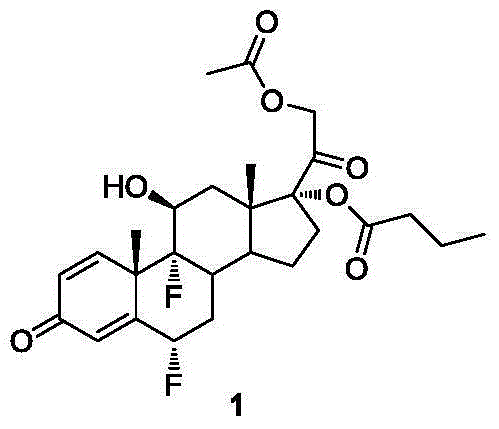
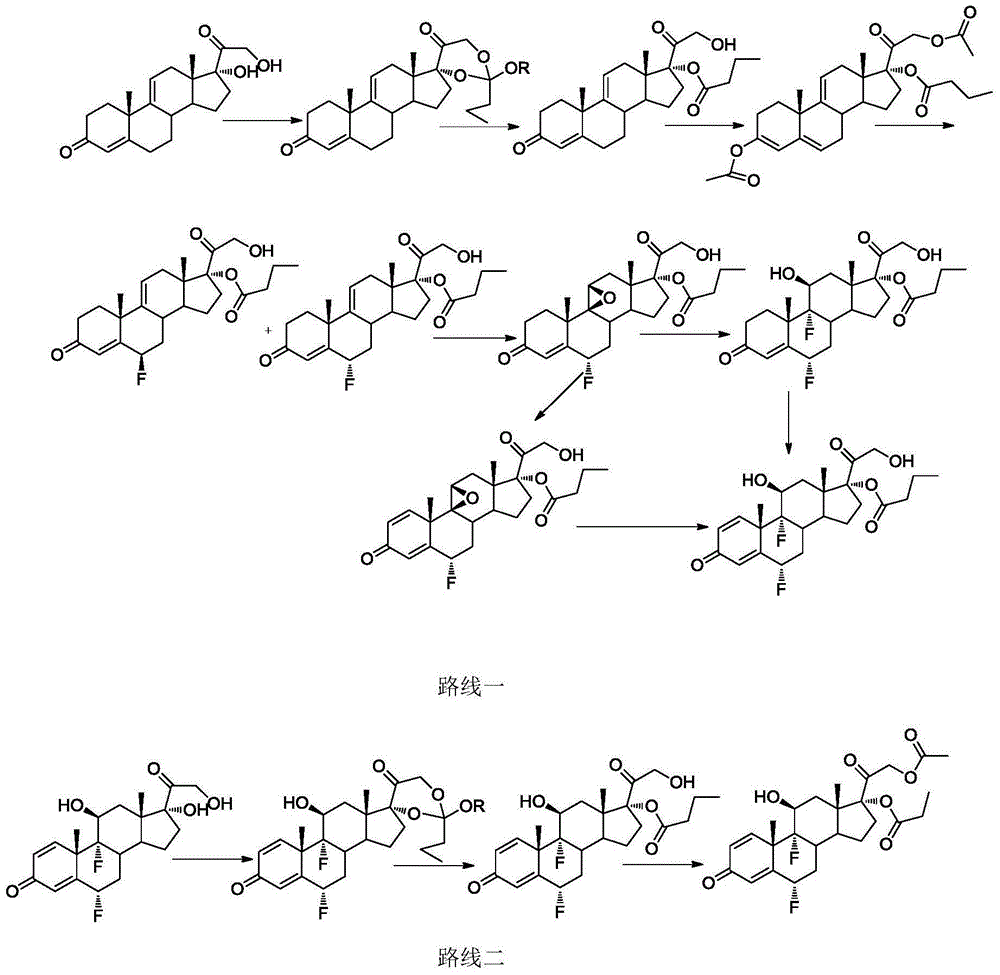
A method for synthesizing difluprednate from sterol fermentation product
A technology of sterol fermentation product and difluprednate, which is applied in the directions of steroids, organic chemistry, etc., to avoid the heavy metal pollutant chromium, the source of raw materials is cheap, and the source of raw materials is easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
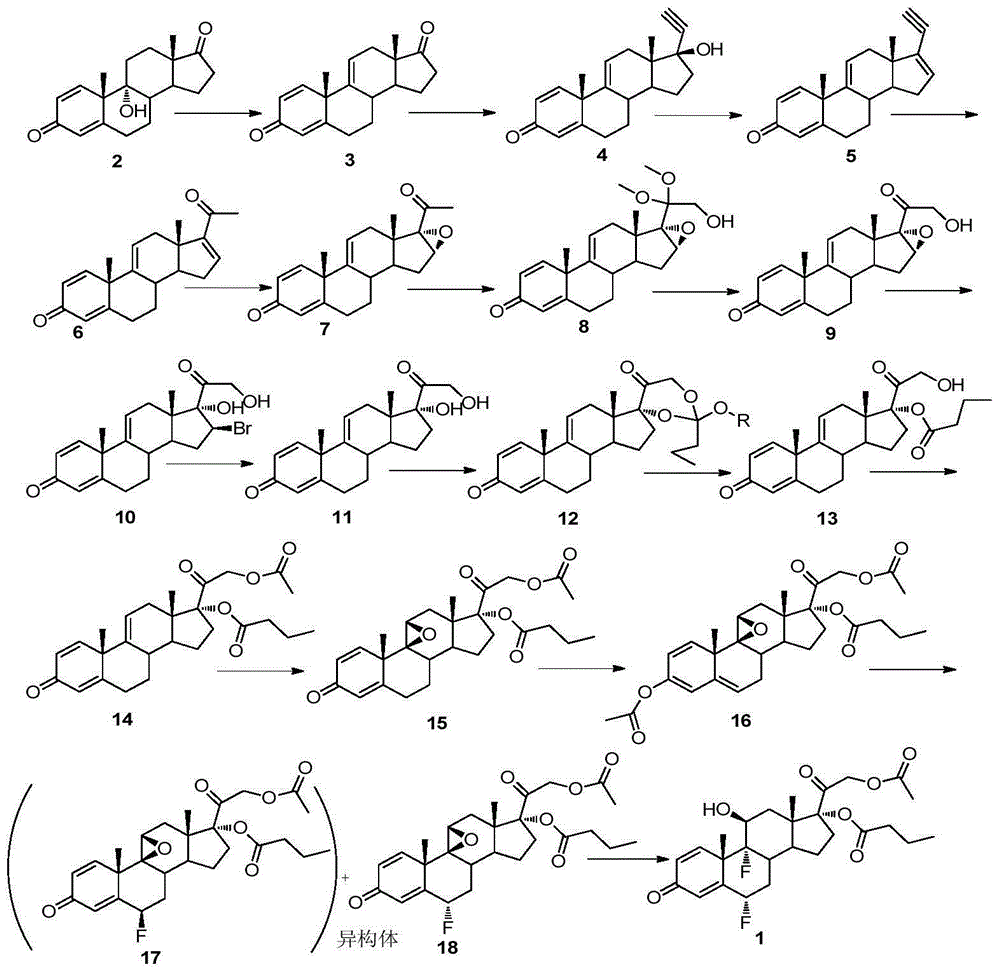
[0054] Embodiment 1: Synthesis of androst-1,4,9(11)-triene-3,17-dione (compound of formula 3)
[0055] Under argon protection, add 15.0g 9α-hydroxy-1,4-diene-3,20-dione (compound of formula 2), 100mL benzene, 35mL (5 times equivalent) of BF to the reaction flask 3 AcOH solution, reflux and stir for 30 minutes, cool at room temperature, add 50mL of water, stir for 30 minutes, let the solution separate, wash the organic phase with water, dry over anhydrous sodium sulfate, and drain to obtain 14.2g of light yellow solid (compound of formula 3) , 90% yield.
[0056] Formula 3 compound: molecular weight, 282.38; 1 HNMR (400MHz, CDCl 3 ): δ7.18(d, J=10.2Hz, 1H, C 1 H),6.28(dd,J=10.2,1.8Hz,1H,C 2 H),6.08(s,1H,C 4 H),5.58(s,1H,C 11 H),1.43(s,3H,C 19 H),0.91(s,3H,C 18 H).
Embodiment 2
[0057] Example 2: Synthesis of 17β-hydroxy-17α-acetylene-pregna-1,4,9(11)-trien-3-one (compound of formula 4)
[0058] Under argon protection, add 10g of potassium hydroxide, 40mL of tetrahydrofuran and 9mL of ethanol into the reaction flask, stir at 60°C for 2 hours, cool to 3°C, and pour pure acetylene gas into the solution for 2 hours (8 times equivalent) , then add 12mL tetrahydrofuran solution containing 5.9g androst-1,4,9(11)-triene-3,17-dione (Formula 3) into the above solution, stir and react for 2 hours, TLC detects that the reaction is complete , then added 60 mL of 10% hydrochloric acid solution to the solution, stirred for 1 hour, filtered, washed with water, dried over anhydrous sodium sulfate, and drained to obtain 6.1 g of light yellow powder (compound of formula 4), with a yield of 95%.
[0059] Formula 4 compound: molecular weight, 308.41; 1 HNMR (400MHz, CDCl 3 )δ7.20(d,J=10.2Hz,1H,C 1 H), 6.26(d, J=10.2Hz, 1H, C 2 H),6.05(s,1H,C 4 H), 5.58(d, J=5.4Hz, 1...
Embodiment 3
[0060] Example 3: Synthesis of 17-acetylene-pregna-1,4,9(11), 16(17)-tetraen-3-one (compound of formula 5)
[0061] Under argon protection, dissolve 308 mg of 17β-hydroxy-17α-acetylene-pregna-1,4,9(11)-trien-3-one (compound of formula 4) in 2 mL of treated benzene, add 1 mL of redistilled Add 0.29mL of phosphorus oxychloride slowly under stirring in an ice-water bath. After the dropwise addition, reflux at 80°C for 6 hours. After cooling, pour the solution into 10mL of 10% hydrochloric acid in an ice-water bath, stir for 30 minutes, and use two After extraction with methyl chloride, the extract was washed with water, saturated brine, and water respectively, dried over anhydrous sodium sulfate, and 197 mg of a white solid (compound of formula 5) was obtained by column chromatography, with a yield of 67.5%.
[0062] Formula 5 compound: molecular weight, 290.40; 1 HNMR (400MHz, CDCl 3 ):δ7.20(d,J=10.2Hz,1H,C 1 H), 6.29(d, J=10.2Hz, 1H, C 2 H),6.11(s,1H,C 4 H),6.07(s,1H,C 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com