Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "D-mannitol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stabilized human monoclonal antibody preparation
InactiveUS6165467ASufficient stabilizationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsD-mannitolSolution state
A stabilized human monoclonal antibody preparation containing 1 to 20 mg of D-mannitol per 1 mg of a human monoclonal antibody. This preparation is excellent in stability in a solution state, a freeze drying state and a freezing state, particularly stability against aggregation and precipitation of the human monoclonal antibody at the time of redissolution after freeze drying.
Owner:HAGIWARA HIDEAKI
Tablets quickly disintegrating in oral cavity
InactiveUS20050147666A1Disintegrates quicklyLoses its shapeMetabolism disorderInorganic non-active ingredientsCompression moldingD-mannitol
According to the present invention, provided are an intraorally rapidly disintegrable tablet which comprises D-mannitol and a disintegrator in addition to fine granules prepared by granulating a mixture of a water-soluble pharmacologically active ingredient and an adsorbent; a process for producing an intraorally rapidly disintegrable tablet which comprises mixing D-mannitol and a disintegrator with fine granules prepared by granulating a mixture of a water-soluble pharmacologically active ingredient and an adsorbent to yield a material for compression molding, and subjecting the material to compression molding; and an intraorally rapidly disintegrable tablet which is prepared by mixing D-mannitol and a disintegrator with fine granules prepared by granulating a mixture of a water-soluble pharmacologically active ingredient and an adsorbent to yield a material for compression molding, subjecting the material to compression molding.
Owner:KYOWA HAKKO KIRIN CO LTD
Plukenetia volubilis linneo health-care nougat
ActiveCN103039687APrevent oxidationPrevent rancidityConfectionerySweetmeatsAdditive ingredientNutrient
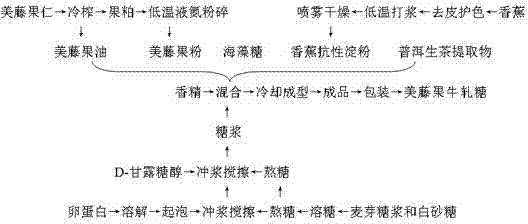
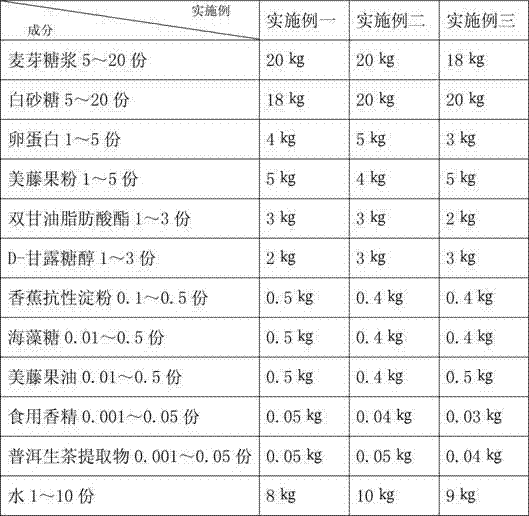
The invention discloses plukenetia volubilis linneo health-care nougat. The nougat comprises maltose syrup, white granulated sugar, ovalbumin, plukenetia volubilis linneo powder, diglycerol fatty acid ester, D-mannitol, banana resistant starch, trehalose, plukenetia volubilis linneo oil, edible essence, Pu-Er raw tea extract and water. The nougat is prepared by the following steps of: extruding plukenetia volubilis linneo kernels by a cold press to prepare the plukenetia volubilis linneo oil, crushing plukenetia volubilis linneo dregs into the plukenetia volubilis linneo powder by a low-temperature liquid nitrogen crushing technology, preparing the banana resistant starch by a spray drying technology, and mixing and boiling the nutrients including the trehalose, the plukenetia volubilis linneo oil, the plukenetia volubilis linneo powder, the banana resistant starch and the Pu-Er raw tea extract and other accessories. The nougat is stable in preservation, delicate in mouthfeel during eating, moderate in toughness, non-sticky, high in sweetness and low in calorie, has less possibility of softening, has the effects of clearing free radicals in body, improving the immunity of organism, regulating the level of blood sugar and the like, can prevent human osteoporosis and promote proliferation of human bifidobacteria, and is beneficial to gastrointestinal health.
Owner:普洱联众生物资源开发有限公司
Fast water-dispersible domperidone tablets
InactiveUS20060051414A1Improves Structural IntegrityPleasant tasteBiocideDispersion deliverySolubilityWater dispersible
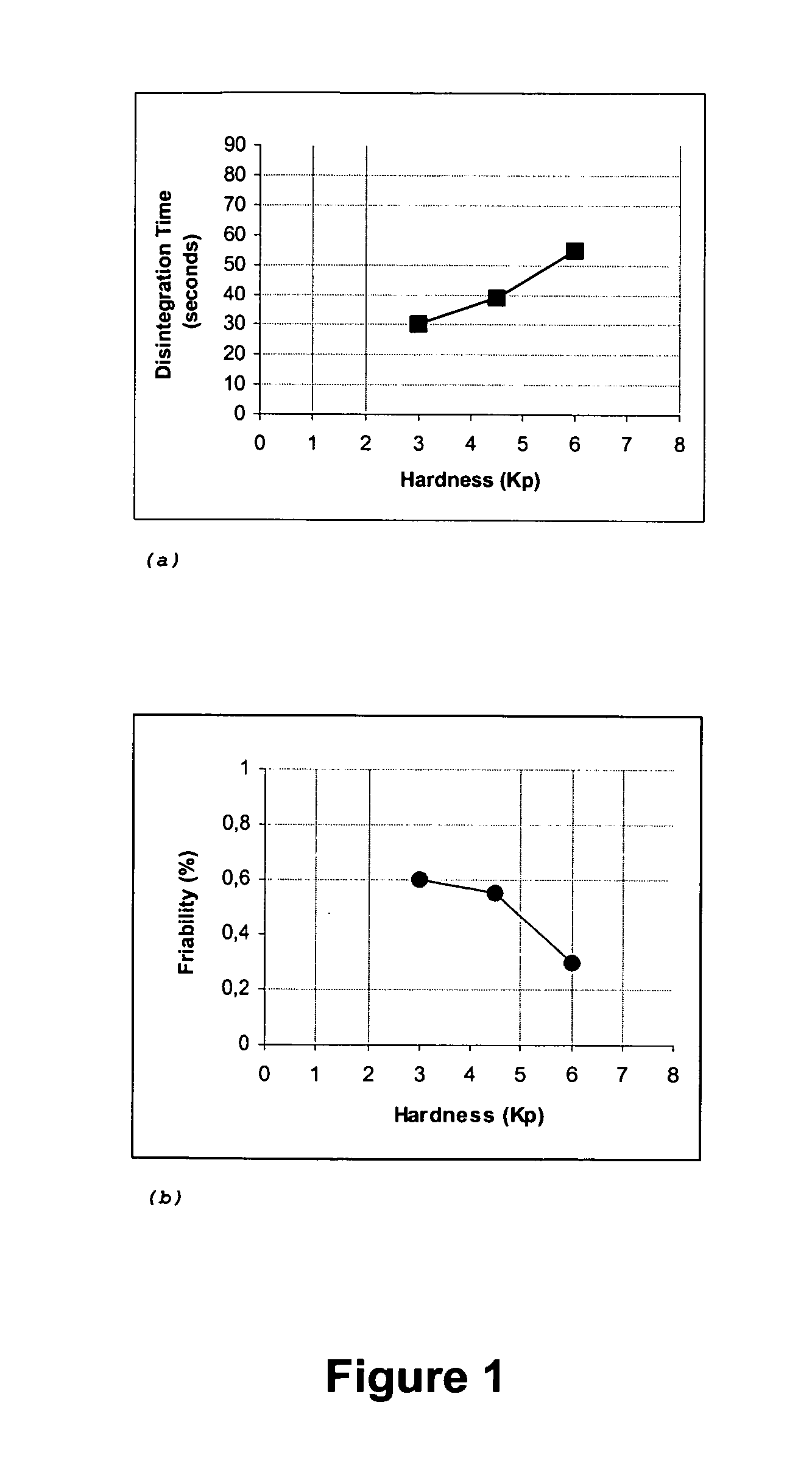
The present invention relates to fast water-dispersible tablets containing domperidone for oral administration. The formulations comprise domperidone or pharmaceutically acceptable salts thereof, about 60-80% of a “auxiliary” granulate (w / w), and about 10-30% of microcrystalline cellulose (w / w), expressed in relation to the total weight of the tablets, a sweetener, a flavouring agent and a lubricant. The “auxiliary” granulate is obtained by wet granulation of D-mannitol and maize starch gum in a high shear granulator, it facilitates the flowability and the compressibility of the mixture and, because of its high solubility in water, contributes to the fast dispersion of the tablet. The formulations have an enhanced structural integrity, for instance having a friability lower than 1.0% and hardness values between 3 and 6 Kp, and are able to disperse in water within 3 minutes, preferably within 2 minutes and most preferably within 1 minute, to provide a dispersion that passes through a 710 μm diameter mesh size sieve and presents a pleasant taste and the absence of perceptible granules in the mouth. This invention also refers to the process for the preparation of said pharmaceutical preparations.
Owner:LAB MEDINFAR PROD FARMS
Phase-change microcapsule composition material and preparation method thereof
InactiveCN104877642AIncrease coverageImprove sealingHeat-exchange elementsMicroballoon preparationD-mannitolPolymer science
Owner:NANJING UNIV OF TECH
Compound nutritious food capable of improving whole digestive tract
InactiveCN105685970APromote colonizationPromote proliferationFood ingredientsDiseaseIsomaltooligosaccharide
The invention provides compound nutritious food capable of improving the whole digestive tract. The compound nutritious food capable of improving the whole digestive tract is prepared from the following raw materials in parts by weight: 5-15 parts of xylooligosaccharide, 2-20 parts of fructo-oligosaccharide, 3-20 parts of isomaltooligosaccharide, 3-30 parts of stachyose, 5-20 parts of inulin, 6-20 parts of concentrated whey protein, 1-15 parts of chitosan oligosaccharide, 0.5-10 parts of saccharomyces boulardii powder and 0.5-15 parts of lactobacillus plantarum powder; and according to dosage form requirements, the compound nutritious food capable of improving the whole digestive tract also comprises the following auxiliary materials in parts by weight: 10-80 parts of maltodextrin, 5-20 parts of D-mannitol, 0.5-1.5 parts of guar gum, 0.5-1 part of citric acid and 0.3-1 part of silicon dioxide, wherein the preservation number of lactobacillus plantarum is CGMCC NO.11763. The compound nutritious food provided by the invention has the advantages that high-performance dominant probiotics and multiple nutrient substances are comprehensively utilized and combined, the whole digestive tract is improved, microecological balance in the human body is kept by regulating gastrointestinal florae, and further food digestion and nutrition absorption are promoted, growth of harmful bacteria and toxin in the digestive tract is inhibited and diseases of the digestive tract are prevented, so that comprehensive improvement of health condition of the digestive tract and significant enhancement of immunity of the organism are realized.
Owner:北京东方兴企食品工业技术有限公司
Sugar-free products with improved characteristics
InactiveUS6855361B2Improve solubilityImprove powerOrganic active ingredientsConfectioneryD-SorbitolD-Glucopyranose
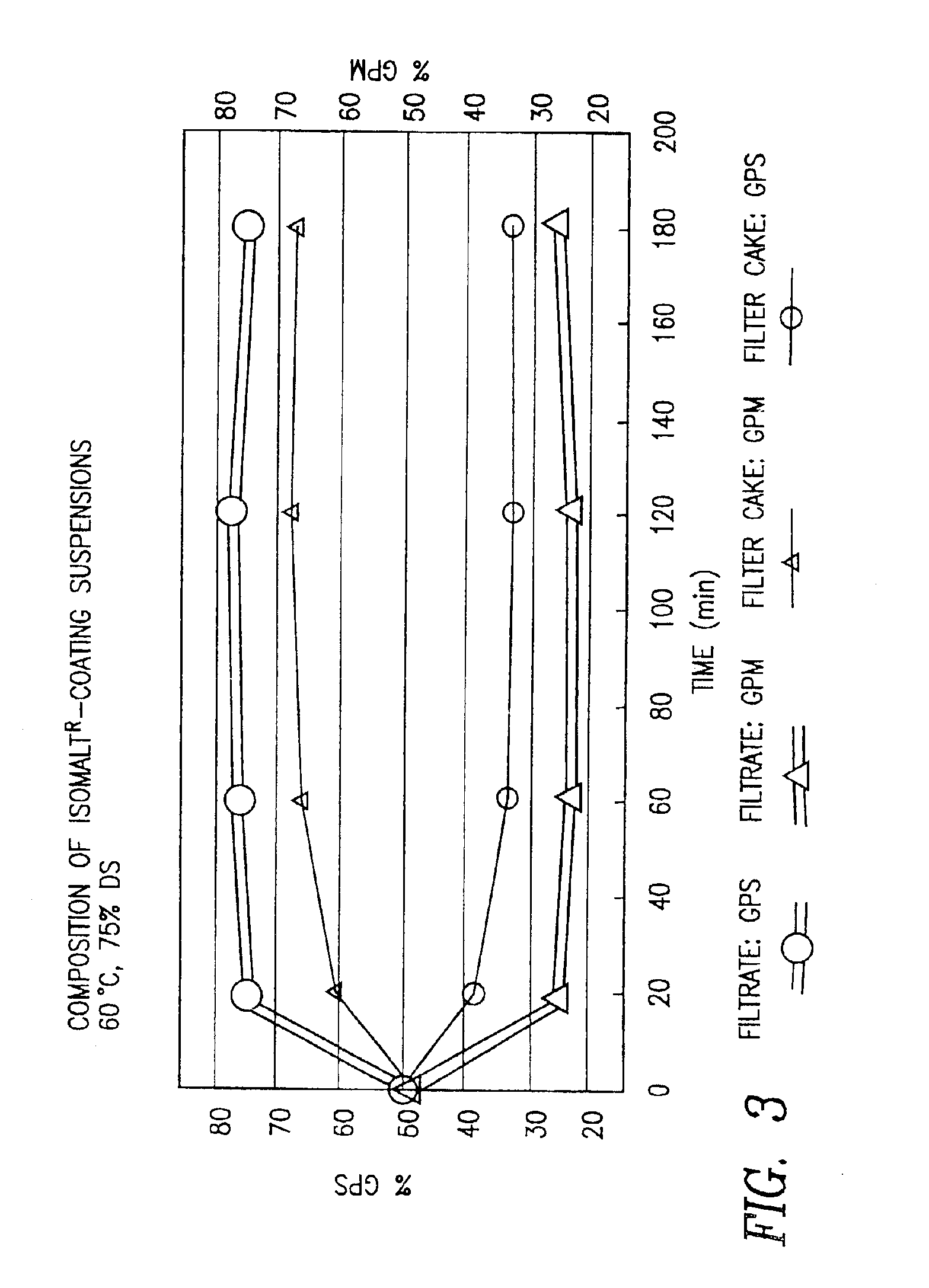
The invention concerns improved sugar-free products, their production and use, in particular, coated products, their production and use. The products are characterized by their content in enriched mixtures of 1-O-α-D-glucopyranosyl-D-mannitol (1,1-GPM) and 6-O-α-D-glucopyranosyl-D-sorbitol (1,6-GPS).
Owner:SUDZUCKER AG MANNHEIM OCHSENFURT
Polyesters comprising 2,5-furandicarboxylate and saturated diol units having a high glass transition temperature
A polyester including at least one furandicarboxylate unit, at least one saturated, linear or branched, diol unit including from 2 to 10 carbon atoms, and at least one bicyclic diol unit, the said bicyclic diol being chosen from: isosorbide, isoidide, isomannide, 2,3:4,5-di-O-methylene-galactitol, and 2,4:3,5-di-O-methylene-D-mannitol. The glass transition temperature of the polyester is greater than or equal to 90° C. Processes for preparing this polyester are also described.
Owner:FURANIX TECH BV +1
Cordyceps liquor and method of producing the same
The invention discloses a worm grass wine and preparation process, wherein, the worm grass wine belongs to the health medicine wine. The preparation process includes that: the worm grasses are bred in various wine containers through artificial fungus inoculation and moss silk reproduction by taking advantage of artificial broth bases, and then are added with wine to produce the worm grass wine. The worm grass wine includes 21 trace elements, a plurality of vitamins, cordycepin, cordycepicacid (d-mannitol), SOD enzyme, nucleic acid derivatives, uracil, adenine, inosine and ergo-sterol, and the content of the protein is 1.6 times that of the worm grasses. The worm grass wine can keep the worm grasses fresh, ensures the nourishment is not destructed and lost; moreover, the formation of the worm glass wine is beautiful and vivid like living creatures in the water due to the fact that the worm glass wine is bred in wine bottles through artificial broth bases with high values of medicine, appreciation, collection and so on.
Owner:张笑容
Procyanidin capsules and process for manufacturing same
InactiveCN101642445AEasy to eatPromote absorptionOrganic active ingredientsCapsule deliveryWrinkle skinMagnesium stearate
The invention discloses a procyanidin capsules and a process for manufacturing the same. The capsules are manufactured by taking procyanidin and astragalus polysaccharide as main materials and caseinphospho peptides or D-mannitol, magnesium stearate and gelatin as auxiliary materials. The capsule has the advantages of being conveniently taken, avoiding absorbing moisture and having functions of immunoregulation, anti-ageing and radiation hardening, protecting cardiovascular health by removing free radicals in vivo, and preventing crosslinking of skin collagens, inhibiting pigmentation, dispelling wrinkles and whitening the skin, keeping skin elasticity and delaying ageing.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Sugar-free pharmaceutical products
The invention concerns improved sugar-free products, their production and use, in particular, coated products, their production and use. The products are characterized by their content in enriched mixtures of 1-O-α-D-glucopyranosyl-D-mannitol (1,1-GPM) and 6-O-α-D-glucopyranosyl-D-sorbitol (1,6-GPS).
Owner:SUDZUCKER AG MANNHEIM OCHSENFURT
Pharmaceutical health preparation for improving sleeping and preparation method of pharmaceutical health preparation
InactiveCN108543020AImprove sleepingPromote absorptionNervous disorderHydroxy compound active ingredientsSide effectTaurine
The invention provides a pharmaceutical composition for improving sleeping. The pharmaceutical composition contains the following raw material components: nepenthe, inulin, D-mannitol, lotus seeds, roses, spina date seeds, gastrodia elata, American ginseng, lucid ganoderma, stigma croci, gamma-aminobutyric acid, phosphatidylserine, taurine andcalcium glycinate, and the raw materials generate a mutual synergistic effect and a common effect at a specific proportion, so that the sleeping improvement effect is achieved; and ultrafine powder is easily absorbed by a human body, so that the bioavailability is high. Clinical statistical materials show that a pharmaceutical preparation provided by the invention has a remarkable effect on the improvement of sleeping, has no toxic or side effect, andis very high in safety, simple in constitution, relatively low in cost and convenient and fast to use.
Owner:朱永红
Method for preparing gemcitabine hydrochloride
The invention discloses the compounding method for hydrochloric acid Gemcitabine. It uses D-mannitol as raw material and takes the processes of hydroxyl protection, oxidation, and addition of reformatsky, hydroxy benzoylation, hydrolysis, hydroxyl sulfonylation, ring closure, carbonyl reduction, hydroxyl sulfonylation, condensation, hydrolysis and crystallization to gain hydrochloric acid Gemcitabine. It has the advantages of high yield, simple operation, and is suitable to industrial producing.
Owner:HUBEI YITAI PHARMA
Tablet candy comprising endothelium corneum gigeriae galli, hawthorns and malts and preparation method of same
InactiveCN106234738ASweet and sour tasteGood for stomach and digestionConfectionerySweetmeatsD-mannitolCrataegus nigra
A tablet candy comprising endothelium corneum gigeriae galli, hawthorns and malts and a preparation method of same. The tablet candy is prepared from, by weight, 1-5 parts of an extract of endothelium corneum gigeriae galli, 5-15 parts of an extract of hawthorns, 5-15 parts of an extract of malt, 10-20 parts of an extract of Chinese yam, 2-10 parts of an extract of jujube, 1-5 parts of an extract of pericarpium citri reticulatae, 15-25 parts of maltodextrin, and 40-80 parts of D-mannitol. The tablet candy is prepared from various natural plant extracts, has a refreshing taste, and has the effects of fortifying stomach to promote digestion and treating deficiency of the spleen and stomach and dyspepsia.
Owner:合肥远志医药科技开发有限公司
Oral disintegrating tablet
InactiveUS20100098756A1Good effectAppropriate strengthBiocidePharmaceutical non-active ingredientsCrospovidonesD-mannitol
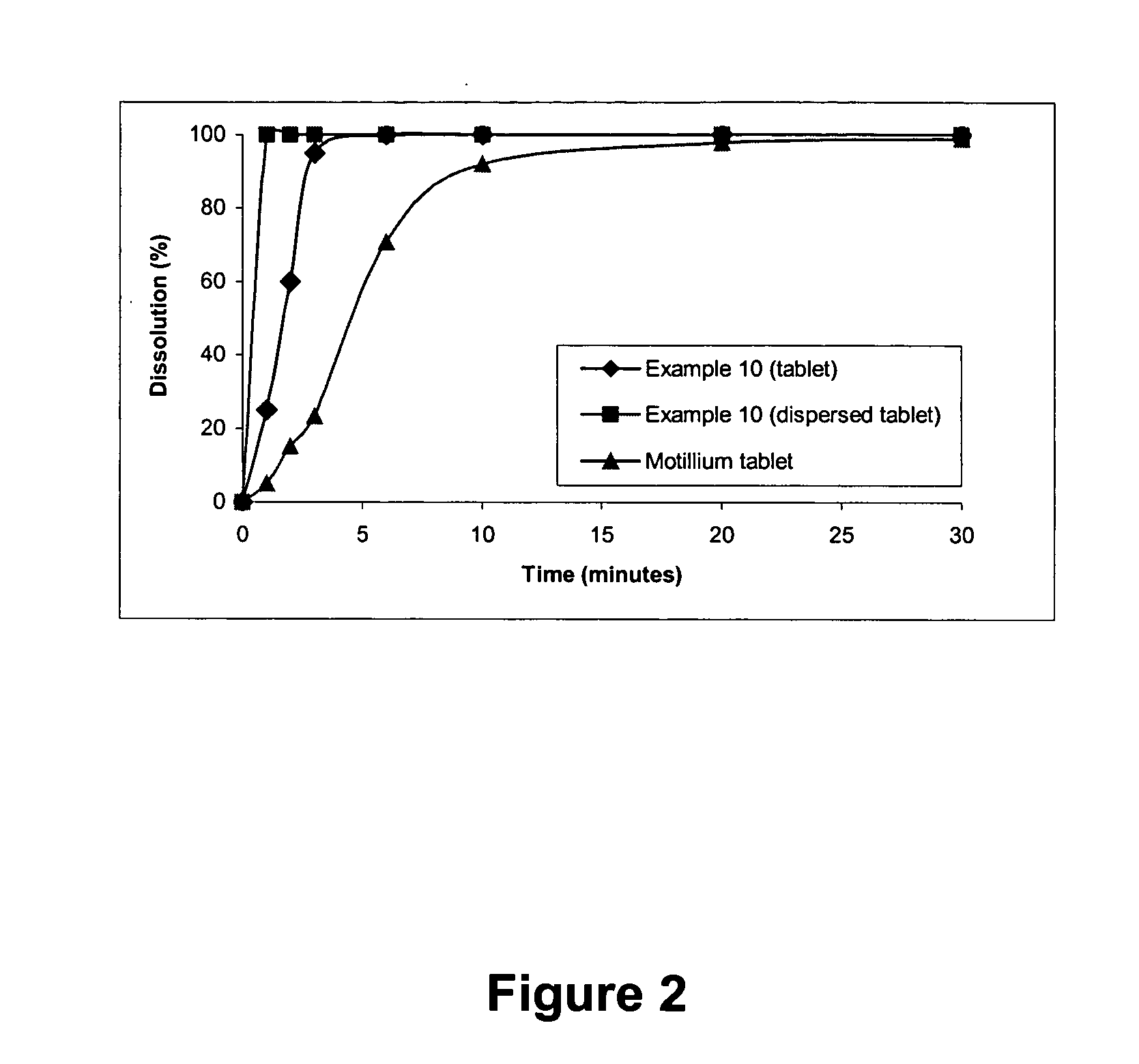
An oral disintegrating tablet containing (1) D-mannitol, (2) an active ingredient, (3) one or more disintegrating agents selected from the group consisting of crospovidone and carmellose, and (4) one or more lubricants selected from the group consisting of sodium stearyl fumarate and sucrose esters of fatty acids. The oral disintegrating tablet of the present invention has some excellent properties of (1) allowing easy production in a common facility without necessitating a specialized pharmaceutical technique, (2) having an appropriate strength that does not breakdown in the process of distribution, (3) having a fast disintegrating ability in the oral cavity, and (4) also having excellent ingestion feel such as greatly reduced bitterness or gritty feel; therefore, the tablet can be suitably used as a dosage form that is suitable for aged individuals, children, and seriously ill patients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Pressed candy and making method thereof
InactiveCN107279420AImprove immunityPractical and convenientConfectionerySweetmeatsSide effectLiver and kidney
The invention discloses pressed candy and a making method thereof, and belongs to the technical field of health-care food. The pressed candy is made from ginseng, cinnamomi cortex, semen euryales, rhizoma polygonati, male flowers of eucommia ulmoides, oysters, poria cocos, rape pollen, endothelium corneum gigeriae galli, dandelions, common yam rhizomes, corn starch, dextrin, D-mannitol, magnesium stearate, phytosterol and xylitol according to the certain weight proportion. Accordingly, the formula is scientific and reasonable, effects of all raw materials cooperate with one another, mutual promotion is achieved, and the product has the multiple effects of clearing heat, promoting diuresis, removing toxicity, beautifying skin, tonifying the liver and kidney and improving organism immunity. The pressed candy is practical, convenient, good in taste and free of toxic and side effects.
Owner:李钦超
Preparation method for 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribono-gamma-lactone
ActiveCN106083773ALow priceReduce manufacturing costOrganic chemistryBulk chemical productionPhenacylPotassium fluoride
The invention discloses a preparation method for 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribono-gamma-lactone. The preparation method comprises the following steps: subjecting a starting raw material D-mannitol to acetone protection and sodium periodate oxidation; then subjecting the treated D-mannitol and a self-made ylide reagent to the Witting reaction; then carrying out selective oxidation by using an aqueous sodium permanganate solution; then successively carrying out sulfonylation, fluorination with potassium fluoride, and ring closing with a concentrated protective group protective group; protecting the hydroxyl group by using benzoyl chloride; and then carrying out purification so as to obtain the final product 3,5-dibenzoyl-2-deoxy-2-fluoro-2-methyl-D-ribono-gamma-lactone. The preparation method provided by the invention uses easily available and cheap raw materials, so production cost is greatly reduced; and the operation of the preparation method is coherent and simple, and the quantity of waste gas, waste water and industrial residues is lower than the quantity of waste gas, waste water and industrial residues reported in the prior art.
Owner:杭州惠诺医药科技有限公司
Quickly disintegrating solid preparations
Quickly disintegrating solid preparations which contain: a) an active ingredient; b) D-mannitol having an average particle size of 30 μm to 300 μm; c) a disintegrating agent; and d) celluloses.
Owner:OHKOUCHI KAZUHIRO +1
Composite cordyceps militaris probiotic enzyme and preparation method thereof
InactiveCN105725195AAchieve native diversityAchieve conversionFood ingredient functionsBiotechnologyD-mannitol
The invention discloses a composite cordyceps militaris probiotic enzyme and a preparation method thereof. The method includes the following six steps of preparation of a strain liquid culture medium, preparation of a fermentation strain, preparation of a fermentation substrate, first-time fermentation, second-time fermentation and product obtaining. The preparation method adopts step-by-step two-time fermentation of a cordyceps militaris strain and the composite enzyme, utilizes a cordyceps militaris strain fermentation product as the substrate to perform second-time fermentation of the composite enzyme, native diversity of enzyme components is achieved, the composite cordyceps militaris probiotic enzyme contains the effective components of the cordyceps militaris and effective components of the composite enzyme, and meanwhile D-mannitol produced by the cordyceps militaris is converted into D-mannose which can be absorbed by human. The two times of fermentation are not influenced mutually and can be performed continuously and be continuously performed, the efficiency is high, and the composite cordyceps militaris probiotic enzyme and the preparation method are very suitable for large-scale production.
Owner:HESHAN ZHONGCHUN BIOTECH
Astaxanthin and hippophae rhamnoides fruit powder tabletting sweets and preparation method thereof
InactiveCN105767417AImprove antioxidant capacityStrong Antioxidant FunctionConfectionerySweetmeatsD-mannitolHippophae rhamnoides fruit
The invention discloses astaxanthin and hippophae rhamnoides fruit powder tabletting sweets and a preparation method thereof. The tabletting sweets are prepared from the following raw materials and auxiliary materials in parts: 30-50 parts of astaxanthin, 30-250 parts of hippophae rhamnoides fruit powder, 90-250 parts of beta-cyclodextrin, 30-300 parts of D-mannitol, 1-12 parts of citric acid, 1-150 parts of microcrystalline cellulose, 6-12 parts of calcium chloride, and 1-10 parts of magnesium stearate. According to the astaxanthin and hippophae rhamnoides fruit powder tabletting sweets disclosed by the invention, through experiments, a formula proportion of the astaxanthin to the hippophae rhamnoides fruit powder, an embedding proportion of the astaxanthin to an embedding medium, an addition amount of correctant citric acid and the like are preferably selected, and appropriate technological parameters are also preferably selected, so that antioxidative activity in the astaxanthin and the hippophae rhamnoides fruit powder is reserved to the maximum extent, and a strong antioxidative function that the function of the tabletting sweets is better than the function of the astaxanthin and the function of the hippophae rhamnoides fruit powder is generated, and the problem that the mouth feel is poor is solved.
Owner:SHANDONG JINHE DRUG RES DEV
Composition containing lutein and lutein ester, pressed lutein candy, preparation method and application
PendingCN109645201AEffective absorptionAbsorption lastsConfectionerySweetmeatsD-mannitolProtecting eye
The invention discloses a composition containing lutein and lutein ester, pressed lutein candy, a preparation method and application. The composition comprises lutein, lutein ester, zeaxanthin, blueberry powder, natural carotene, trehalose, D-mannitol and the like. The composition is used for preparing the pressed lutein candy, and the preparation method comprises the steps of embedding natural carotene and the like, mixing the natural carotene with remaining materials except trehalose and D-mannitol, and pulverizing and sieving the mixture to obtain powder; using the powder as a core material, coating the core material with a wall material solution, and conducting granulating, drying, tabletting and coating to obtain a final product. According to the method, specific components and content are combined together, and therefore the absorption dose demanded by the human body can be achieved; each component plays a good synergistic role in eye protection; through a specific technologicalprocess, the final product is stable and has a slow release function, correspondingly people can effectively and continuously absorb effective components, and the problem of absorption limitation is solved.
Owner:四川益康天成生物科技开发有限公司 +1
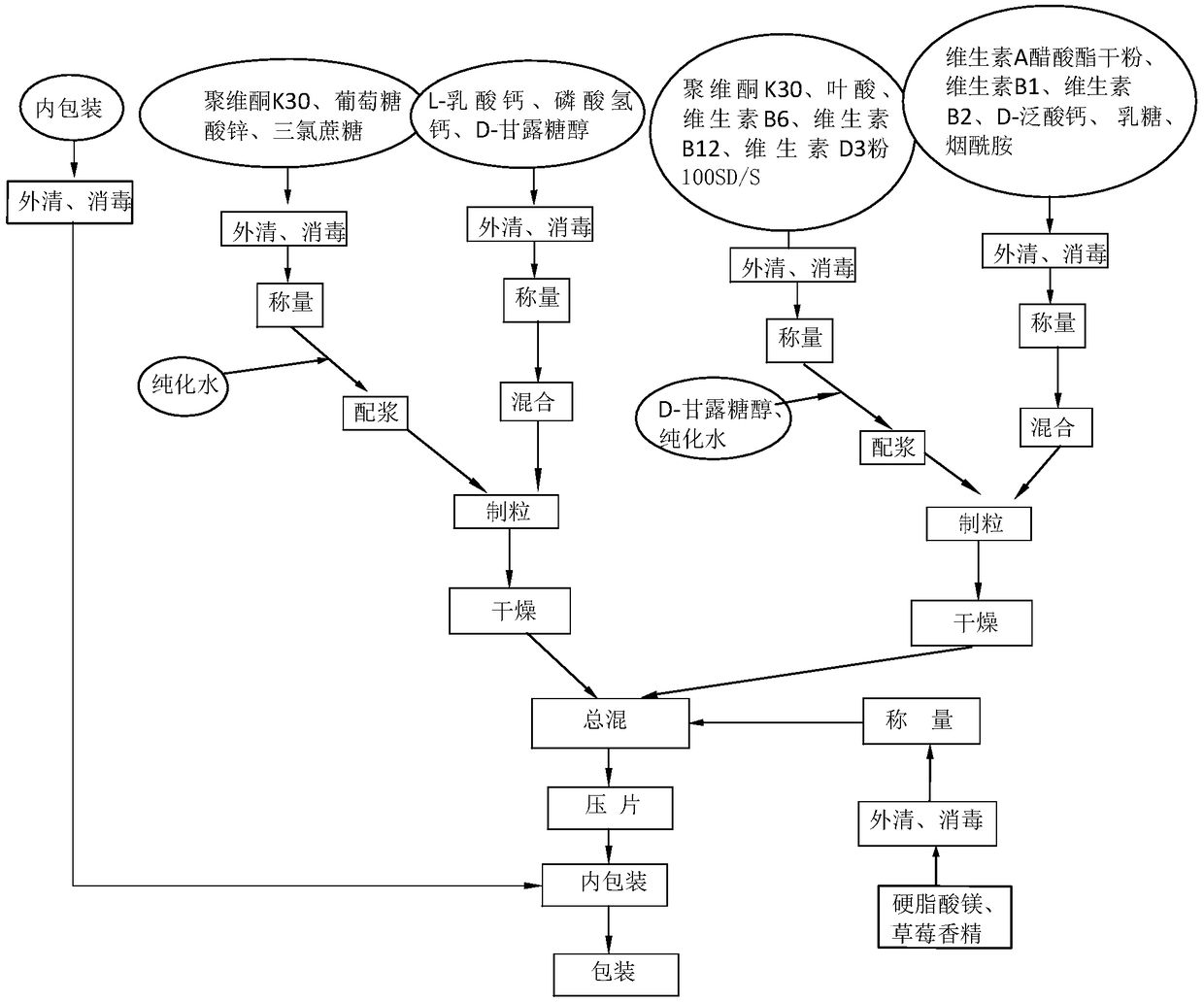
Multi-vitamin calcium-zinc chewable tablets and preparation method thereof
InactiveCN108653324AReduce wastePrevent diseaseMetabolism disorderPill deliveryMagnesium stearateGluconic acid
The invention relates to multi-vitamin calcium-zinc chewable tablets which are prepared from povidone K30, zinc gluconate, sucralose, calcium hydrogen phosphate, L-calcium lactate, D-mannitol, folic acid, vitamins, D-calcium pantothenate, lactose, nicotinamide, strawberry essence and magnesium stearate. More specifically, the multi-vitamin calcium-zinc chewable tablets provided by the invention are vitamin B tablets; compared with common vitamin B chewable tablets which are added with some trace elements that the human body lacks and need to be supplemented by food, a simple preparation can replace the preparations such as vitamin B, calcium tablets and zinc gluconate oral liquid while supplementing vitamin B, calcium and zinc; moreover, the preparation technology is simple and easy to implement, the product performance is stable, the production cost is relative low, and the effect of the product is relatively obvious.
Owner:海南金贝康制药有限公司
Water-based flocking adhesive and preparation method thereof
InactiveCN110144189AImprove water resistanceWith wear resistancePolyureas/polyurethane adhesivesGraft polymer adhesivesWater basedAdhesive
The invention relates to a water-based flocking adhesive. The flocking adhesive is characterized by being prepared from the following raw materials, in parts by weight: 20-30 parts of a water-solubleterminal acrylate hyperbranched polyester, 5-10 parts of 3-chloro-2-hydroxypropyl acrylate ionized modified hyperbranched polyethyleneimine, 30-40 parts of hydroxyl terminated water-based polyurethane, 1-5 parts of a blocked isocyanate crosslinking agent, 1-3 parts of D-mannitol-1,6-bis(2-isocyano-3-methyl-2-butenoate), 0.5-1 part of a fluorine-containing trihydroxysilane compound, 2-5 parts of anemulsifying agent, 0.1-0.5 part of an initiator, and 50-60 parts of water. The invention also discloses a preparation method and use method of the water-based flocking adhesive. The water-based flocking adhesive disclosed by the invention has the advantages of excellent water resistance, wear resistance and weather resistance, high bonding strength, excellent stability, water resistance, hand feeling and air and moisture permeability, and safe, green and environmentally-friendly use.
Owner:汕头市易贴包装材料有限公司
Natural type oil-water compatible edible essence and preparation method thereof
InactiveCN103734658AImprove solubilityGood antibacterial and bactericidal effectFood preparationSolubilityGlycerol
The invention relates to a natural type oil-water compatible edible essence and a preparation method thereof. The natural type oil-water compatible edible essence has oil solubility and water solubility and is prepared by uniformly mixing and dissolving following components in parts by weight: 5-10 parts of limonene, 10-20 parts of glycerol, 5-10 parts of phospholipid, 1.5-2.5 parts of D-mannitol, 1-2 parts of polydextrose and 2-4 parts of maltitol. According to the product, the adopted main material limonene is produced from natural tangerine and orange peels and dreg, is natural, has no stimulation and has a lasting aroma; the raw materials are recyclable and renewable and wide in sources so that wastes are changed into valuable things. The edible essence has many functions, a sweet taste and good biological degradability; the manufacturing process is simple, the operation is convenient, the conditions of a production environment are not strict, and no pollution is caused; special equipment and a matched pollution discharging facility are not needed and the practical value and the economic benefit are obvious. The use amount of the product can be adjusted according to an actual production condition; the seasoning effect is very good and the quality guaranteeing time is long; the natural type oil-water compatible edible essence is convenient to transport and storage, can be widely applied to food processing fields of candies, bakery products, beverages and the like, and is convenient to use.
Owner:TIANJIN LIUHONG TECH DEV
Tabletted sweets capable of alleviating asthenopia as well as preparation method and application of tabletted sweets
The invention relates to the technical field of foods, and discloses tabletted sweets capable of alleviating asthenopia. The tabletted sweets comprise the following raw materials in parts by weight: 40-50 parts of blueberry fruit juice powder, 1-5 parts of chrysanthemum powder, 20-30 parts of Chinese wolfberry fruit powder, 1-5 parts of grape seeds, 0.1-1 part of xanthophyll, 5-10 parts of dietaryfibers, 10-20 parts of D-mannitol, 5-10 parts of maltodextrin and 0.1-1 part of magnesium stearate. The invention further discloses a preparation method of the tabletted sweets and an application ofthe tabletted sweets in the respect of alleviating asthenopia. The tabletted sweets have the beneficial effects of being comprehensive in nutrient components, an original ecology low-temperature processing technology is adopted, the tabletted sweets conform to the proportion of promoting blood circulation for removing blood stasis, nourishing liver and kidney and removing liver fire for improvingeyesight in traditional Chinese medicines, and nutrient components of the raw materials are guaranteed not to be destroyed, so that the efficacy of promoting blood circulation for removing blood stasis and removing liver fire for improving eyesight can be achieved.
Owner:江苏海王健康生物科技有限公司
Skin-brightening freeze-drying powder containing oligopeptides and 3-O-ethyl ascorbic acid
ActiveCN109646320APenetrate fastIncrease brightnessCosmetic preparationsToilet preparationsVitamin CPullulan
The invention relates to the technical field of cosmetics, in particular to a skin-brightening freeze-drying powder containing oligopeptides and 3-O-ethyl ascorbic acid. The skin-brightening freeze-drying powder is prepared from, by weight, 85-95 parts of deionized water, 3-8 parts of D-mannitol, 2-7 parts of a vitamin C derivative, 0.7-1.4 parts of a sugar composition and 0.0008-0.0018 parts of oligopeptides. The oligopeptide refers to oligopeptide-5, the vitamin C derivative refers to 3-O-ethyl ascorbic acid, and the sugar composition includes trehalose and pullulan polysaccharide; the obtained freeze-drying powder can penetrate the skin rapidly, inhibit the tyrosinase activity, inhibit melanin formation, and can effectively enhance the skin brightness and effectively and continuously brighten the skin; the components in a raw material formula complement one another, the obtained freeze-drying powder has the synergistic effect and the synergistic effect is especially obvious in skinbrightness enhancing and brightening lasting.
Owner:广州市美夫兰化妆品有限公司
Vitamin C effervescent tablet
InactiveCN108157975AImprove effervescenceImprove stabilityFood shapingFood ingredient functionsDiseaseVitamin C
The present invention relates to an effervescent tablet, and particularly relates to a vitamin C effervescent tablet. The invention belongs to the technical field of health food tablet formulation. Vitamin C, one of the essential vitamins of the human body, has a preventive effect on many diseases. Vitamin C can also be used in combination with many other drugs in the treatment of certain diseases. Effervescent tablet is a novel tablet developed and applied abroad in recent years, and the effervescent tablet is usually prepared by acid and alkali separate wet granulation, followed by being mixed with other raw materials for tablet compressing. In order to shorten the production process and further improve the product stability, the invention adopts the mixed use of alpha- and beta-crystalform D-mannitol, which reduces the fragility of the tablets on the basis of ensuring the effervescent effect of the tablets. The formula of the invention is suitable for the direct tablet compressingprocess, free of the addition of water or other wetting agents in the production process and capable of reducing the occurrence of material pollution, improving the production efficiency, reducing theprocess flow and decreasing and reducing the energy consumption. In addition, the addition of sodium caseinate can improve the effervescent effect of the tablets.
Owner:SHANDONG TIANLI PHARMA
Peptide pharmaceutical formulations
InactiveUS20060183685A1Improve stabilityImprove toleranceBiocidePeptide/protein ingredientsAcetic acidD-mannitol
A pharmaceutical composition for administration to a mammal is disclosed. The composition includes a therapeutically effective amount of a peptide, such as a GLP-1 molecule, a PTH molecule, or a GRF molecule. The composition further includes a buffer including a weak acid having an acid dissociation constant value of greater than about 1×10−5, such as acetic acid. The composition also includes an excipient for making the composition generally isotonic, such as D-mannitol.
Owner:JEFFERSON PHARMA
Extinction type polyester resin for HAA system as well as preparation method and application thereof
InactiveCN110483751APowdery paintsPolyester coatingsNaphthalenetetracarboxylic dianhydrideD-mannitol
The invention relates to extinction type polyester resin for an HAA system. Terephthalic acid, tetrahydrophthalic acid, 2-amino-4,6-dihydroxypyrimidine, neopentyl glycol, 1,10-decanediol, diacetone-D-mannitol, 1,4,5,8-naphthalenetetracarboxylic dianhydride, 5-methyl isophthalic acid and trimethyl citrate are adopted as raw materials. All the components are matched with one another and have a synergistic effect so that the molecular weight distribution of the finally obtained polyester resin is not uniform and polybasic acids for end capping are different in type, and therefore, when the polyester resin with different end-capping functional groups and HAA are cured, the reaction activity difference is large, the curing process cannot be synchronously carried out, the cured coating surface shrinks, and the surface gloss is effectively low. Experimental data show that when the prepared extinction type polyester resin is used for preparing extinction type powder coating of an HAA system, acoating film with the glossiness smaller than 15% can be obtained, and excellent comprehensive performance is achieved.
Owner:HUANGSHAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com