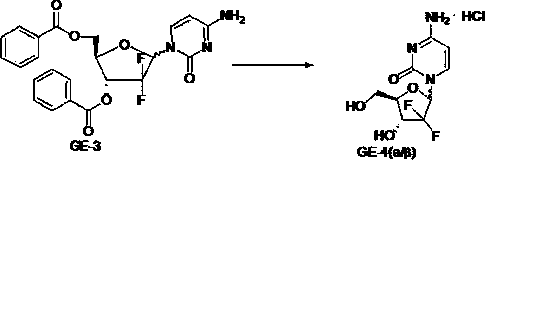
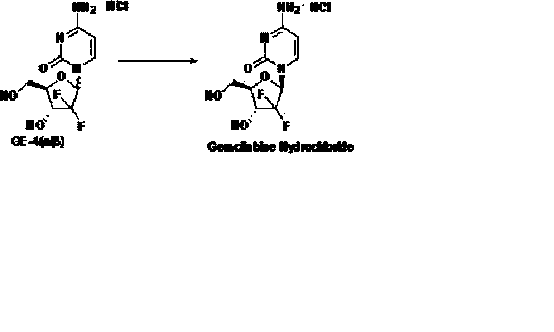
The invention discloses the compounding method for hydrochloric acid Gemcitabine. It uses D-mannitol as raw material and takes the processes of hydroxyl protection, oxidation, and addition of reformatsky, hydroxy benzoylation, hydrolysis, hydroxyl sulfonylation, ring closure, carbonyl reduction, hydroxyl sulfonylation, condensation, hydrolysis and crystallization to gain hydrochloric acid Gemcitabine. It has the advantages of high yield, simple operation, and is suitable to industrial producing.
Owner:HUBEI YITAI PHARMA
Method for preparing gemcitabine hydrochloride
ActiveCN102603838ASugar derivativesSugar derivatives preparationThree levelGemcitabine Hydrochloride
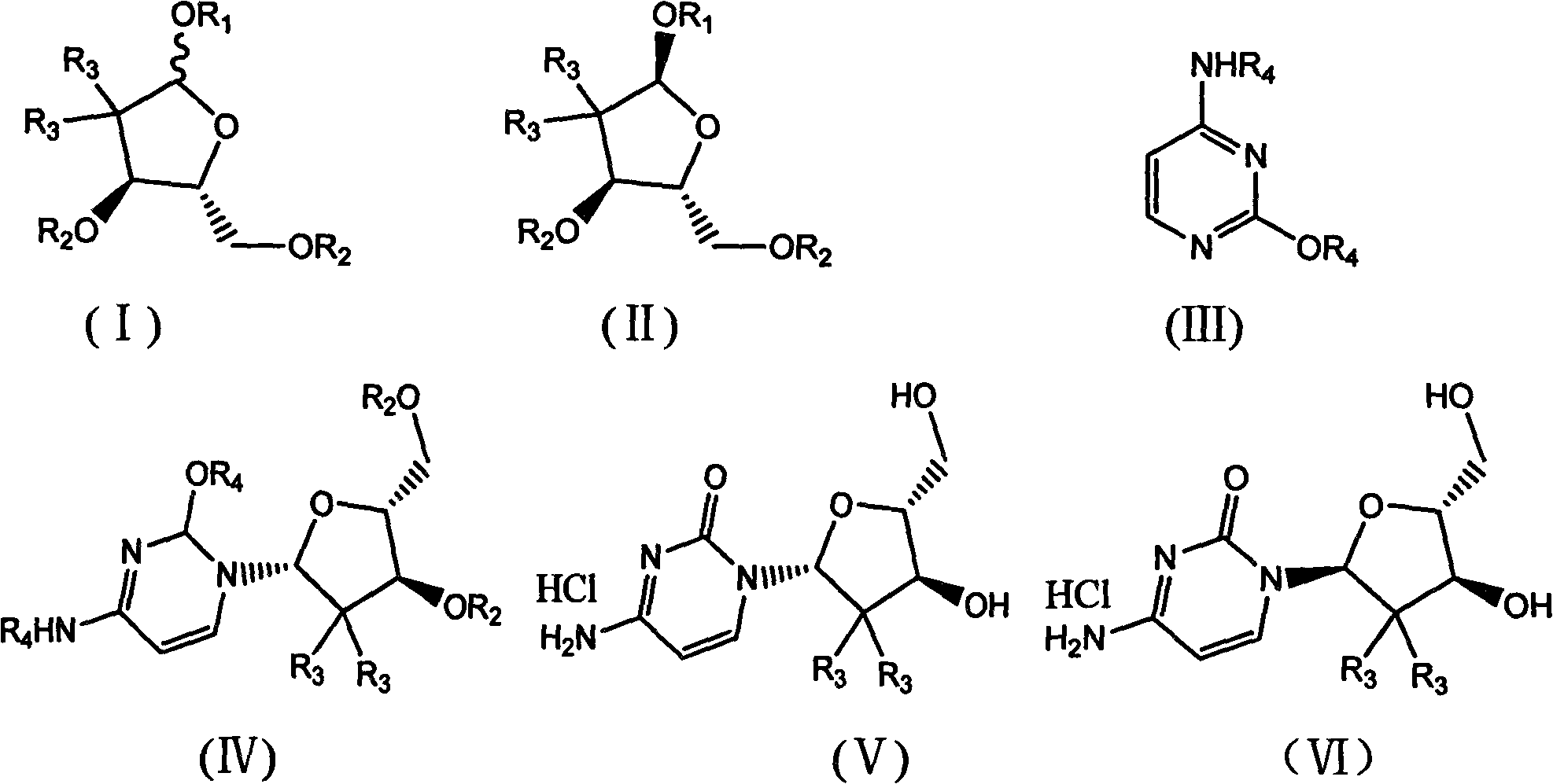
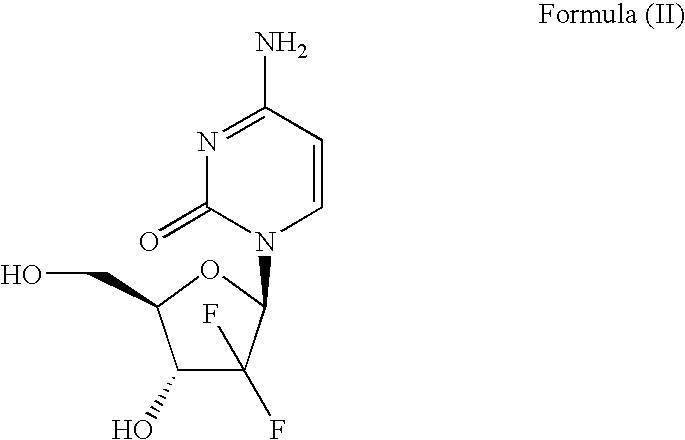
The invention belongs to technical field of pharmacy and provides a method for preparing gemcitabine hydrochloride, which solves the problems that the industrialization cost is high and the total yield is low in the original preparation method of gemcitabine hydrochloride. The method comprises the steps of a. converting and extracting alpha anomer of a formula (II) from a formula (I); b. coupling the formula (II) and a formula (III); and c. obtaining the gemcitabine hydrochloride of a formula (V). The step a comprises the steps of (1) dissolving the compound with the formula (I) in an aprotic solvent at least containing R1OH and three-level organic amine salified by R1OH, heating and reflowing, then reducing temperature so as to increase the mole ratio of the Alpha anomer of the formula (II) in the Alpha anomer mixer of the formula (I); (2) extracting the compound with the formula (I), reducing the temperature under recrystallization liquid so as to recrystallize the extracted product to obtain a solid Alpha anomer of the formula (II) and recrystallization liquid of the formula (I).
Owner:江苏八巨药业有限公司
Method for preparing gemcitabine hydrochloride and intermediate thereof with high selectivity
InactiveCN102153601AReduce corrosionLow costSugar derivativesSugar derivatives preparationFuranGemcitabine Hydrochloride
The invention discloses a method for synthesizing an important intermediate 2-deoxy-2, 2-difluorofuran ribose compound of gemcitabine hydrochloride and a method for synthesizing the gemcitabine hydrochloride by using the compound as a raw material under the catalysis of cytosine. The method has good stereo selectivity, a mild reaction condition, easily obtained raw materials, low pollution and high yield, and is suitable for industrialized production.
Owner:湖南欧亚药业有限公司
Gemcitabine hydrochloride or gemcitabine composition
InactiveCN101428035AExtended half-lifeSmall toxicityOrganic active ingredientsPowder deliveryGemcitabine HydrochlorideCurative effect
The invention belongs to the field of pharmacy, which discloses hydrochloric gemcitabine or gemcitabine composition. The composition comprises components in the following weight ratio: the ratio of hydrochloric gemcitabine or gemcitabine, polymer and protein is 0.1 to 20:0.00 to 50:0.00 to 20, wherein, the weight of polymer and protein is not zero at the same time. The composition can effectively prevent hydrochloric gemcitabine or gemcitabine from loosing the activity in vivo, and has the advantages of good stability, low toxicity and good efficacy.
Owner:常州安孚立德药业技术有限公司
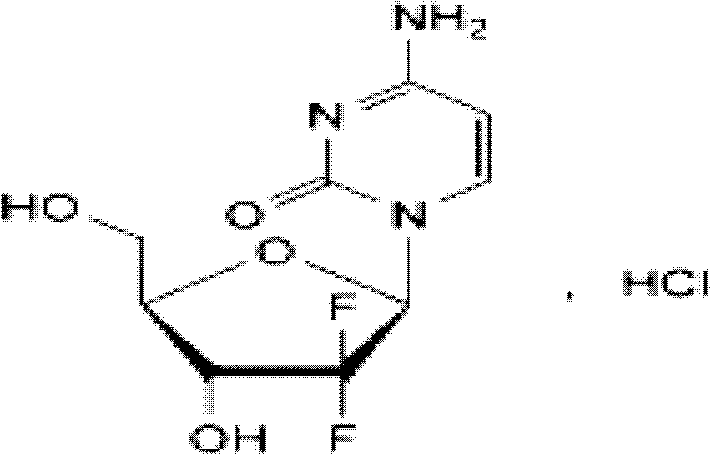
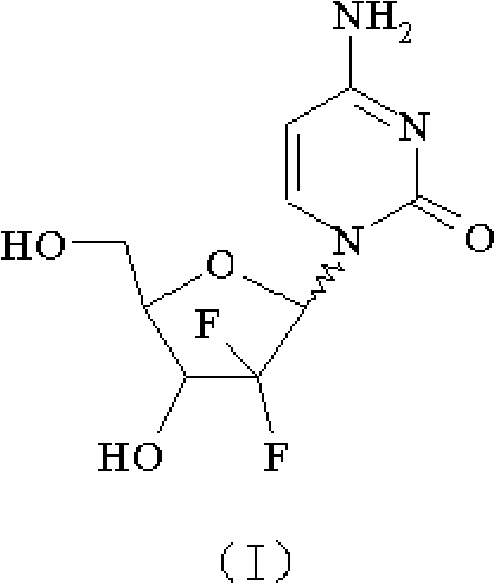
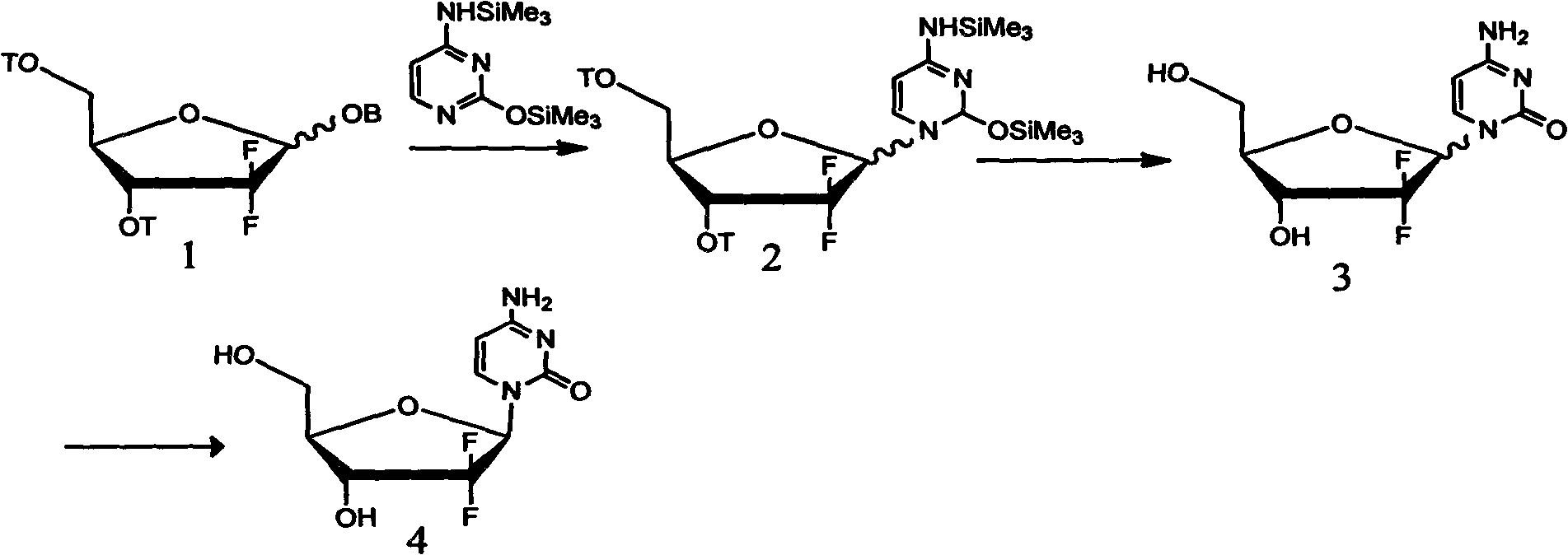
Method for preparing 2-deoxidized-2, 2-hydrochloric acid difluoro deoxycytidine
InactiveCN102617677ASimple and fast operationHigh yieldSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideChemistry
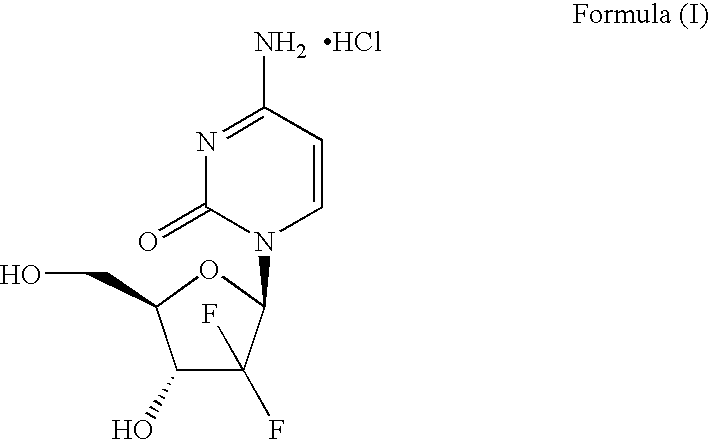
The invention relates to a 2-deoxidized-2, 2-hydrochloric acid difluoro deoxycytidine compound as shown in a formula (I), and discloses a method for preparing gemcitabine hydrochloride. The method is simple in process, high in yield and quite suitable for industrial production, the purity of a product is fine, and harsh reaction conditions are omitted.
Owner:JIANGSU HANSOH PHARMA CO LTD
Synthesis process of the industrial production of gemcitabine hydrochloride
ActiveCN101492482AImprove stabilityEasy to separate and purifySugar derivativesSugar derivatives preparationCytosineGemcitabine Hydrochloride
The invention relates to a synthesis process of industrial production of gemcitabine hydrochloride and pertains to the field of chemical product synthesis process. The invention takes 2, 3-oxygen-isopentylidene-D-glyceraldehyde as the raw materials and respectively uses TBDPSCl and benzoyl chloride to protect hydroxyl to produce an intermediate after addition, ring opening and cyclization. The intermediate is condensed with cytosine after reduction and methyl sulfonylation and then acquires gemcitabine hydrochloride after de-protection and salification. The invention has the advantage that TBDPSCl is adopted to protect one of hydroxyls, thus increasing the stability of the intermediate double benzoyl and facilitating the separation and purification of the product. The invention solves the problem in the prior gemcitabine hydrochloride production process that the intermediate double benzoyl protector is unstable and easily de-protected in alkalescence, thus affecting the product quality and yield. The total yield of the synthesis process is 17.9 percent and the purity is above 99.8 percent, thus being particularly applicable to the industrial production of gemcitabine hydrochloride.
Owner:NENTER & CO
Gemcitabine hydrochloride purifying method
ActiveCN102659884AHigh purityImprove product qualitySugar derivativesSugar derivatives preparationGemcitabine HydrochlorideActivated carbon
The invention relates to a gemcitabine hydrochloride purifying method. The gemcitabine hydrochloride purifying method comprises the following steps: a), dissolving a gemcitabine hydrochloride coarse product into water, regulating the pH of the solution to 8-9 by adding alkali, stirring and reacting, and extracting to obtain gemcitabine; b), dissolving the gemcitabine into an alcohol solution, with the concentration of 0.1-0.2g / mL, adding activated carbon, filtering, and collecting filtrate; c), separating the filtrate with a preparing chromatographic column, wherein a fluid phase used by the chromatographic column is acetone or acetonitrile and a hydrochloric acid solution, the volume ratio of acetone or acetonitrile to the hydrochloric acid solution is 30-50: 70-50, and a stationary phase filler is silica or aluminum oxide; collecting filtrate; and drying to obtain a gemcitabine hydrochloride refined product. Through acid-base reaction, activated carbon adsorption, and separation and purification of the preparing chromatographic column, the purity of the gemcitabine hydrochloride is improved, the quality of a prepared product is optimized and the safety of clinical medicine is ensured; and the method is simple in process and low in cost and is applicable to industrial production.
Owner:NANJING CHENGONG PHARM CO LTD
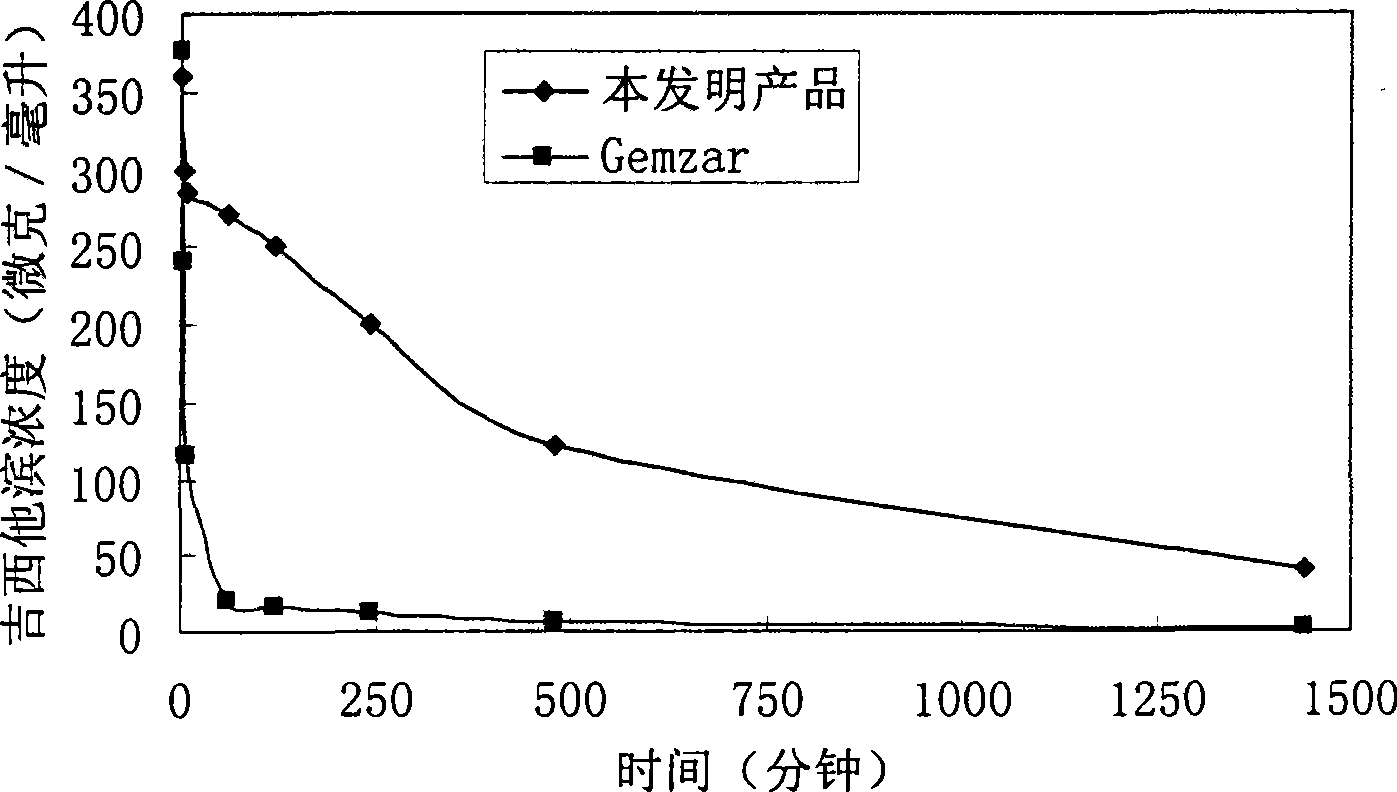
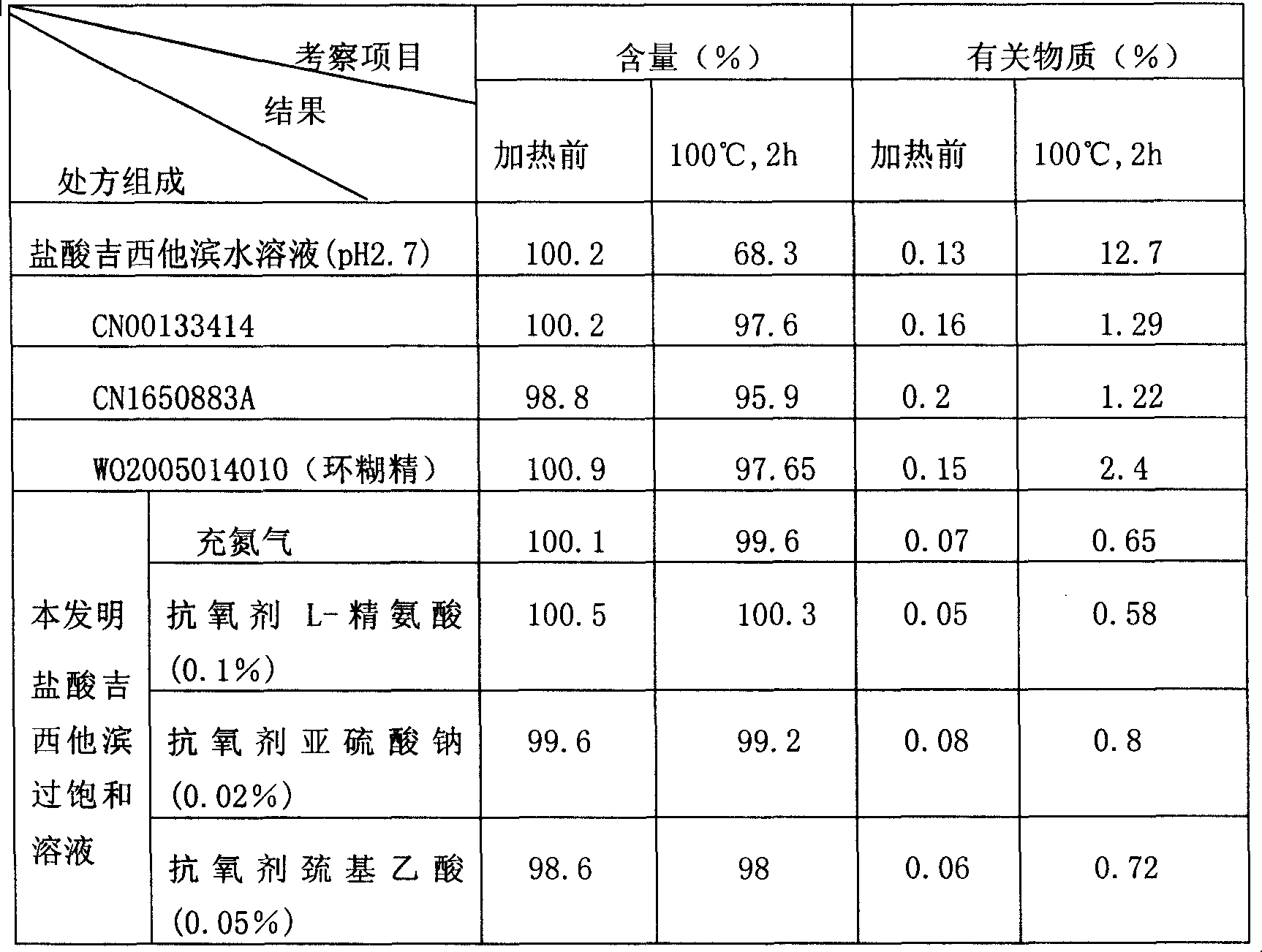
Stable supersaturated gemcitabine hydrochloride solution and its prepn process
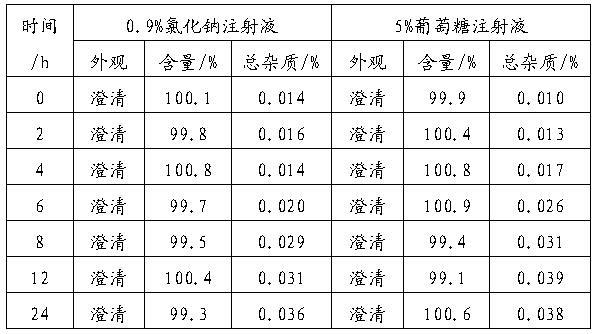
ActiveCN101088492AIncrease concentrationImprove stabilityOrganic active ingredientsInorganic non-active ingredientsGemcitabine HydrochlorideNitrogen
The present invention discloses stable supersaturated gemcitabine hydrochloride solution and its preparation process. Gemcitabine hydrochloride in certain amount is heated in pH 4-8 condition for being dissolved completely to obtain supersaturated gemcitabine hydrochloride solution of 45 g / l concentration, higher than the saturation concentration at 23 deg.c, and the supersaturated gemcitabine hydrochloride solution is packed in single dosage container while the solution is still hot. The supersaturated gemcitabine hydrochloride solution may be maintained at room temperature without separating out, and filling nitrogen and / or adding antioxidant can further raise the chemical stability. It may be used in preparing gemcitabine hydrochloride preparation for intravenous injection.
Owner:QILU PHARMA HAINAN
Gemcitabine hydrochloride lyophilized composition and preparation method thereof
ActiveCN102579372AReduce dosageReduce manufacturing costPowder deliveryOrganic active ingredientsGemcitabine HydrochlorideCyclodextrin Derivatives
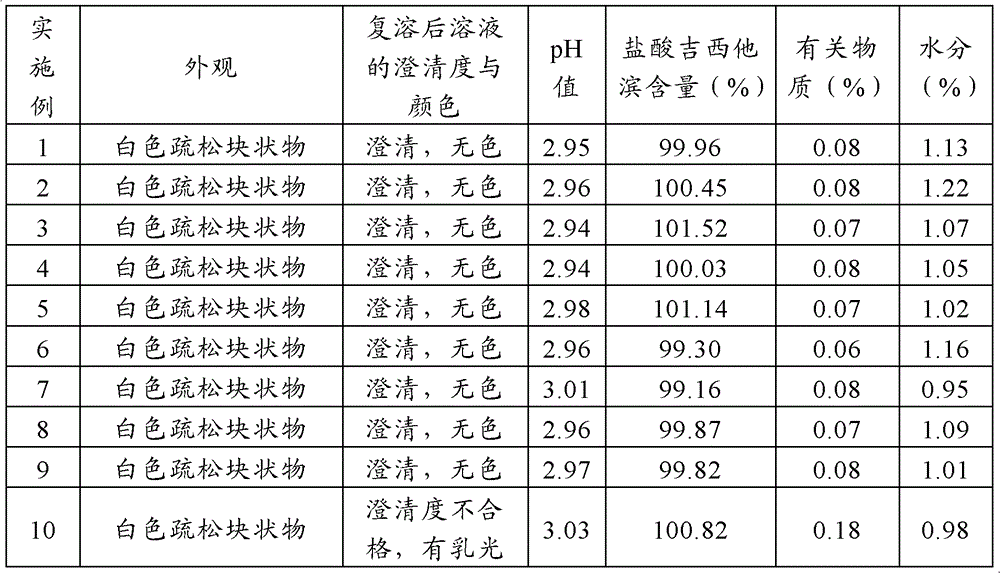
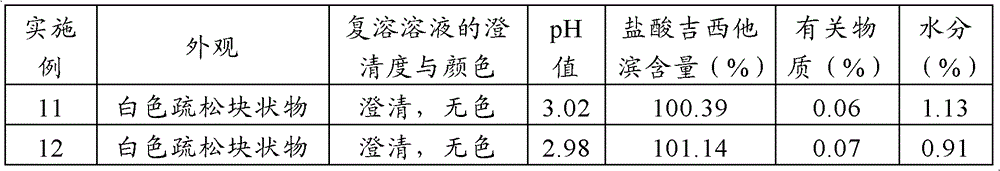
The invention relates to a gemcitabine hydrochloride lyophilized composition and a preparation method thereof, and aims to solve the technical problems of poor stability and long production cycle in the current gemcitabine hydrochloride lyophilized preparation. The gemcitabine hydrochloride lyophilized composition comprises the following components: gemcitabine hydrochloride, cyclodextrin or cyclodextrin derivative, lyophilized excipient and pH regulator, wherein the weight proportion of the components is 1:0.2-0.55:0.2-0.7:0.01-0.1. The gemcitabine hydrochloride lyophilized composition and the preparation method thereof have the benefits that a proper amount of solubilizer (cyclodextrin or cyclodextrin derivative) is added to the gemcitabine hydrochloride lyophilized composition, so thatthe solubility of gemcitabine hydrochloride can be enhanced, the preparation has good reconfigurability and stability, the production cycle of medicine is shortened, the energy consumption is reduced, the gemcitabine hydrochloride lyophilized composition is convenient to store and transport, and security is provided for clinical medication.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Process for Preparation of Gemcitabine Hydrochloride
A process for isolating β-anomer enriched Gemcitabine hydrochloride by converting Gemcitabine base into Gemcitabine hydrochloride followed by its purification using solvents from the series of water soluble ethers like 1,4-dioxane or Monoglyme.
Owner:ARCH PHARMALABS LTD
Gemcitabine hydrochloride lyophilized preparation
ActiveCN102302462BFix stability issuesGuarantee product qualityPowder deliveryLyophilised deliveryGemcitabine HydrochlorideAntioxidant
The present invention discloses a gemcitabine hydrochloride lyophilized preparation. The lyophilized preparation comprises, by weight, 20-70 parts of gemcitabine hydrochloride, 15-60 parts of a lyoprotectant and 0.005-0.5 parts of an antioxidant. Compared to the prior art, with the gemcitabine hydrochloride lyophilized preparation provided by the present invention, the problem of the stability ofthe gemcitabine hydrochloride is solved; the product quality is ensured; the risk of drug using is reduced; the preparation process is simple, the cost is low; and the preparation is suitable for theindustrial production, and has practical value.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD
Preparation method of gemcitabine hydrochloride
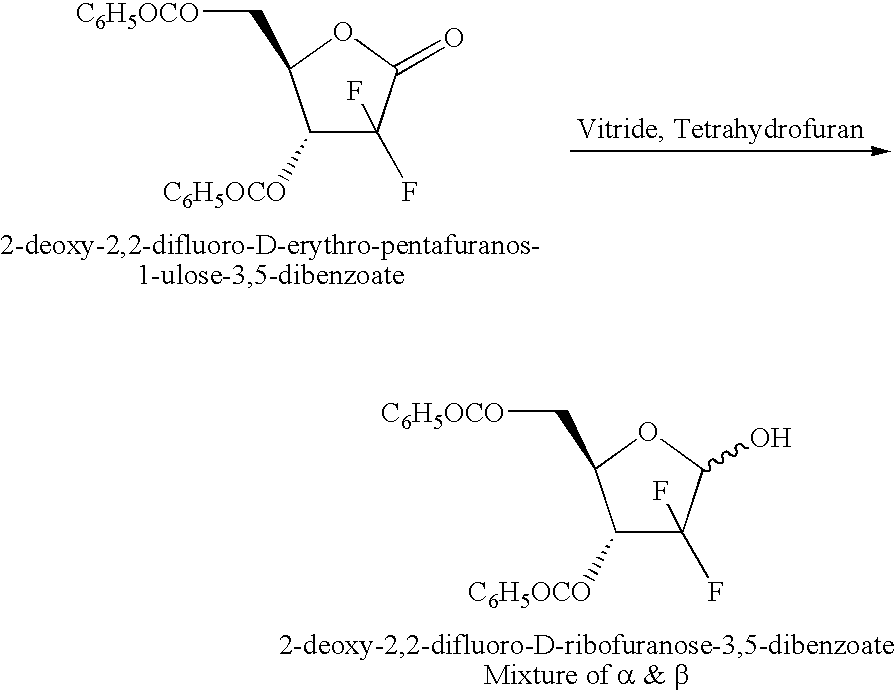
ActiveCN104109182AHigh purityHigh yieldSugar derivativesSugar derivatives preparationBenzoic acidFuran
The invention belongs to the drug synthesis field, and provides a novel method for synthesizing gemcitabine hydrochloride. In the method, 2-deoxy-2,2-difluoro-D-erythro-pentafuranous-one-3,5-dibenzoate (bifluoro-sugar for short) is taken as the primary raw material, and then the primary raw material is subjected to steps of reduction, methyl-sulfonylation, condensation, deprotection, salt forming, separation, and refinement so as to obtain the finished product. The method has the advantages of simple technology, high yield, high purity (which can reach 99.8% or more), and more suitability for industrial production.
Owner:NAT INST OF PHARMA R & D CO LTD
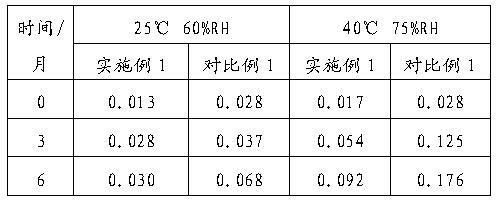
Gemcitabine hydrochloride lyophilized powder for injection and preparation method thereof
ActiveCN102697740AImprove stabilityImprove securityOrganic active ingredientsPowder deliveryGemcitabine HydrochlorideLight irradiation
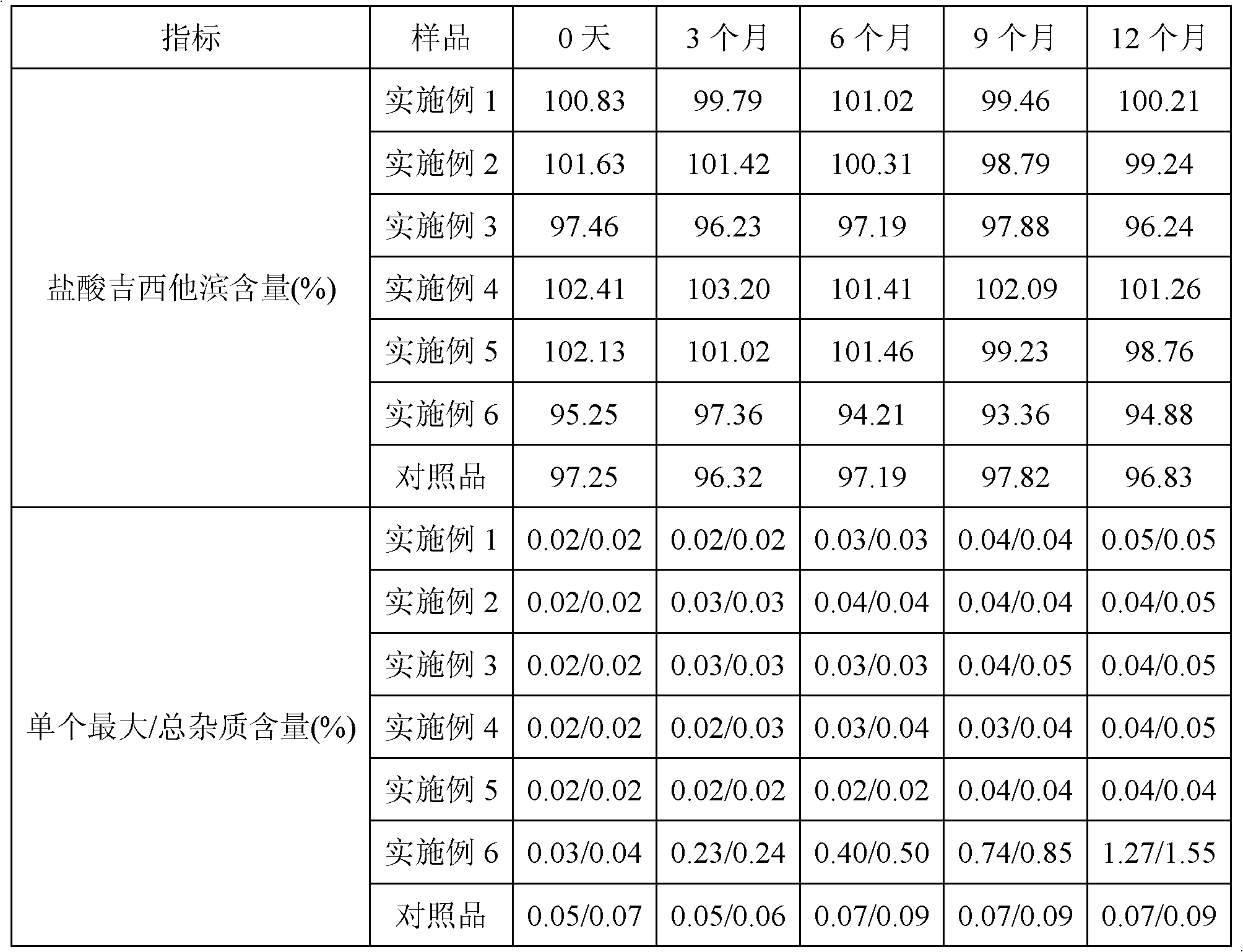
The invention discloses gemcitabine hydrochloride lyophilized powder for injection. The gemcitabine hydrochloride lyophilized powder consists of the following components in parts by mass: 100 parts of gemcitabine hydrochloride, 160-180 parts of lactose and 3-6 parts of sodium citrate. As proved by influencing factor testing investigation, each detection index the gemcitabine hydrochloride lyophilized powder for injection prepared by adopting a formula and a process provided by the invention does not change remarkably in a high-temperature, high-humidity and strong-light-irradiation environment, and high stability is achieved.
Owner:NANJING CHIA TAI TIANQING PHARMA
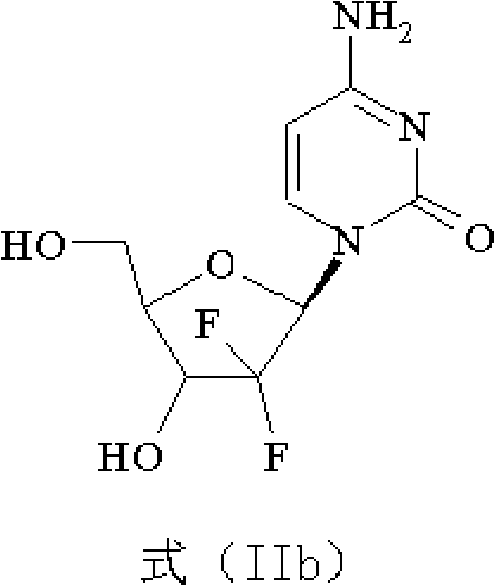
Intermediate and process for preparing of beta- anomer enriched 21-deoxy,21,21-difluoro-D-ribofuranosyl nucleosides
InactiveUS20060217547A1Reduce usageSimple and cost-effectiveEsterified saccharide compoundsBiocideGemcitabine HydrochlorideImide
The present invention provides a highly stereoselective, simple and economical glycosylation process for preparation of β-anomer enriched 21-deoxy-21,21-D-ribofuranosyl difluoronucleosides of formula (II), and physiologically acceptable slats thereof, in particular, the β-enriched anomer of gemcitabine hydrochloride of formula (IIb) in purity of >99% is provided through utilization of a novel trichloroacetimidate of formula (I).
Owner:FRESENIUS KABI ONCOLOGY LTD
Industrialized gemcitabine hydrochloride synthesis method
InactiveCN103232508AExtended crystallization timeHigh reaction yieldSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideSynthesis methods
The invention relates to an industrialized gemcitabine hydrochloride synthesis method, and belongs to the field of chemical product synthesis process. According to the invention, 2'-deoxy-2',2'-difluorocytidine-3',5'-dibenzoate is subjected to benzoyl protecting group removing in a methanol solution comprising ammonia water, and salt-formation is proceeded, such that 2'-deoxy-2',2'-difluorocytidine hydrochloride is formed; and crystallization and separation is carried out in an acetone-water mixed solvent, such that a gemcitabine hydrochloride pure product is obtained. According to the invention, a commonly used method for adding ammonia gas in the step 1 is changed into adding concentrated ammonia water; and a freezing crystallization overnight operation is adopted in subsequent purification process, such that process operability is increased, and reaction yield is improved. The synthesized process product has high refined rate. The purity of the product is maintained above 99.8%.
Owner:湖北一半天制药有限公司
Method for preparing gemcitabine hydrochloride
ActiveCN102617678BFew stepsSimple and fast operationSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideProtecting group
The invention relates to a compound in a formula (I), and discloses a method for preparing gemcitabine hydrochloride. The method includes preparing an intermediate type (III) compound by means of realizing Reformatsky reaction, removing a protecting group and realizing lactonization and double benzoylation; obtaining a compound in a formula (VI) via reduction, methyl sulfonylation and condensation; and finally removing a protecting group, saltifying and realizing crystallization to obtain a final product. The method is simple in process, high in yield and quite suitable for industrial production, the purity of the product is fine, and harsh reaction conditions are omitted.
Owner:JIANGSU HANSOH PHARMA CO LTD
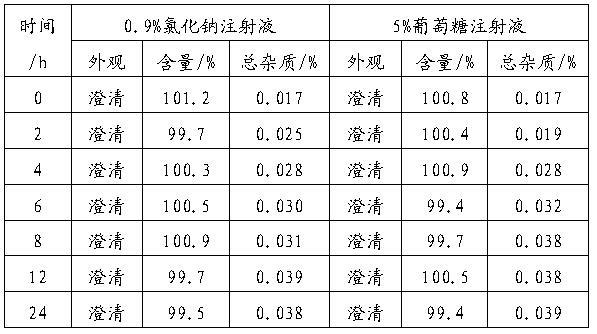
Gemcitabine hydrochloride injection and preparation method thereof
ActiveCN102949337AImprove stabilityEasy to useOrganic active ingredientsPharmaceutical delivery mechanismGemcitabine InjectionGemcitabine Hydrochloride
The invention relates to gemcitabine hydrochloride injection and a preparation method of the gemcitabine hydrochloride injection. Specifically, 1ml of gemcitabine hydrochloride injection comprises the following components of 30-70mg of gemcitabine hydrochloride, 1-5mg of NaCl, 10-50mg of hydroxypropyl-beta-cyclodextrin and 1-10mg of pH (Potential of Hydrogen) regulator. The gemcitabine hydrochloride injection provided by the invention is better in stability, simple in preparation and convenient in use, and can reduce the pollution ways of drugs.
Owner:JIANGSU HANSOH PHARMA CO LTD
Preparation method of a gemcitabine hydrochloride freeze-dried powder injection
ActiveCN106265545AReduce peelingInefficient freeze-dryingPowder deliveryOrganic active ingredientsPressure riseGemcitabine Hydrochloride
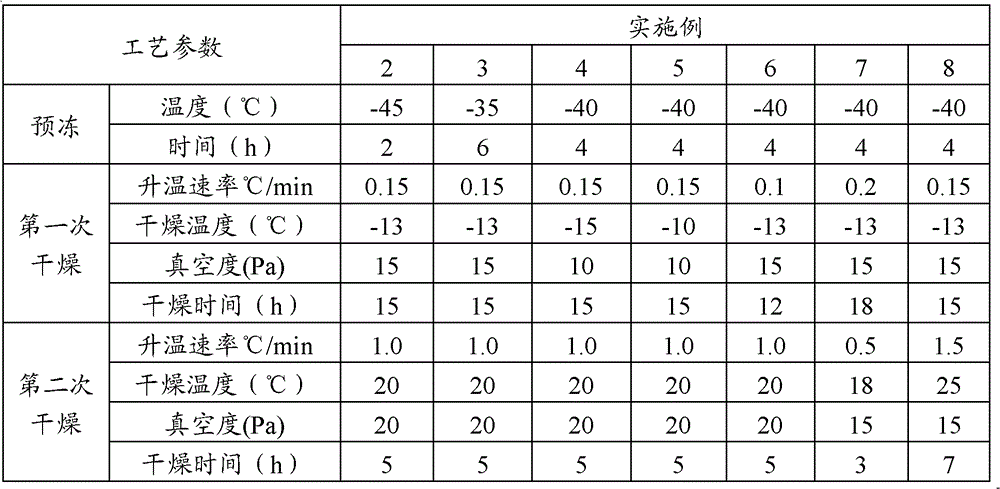
The invention discloses preparation method of a gemcitabine hydrochloride freeze-dried powder injection, which comprises the following steps of: Controlling the inlet temperature of the conducting oil within 20oC+-5 oC and putting the gemcitabine hydrochloride solution into the freeze-drying box; Conductng pre-freeze; Reducing the conduction oil inlet temperature at a full speed to - 2oC and keeping the temperature for 2 hours. Reducing the conduction oil inlet temperature at a full speed to -45oC and keeping the temperature for 4 hours. After the pre-freeze is completed, confirming that the condenser temperature has reduced to be lower than 45oC. Then pumping ultimate vacuum. When the vacuum degree reaches 10Pa , reducing the conduction oil inlet temperature from -45 oC to 20 oC and opening the vacuum control. Adjusting the vacuum control range of the front box to 35+-5Pa and keeping the temperature for 4 hours till the water trace disappears. Heating up the plate at a full speed to 30oC and maintaining it for 4-6 hours. Then stopping the vacuum control. Keeping maintaining the temperature for 1 hour. Testing that the pressure rise range does not exceed 2Pa within 15 minutes. Taking the gemcitabine hydrochloride solution out of the box. This preparation method can reduce the cost of freeze-drying, improve production efficiency and increase the production capacity to the utmost.
Owner:NANJING CHIA TAI TIANQING PHARMA
Method for preparing gemcitabine hydrochloride lyophilized powder
ActiveCN102793677AAvoid dehydrochlorinatioAvoid efficiencyOrganic active ingredientsPowder deliveryGemcitabine HydrochlorideLow vacuum
The invention relates to the field of pharmaceutic preparations and discloses a method for preparing gemcitabine hydrochloride lyophilized powder. According to the method for preparing the gemcitabine hydrochloride lyophilized powder, a primary pre-freezing process and a stage drying process are used for lyophilization; appropriate vacuum degrees and temperature are matched at all drying stages to avoid the problem that hydrochloric acid is removed from gemcitabine hydrochloride under high vacuum degree, and drying efficiency is low due to low vacuum degree; meanwhile, temperature is slowly raised at different speed at different drying stages, and the problem that the gemcitabine hydrochloride is deacidified due to too high heating speed can be solved. Compared with the prior art, the method for preparing the gemcitabine hydrochloride lyophilized powder has the advantages that the method is easy to operate, lyophilization time is short, and drying temperature is low; the prepared gemcitabine hydrochloride lyophilized powder product is high in outer structural quality and redissolution performance; a redissolution solution is clear and transparent, and opalescence is avoided; relative substances are low in content, and the prepared gemcitabine hydrochloride lyophilized powder is safe and reliable in quality; and the method is suitable for preparing the gemcitabine hydrochloridelyophilized powder and can be widely applied to large-scale production of the gemcitabine hydrochloride lyophilized powder.
Owner:CHONGQING LUMMY PHARMA
Gemcitabine hydrochloride liposome injection
InactiveCN102716089BWon't breakHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsSolubilityRetention time
The invention discloses a gemcitabine hydrochloride liposome injection and a preparation method thereof. The gemcitabine hydrochloride liposome injection is prepared from the following components in specific percentage by weight: gemcitabine hydrochloride, cholesterol, egg yolk phosphatidylinositol, phosphatidylcholine, tween 80, trehalose, mannitol and polyvinylpyrrolidone. The liposome injection is high in stability and cannot be cracked due to fusion, ice crystal and the like during freezing; and after long-term storage, liposome is still high in package rate. According to the gemcitabine hydrochloride liposome injection, the solubility of gemcitabine hydrochloride is improved; the quality of a product is improved; the toxic and side effects are reduced; the retention time of medicines during general circulation is prolonged; the biological utilization rate of the medicine is increased; the treating effect is obviously enhanced; the preparation method is simple; and the gemcitabine hydrochloride liposome injection is suitable for industrial large-scale production.
Owner:灵康药业集团股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com