Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Intermediate type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
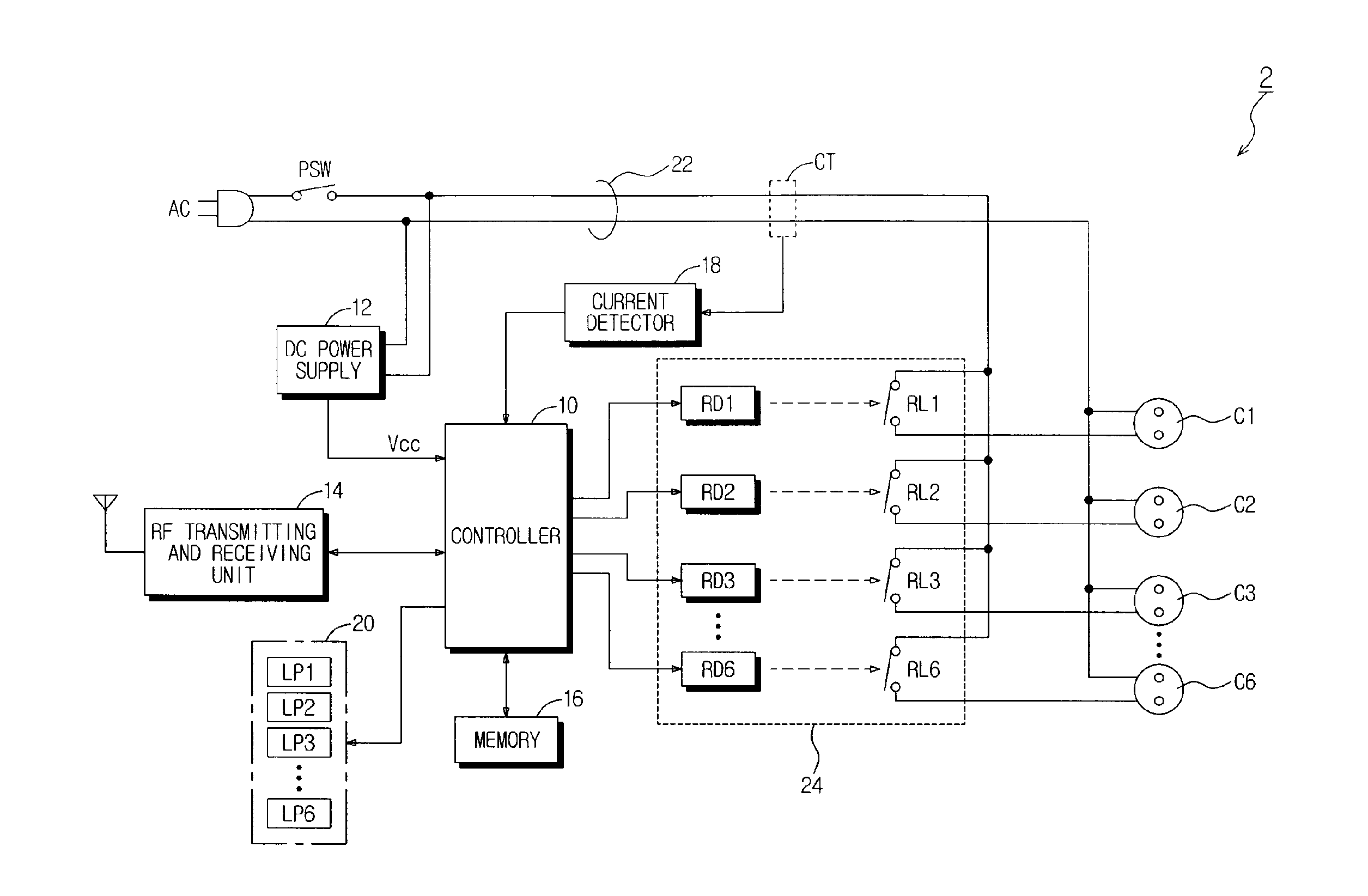
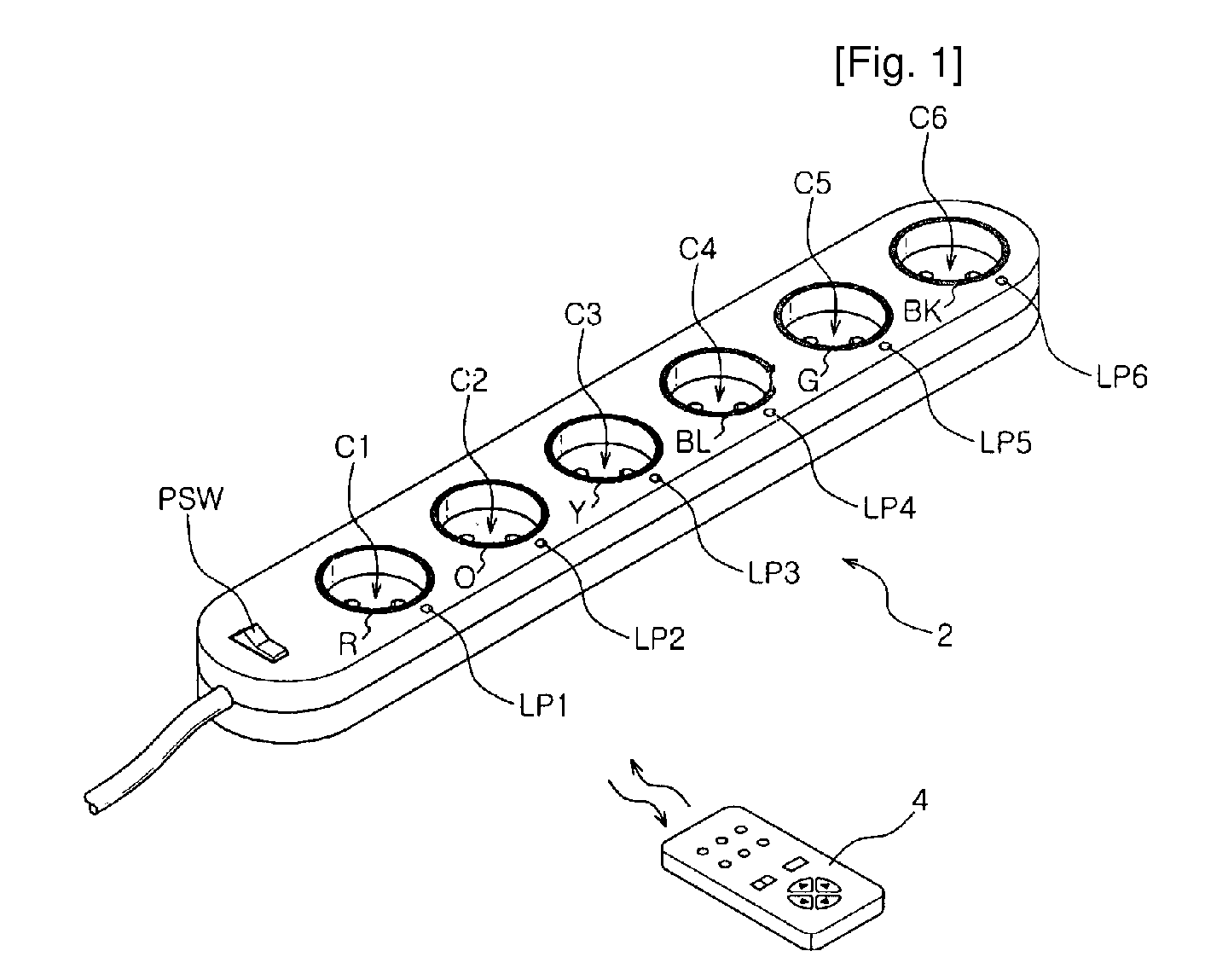
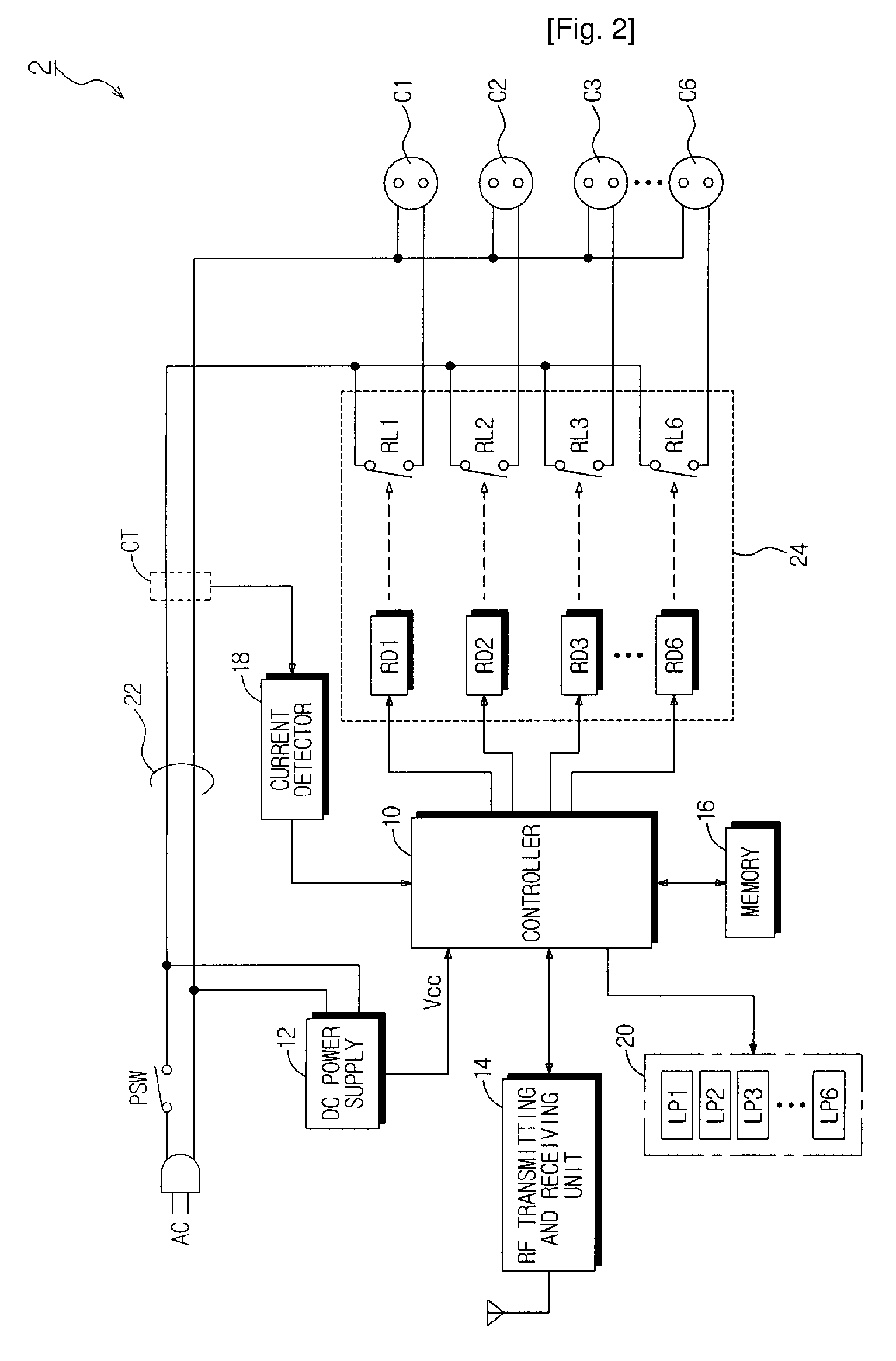
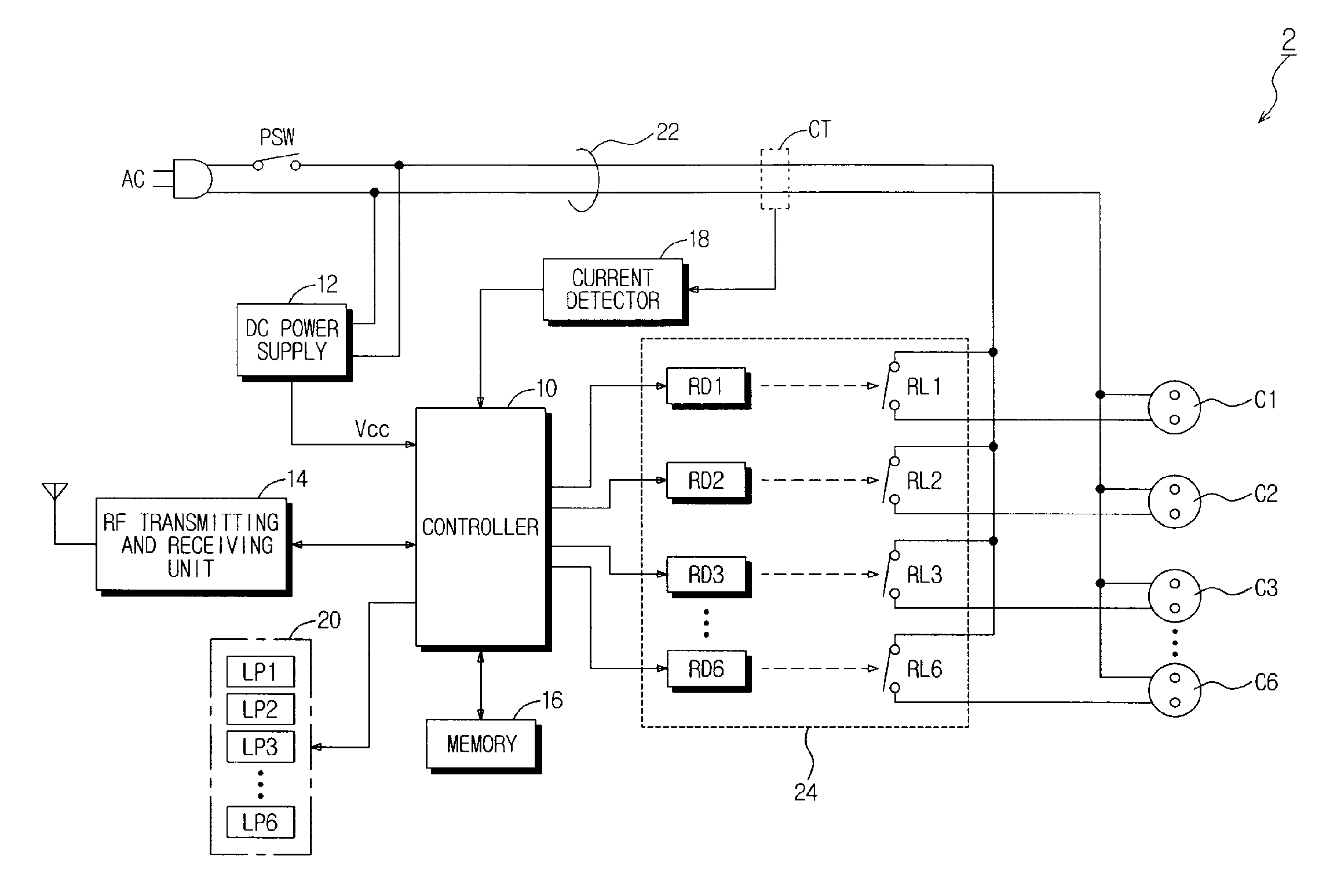
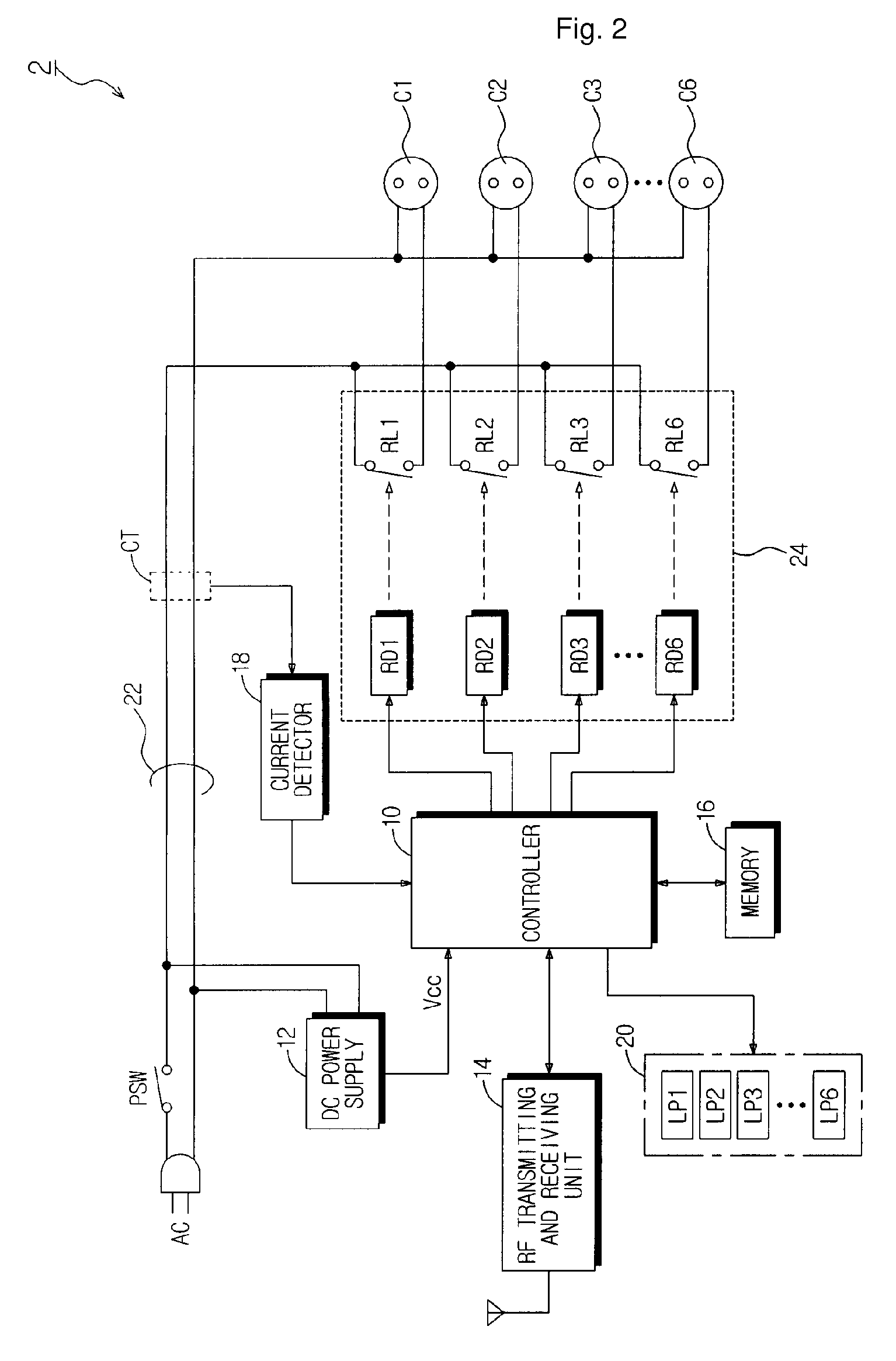
Power-Controllable Outlet Receptacle
InactiveUS20080309164A1Prevent overloadDc network circuit arrangementsBoards/switchyards circuit arrangementsEngineeringPower strip
A multi-type power strip includes a main body having connecting sockets with surfaces indicated by priority of supplying electric power to the connecting sockets, operation lamps, and an electric circuit installed in the main body. The circuit interfaces with a remote controller through RF, and checks statuses of loads connected to the main body to switch plural switches based on the priority when the load exceeds a threshold. An intermediate type power strip includes intermediate socket main bodies inserted into sockets and respectively having at least one connecting socket, and a remote controller to individually control the intermediate socket main bodies in remote through RF communication. The surfaces of the intermediate socket main bodies are distinguished by indicators. When switch status of the intermediate socket main bodies is requested by the remote controller, the status is displayed by lamps of a socket controlling button of the remote controller.
Owner:LIM CO LTD
Power-controllable outlet receptacle
InactiveUS7843081B2Prevent overloadDc network circuit arrangementsBoards/switchyards circuit arrangementsElectric powerPower strip
A multi-type power strip includes a main body having connecting sockets with surfaces indicated by priority of supplying electric power to the connecting sockets, operation lamps, and an electric circuit installed in the main body. The circuit interfaces with a remote controller through RF, and checks statuses of loads connected to the main body to switch plural switches based on the priority when the load exceeds a threshold. An intermediate type power strip includes intermediate socket main bodies inserted into sockets and respectively having at least one connecting socket, and a remote controller to individually control the intermediate socket main bodies in remote through RF communication. The surfaces of the intermediate socket main bodies are distinguished by indicators. When switch status of the intermediate socket main bodies is requested by the remote controller, the status is displayed by lamps of a socket controlling button of the remote controller.
Owner:LIM CO LTD
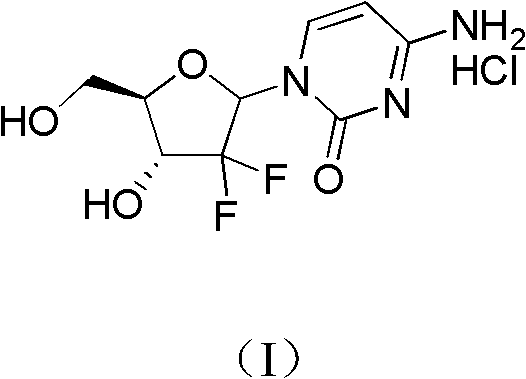
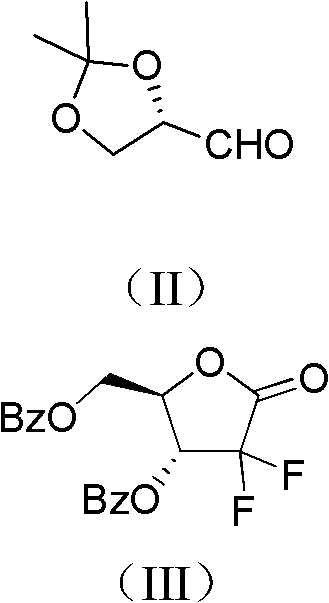
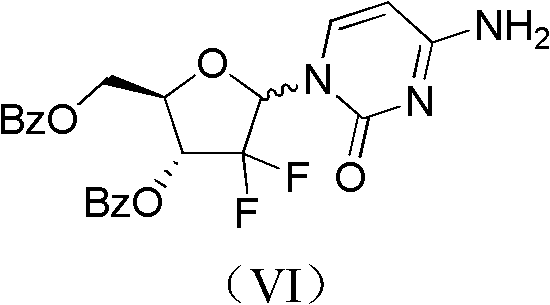
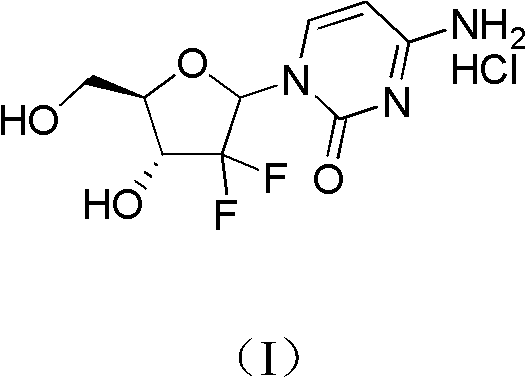
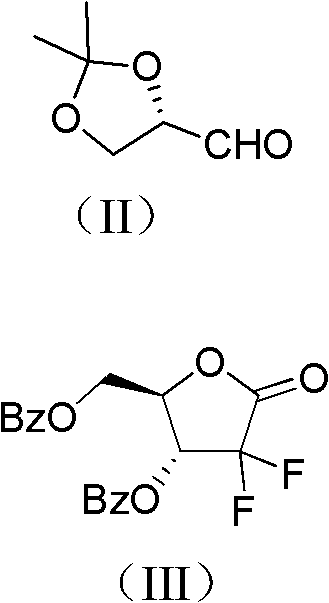
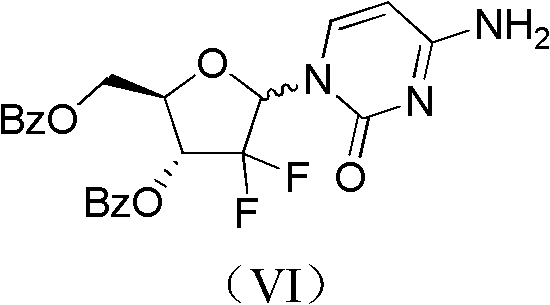
Method for preparing gemcitabine hydrochloride
ActiveCN102617678AFew stepsSimple and fast operationSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideProtecting group
The invention relates to a compound in a formula (I), and discloses a method for preparing gemcitabine hydrochloride. The method includes preparing an intermediate type (III) compound by means of realizing Reformatsky reaction, removing a protecting group and realizing lactonization and double benzoylation; obtaining a compound in a formula (VI) via reduction, methyl sulfonylation and condensation; and finally removing a protecting group, saltifying and realizing crystallization to obtain a final product. The method is simple in process, high in yield and quite suitable for industrial production, the purity of the product is fine, and harsh reaction conditions are omitted.
Owner:JIANGSU HANSOH PHARMA CO LTD
Power-controllable outlet receptacle
InactiveCN101208840APrevent overloadPower network operation systems integrationCoupling device detailsEngineeringElectric power
The present invention provides electronic-controlling sockets. The power panel comprises: the body linking with the sockets, the surface of the said sockets indicts the priority of supplying power to the linking sockets, operation lamp, and the circuit install in the said body. The said circuits communicate with the remote controller via RF, and determine the status of the load linking with the said body, and when the load is over the threshold, change-over switch according to the priority. The middle electronic panel comprises: the socket body inserted into the sockets and has at least one linking socket, and remote controller used to control the middle socket body via RF communication. The surface of said middle socket body can be distinguished by the indicator. When the remote controller asks for the switch status of the middle socket body, the status can be displayed by the lamp of the socket controlling button of the said remoter controller.
Owner:林成珪
Design and process method for profile and structure of streamline locomotive
InactiveCN1634735AReduce labor intensityRealize shape designRailway transportAxle-box lubricationIntermediate typeCoating
This invention relates to train body parts kinds in railway trains and especially to streamline trains, which comprises railway speeding train, speeding gas train set and speeding automobiles or high speed magnetic floating train head part or structure design. The invention is characterized by the following: the streamline train head is composed of multiple NUBRS curved pieces to form the train head outline through dynamic automatic generation or piece adjusting; then welding the space net structure plane of the head structure with the coating iron into the streamline train head.
Owner:CENT SOUTH UNIV
Interback-type substrate processing device
InactiveUS7407358B2Improve productivityGreat intricacyCellsStatic indicating devicesEngineeringVacuum chamber
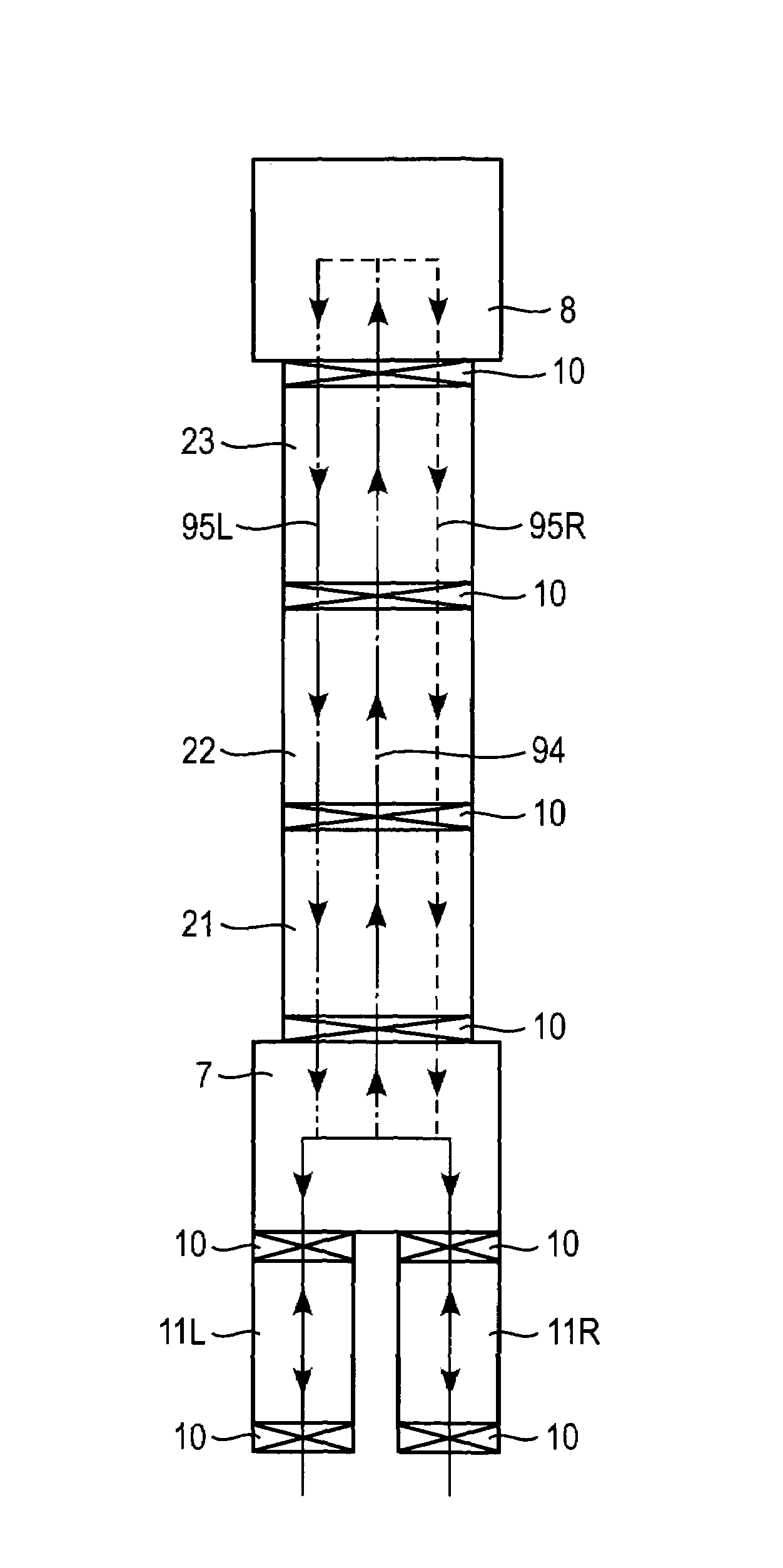
An interback-type device in which a substrate 9 is carried into the device from one side of the device and is inverted in the device to be carried out and returned to the same side, a plurality of vacuum processing chambers 21, 22, 23 are longitudinally-provided and hermetically connected, and a carry system which passes through these vacuum chambers for carrying the substrate along established carry lines 94, 95L, 95R is provided. The carry line includes an outward carry line 94 toward an inversion position and return carry lines 95L, 95R returning from the inversion position, and the outward line 94 and return carry lines 94, 95L, 95R are different parallel paths, and the return carry lines 95L, 95R are branched in plurality. The outward carry line 94 and return carry lines 95L, 95R are established to pass through the same three processing chambers 21, 22, 23.
Owner:CANON ANELVA CORP
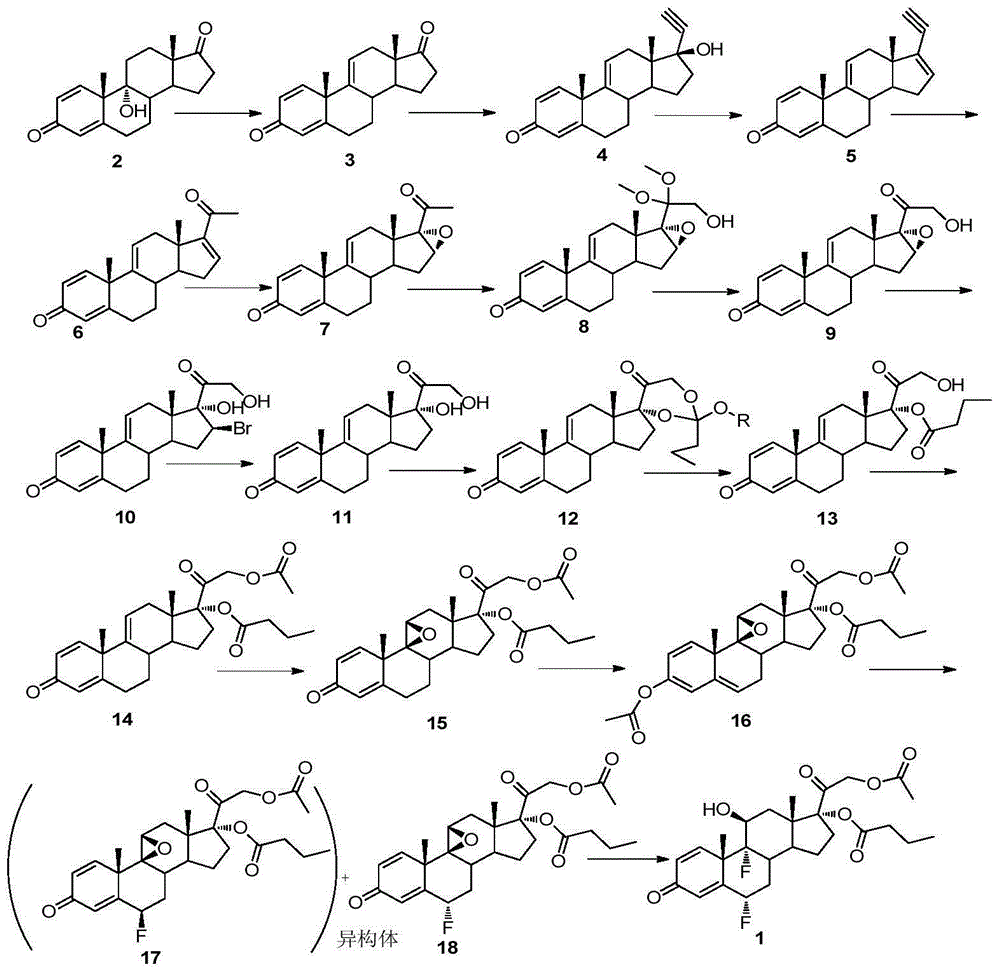
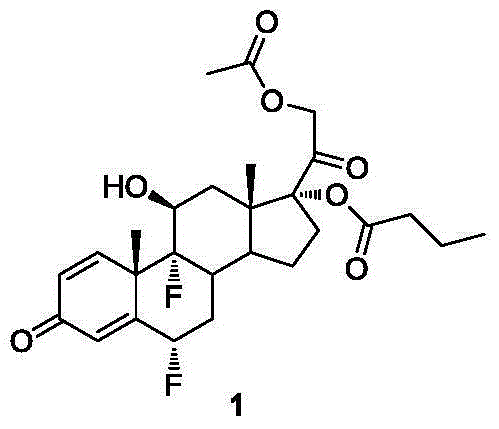
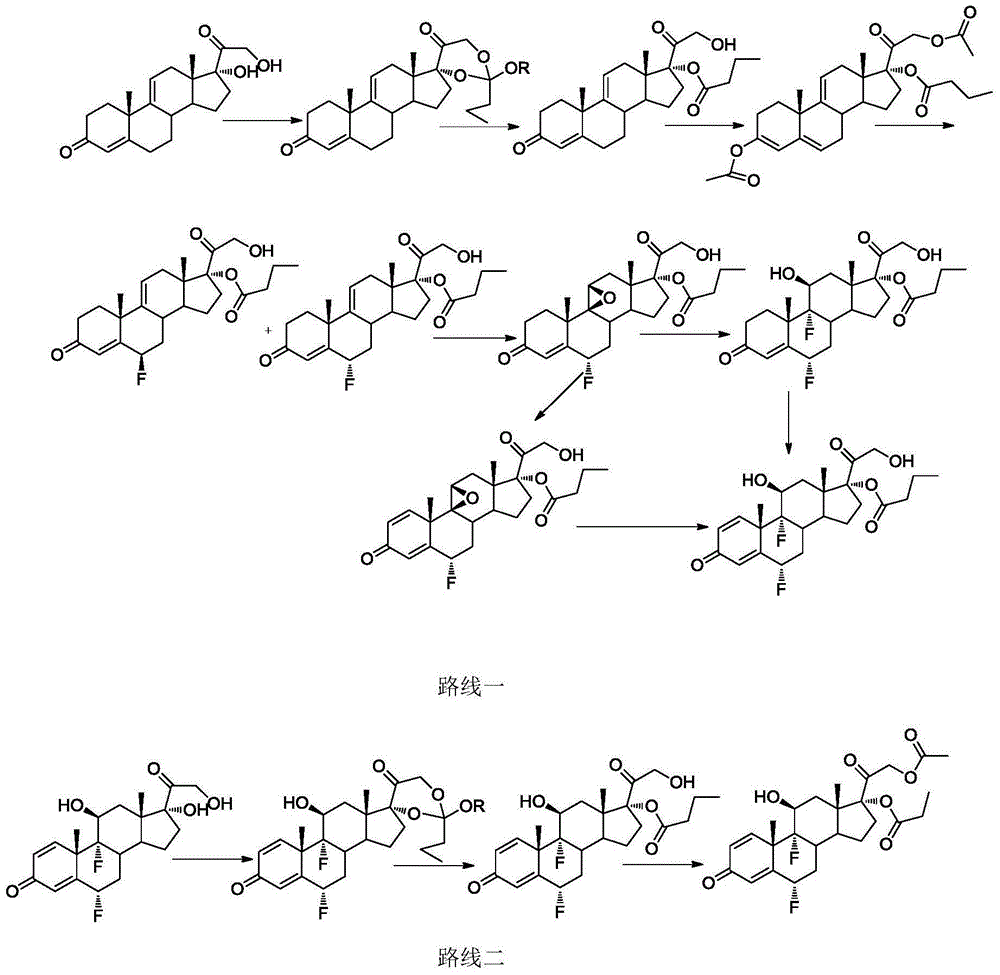
Method for synthesizing difluprednate from sterol fermentation product
The invention provides a method for synthesizing difluprednate from a sterol fermentation product. The sterol fermentation product, namely 9 Alpha-hydroxyl-androstane-1,4-diene-3,17-diketone (9 Alpha-OH-AD) obtained by fermenting phytosterol of which the content in byproducts of the grease industry is very high, serves as a starting raw material. The method comprises the following 15 reaction steps in total: dehydrating steride 9-hydroxyl to form a double-bond; adding 17-carbonyl with acetylene; dehydrating; producing 21-copper carbonyl under an acid condition; epoxidizing 16,17-double bond; oxidizing periodide and introducing 21-hydroxyl; performing ring opening on 16,17 Alpha-epoxy hydrobromate; hydrogenating for removing 16 Beta bromine; forming a ring on orthoester; performing ring opening; esterifying; epoxidizing 9,11-double bond-Beta; enolizing and esterifying; performing ring opening on 6-electrophilic fluoro; and performing ring opening on 9,11-epoxy fluoro. According to the method, steride 17 Alpha and 21-dyhydroxyl are efficiently built by means of periodide oxidization, epoxide ring-opening and debromination and an important intermediate type 11 compound is obtained; in the whole process, a large quantity of heavy metal pollutant chromium which is generated when producing corticoid medicines in the traditional industry is effectively avoided, so that the method is green, environment-friendly and suitable to industrialized production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Efficiently correlating nominally incompatible types
ActiveUS9201874B2Digital data information retrievalSpecial data processing applicationsProgramming languageSource type
A nominal type framework can be configured to efficiently correlate different nominal types together based on a minimum set of common type shapes or structures. In one implementation, a developer identifies a number of different nominal types of interest (source types), and identifies the minimum set of common type shapes to be accessed by an application program. The minimum set of common type shapes can then be used to create an intermediate type (target type) to which each of the other different source types can be mapped. For example, one or more proxies can be created that map shapes of the one or more source types to corresponding shapes of the created target type. The application program created by the developer, in turn, can access, operate on, or otherwise use the mapped data of each different source type through a single target type.
Owner:MICROSOFT TECH LICENSING LLC
Locating locking fixture for preventing upper and lower static contact baffles in switch cabinet from being opened
InactiveCN101692539AAdjust the gapCan not be damagedSwitchgear detailsSwitchgear shutters/guardsSwitchgearIntermediate type
The invention discloses a locating locking fixture for preventing upper and lower static contact baffles in a switch cabinet from being opened, which is characterized in that the fixture is provided with a rotary stopping handle comprising an insulated handle (1) and a handle screw (2). The handle screw (2) is movably connected with a locating rod (8) which is provided with a locating tube (3). Two opposite cutting sleeve adjusting screws (5) are inserted in the locating tube (3), wherein one cutting sleeve adjusting screw (5) is sleeved with a spring (7) and the other cutting sleeve adjusting screw (5) is provided with a stopping pin (6). Each cutting sleeve adjusting screw (5) is connected with an adjusted cutting sleeve (4). An adjusting screw (10) is movably connected at the front end of the locating rod (8). The fixture is simple in structure, safe and reliable, is suitable for different kinds of 10kV and 35kV intermediate type switch cabinets, can realize preventing upper and lower static contact baffles in the switch cabinet from being opened, prevent the dangerous points in the work and ensure the safety of the workers.
Owner:YANTAI POWER SUPPLY COMPANY OF STATE GRID SHANDONG ELECTRIC POWER +1
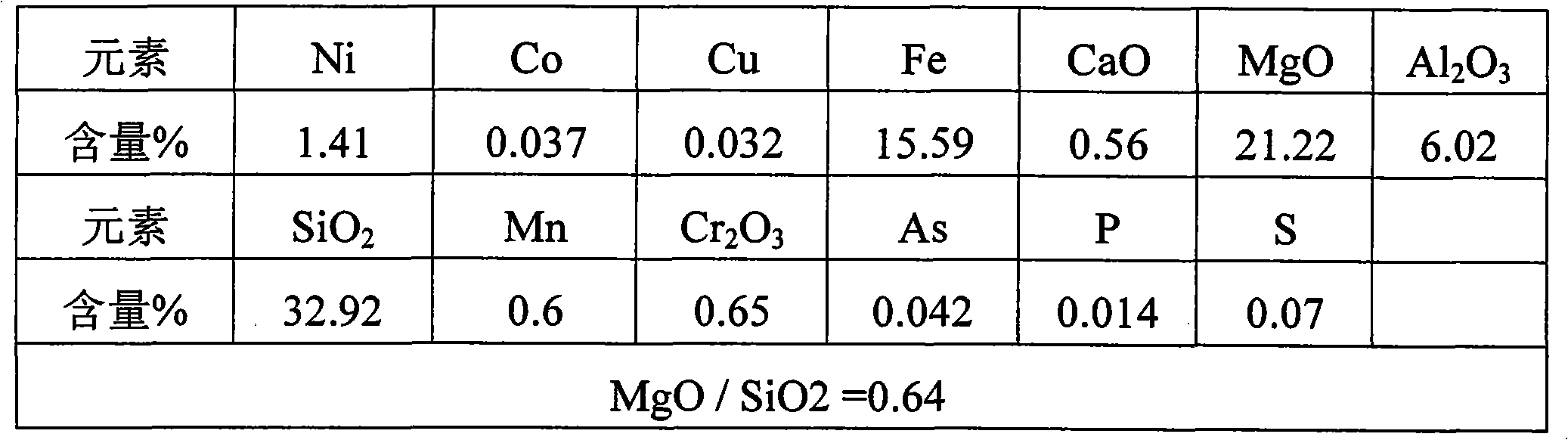
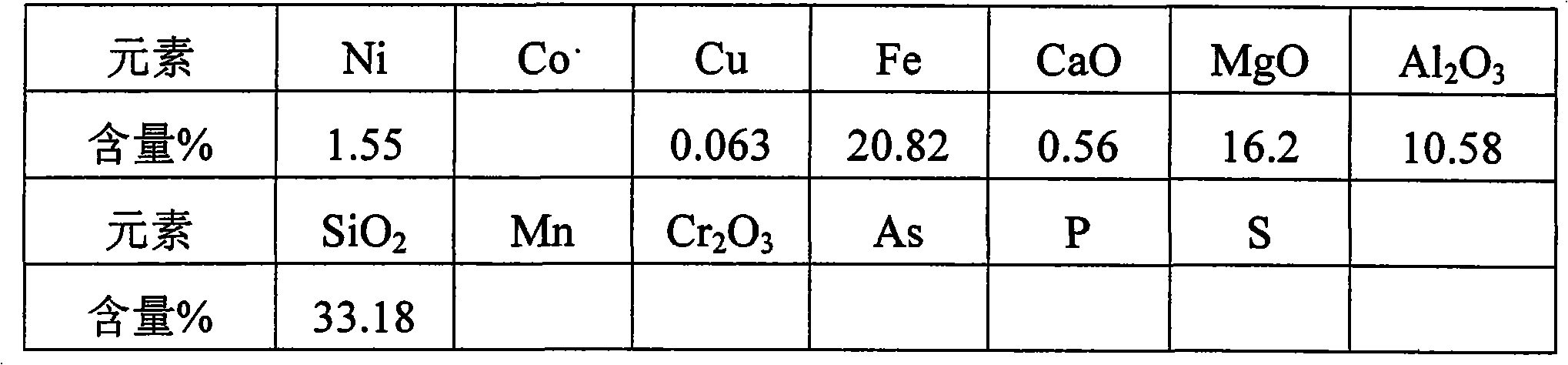
Method for producing ferronickel by utilizing low-magnesium intermediate type laterite nickel ore
The invention discloses a method for producing ferronickel by utilizing low-magnesium intermediate type laterite nickel ore, which sequentially comprises: (1) roasting pre-reduction: after pulverization, sieving, pelletization and drying, dry-based laterite nickel ore with moisture of less than 20 percent is mixed with 10 weight percent of anthracite and 2 to 7 weight percent of CaO; the mixture is subjected to pre-reduction roasting treatment; potency dimension of ore particles is between 5 and 10 mm; time is between 30 and 50 minutes; the temperature is between 800 and 900 DEG C; and reducing degree of iron is controlled to be between 70 and 80 percent; (2) smelting: roasted sand and reclaimed soot are mixed with 1 to 3.5 weight percent of a reducer; and the mixture is delivered to a transferred arc direct current furnace and subjected to open-arc-melting; and (3) slag separation: a ferronickel smelted product is obtained. The method uses direct current for power supply, generates electric arc with directional electronic flow between a cathode and an anode of an electric furnace, generates Joule heat through an electric arc resistor to heat a charging material so as to realize melting, can realize open-arc-melting or submerged-arc-melting according to the metallurgical characteristic of the charging material, is in particular suitable for melting the ferronickel by the low-magnesium intermediate type laterite nickel ore and has high production efficiency.
Owner:巧家奥鑫资源再生利用有限公司
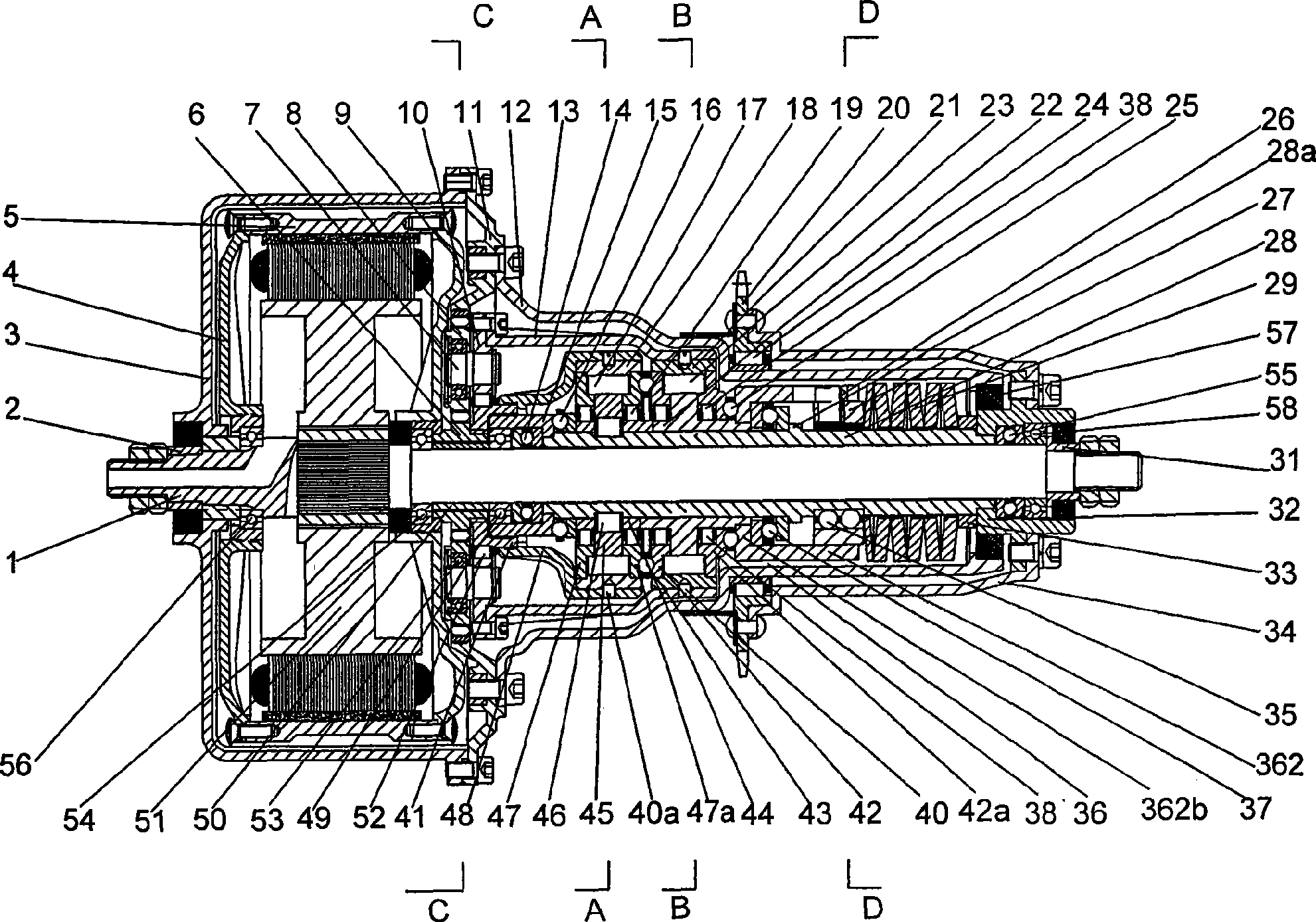
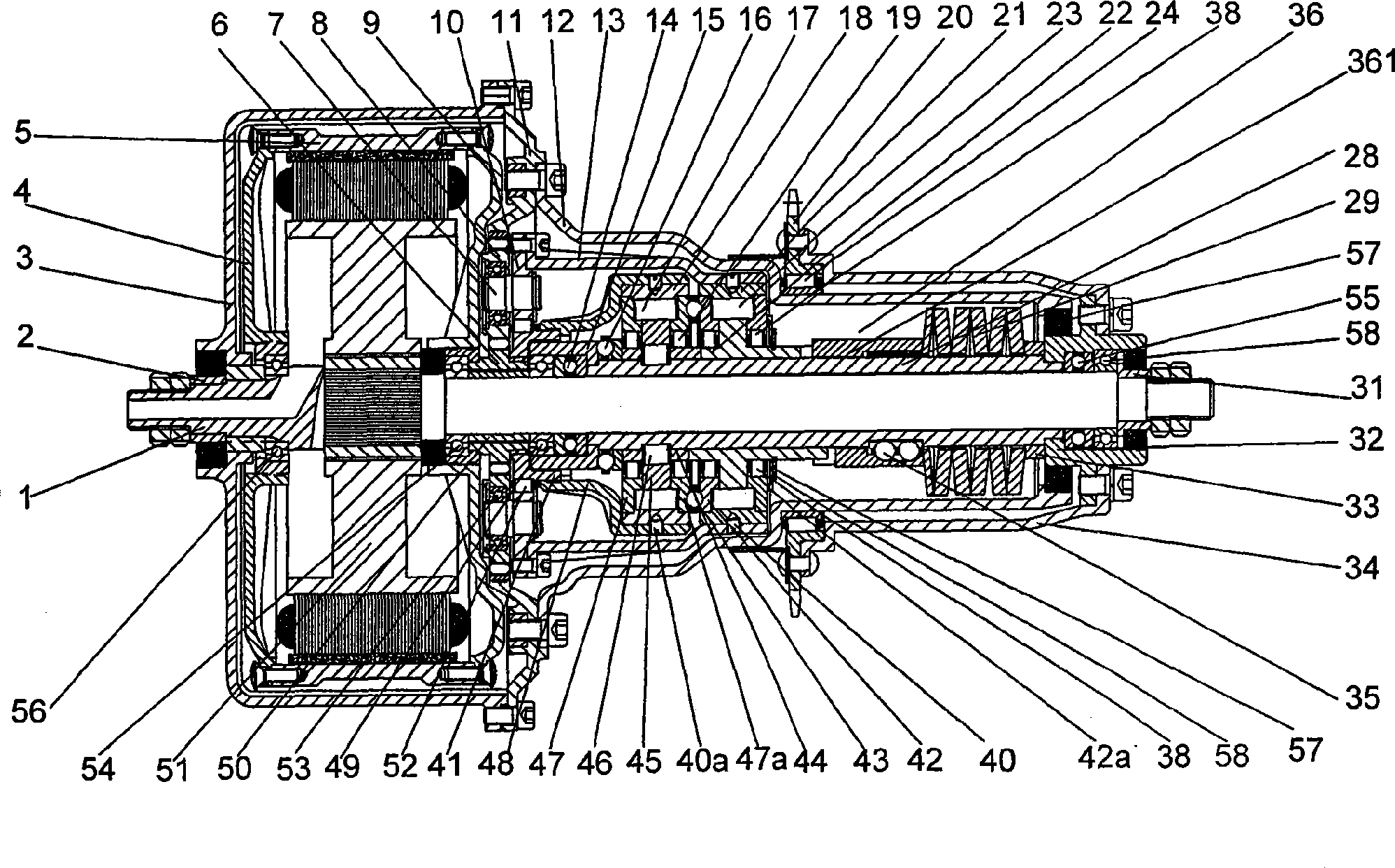
Middle arranging type planetary gear two-shift automatic speed variator
ActiveCN101377228AImprove powerImprove economyToothed gearingsGearing controlGear wheelGravity center
The present invention discloses a two-gear automatic transmission of an intermediate type planetary gear, and comprises a power device, a fixed shaft which is fixed with the stator of the power device, a transmission sleeve which is sheathed on the fixed shaft and matches the transmission of the fixed shaft, and a power output wheel which is fixedly connected with the transmission sleeve. The two-gear automatic transmission also comprises a planetary gear speed change mechanism and a self-adaptive automatic gear shifting transmission assembly; wherein, the self-adaptive automatic gear shifting transmission assembly comprises a cam push rod type overrunning clutch, a low gear overrunning clutch and a variable speed spring self-adaptive annular axial cam mechanism. The present invention realizes the non-gear rigid transmission, self-adaptively and automatically shifts the gears for the speed change along with the changes of the running resistance when the driving force is not cut off, and satisfies the requirements for utilization under the condition with the mountainous area, the hill and the heavy load. The speed change is stable and slow. Simultaneously, the present invention has the advantages of small volume, compact structure and intermediate installation method; the center of gravity does not lean back, which facilitates the safe running.
Owner:SOUTHWEST UNIV
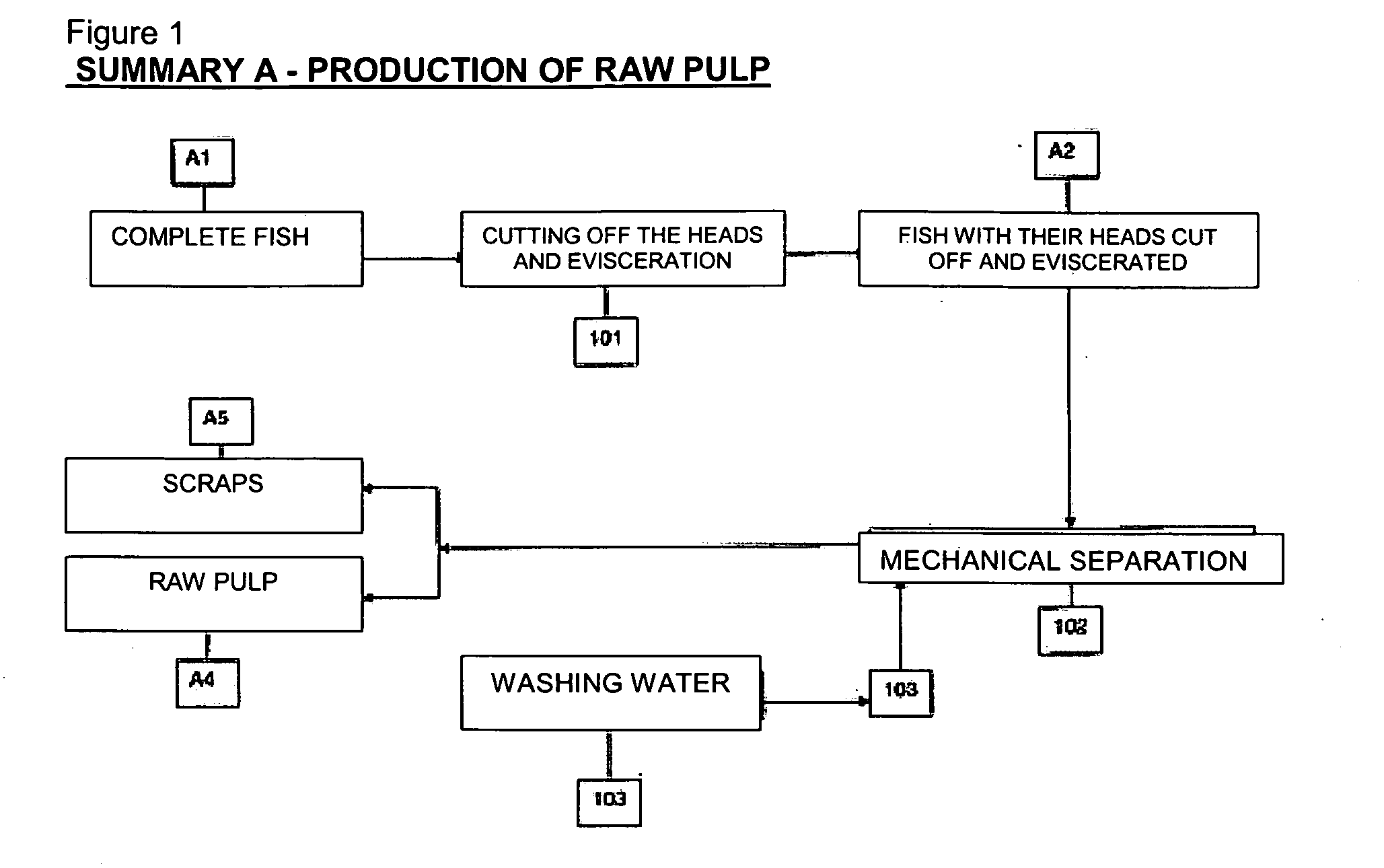
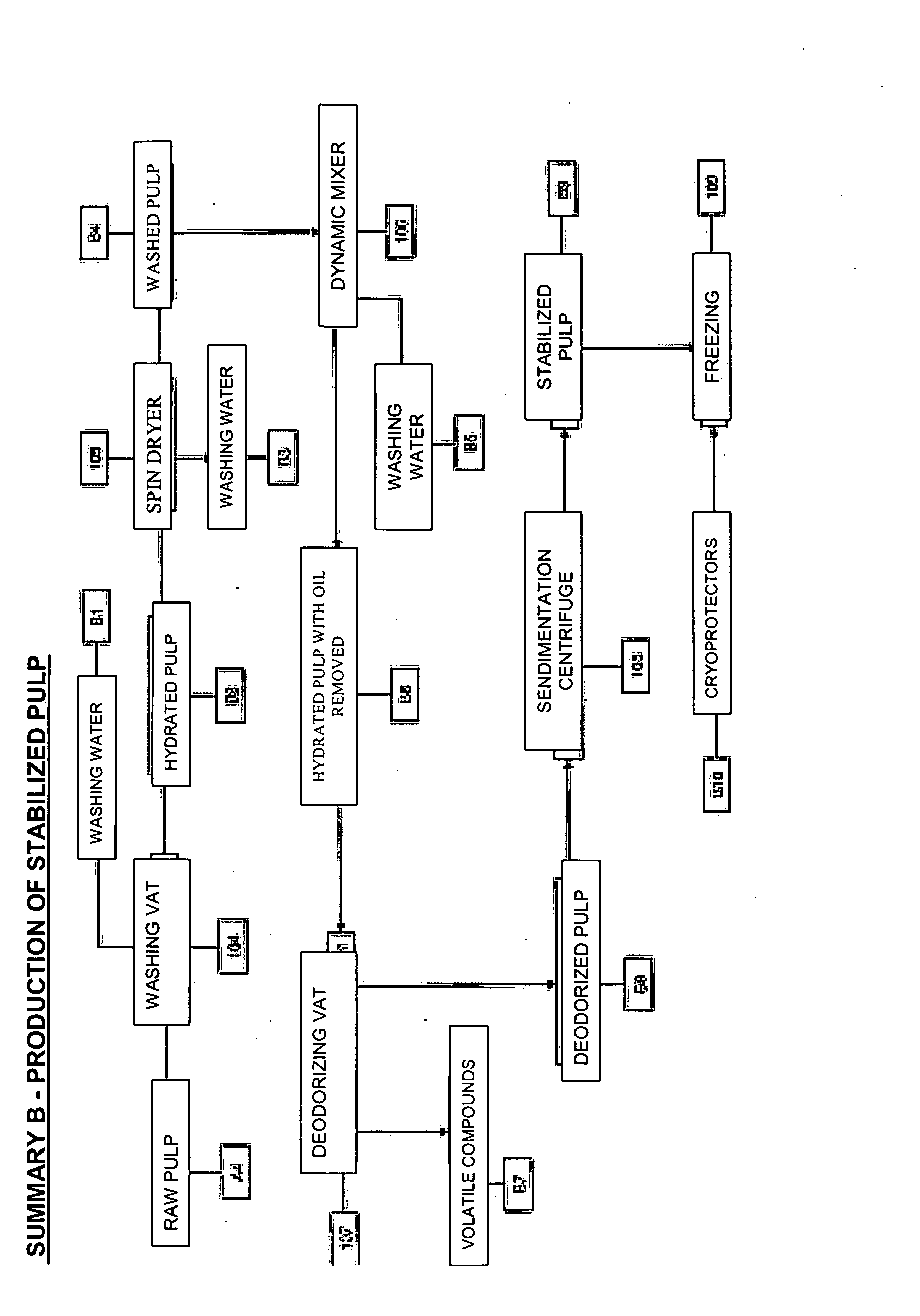
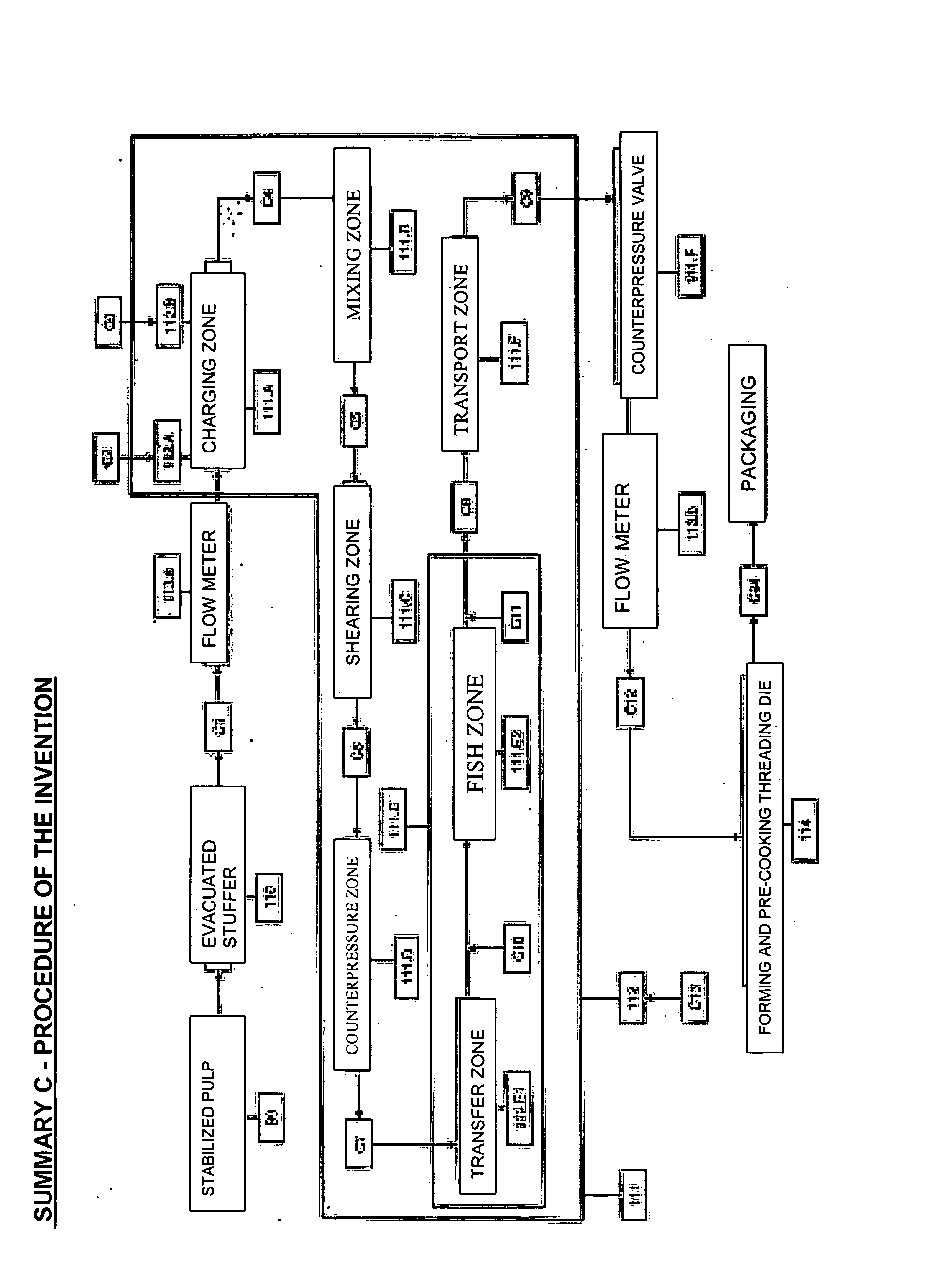
Process for manufacturing, by cold extrusion, puffed intermediate food products which are stable to heat treatment, from hydrated animal proteins
Manufacture of intermediate food products and / or of textured finished products that have been puffed to a greater or lesser extent from hydrated animal proteins without the addition of additives or emulsifiers and / or texturizing agents, which are stable to heat treatment, involving the following succession of steps: (1) a raw initial pulp (A4) is prepared from fish fillets; (2) a series of operations is performed to lead to the production of stabilized pulp (B9) which may or may not be kept in the frozen state; (3) this stabilized pulp is introduced into an evacuated stuffer (110) to be fed into a cold-regulated twin-screw extruder (111) with contra-rotating screws, in which a succession of steps of filling (111A) which may or may not be accompanied by the incorporation of mixing additives (112A / B), of mixing (111B) with a view to homogenizing the ingredients, of shearing (111C) so as to increase the number of potential protein re-attachment sites, of puffing (111 E2with incorporation of air or gas, then of conveying (111F) are carried out in such a way as to obtain a textured food product which has been puffed to a greater or lesser extent and may or may not include inclusions (C11) which is stable to heat treatment and the organoleptic and structural properties of which can be adapted and can differ from those of the raw material used.
Owner:AYAM
Fabric, method for manufacturing same and application of fabric
InactiveCN104342822ADelicate bump effectEasy to processFibre typesHeating/cooling textile fabricsYarnPolyester
The invention discloses a fabric, a method for manufacturing the same and application of the fabric. The method includes at least weaving interlacing composite yarns to obtain grey fabrics; performing processes including scouring desizing, intermediate type approval, calendaring, liquid flow dyeing and finishing type approval on the grey fabrics to obtain a product. The interlacing composite yarns comprise polyester fully-drawn yarns and polyester pre-oriented yarns. The fabric, the method and the application have the advantages that the finished product has the similarly snakeskin-shaped natural appearance, feels soft and is suitable for manufacturing fashion dress and casual clothes.
Owner:TORAY FIBER RES INST(CHINA) CO LTD
Breeding method and propagation method for indica-japonica intermediate type sterile line Chunjiang 35A with early flowering time and high stigma exsertion rate
InactiveCN107360966AImprove qualityStrong stress resistanceHorticulture methodsPlant genotype modificationAgricultural scienceHeterosis
The invention discloses a breeding method and a propagation method for an indica-japonica intermediate type sterile line Chunjiang 35A with early flowering time and a high stigma exsertion rate, and belongs to the field of utilization heterosis in hybrid rice. The breeding method disclosed by the invention comprises the following steps of selecting an indica-doped type late japonica rice maintainer line Chunjiang 19B with the characteristics of high stigma exsertion rate, early flowering time, resistance to the stripe virus disease and the like and a partial indica type maintainer line Chunjiang 23B with the characteristics of high stigma exsertion rate, very early flowering time and the like to carry out hybridization; then carrying out backcross by using a late japonica rice sterile line Chunjiang 16A with the characteristics of high stigma exsertion rate, very early flowering time and the like as a male parent; planting a backcross descendant, until B6F1 generation is low and the backcross descendant is stable, naming the backcross descendant as Chunjiang 35A. The indica-japonica intermediate type sterile line Chunjiang 35A bred by the method is early in flowering time, high in stigma exsertion rate, high in allogamous seed setting rate, high stress resistance (to be specific) and excellent in quality.
Owner:CHINA NAT RICE RES INST
Method for preparing gemcitabine hydrochloride
ActiveCN102617678BFew stepsSimple and fast operationSugar derivativesSugar derivatives preparationGemcitabine HydrochlorideProtecting group
The invention relates to a compound in a formula (I), and discloses a method for preparing gemcitabine hydrochloride. The method includes preparing an intermediate type (III) compound by means of realizing Reformatsky reaction, removing a protecting group and realizing lactonization and double benzoylation; obtaining a compound in a formula (VI) via reduction, methyl sulfonylation and condensation; and finally removing a protecting group, saltifying and realizing crystallization to obtain a final product. The method is simple in process, high in yield and quite suitable for industrial production, the purity of the product is fine, and harsh reaction conditions are omitted.
Owner:JIANGSU HANSOH PHARMA CO LTD
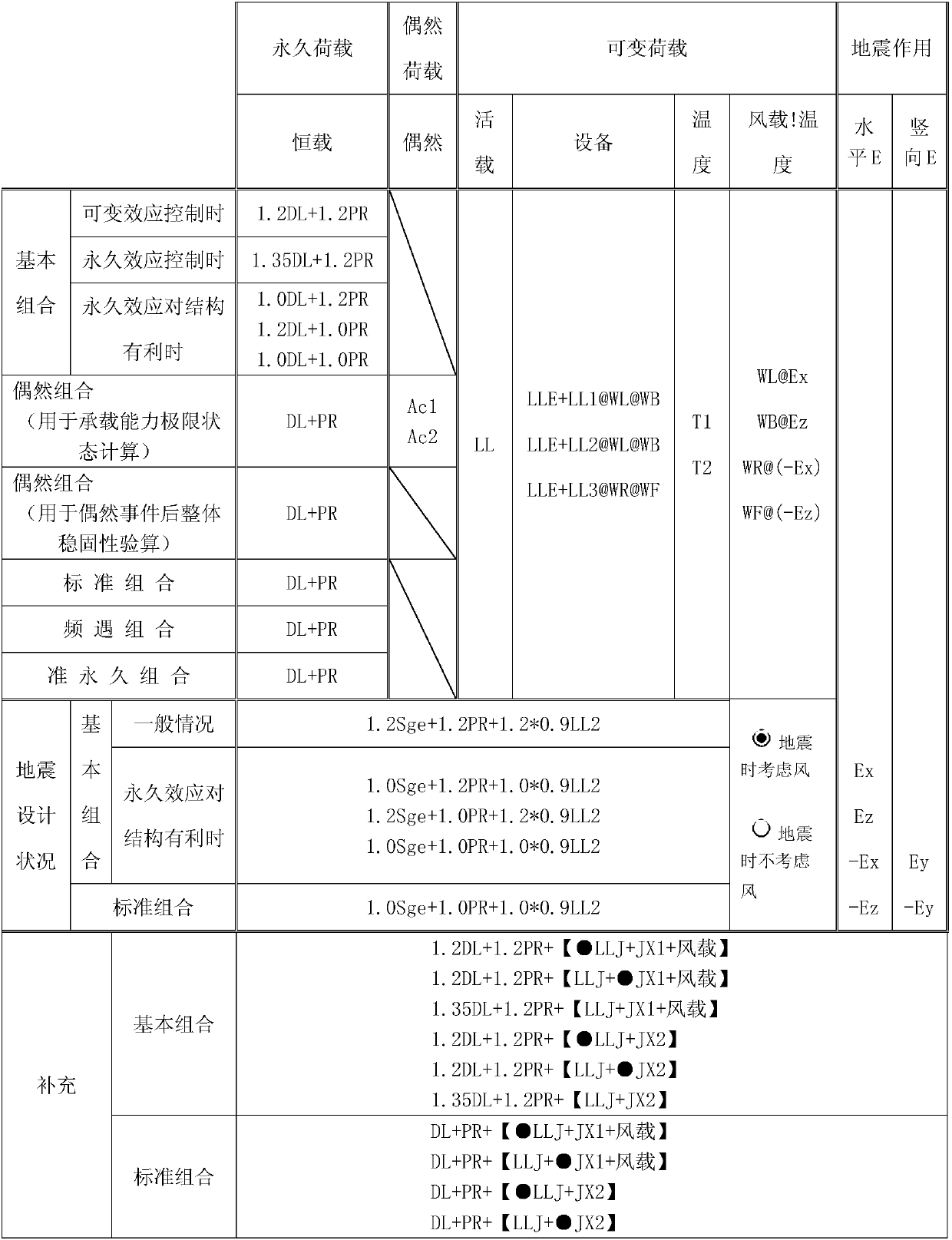
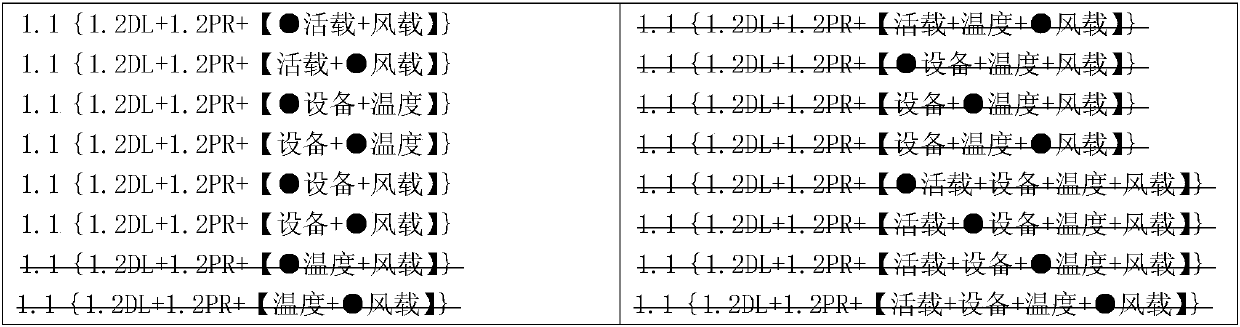
Load combination forming method
ActiveCN107644135AEasy to understandEasy to useSpecial data processing applicationsComputer architectureHouse building
The invention relates to the field of house building structures and discloses a load combination forming method. The method comprises the processes that load conditions are created, and names, numbers, categories and coefficients needed for participating in combination are set; after the load conditions capable of being merged are merged, all the load conditions capable of serving as independent conditions to participate in load combination are intermediate conditions; the intermediate conditions which belong to the same category and are mutually exclusive are arranged together to be load intermediate types; a logic relation among the intermediate types and a logic relation among the intermediate conditions are set; intermediate type combination formulas are created automatically in a classified mode, and the combination formulas not meeting the logic relation among the intermediate types are deleted; the intermediate type combination formulas are traversed, the intermediate conditionsare automatically substituted into the intermediate types to form all possible intermediate condition combination formulas, and the combination formulas not meeting the logic relation among the intermediate conditions are deleted; the coefficients of the load conditions are filled up completely according to the categories of the combination formulas automatically; and the coefficients of the loadconditions in the combination formulas are calculated automatically, and repeated load combinations are removed in a classified mode.
Owner:SOUTHWEST ELECTRIC POWER DESIGN INST OF CHINA POWER ENG CONSULTING GROUP CORP
Breeding method for intermediate type light/temperature-sensitive genic sterile line of japonica rice
The invention belongs to the field of rice breeding, discloses a breeding method for an intermediate type light / temperature-sensitive genic sterile line of japonica rice and relates to the utilization for the advantages of two-line japonica hybrid of rice. The invention aims to utilize a light / temperature-sensitive genic sterile gene to breed the intermediate type light / temperature-sensitive genic sterile line of japonica rice through the japonica hybrid. The two-line sterile line has japonica rice gene components at a certain ratio and can be matched with a hsien rice restoring line for breeding the two-line japonica hybrid of rice with strong heterosis. The proportion of the japonica rice gene components is less, the light sensitivity is weak and the seed production risk is reduced. On the basis of the characteristics of freedom and being not constrained by the restoring and maintaining relationship of the two-line japonica hybrid of rice, the application of the genic sterile line for breeding the two-line japonica hybrid of rice is capable of realizing the utilization for the heterosis of the two-line japonica hybrid of rice and greatly widening the limitation for the advanced genetic resource utilization of the two-line japonica hybrid of rice.
Owner:ZHEJIANG NONGKE SEED IND
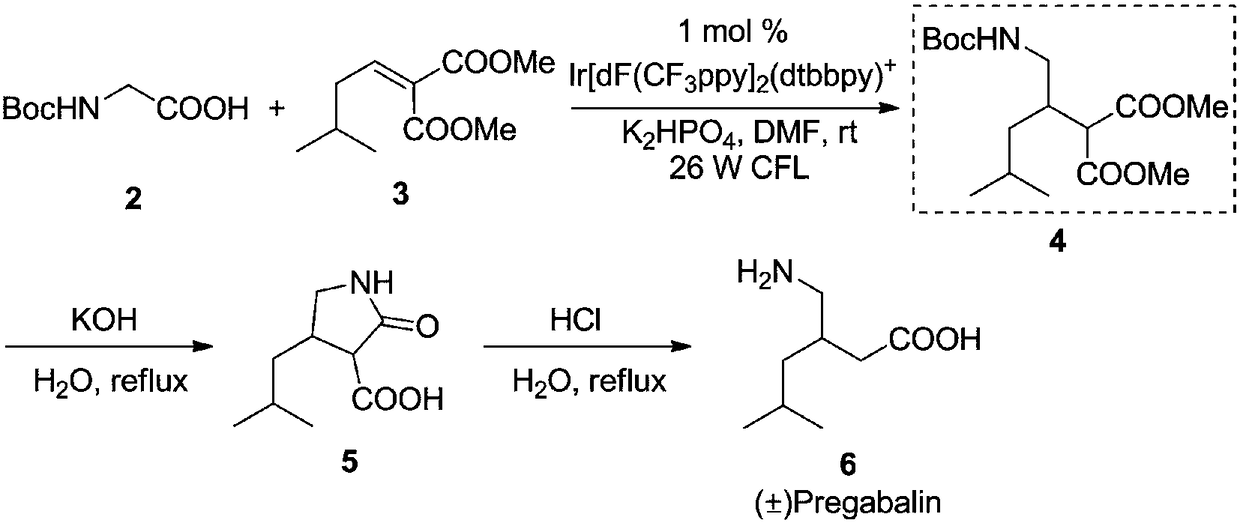
Preparation method of pregabalin
ActiveCN108358799ALow priceRaw materials are easy to getUrea derivatives preparationCarbamic acid derivatives preparationPregabalinDecarboxylation
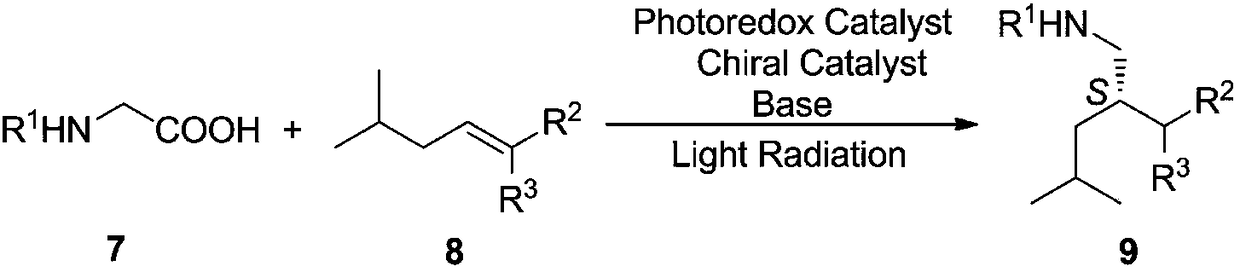
The invention discloses a preparation method of pregabalin. The method is characterized in that a compound shown as a formula 7 and a compound shown as a formula 8 are used as raw materials; a photooxidative reducing agent and a chiral organic catalyst are used under the illumination condition for combined catalysis of asymmetric free radical decarboxylation Michael addition reaction for synthesizing a chiral intermediate type compound shown as a formula 9; then, the compound shown as the formula 9 is hydrolyzed under the acidic or alkaline basic conditions to prepare a pregabalin type compound shown as a formula 1. The formulas 7, 8, 9 and 1 are shown in the description, wherein the R1 is Cbz, COOEt, COOMe, COOPr, PhNHCO or Boc; the R2 is COOEt, COOMe, COOBu, COOPr, COOBn, COOPh, COOAll or CN; the R3 is H or is identical to the R2.
Owner:GUIZHOU NORMAL UNIVERSITY
Production method of rice seeds
InactiveCN104871962AEasy to achieve super high yieldIncrease productionPlant genotype modificationGermplasmHeterosis
The invention discloses a production method of rice seeds. The method is characterized in that rice seeds produced by indica / japonica intermediate type restoring series R900 are adopted, wherein the indica / japonica intermediate type restoring series R900 are the intermediate materials which are prepared through wide spectrum and wide compability gene according to the high heterosis of indica / japonica subspecies; the intermediate materials are large in capacity, sufficient in source, high in ripening rate, and good in circularity; then the high pressure sieving is carried out to select multi-antibody seedling germplasm with polymerized indica / japonica stress resistance genes; the new rice spicy with high output, high quality and unified multi-antibody performances can be selected by rice inspection.
Owner:ANHUI HUIDA AGRO
Seedling identification method of purity of palmatum leaf pigment gland double labeled hybrid cotton seeds
InactiveCN102640591ASolve the purity identification problemGuarantee product qualitySeed and root treatmentPlant genotype modificationHybrid seedAgricultural science
The invention relates to a seedling identification method of the purity of palmatum leaf pigment gland double labeled hybrid cotton seeds, which comprises the following steps of: respectively sowing pigment gland labeled hybrid cotton F1 and male and female parent seeds thereof, and observing the presence and the amount of pigment glands on seedling necks and cotyledon, wherein pigment-glandless seeds are male parent seeds or seeds mixed with other pigment-glandless cotton, seeds with the pigment gland density between male parent seeds and female parent seeds are F1 hybrid seeds, and seeds with higher pigment gland density are female selfing parents or seeds mixed with other multi-pigment gland cotton; and determining the purity of F1 hybrid seeds according to the total number of measured cottonseeds and the quantity of seedlings with male parent, female parent and intermediate type pigment gland characters. The method provided by the invention is simple and easy to implement, fast and reliable, and solves the identification difficult problem of the purity of cotton hybrid seeds. The seed purity can be sampled for inspection at any time in the processes of acquisition, processing, storage, transportation and sale of hybrid cotton seeds according to needs, so that the production quality of the seeds is ensured, and the legitimate rights and interests of producers, sellers and users of the hybrid cotton seeds are protected.
Owner:HENAN ACAD OF AGRI SCI
Intermediate type nasopharynx expanding and bleeding-stopping bag and use method thereof
The invention discloses an intermediate type nasopharynx expanding and bleeding-stopping bag and a use method thereof. The intermediate type nasopharynx expanding and bleeding-stopping bag comprises a flexible rubber hose, wherein a blind tube is arranged at one end of the flexible rubber hose while a valve is arranged at the other end of the flexible rubber hose; a soft rubber bag is fixedly arranged at the middle position of the flexible rubber hose; the flexible rubber hose runs through the soft rubber bag; the soft rubber bag and the flexible rubber hose are sealed to form an integral structure; and the flexible rubber hose in the soft rubber bag is provided with a small hole serving as a channel which allows air and a liquid to flow in and out. The intermediate type nasopharynx expanding and bleeding-stopping bag, disclosed by the invention, has a simple structure, is easy to process and produce, has a low cost, can protect the surface of a wound of the nasopharynx part safely, can stop bleeding and prevent infection efficiently and can prevent adhesion and bleeding of the nasopharynx part after an operation.
Owner:GENERAL HOSPITAL OF PLA
Rice seed production method
InactiveCN106106129AEasy to achieve super high yieldIncrease productionHorticulture methodsPlant tissue cultureAgricultural scienceSubspecies
The invention discloses a rice seed production method. The method is characterized in that rice species seeds generated by the Indica-Japonica intermediate type restorer 'R900' are adopted; the Indica-Japonica intermediate type restorer 'R900' is obtained in the manner that an intermediate material large in inventory, sufficient in source, high in grain setting rate and good in grain plumpness is bred with wide-spectrum and wide-compatibility genes by utilizing the strong heterosis of Indica-Japonica subspecies, a multi-resistance pioneer germplasm with Indica-Japonica stress resistance genes polymerized is selected through high-pressure screening, and finally a high-yield, good-quality and multi-resistance-unified new rice species is selected through rice quality inspection.
Owner:ANHUI JINPEIYIN TECH
Efficient separation and purification method for F2-generation chili hybrid variety
InactiveCN107711467AShorten the timeLow costBioloigcal waste fertilisersAgriculture gas emission reductionPurification methodsMicrobiology
The invention discloses an efficient separation and purification method for F2-generation chili hybrid variety. The efficient separation and purification method comprises the steps of culturing a chili seedling, cultivating the chili seedling, and carrying out descendant screening. The expanding propagation and acceleration generation are carried out through soilless cultivation of a chili matrix,the front four generations are not screened, and at least four generations can be cultivated in one year, so that the material purification time is greatly shortened, and the purification cost is lowered; a fifth generation is cultivated through pot culture, so that the characters of a chili material is adequately expressed; a contrast is added, more than 95% of intermediate plants are removed byvirtue of a two-end selection method; and a sixth generation is subjected to ground screening in a field, and high-homozygous chili material with target character can be screened. The efficient separation and purification method is simple in operation, short in time and high in efficiency, a large amount of cost is saved, and the screened chili material is high in purity and good in character.
Owner:JIANGSU XUHUAI DISTRICT HUAIYIN AGRI SCI RES INST
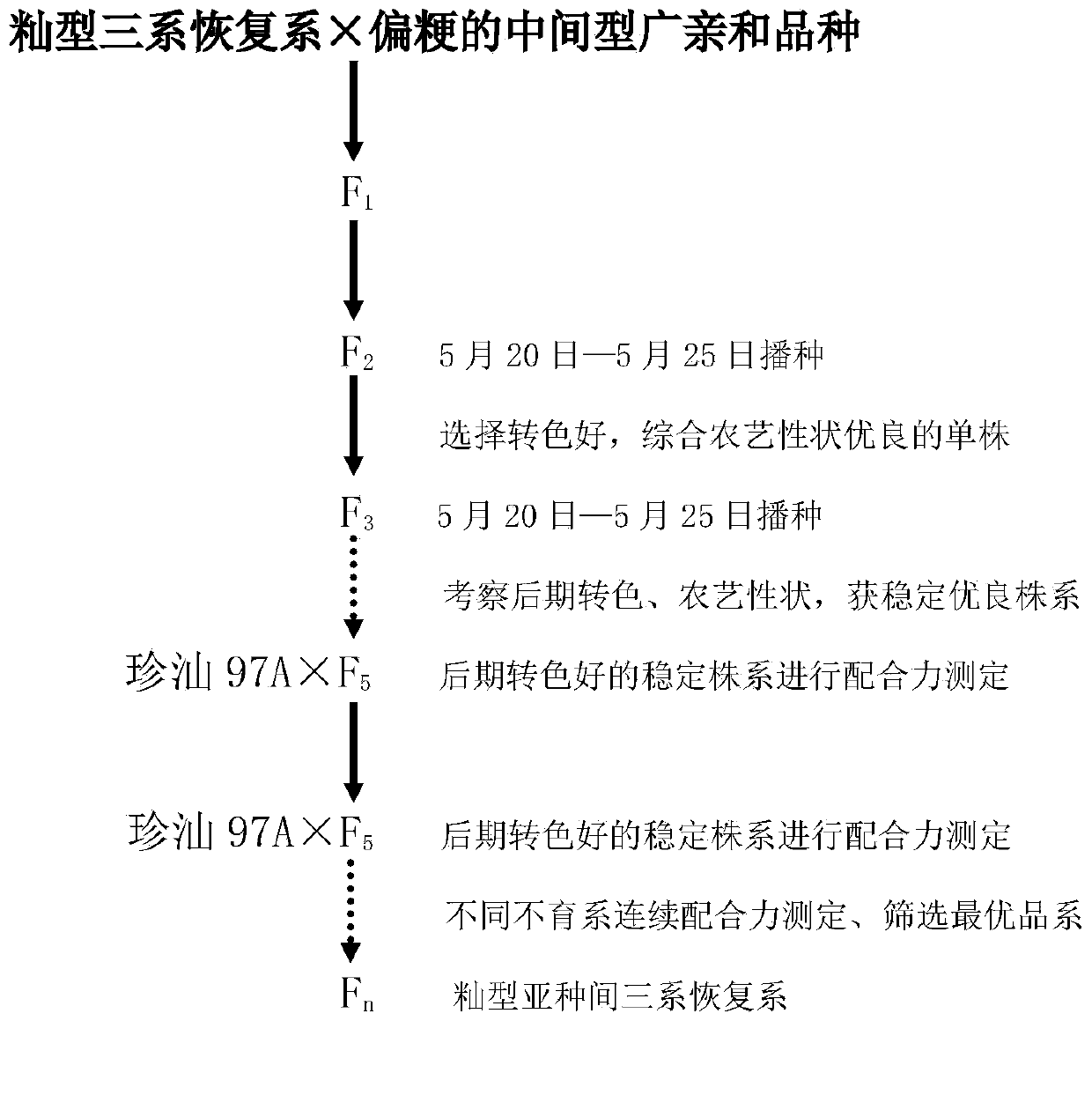
Intersubspecific indica type three-line hybrid rice restorer line breeding method
ActiveCN103461093AGood plant typeStrong plant typePlant genotype modificationAgricultural scienceVitality
The invention discloses an intersubspecific indica type three-line hybrid rice restorer line breeding method, which employs the indica type three-line hybrid rice restorer line as female parent and the japonica-clinous intermediate type wide-compatible variety as male parent for hybridization; F2 and filial generations are sown during May 20th-25th, and the colouring at later period is used as a first elimination index, and a pedigree method with other integrated agronomic characters is applied for selection till a stable line with good colouring at later period and excellent integrated agronomic characters are acquired; a 2-3 years combining ability determination is carried out for the stable line, and colouring of the test cross F1 at later period is observed; the selected indica type intersubspecific three-line hybrid rice restorer line is required to have good colouring at later period with excellent integrated agronomic characteristics, and the corresponding test cross variety is required to have superior advantages and good colouring at later period. The invention has the advantages that the restorer line and the hybrid rice combination configured using the restorer have strong vitality of root, lasting vitality of functional leaf, difficult premature senility, good colouring at later period, good strain shape, and superior advantage.
Owner:ZHENJIANG AGRI SCI INST JIANGSU HILLY AREAS
Process for preparing steel-based die by spraying
InactiveCN107737930ASuccessfully preparedCounteract volume shrinkageAdditive manufacturing apparatusMolten spray coatingMartensite transformationMartensitic stainless steel
The invention provides a process for preparing a steel-based die by spraying. The process for preparing the steel-based die by spraying includes the steps that firstly, a rapid prototyping is prepared; secondly, an intermediate type is prepared; and thirdly, the die is prepared in a spraying manner. The process for preparing the steel-based die by spraying has the following beneficial effects thatarc spraying and the rapid prototyping technology are combined, and the steel-based drawing die is successfully prepared; in the process of the preparing the die in the spraying manner, preparation of a ceramic model is the most critical technical link. Al2O3 ceramic powder is adopted and is matched with a proper sintering process, and the ceramic intermediate type with the strength and the sizeprecision both meeting requirements is prepared; and 3Cr13 martensitic stainless steel and 65Mn, 70, 82B high-carbon steel are adopted, by controlling the cooling speed of a deposition layer, a spraying deposition layer with a martensitic structure is obtained, and by means of phase change expanding of martensite, volume shrinkage during cooling is effectively counteracted.
Owner:天津汇友连众精密模具股份有限公司
Quick oriented selection and breeding technology for hybrid japonica rice restoration line
InactiveCN1843090AShorten the breeding periodReduce artificial emasculationPlant genotype modificationBiotechnologySeeds source
The invention relates to a technology of fast and directional breeding recovery strain of round-shaped hybrid rice, essentially comprising: leading recovery gene into the intermediate material got in high-yield normal strain breeding process, which can shorten the time for recovery strain breeding, then directional breeding the normal strain through backcross-testcross, finally breeding the new recovery strain possessing most advantage of normal strain. The invention makes use of the intermediate material of normal sterile rice in backcrossing process to breed recovery strain, which provides a new seed source for recovery strain gene, and eliminates artificial emasculation process. The invention provides technological support for yield increase of hybrid round-shaped rice on base of normal round-shaped rice yield.
Owner:辽宁省稻作研究所
Method for identifying phytochemical type of marihuana by virtue of one-step multiplex PCR
InactiveCN104293934AEasy to operateShort cycleMicrobiological testing/measurementAgarose electrophoresisElectrophoresis
The invention discloses a method for identifying a phytochemical type of a marihuana by virtue of one-step multiplex PCR. The method for identifying the phytochemical type of the marihuana by virtue of one-step multiplex PCR comprises the following steps: 1) extracting genome DNA of a marihuana plant; 2) designing a multiplex PCR primer; 3) preparing a PCR reaction system; 4) carrying out PCR amplification; 5) carrying out agarose electrophoresis; and 6) observing an electrophoretic band of a PCR product and judging a chemical type. By virtue of the one-step PCR reaction, a drug type marihuana can be amplified to obtain a specific THC stripe with the size being about 550bp, a fiber type marihuana can be amplified to obtain a CBD stripe with the size being about 1050bp, and an intermediate type marihuana can be amplified the THC stripe with the size being about 550bp and the CBD stripe with the size being about 1050bp at the same time, so that chemical type separation of a tested marihuana sample can be visually judged according to amplification stripes. The method for identifying the phytochemical type of the marihuana by virtue of the one-step multiplex PCR is not restricted by sampling time, a sampling site and the like in the traditional chemical detection, has the characteristics of short period, low cost and the like, is easy to operate, can be used for quickly identifying the phytochemical type of the marihuana and can be used by a public security drug detection department, an agriculture breeding department and the like, so that the method for identifying the phytochemical type of the marihuana by virtue of the primary multiplex PCR has a good application prospect.
Owner:云南省农业科学院经济作物研究所
High-yield cultivation method of non-pollution intermediate type cucumis melo in greenhouse
InactiveCN109076897AGuaranteed resultsGuaranteed fruit set rateFruit crop cultivationDiseaseFruit set
The invention discloses a high-yield cultivation method of non-pollution intermediate type cucumis melo in a greenhouse. The high-yield cultivation method of the intermediate type cucumis melo has theadvantages that the fruit quality is good, the yield is high, the influence of adverse growth environments of high temperature, high humidity and multiple diseases and insect pests can be effectivelyovercome, water and fertilizers can be effectively saved, economic benefits are increased, scientific field management is carried out according to the growth and development law, the fruiting rate and fruit-setting rate of cucumis melo are ensured by adopting artificial pollination, control methods of pest and disease damage, melons do not go bad easily, the marketing time of the melons is advanced, the economic benefits are good, and the income of the melon farmers is increased.
Owner:丁广礼
A method for synthesizing difluprednate from sterol fermentation product
InactiveCN103965277BSources are cheap and readily availableSuitable for industrial productionSteroidsIodideDouble bond
The invention provides a method for synthesizing difluprednate from a sterol fermentation product. The sterol fermentation product, namely 9 Alpha-hydroxyl-androstane-1,4-diene-3,17-diketone (9 Alpha-OH-AD) obtained by fermenting phytosterol of which the content in byproducts of the grease industry is very high, serves as a starting raw material. The method comprises the following 15 reaction steps in total: dehydrating steride 9-hydroxyl to form a double-bond; adding 17-carbonyl with acetylene; dehydrating; producing 21-copper carbonyl under an acid condition; epoxidizing 16,17-double bond; oxidizing periodide and introducing 21-hydroxyl; performing ring opening on 16,17 Alpha-epoxy hydrobromate; hydrogenating for removing 16 Beta bromine; forming a ring on orthoester; performing ring opening; esterifying; epoxidizing 9,11-double bond-Beta; enolizing and esterifying; performing ring opening on 6-electrophilic fluoro; and performing ring opening on 9,11-epoxy fluoro. According to the method, steride 17 Alpha and 21-dyhydroxyl are efficiently built by means of periodide oxidization, epoxide ring-opening and debromination and an important intermediate type 11 compound is obtained; in the whole process, a large quantity of heavy metal pollutant chromium which is generated when producing corticoid medicines in the traditional industry is effectively avoided, so that the method is green, environment-friendly and suitable to industrialized production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +1
Combined electric bicycle
InactiveCN101417683AEasy to installPromote decompositionConvertible cyclesMotorcyclesSingle vehicleMechanical engineering
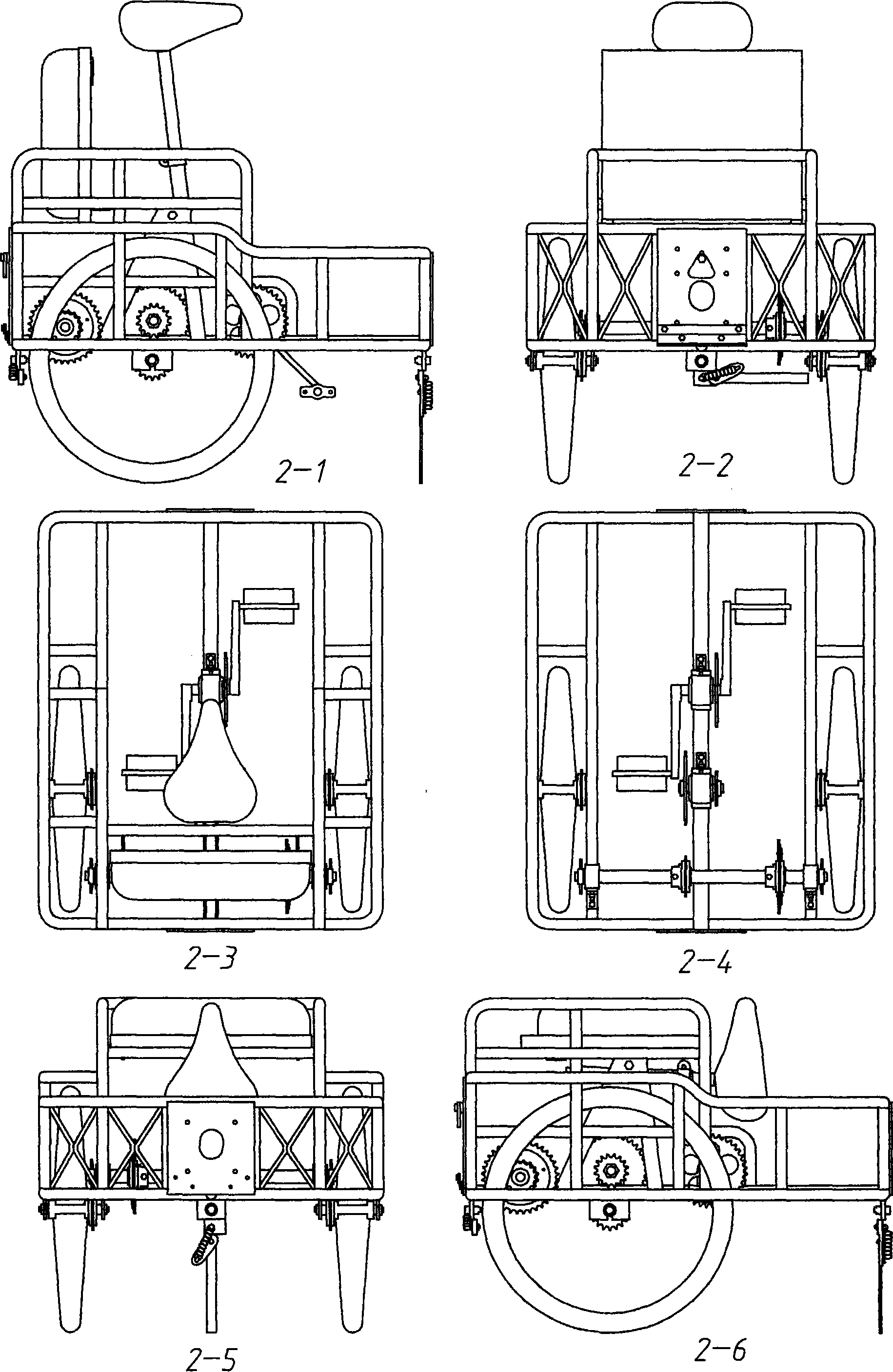
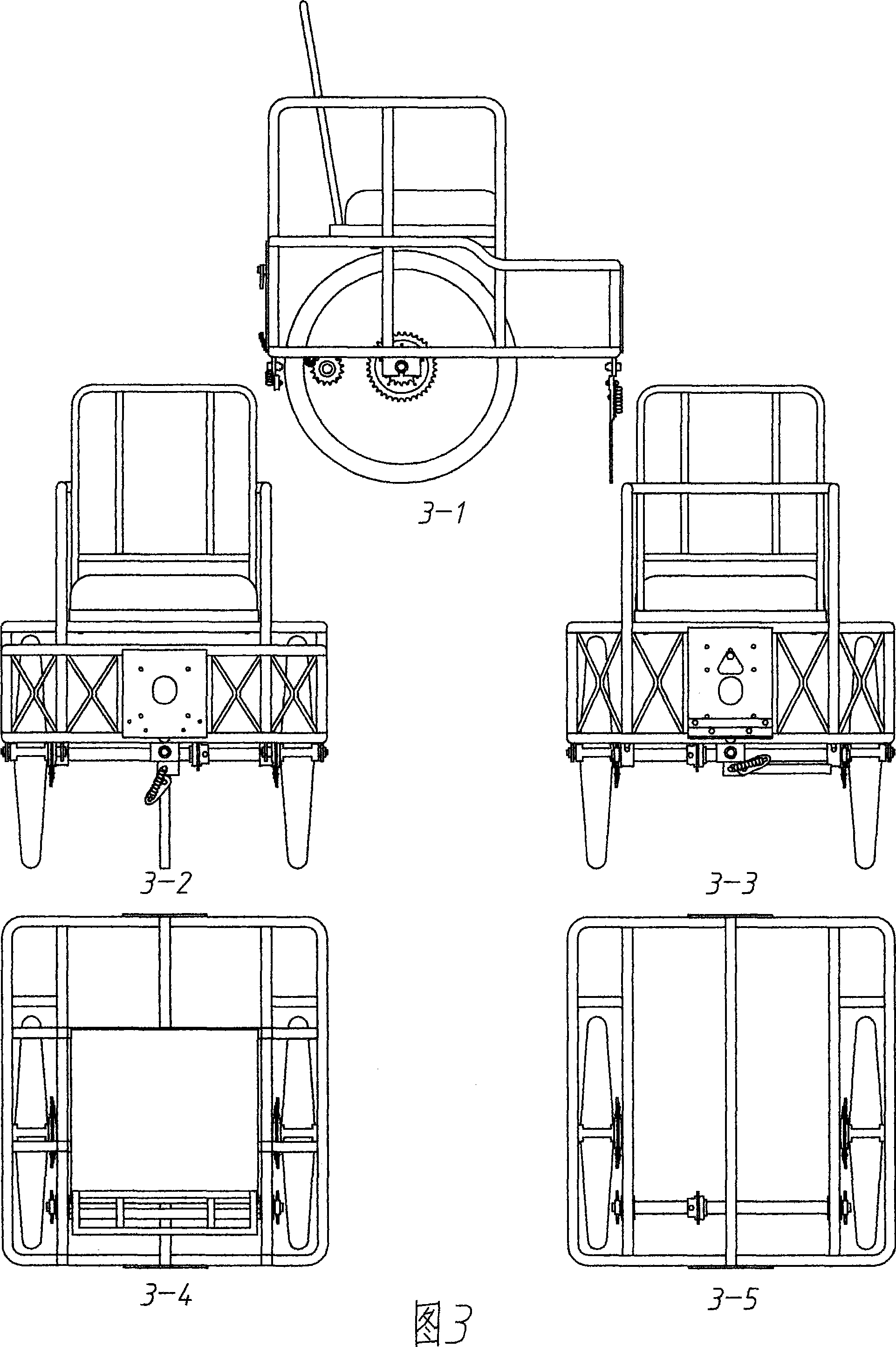
The invention discloses a combined electric bicycle as a common vehicle which is portable and stable on performance, simple on structure, suitable for both rural and urban districts and easy for operation. The conception of the electric bicycle takes the integrity into account and the design of the bicycle takes the wheel diameter and human body as standards and the vehicle type can be classified as: type-A direction bicycle; type-B push-bicycle; type-C soft-seat bicycle; type-D relaxing bicycle and type-E luggage bicycle. All the types of bicycle adopt the frame structure and two wheels are fixed inside in a parallel manner; the connecting part on both the front and back parts of the bicycle are unified and provided with the multi-direction turning function so as to lead each wheel to be connected with grounded connectors to form integrated four-wheel or multi-wheel vehicles. All the types of vehicle can be quickly assembled and disassembled and the intermediate-type and mini-type vehicles can be carried into elevators or rooms so as to bring convenience for charging and storage. Moreover, all the types of vehicle can be provided with components of parallel rotary rods and the like and then the parallel wheels can turn synchronously when the pedal or power is adopted, thus being suitable for the travelling by riding bicycle on the common roads and meeting the environment protection requirement. The main parts of the electric bicycle adopt the standard bicycle parts and standard steel pipes and the electric bicycle can be manufactured by the existing bicycle manufacturers.
Owner:陈冀鲁
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com