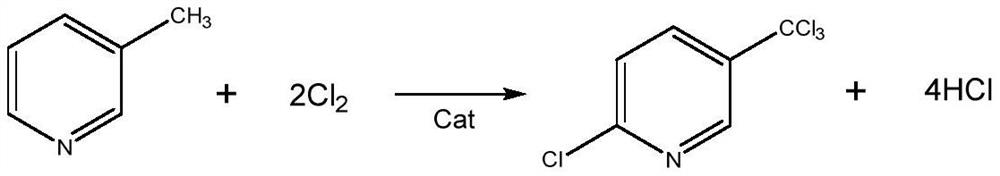
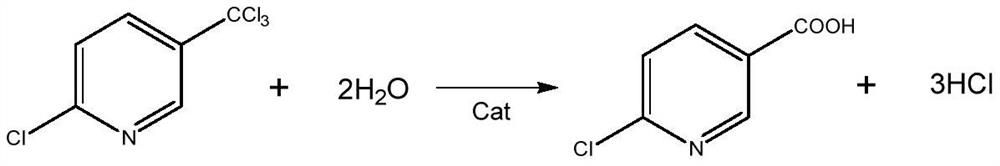
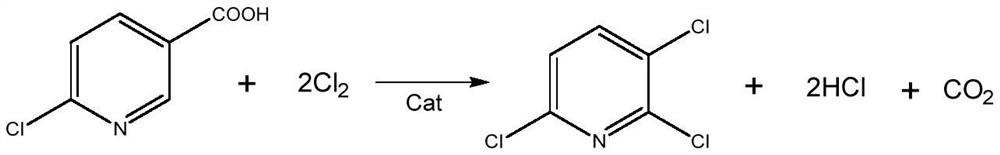
A kind of preparation method of 2,3,6-trichloropyridine
A technology of trichloropyridine and picoline, which is applied in the field of preparation of 2,3,6-trichloropyridine, can solve the problems of low selectivity of target products, low conversion rate of raw materials, harsh equipment requirements, etc., and achieve good industrial application Foreground, source is cheap and easy to get, the effect of strong processing capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take 10g of hydrogen-type ZSM-5 powder with a silicon-aluminum ratio of 80, 30g of γ-Al 2 o 3 , 0.5g scallop powder, add 0.5g nitric acid (68wt%, the same below) and 10g water, mix uniformly, extrude and form through screw extruder, the catalyst after forming is dried at 120 DEG C for 5h, roasted at 550 DEG C for 6h, after roasting The catalyst was crushed and sieved to obtain a granular catalyst A of 20-40 mesh.
[0032] Get above-mentioned 10g catalyst A, pack in the cylindrical fixed-bed reactor of internal diameter 25mm, plunger pump pumps the carbon tetrachloride solution of 3-picoline, the mass ratio of 3-picoline to carbon tetrachloride is 1 :10, 3-methylpyridine weight hourly space velocity is 6h -1 , the weight hourly space velocity of water is 3h -1 , the chlorine gas flow rate is 2L / min, 3-picoline, water, and chlorine gas are vaporized by the preheating tube and then passed into the fixed-bed reactor at the same time. The fixed-bed reactor is heated by re...
Embodiment 2
[0034] Get catalyst A in 10g embodiment 1, pack in the cylindrical fixed-bed reactor of internal diameter 25mm, plunger pump pumps the carbon tetrachloride solution of 3-picoline, 3-picoline and carbon tetrachloride mass The ratio is 1:10, and the space velocity of 3-picoline is 6h -1 , the weight hourly space velocity of water is 3h -1 , the flow rate of chlorine gas is 2L / min, 3-picoline, water, and chlorine gas are vaporized by the preheating tube and then passed into the fixed bed reactor at the same time. Obtain 2866g of the reaction product mixture in the receiving tank, add ammonia water to neutral, and separate the liquid with a pear-shaped funnel to obtain an organic solution phase, add 30g of anhydrous magnesium sulfate, suction filter, and then rotate evaporate. Remove carbon tetrachloride to obtain white crystals, namely 2,3,6-trichloropyridine. After analysis and detection, the purity of 2,3,6-trichloropyridine is 87%, and the yield is 90%.
Embodiment 3
[0036] Referring to Example 1, catalyst B was prepared by replacing the hydrogen-form ZSM-5 raw powder with a silicon-aluminum ratio of 80 with the hydrogen-form ZSM-5 raw powder with a silicon-aluminum ratio of 230.
[0037] Get 10g catalyst B, pack in the cylindrical fixed-bed reactor of internal diameter 25mm, plunger pump pumps the carbon tetrachloride solution of 3-picoline, 3-picoline and carbon tetrachloride mass ratio are 1: 10,3-Methylpyridine has a weight hourly space velocity of 6h -1 , the weight hourly space velocity of water is 3h -1, the flow rate of chlorine gas is 2L / min, 3-picoline, water, and chlorine gas are vaporized by the preheating tube and then passed into the fixed bed reactor at the same time. Obtain 2890g of the reaction product mixture in the receiving tank, add ammonia water to adjust to neutrality, separate the liquid with a pear-shaped funnel, and obtain the organic phase, add 30g of anhydrous magnesium sulfate, suction filter, and then rotary ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com