Gemcitabine hydrochloride purifying method
A technology of gemcitabine hydrochloride and a purification method, which is applied in the field of purification of antitumor drug gemcitabine hydrochloride, can solve problems such as difficulty in purifying gemcitabine hydrochloride crude product, and achieve the effects of optimizing product quality, improving purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
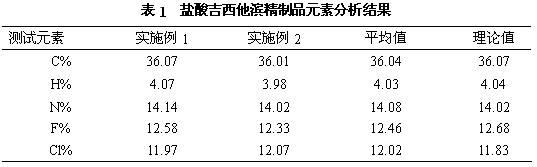
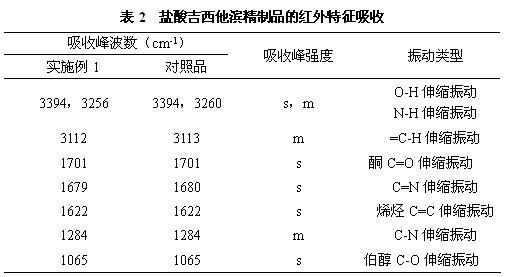
[0020] a) Dissolve 100g (0.33mol) of crude gemcitabine hydrochloride in 1000mL of water, slowly add saturated ammonia water, stir the reaction until the pH is 8.5, and produce gemcitabine precipitate, filter it with suction, and dry it in vacuum at 45°C to obtain 82g (0.31mol) of gemcitabine. Rate 94%;
[0021] b) Dissolve 82g of gemcitabine obtained in the previous step in 410mL of pure methanol, add 4.1g of activated carbon, stir at 30°C for 0.5 hours, filter for decarburization, and collect the filtrate;
[0022] c) The above-mentioned filtrate is separated and purified by a chromatographic column to obtain a gemcitabine hydrochloride refined product, wherein the mobile phase used in the chromatographic column is acetone and an aqueous hydrochloric acid solution with a pH of 2 to 3, the volume ratio of acetone to aqueous hydrochloric acid is 30:70, and the stationary phase The filler is silica gel, the flow rate is 4.6mL / min, and the column temperature is 20°C. The filtrat...
Embodiment 2
[0024] a) Dissolve 100g (0.33mol) of crude gemcitabine hydrochloride in 1000ml of water, slowly add saturated sodium hydroxide solution, stir the reaction until the pH is 9.0, and produce gemcitabine precipitates, filter through suction, and dry under vacuum at 45°C to obtain 84g (0.32mol) of gemcitabine ), yield 97%;
[0025] b) Dissolve 84g of gemcitabine obtained in the previous step in 500mL of ethanol with a purity of 99.5%, add 4.2g of activated carbon, stir at 25°C for 1.0 hour, filter for decarburization, and collect the filtrate;
[0026] c) Separating and purifying the above filtrate with a chromatographic column to obtain refined gemcitabine hydrochloride, wherein the mobile phase used in the chromatographic column is acetonitrile and aqueous hydrochloric acid with a pH of 2 to 3, the volume ratio of acetonitrile to aqueous hydrochloric acid is 50:50, and the stationary phase is The filler is aluminum oxide, the flow rate is 6mL / min, and the column temperature is 25...
Embodiment 3
[0028] a) Dissolve 100g (0.33mol) of crude gemcitabine hydrochloride in 1000mL of water, slowly add sodium methoxide, stir the reaction until the pH is 8, produce gemcitabine precipitate, filter by suction, and dry under vacuum at 45°C to obtain 82g (0.31mol) of gemcitabine. Rate 94%;
[0029] b) Dissolve 82g of gemcitabine obtained in the previous step in 410mL of pure isopropanol, add 4.1g of activated carbon, stir at 20°C for 1.5 hours, filter for decarburization, and collect the filtrate;
[0030] c) The above-mentioned filtrate is separated and purified by a chromatographic column to obtain a gemcitabine hydrochloride refined product, wherein the mobile phase used in the chromatographic column is acetonitrile and an aqueous hydrochloric acid solution with a pH of 2 to 3, the volume ratio of acetonitrile and aqueous hydrochloric acid is 45:55, and the stationary phase The filler is silica gel, the flow rate is 8 mL / min, and the column temperature is 30°C. The filtrate was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com