Gemcitabine hydrochloride lyophilized preparation
A gemcitabine hydrochloride and freeze-dried preparation technology, which is applied in the field of gemcitabine hydrochloride freeze-dried preparations, can solve the problems of inability to reduce the risk of patients, achieve the effects of reducing drug risk, solving stability problems, and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] formula:
[0019] Preparation: First, dissolve the prescribed amount of antioxidant in an enamel or glass container with an appropriate amount of water for injection, filter and add the prescribed amount of gemcitabine hydrochloride, lyoprotectant and appropriate amount of water for injection, stir to dissolve completely, and then add injection Use water to make the total volume 1000ml, then stir to make the mixture even, and finally filter, sub-package, and freeze-dry to obtain.
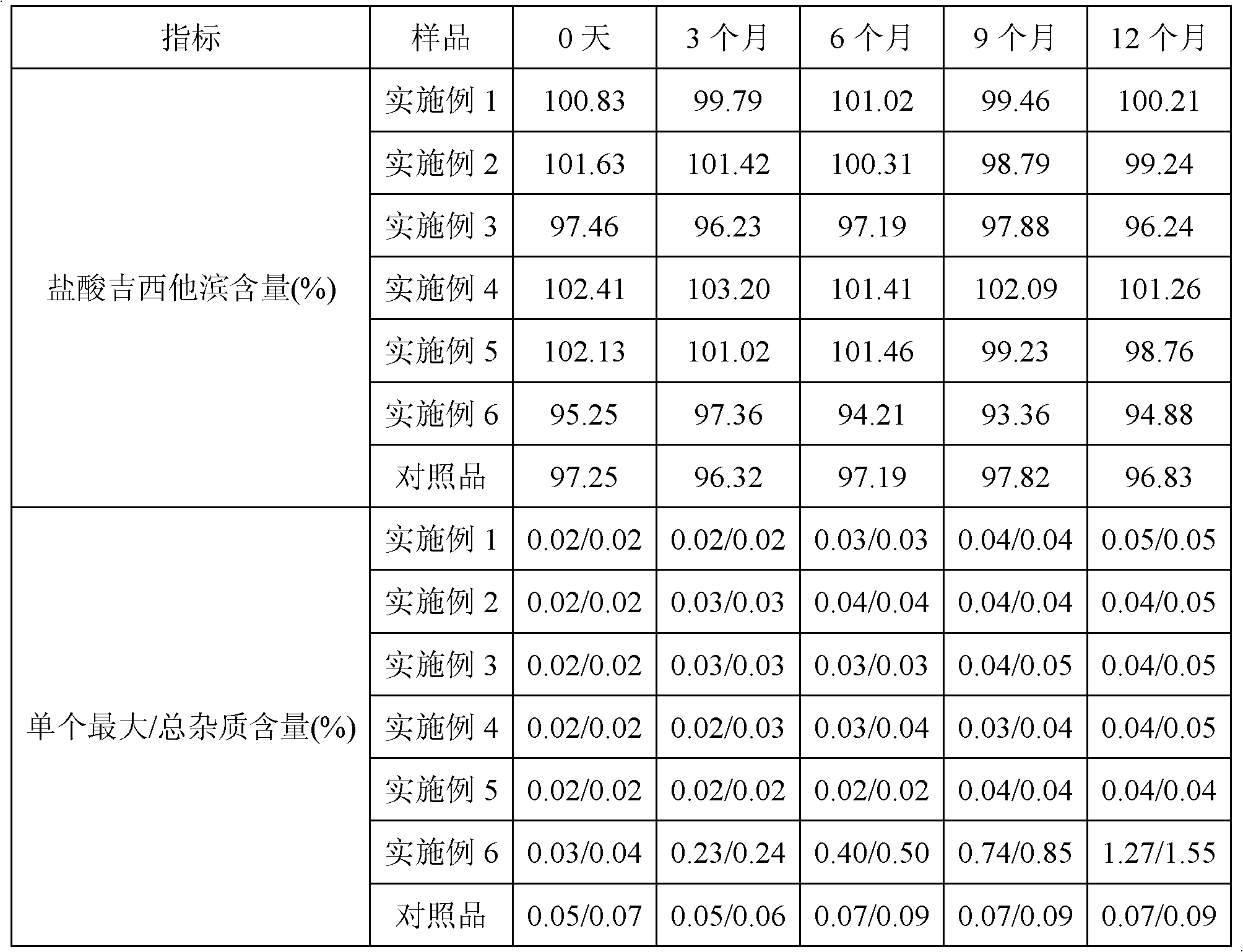
[0020] Stability test: place the finished gemcitabine hydrochloride freeze-dried preparation for 12 months under the conditions of 25°C and relative humidity of 60±5%. , the content of gemcitabine hydrochloride and the content of the single largest / total impurity in the sample were detected every 3 months. The test data are shown in Table 1.
Embodiment 2
[0022] formula:
[0023] The rest of the content is the same as described in Example 1.
Embodiment 3
[0025] formula:
[0026] The rest of the content is the same as described in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com