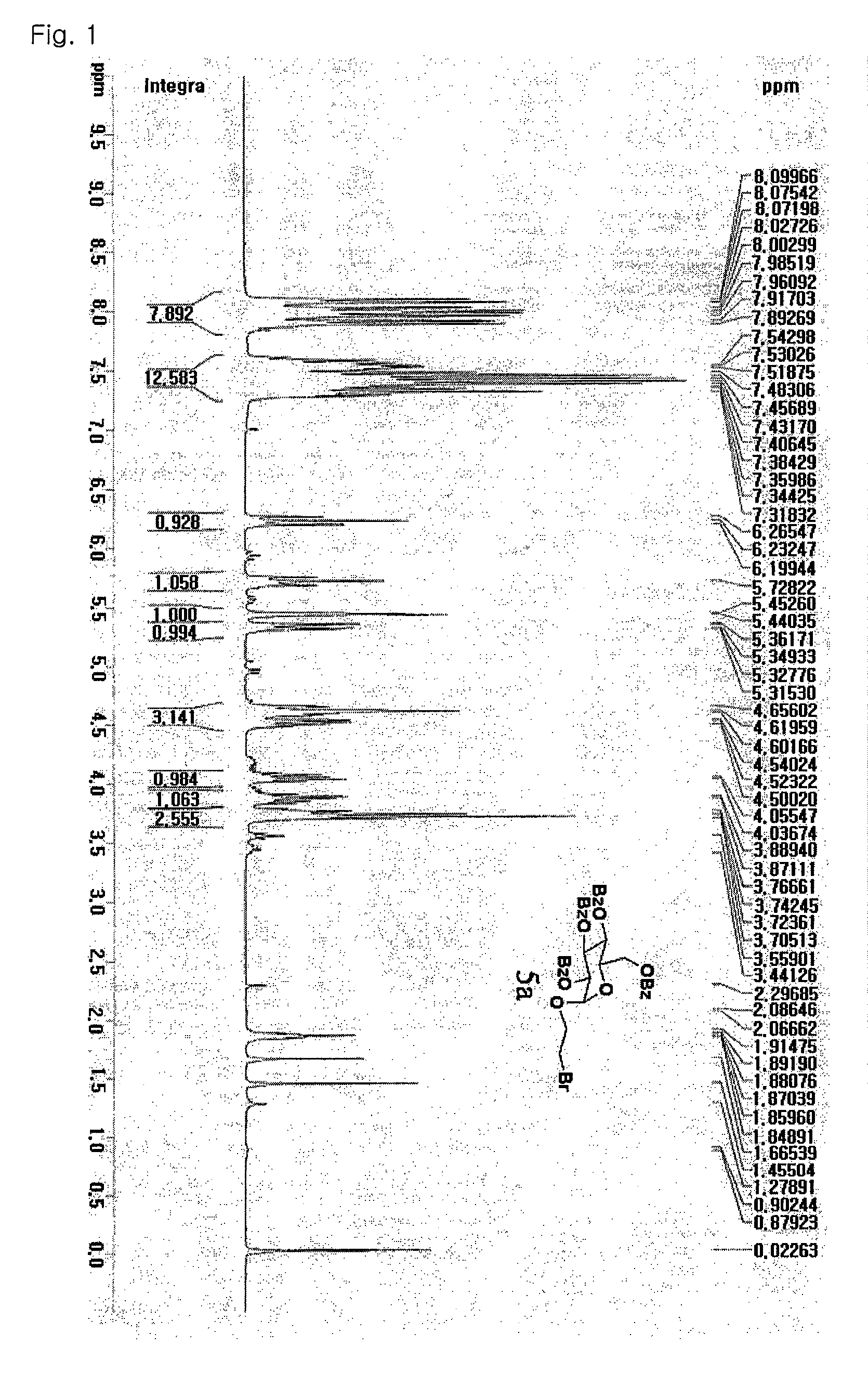
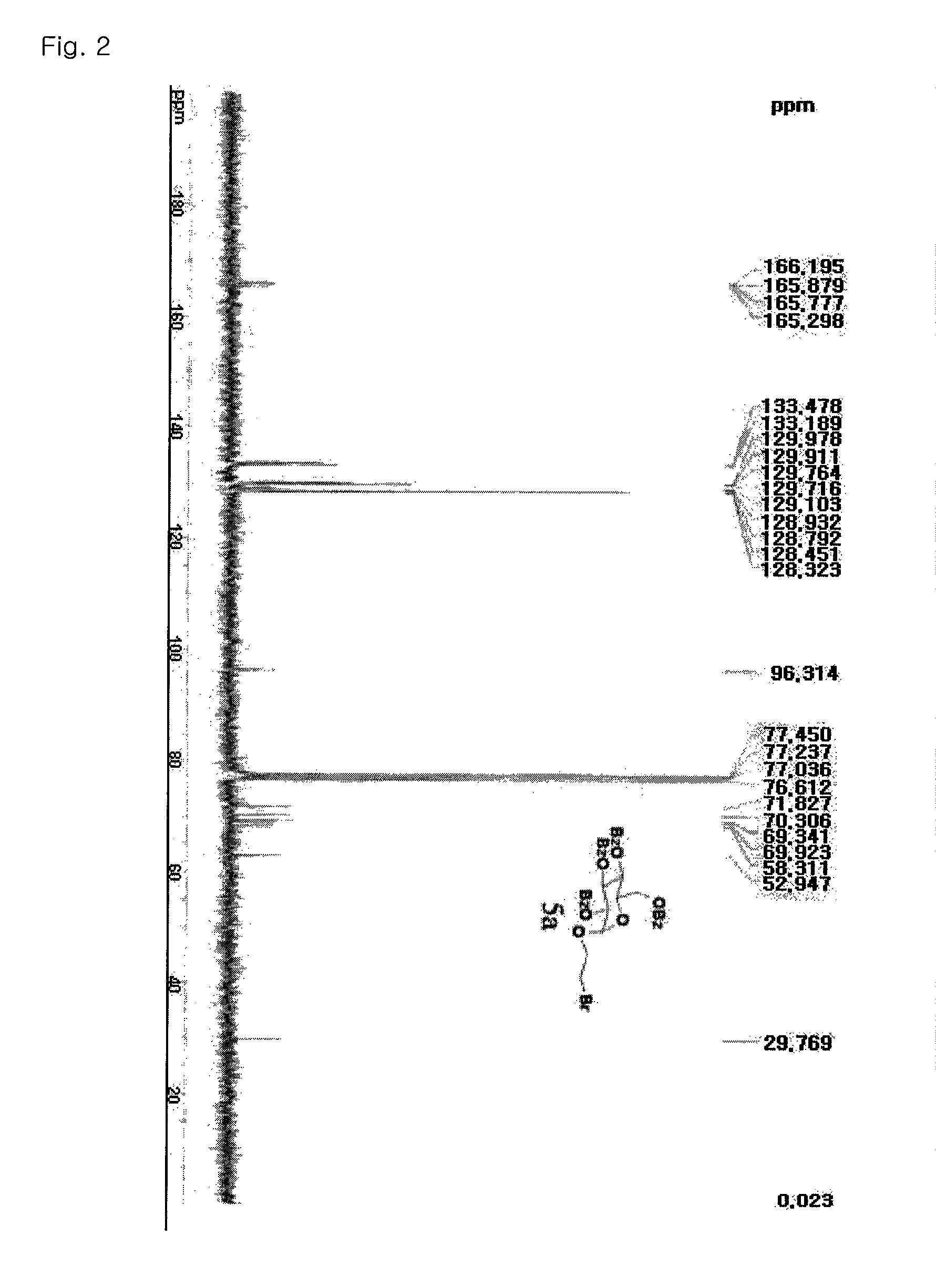
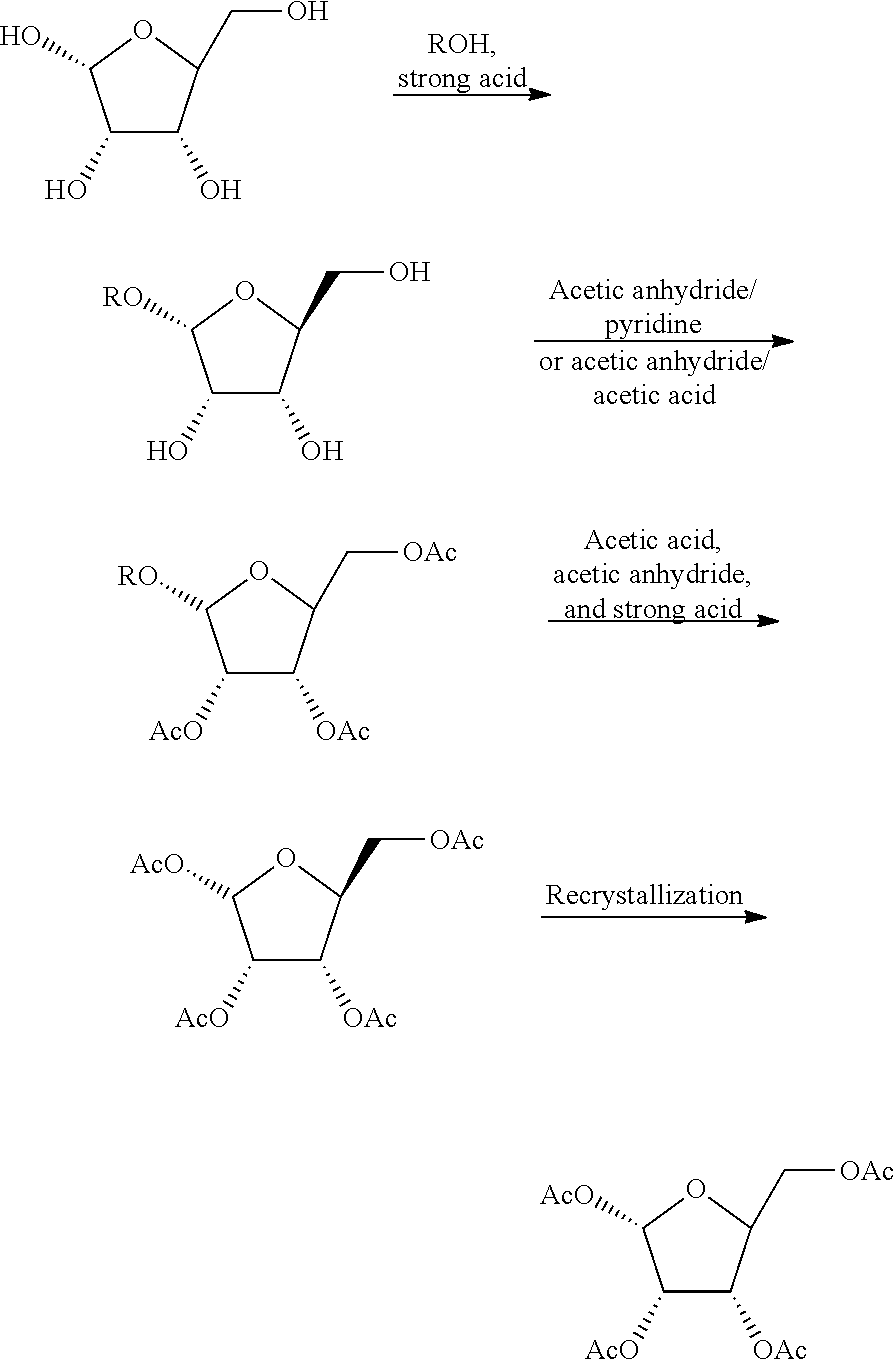
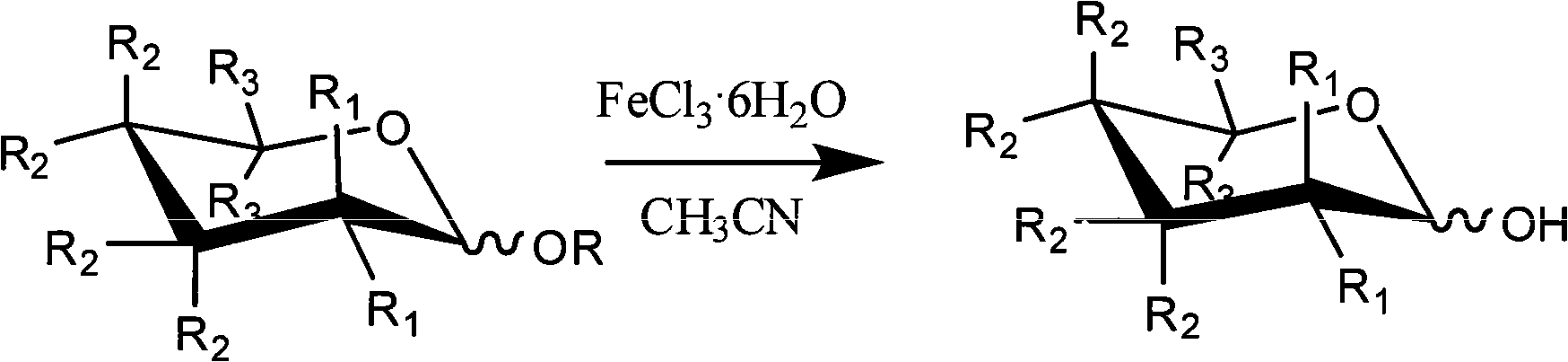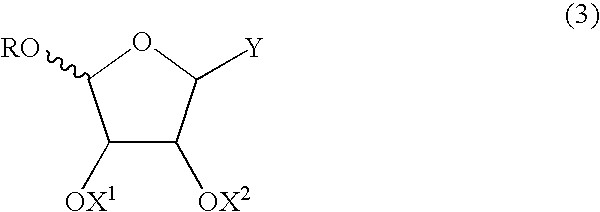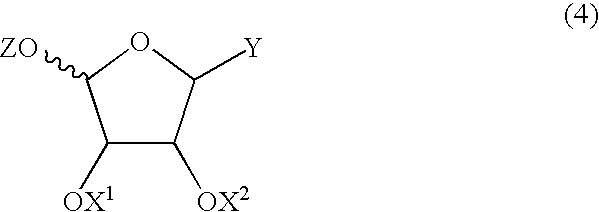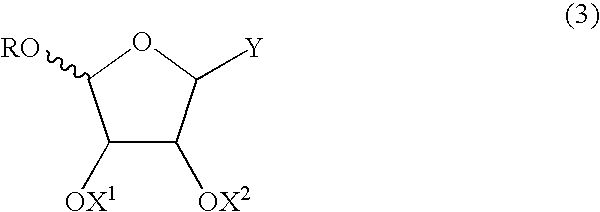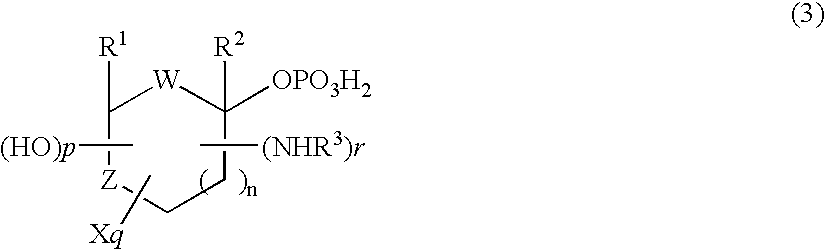Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Anomer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
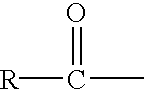
An anomer is a type of geometric variation found at certain atoms in carbohydrate molecules. An epimer is a stereoisomer that differs in configuration at any single stereogenic center. An anomer is an epimer at the hemiacetal/acetal carbon in a cyclic saccharide, an atom called the anomeric carbon. The anomeric carbon is the carbon derived from the carbonyl carbon (the ketone or aldehyde functional group) of the open-chain form of the carbohydrate molecule. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations.
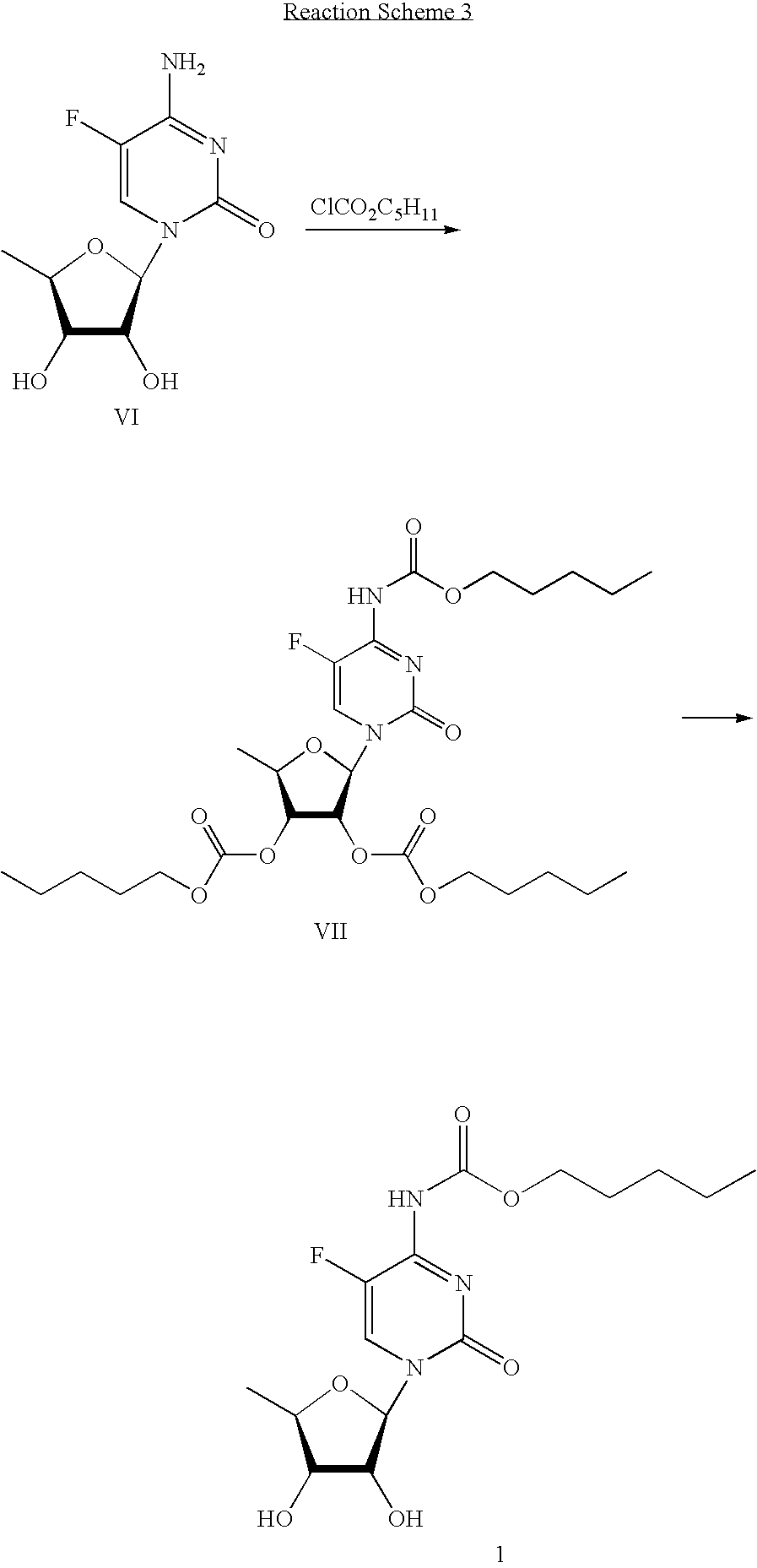
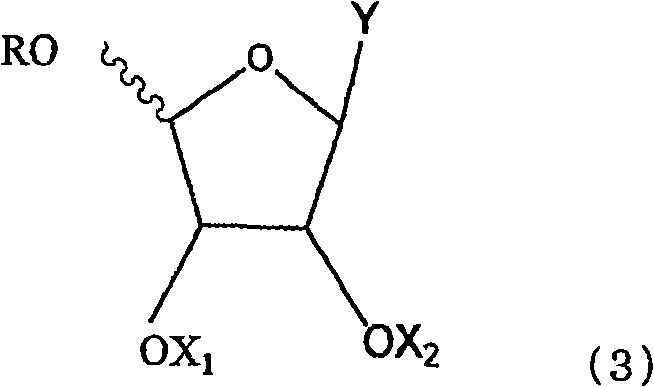
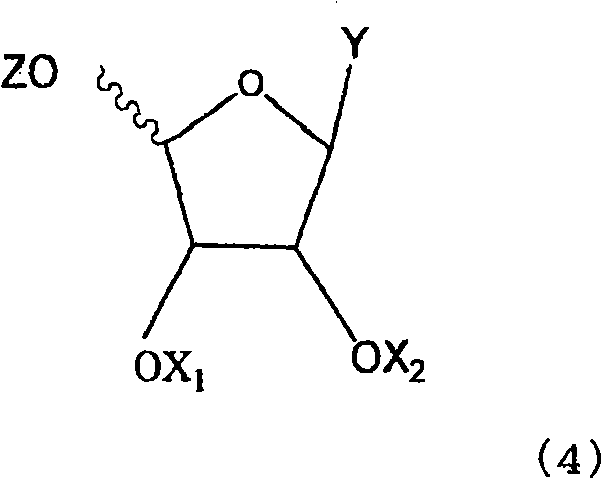
Anti-viral nucleoside analogs and methods for treating viral infections, especially HIV infections
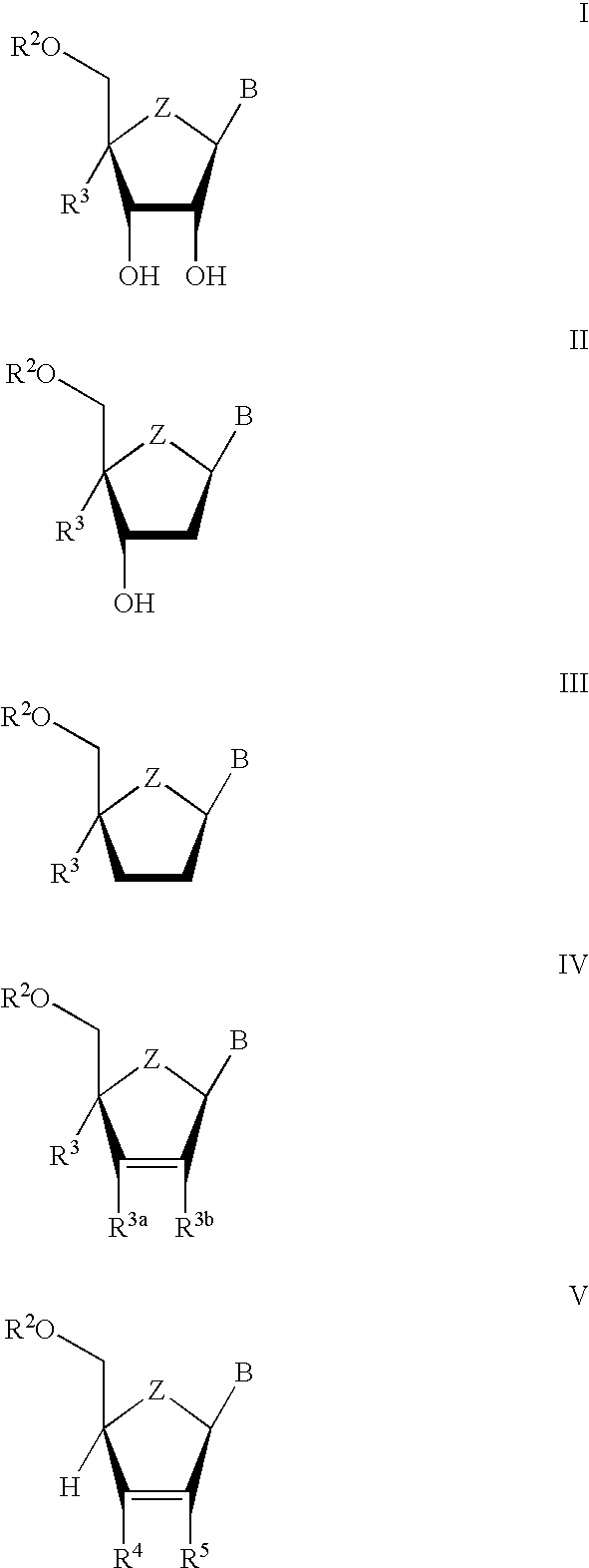
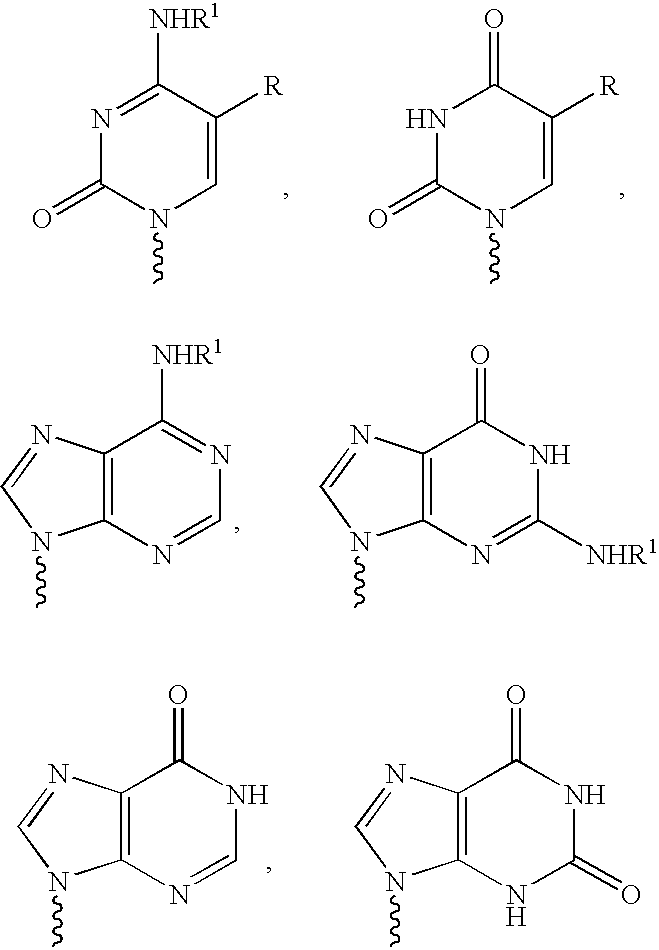
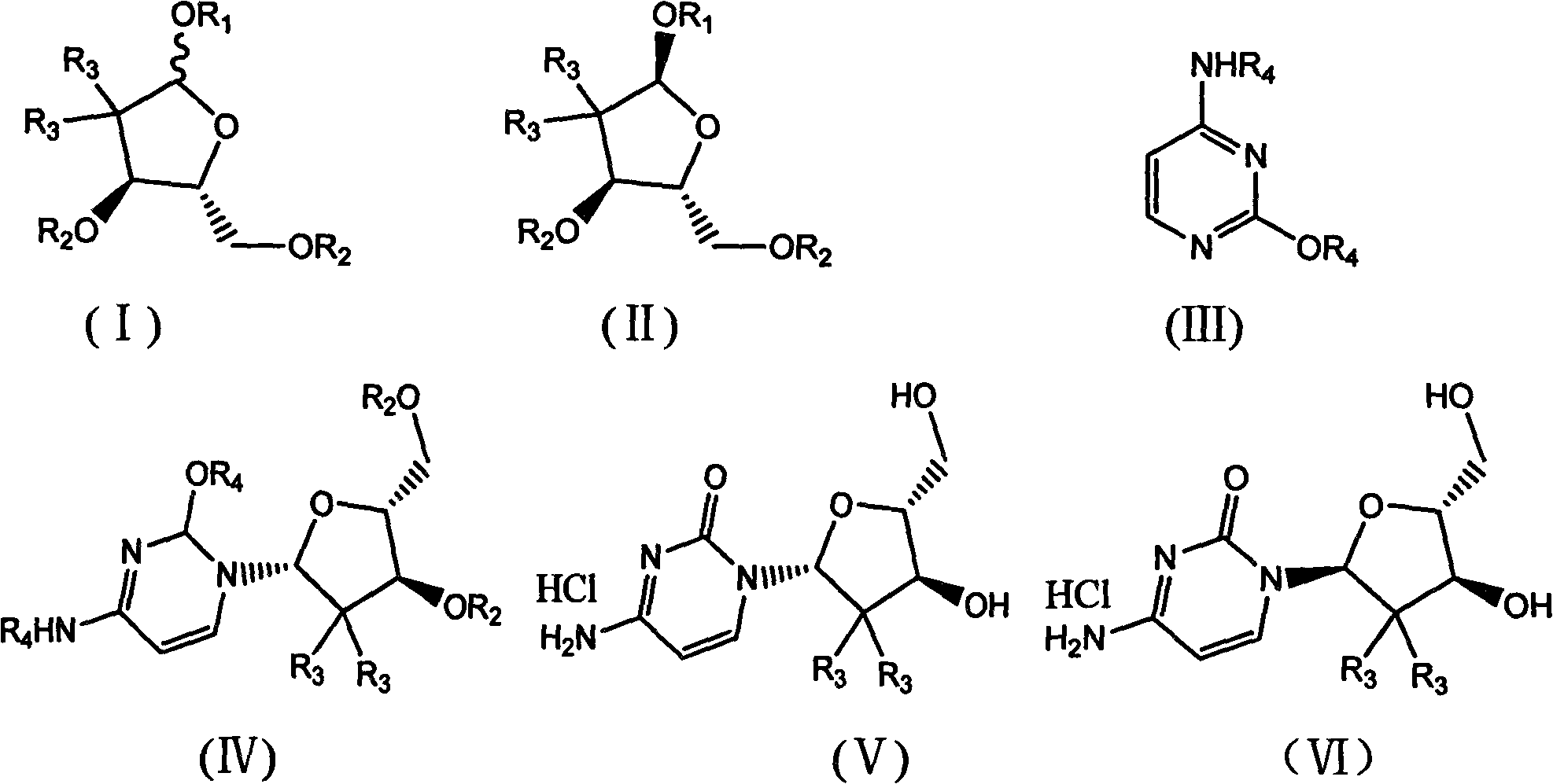
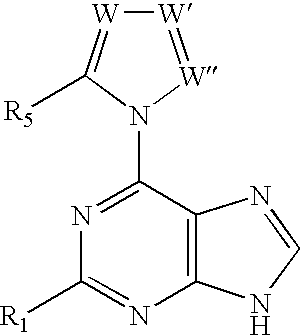
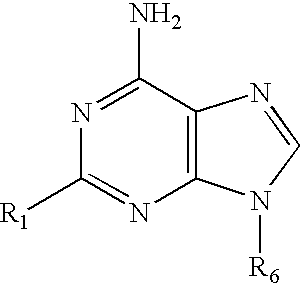
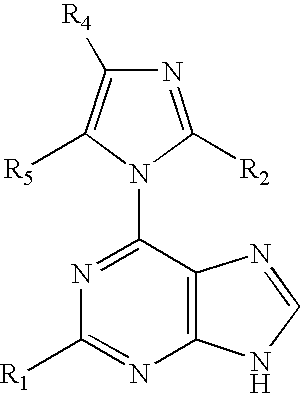
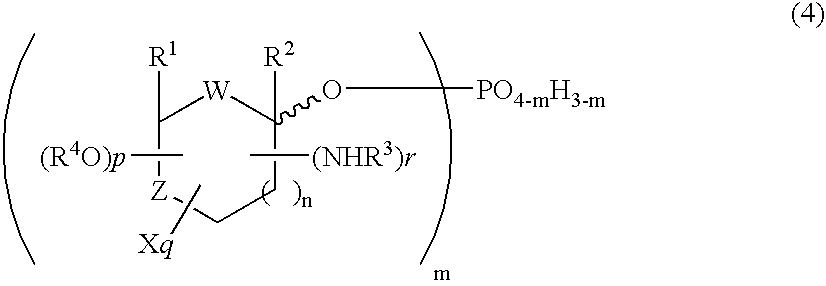
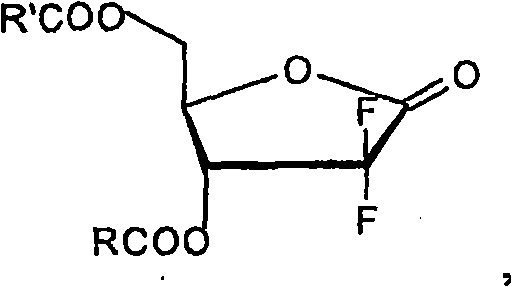
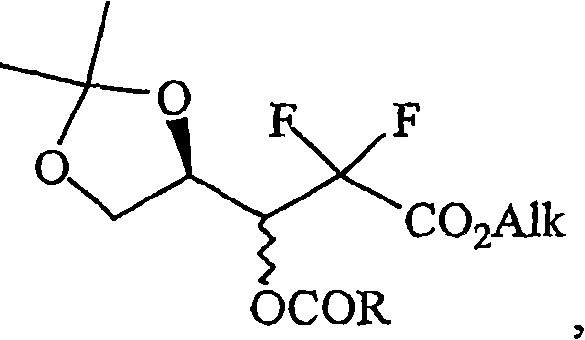
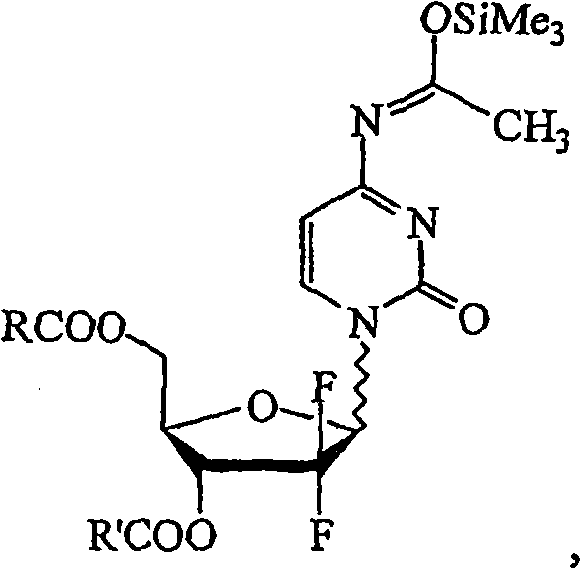
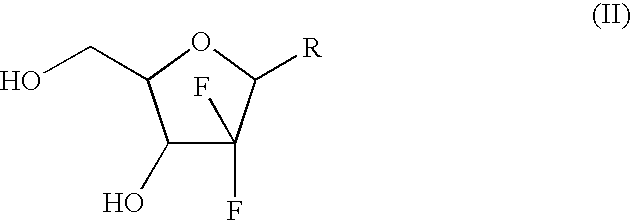
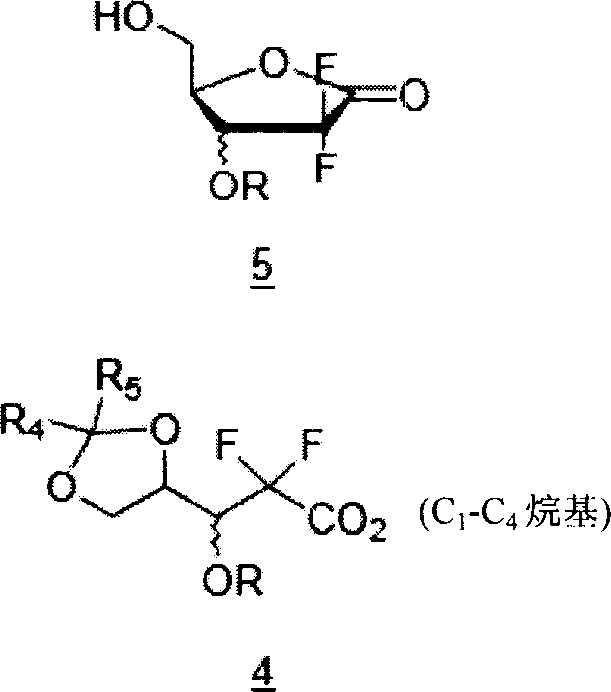
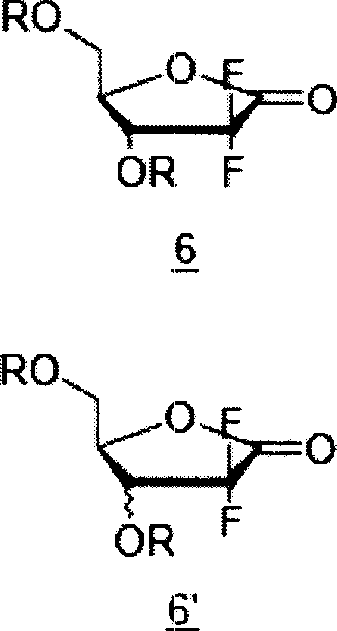
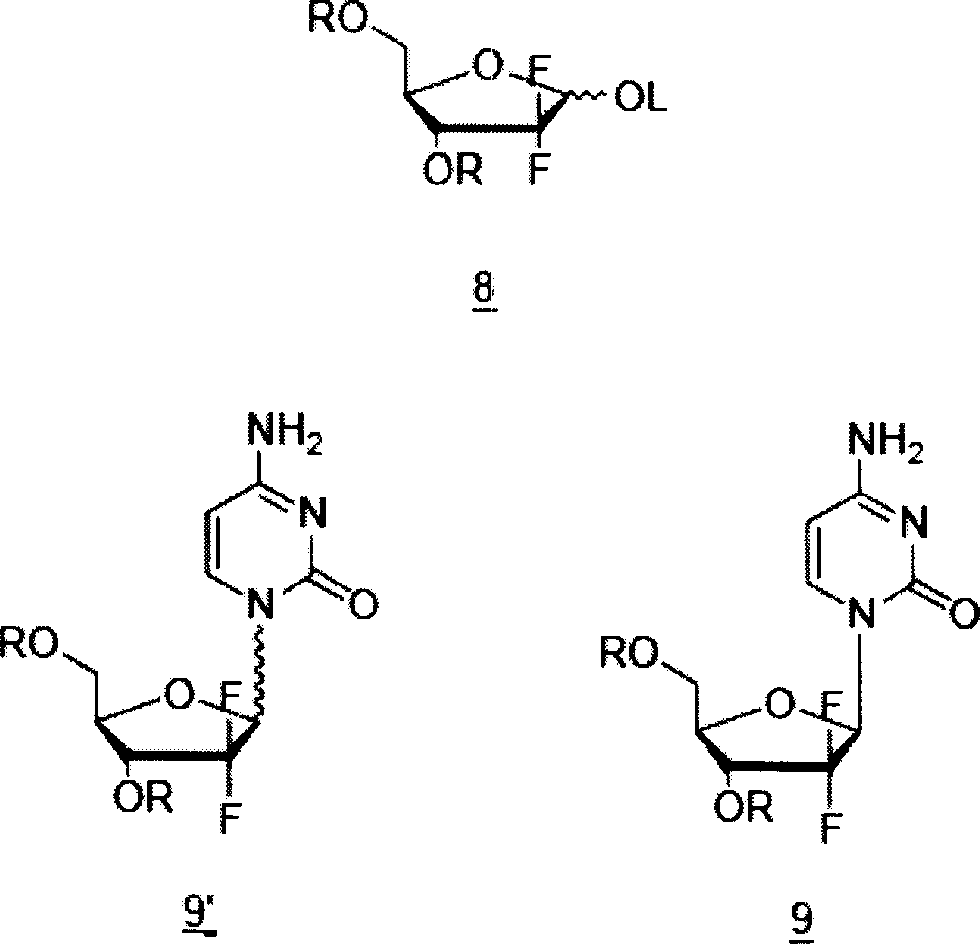
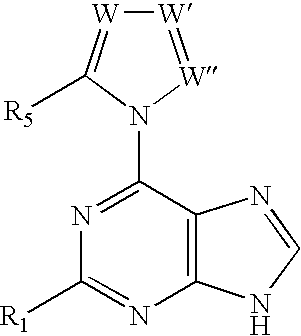
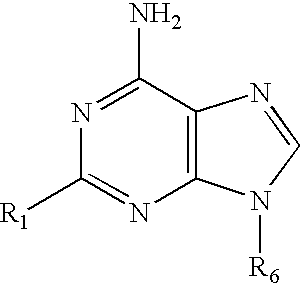
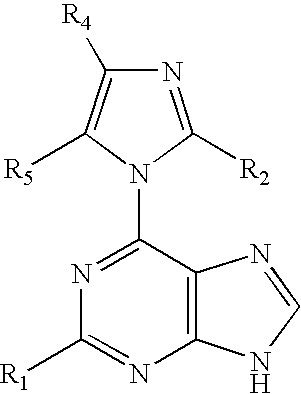
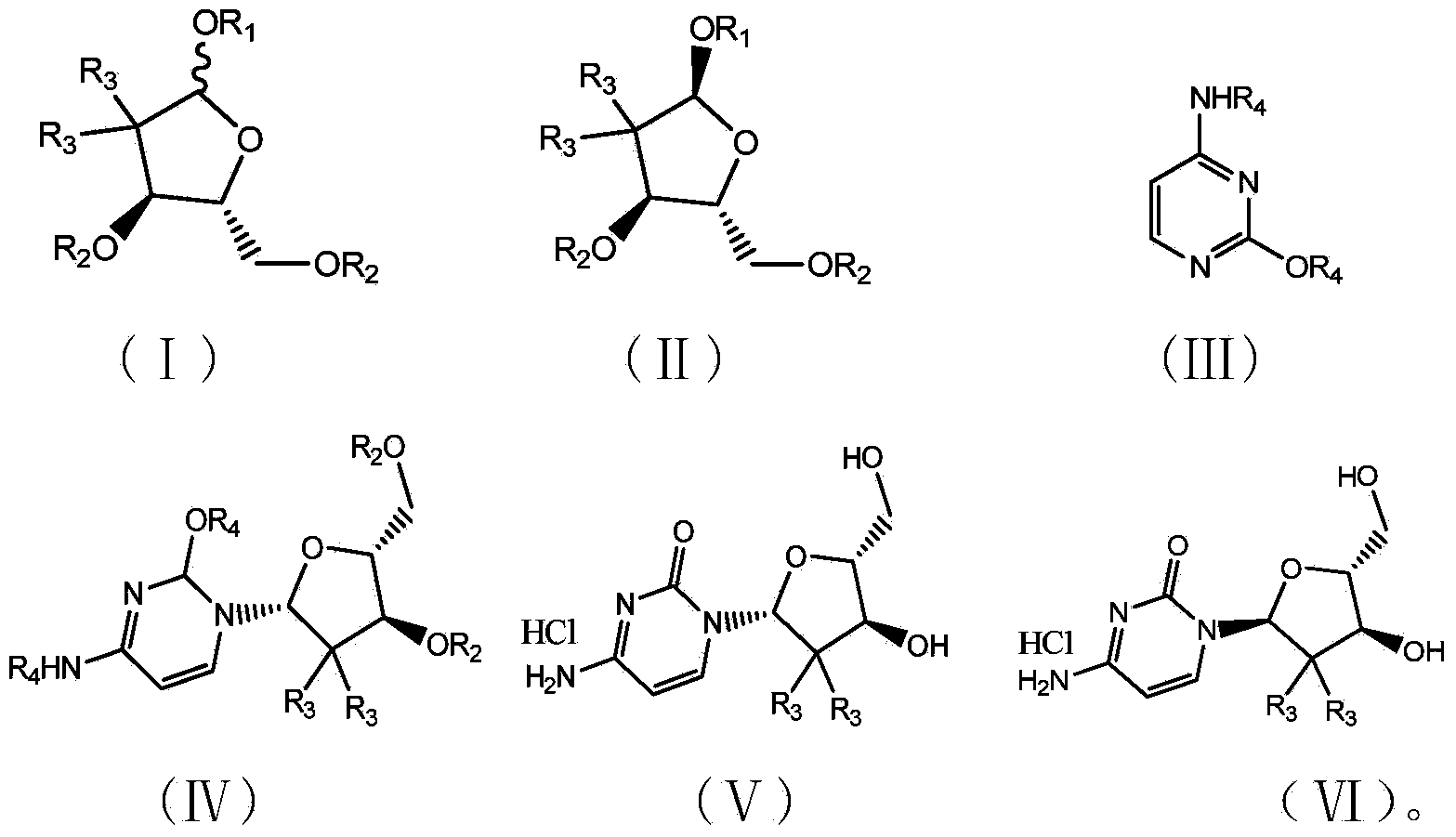
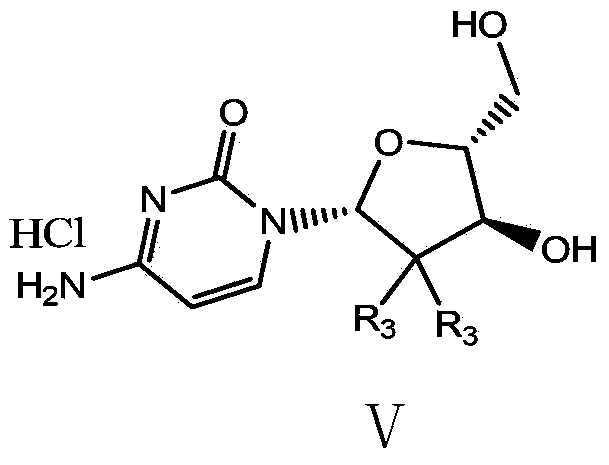
The present invention relates to novel compounds according to the to the general formulas I, II, III, IV or V: wherein B is nucleoside base according to the structure: R is H, F, Cl, Br, I, C1-C4 alkyl (preferably CH3), -C=N, -C=C-Ra, X is H, C1-C4 alkyl (preferably, CH3), F, Cl, Br or I; Z is O or CH2, with the proviso that Z is CH2 and not O when the compound is according to general formula II, R<3 >is -C=C-H and R<2 >is H or a phosphate, diphosphate, triphosphate or phosphotriester group; R<1 >is H, an acyl group, a C1-C20 alkyl or an ether group; R<2 >is H, an acyl group, a C1-C20 alkyl or ether group, a phosphate, diphosphate, triphosphate, phosphodiester group or a group; Nu is a radical of a biologically active antiviral compound such that an amino group or hydroxyl group from said biologically active antiviral compound forms a phosphate, phosphoramidate, carbonate or urethane group with the adjacent moiety; R<8 >is H, or a C1-C20 alkyl or ether group, preferably a C1-C12 alkyl group; k is 0-12, preferably, 0-2; R<3 >is selected from a C1-C4 alkyl (preferably, CH3), -(CH2)n-C=C-Ra, R<3a >and R<3b >are independently selected from H, F, Cl, Br or I; R<4 >and R<5 >are independently selected from H, F, Cl, Br, I, OH, C1-C4 alkyl (preferably, CH3), -(CH2)n-C=C-Ra, with the proviso that R<4 >and R<5 >are not both H; Ra is H, F, Cl, Br, I, or -C1-C4 alkyl, preferably H or CH3; Y is H, F, Cl, Br, I or -C1-C4 alkyl, preferably H or CH3; and n is 0, 1, 2, 3, 4 or 5, preferably 0, 1 or 2; and their anomers, pharmaceutically acceptable salts, solvates, or polymorphs thereof.
Owner:YALE UNIV
2-deoxy-d-ribose derivates, preparation method and use thereof
InactiveCN101311184AEasy to getEasy and efficient to prepareEsterified saccharide compoundsSugar derivativesSulfurBromine
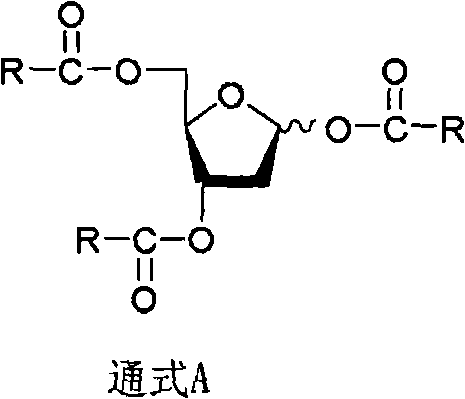
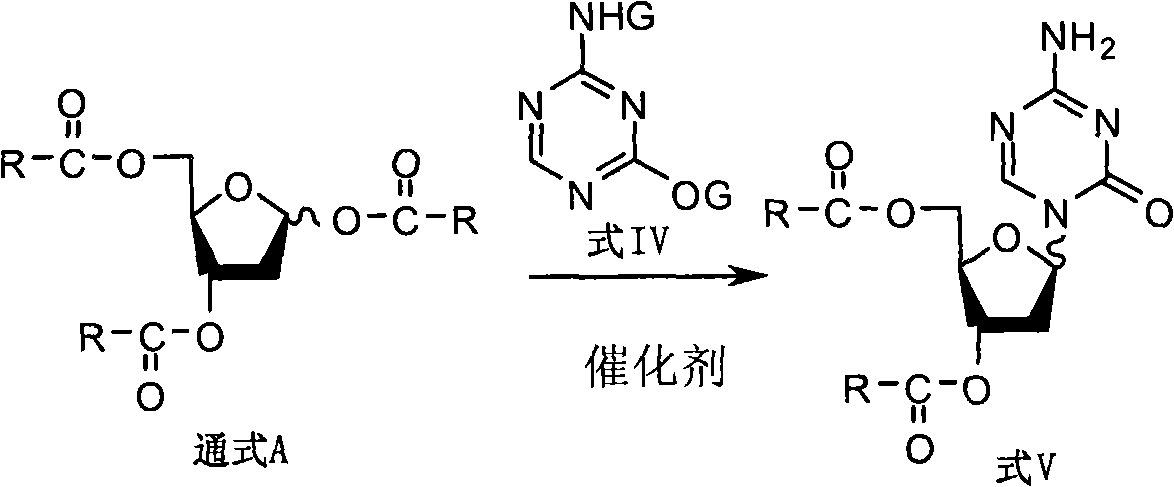
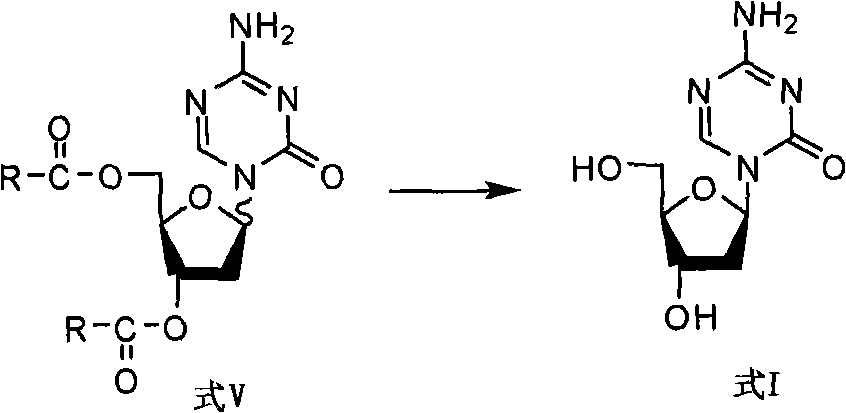
The invention discloses a derivant for 2-deoxy-D-ribose, which is a compound as shown in the constitutional formula A on the right; R is selected from CH3(CH2)n-, CH3(CH2)n-Y-CH2-, X-CH2-, CH3(CH2)nCH(X)-, X2-CH-, CH3(CH2)nC(X)2- or CX3-; wherein, Y is selected from oxygen or sulfur; X is selected from fluorine, chlorine, bromine or iodine; n is equal to 0-5. The invention also provides the preparation method and the application of the compound with the general formula A. The compound with the general formula A includes Alpha anomer, Beta anomer and the mixture thereof.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
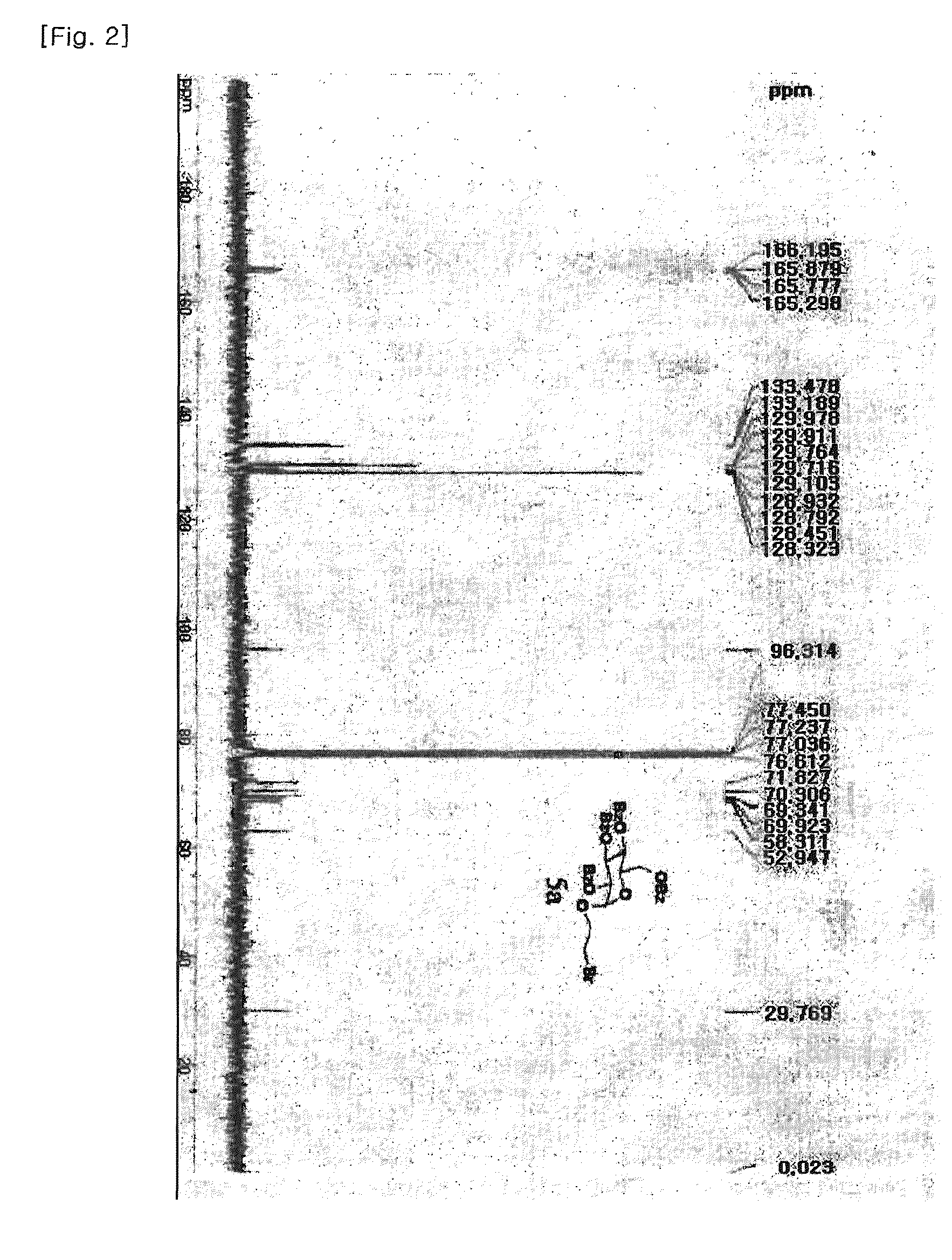
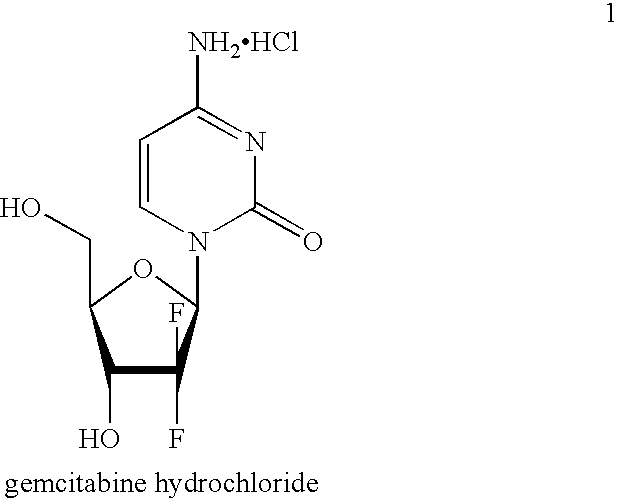
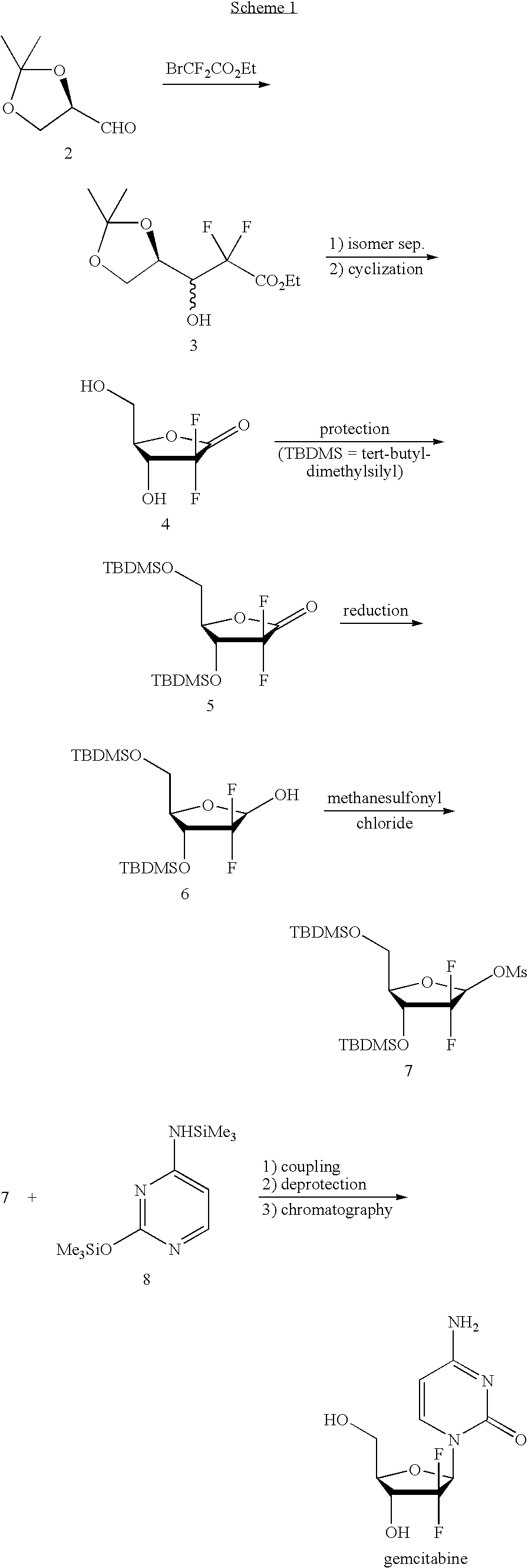
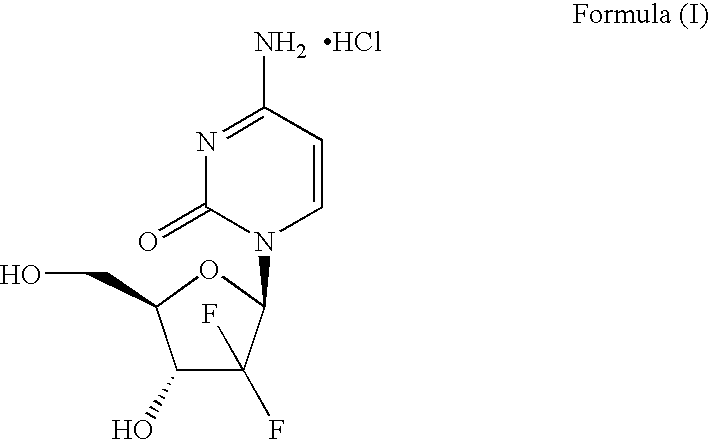
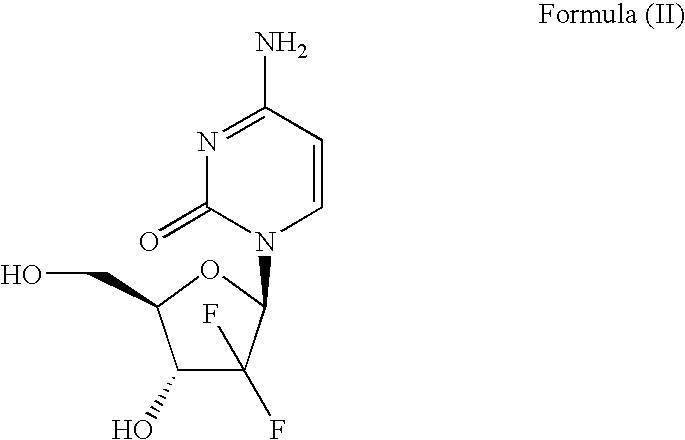
Method for preparing gemcitabine hydrochloride
ActiveCN102603838ASugar derivativesSugar derivatives preparationThree levelGemcitabine Hydrochloride
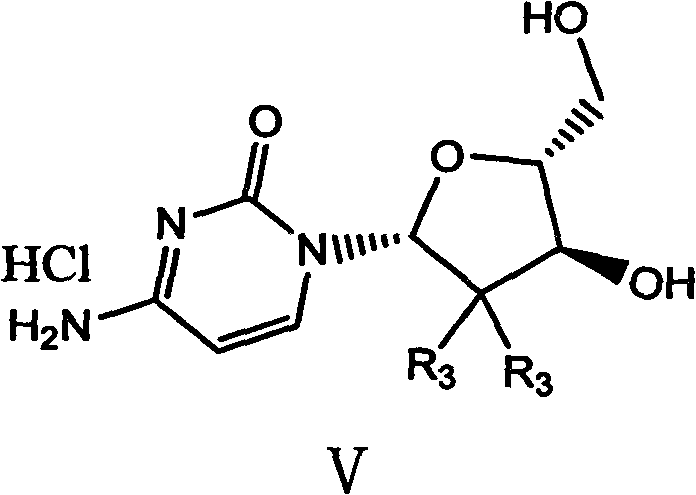
The invention belongs to technical field of pharmacy and provides a method for preparing gemcitabine hydrochloride, which solves the problems that the industrialization cost is high and the total yield is low in the original preparation method of gemcitabine hydrochloride. The method comprises the steps of a. converting and extracting alpha anomer of a formula (II) from a formula (I); b. coupling the formula (II) and a formula (III); and c. obtaining the gemcitabine hydrochloride of a formula (V). The step a comprises the steps of (1) dissolving the compound with the formula (I) in an aprotic solvent at least containing R1OH and three-level organic amine salified by R1OH, heating and reflowing, then reducing temperature so as to increase the mole ratio of the Alpha anomer of the formula (II) in the Alpha anomer mixer of the formula (I); (2) extracting the compound with the formula (I), reducing the temperature under recrystallization liquid so as to recrystallize the extracted product to obtain a solid Alpha anomer of the formula (II) and recrystallization liquid of the formula (I).
Owner:江苏八巨药业有限公司
Methods For Selective N-9 Glycosylation of Purines
A process for providing regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs is described. The introduction of the sugar moiety on to 6-(azolyl)-substituted purine bases is performed so that highly stereoselective formation of the β anomers of only the 9 position regioisomers of the purine nucleoside analogs (either D or L enantiomers) is obtained. This regiospecific and stereoselective introduction of the sugar moiety allows the synthesis of nucleoside analogs, and in particular 2′-deoxy, 3′-deoxy, 2′-deoxy-2′-halo-arabino and 2′,3′-dideoxy-2′-halo-threo purine nucleoside analogs, in high yields without formation of the 7-positional regioisomers. Processes for providing novel 6-(azolyl)purines for the regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs are described. The compounds are drugs or intermediates to drugs.
Owner:BRIGHAM YOUNG UNIV
Cosmetic compositions
InactiveUS6589515B2Avoid or minimise wet patches on their skinAvoidance of high processing temperatureCosmetic preparationsHair cosmeticsHydrogenSoft solids
A cosmetic composition, preferably an antiperspirant composition, in solid or soft-solid form has a continuous phase which contains a water-immiscible liquid carrier and also contains a structurant which is partially or fully esterified maltose of the formulae:which is the beta-anomer, and optionallywhich is the alpha-anomer;wherein each Z is independently hydrogen or an acyl group of the formula:where R denotes a hydrocarbyl group containing from 8 to 31 carbon atoms, with the proviso that not more than half of the Z groups are hydrogen,and the ratio of beta-anomer to alpha-anomer is from 65:35 to 100:0.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Fluorescent dye-labeled glucose bioprobe, synthesis method and usage thereof
ActiveUS20100105149A1Improve performanceSugar derivativesChemiluminescene/bioluminescenceDiseaseSynthesis methods
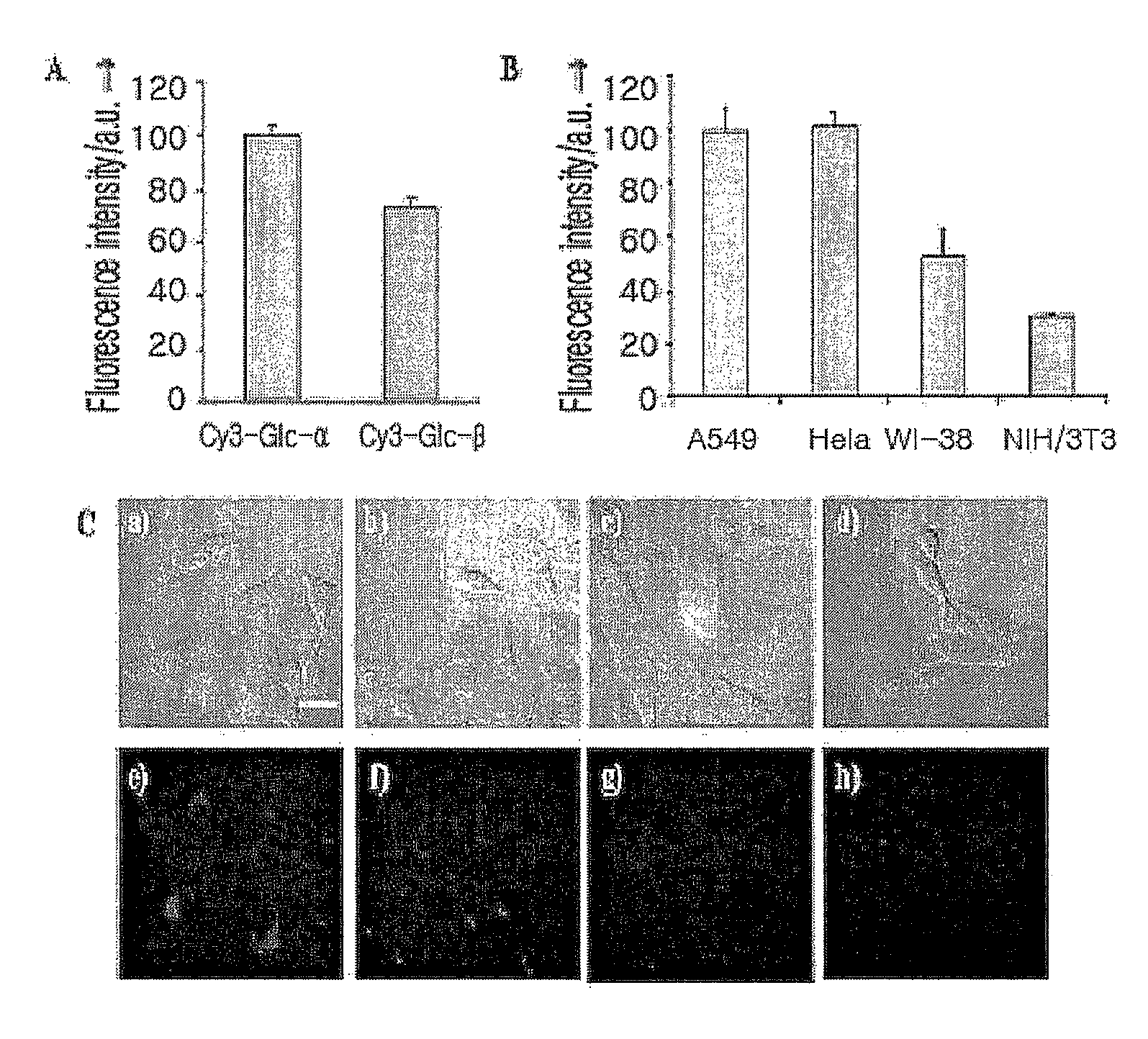
The present invention relates to a fluorescent dye-labeled glucose analog, and a synthesis method and usage of the same, and more particularly, to novel glucose α and β anomers in which a fluorescent dye is labeled by O-1-glycosylation, an asymmetric synthesis method of the anomers, a molecular bioimaging method of the anomers, and a screening method of curing or preventing drugs for diseases related to glucose metabolism.
Owner:SEOUL NAT UNIV R&DB FOUND
Gemcitabine production process
InactiveUS20080262215A1High yieldOrganic active ingredientsSugar derivativesTrimethylsilylProtecting group
Provided is a process for preparing gemcitabine or a salt thereof, which preferably includes selectively precipitating the β-anomer of a 3′,5′-di-O-protected-N4-trimethylsilyl-2′-deoxy-2′,2′-difluorocytidine, removing the protecting groups to produce gemcitabine, and, optionally, converting the gemcitabine into a salt. Preferably, the 3′ and 5′ protecting groups are the same or different, and at least one of the 3′ and 5′ protecting groups is cinnamoyl, naphthoyl, naphthylmethylcarbonyl, 2-methylbenzylcarbonyl, 4-methylbenzylcarbonyl or 9-fluorenylmethyloxycarbonyl. Also provided are methods for enriching the β-anomer from an anomeric mixture of a 3′,5′-di-O-protected-N4-trimethylsilyl-2′-deoxy-2′,2′-difluorocytidine, e.g., a N4-trimethylsilyl-2′-deoxy-2′,2′-difluoro-cytidine-3′,5′-diester, e.g., 3′,5′-dicinnamoyl-N4-trimethylsilyl-2′-deoxy-2′,2′-difluorocytidine, using a slurrying process, and methods for converting the β-anomer-enriched product into gemcitabine or a salt thereof.
Owner:CHEMAGIS
Process for Preparation of Gemcitabine Hydrochloride
A process for isolating β-anomer enriched Gemcitabine hydrochloride by converting Gemcitabine base into Gemcitabine hydrochloride followed by its purification using solvents from the series of water soluble ethers like 1,4-dioxane or Monoglyme.
Owner:ARCH PHARMALABS LTD
Purification method and production method for cellobiose
InactiveUS20090281305A1High yieldIncrease contentSugar derivativesSugar crystallisationFiberPurification methods
The present invention provides a method for purifying cellobiose which comprises the steps of (A) preparing a cellobiose-containing sugar solution; (B) increasing the rate of cellobiose present in the sugar solution relative to the total saccharides present therein up to at least 50% by mass; and (C) crystallizing cellobiose; and a method for preparing cellobiose having a high content of the α-anomer thereof which comprises the step of drying a cellobiose-containing sugar solution having a rate of cellobiose of at least 90% by mass relative to the total saccharides present therein while maintaining the sugar solution at a temperature ranging from 80 to 95° C. These methods of the present invention permit the economical preparation of cellobiose having considerably improved purity and recovery rate, without using any complicated process. Moreover, the present invention also permits the preparation of cellobiose highly soluble in water.
Owner:MATSUTANI CHEM INDS CO LTD +1
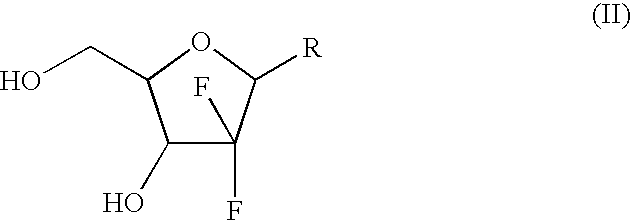
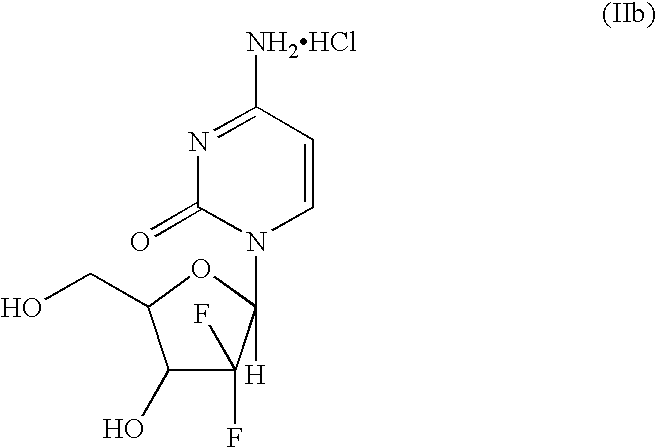
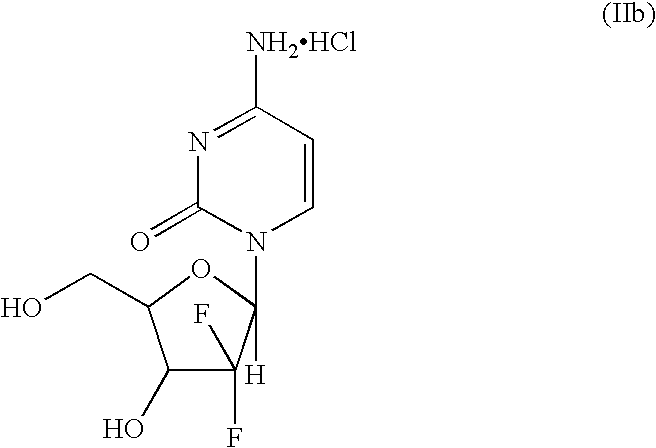
Intermediate and process for preparing of beta- anomer enriched 21-deoxy,21,21-difluoro-D-ribofuranosyl nucleosides
InactiveUS20060217547A1Reduce usageSimple and cost-effectiveEsterified saccharide compoundsBiocideGemcitabine HydrochlorideImide
The present invention provides a highly stereoselective, simple and economical glycosylation process for preparation of β-anomer enriched 21-deoxy-21,21-D-ribofuranosyl difluoronucleosides of formula (II), and physiologically acceptable slats thereof, in particular, the β-enriched anomer of gemcitabine hydrochloride of formula (IIb) in purity of >99% is provided through utilization of a novel trichloroacetimidate of formula (I).
Owner:FRESENIUS KABI ONCOLOGY LTD
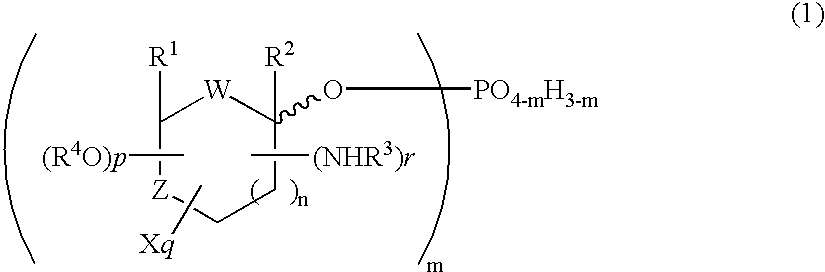
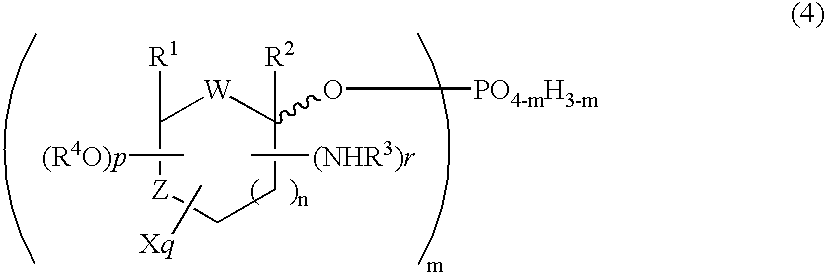
Selective process for producing an anomer of a 1-phosphorylated saccharide derivative and process for producing a nucleoside
InactiveUS20060094869A1Improve conversion rateLow costEsterified saccharide compoundsSugar derivativesIsomerizationPhosphorylation
Owner:MITSUI CHEM INC
Methods for preparing capecitabine and beta-anomer-rich trialkyl carbonate compound used therein
The present invention relates to a method for preparing capecitabine and a method for preparing a β-anomer-rich trialkyl carbonate compound used therein, and a highly pure capecitabine can be efficiently prepared with a high yield by the method of the present invention using the β-anomer-rich trialkyl carbonate compound as an intermediate.
Owner:HANMI SCI CO LTD
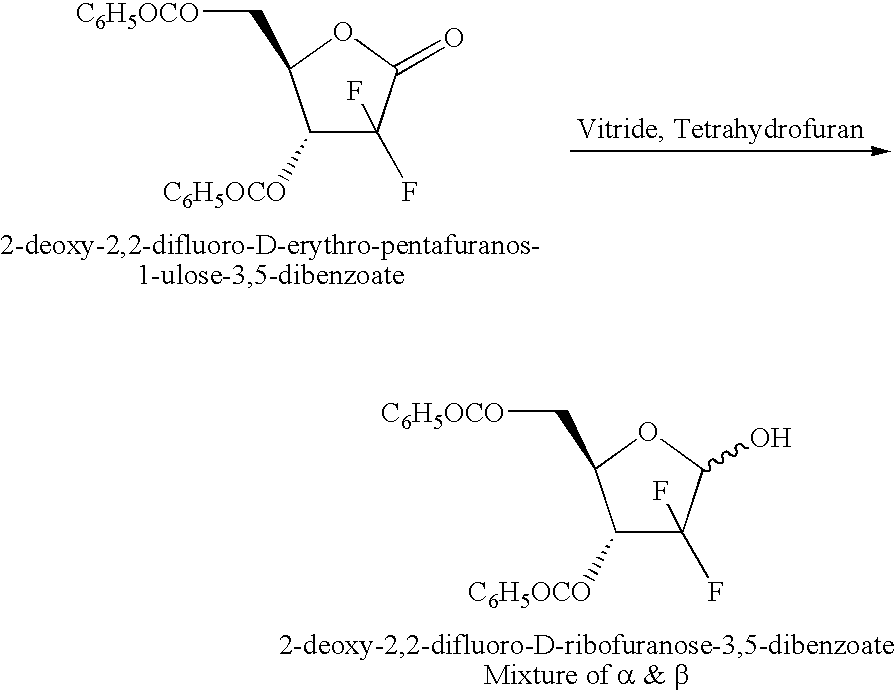
Process for preparing gemcitabine and associated intermediates
InactiveCN101296934APromote total synthesisEasy to useSugar derivativesAntineoplastic agentsPhenacylMethyl benzene
Provided is a process for preparing gemcitabine or a salt thereof, which preferably includes removing at least a substantial portion of the a anomer of a N-1-protected-2'-deoxy-2',2'-difluoro-cytidine-3',5'-diester from an anomeric mixture thereof; removing the 3'-ester, the 5'-ester and the N-protecting group; and optionally forming a salt. The 3'-ester and 5'-ester can be the same or different and at least one of the esters preferably is cinnamoyl, benzoyl, 1-naphthoyl, 1-naphthylmethylcarbonyl, 2-methylbenzylcarbonyl, 4-methylbenzylcarbonyl or 9-fluorenylmethyloxycarbonyl ester. Also provided are novel intermediates, including but not limited to 2-deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-diesters, and methods of producing such intermediates.
Owner:CHEMAGIS
Intermediate and process for preparing of beta- anomer enriched 21-deoxy,21,21-difluoro-D-ribofuranosyl nucleosides
InactiveUS7235647B2Reduce usageSimple and convenient and cost-effectiveEsterified saccharide compoundsBiocideGemcitabine HydrochlorideAnomer
The present invention provides a highly stereoselective, simple and economical glycosylation process for preparation of β-anomer enriched 21-deoxy-21,21-D-ribofuranosyl difluoronucleosides of formula (II), and physiologically acceptable slats thereof, in particular, the β-enriched anomer of gemcitabine hydrochloride of formula (IIb) in purity of >99% is provided through utilization of a novel trichloroacetimidate of formula (I).
Owner:FRESENIUS KABI ONCOLOGY LTD
Methods for preparing capecitabine and beta-anomer-rich trialkyl carbonate compound used therein
The present invention relates to a method for preparing capecitabine and a method for preparing a β-anomer-rich trialkyl carbonate compound used therein, and a highly pure capecitabine can be efficiently prepared with a high yield by the method of the present invention using the β-anomer-rich trialkyl carbonate compound as an intermediate.
Owner:HANMI SCI CO LTD
Method for production of furanose derivative
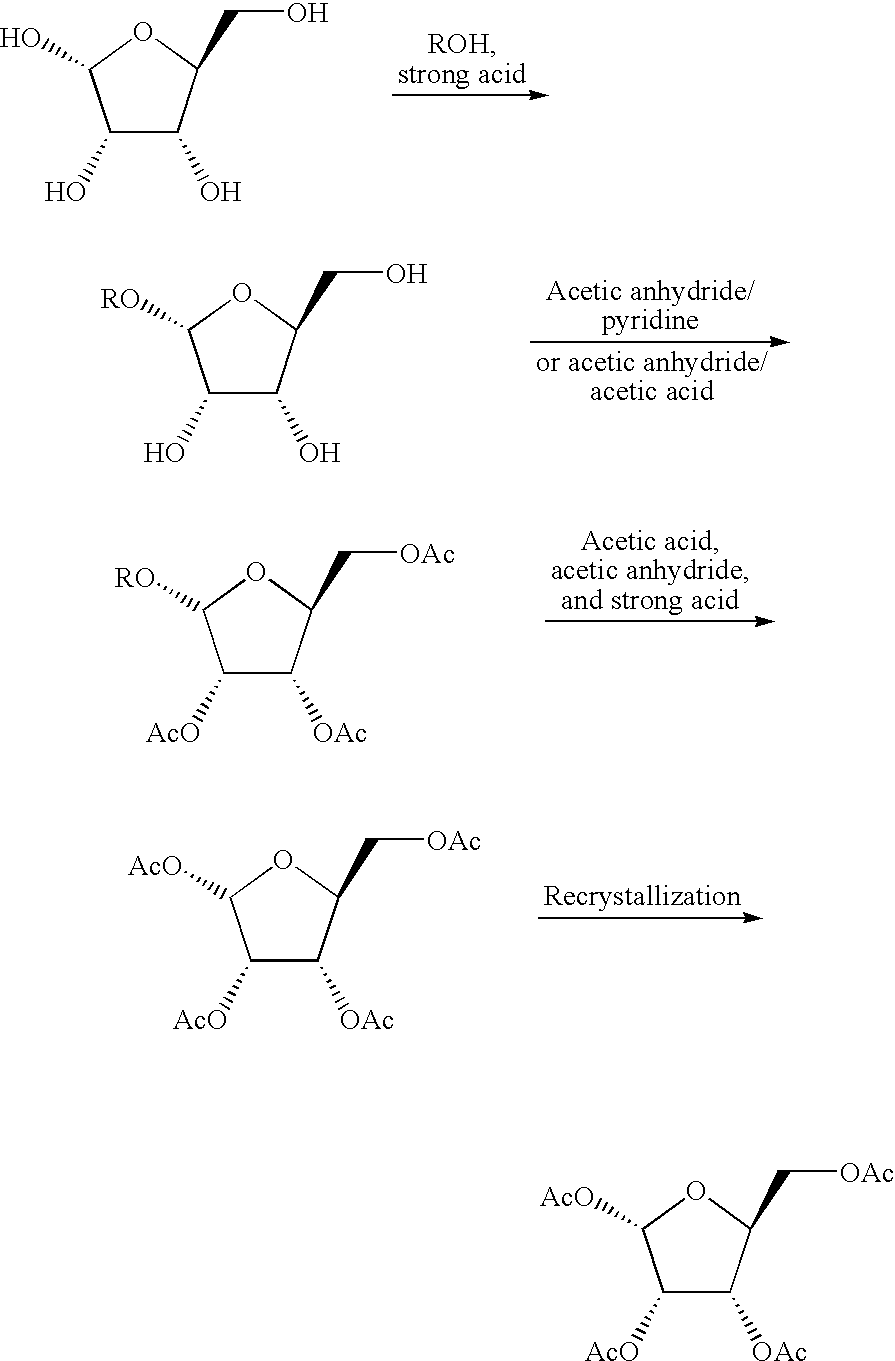
ActiveCN101595117AHigh selectivityImprove efficiencyEsterified saccharide compoundsSugar derivativesAcetic acidFuran
An object of the present invention is to provide a industrially appropriate method for producing the beta-anomers of ribofuranose derivatives in a highly selective manner at a high yield. The present invention provides a method for producing ribofuranose derivatives wherein beta-anomers is precipitated from among the generated furanose derivatives by controlling the amount of a reaction reagent used and / or using a poor solvent in the acetolysis reactions of 2,3,5-tri-O-acyl-1-O-alkyl-ribofuranose and 2,3-di-O-acyl-1-O-alkyl-5-deoxy-ribofuranose.
Owner:API CO LTD
Preparation method of high-anomer 4,4'-diaminodicyclohexylmethane
ActiveCN108516939ASimple production processReduce usageAmino compound purification/separationOrganic chemistry methodsIsomerizationSolvent free
The invention discloses a preparation method of high-anomer 4,4'-diaminodicyclohexylmethane. The content of anomer (PACM50) in the prepared product is 45-55 wt%. The preparation method comprises the following steps: performing a modification reaction on a raw material low-anomer 4,4'-diaminodicyclohexylmethane at 100-110 DEG C under the action of a catalyst and a modifier, raising the temperatureto 120-250 DEG C, and performing an isomerization reaction to prepare the high-anomer 4,4'-diaminodicyclohexylmethane. The method allows the PACM20 conversion rate to reach 99-100% and the PACM50 yield to reach 98-99%. The method has the advantages of realization of a high-temperature catalysis reaction under solvent-free and low-pressure nitrogen conditions without high-pressure nitrogen, simplicity in operation, safety, environmental protection, and suitableness for industrial production.
Owner:WANHUA CHEM GRP CO LTD
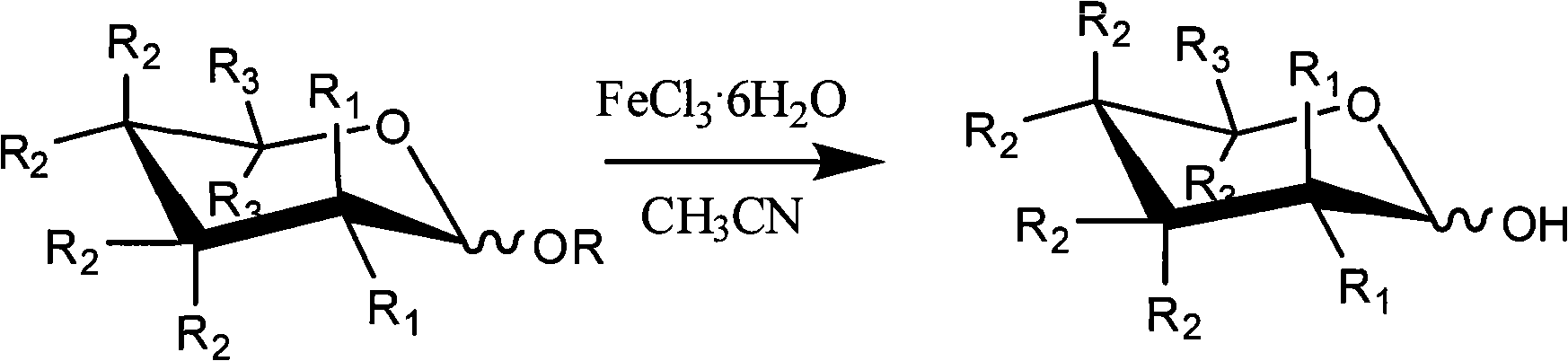
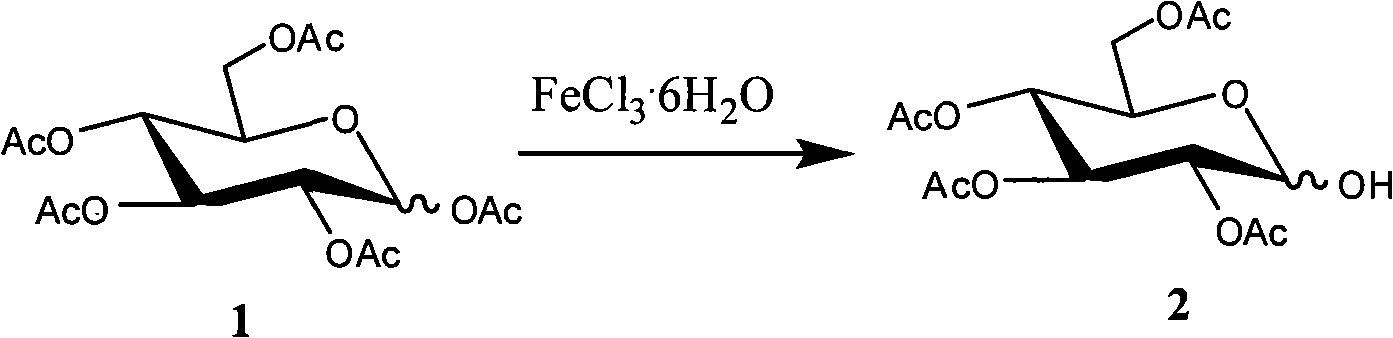
Method for selectively removing carbohydrate compound anomer carbonyl by FeCl3.6H2O
InactiveCN101555262AReduce pollutionSimple methodEsterified saccharide compoundsSugar derivativesAnomerCarbohydrate
The invention provides a method for selectively removing carbohydrate compound anomer carbonyl by FeCl3.6H2O, which is shown as a formula I. The method can play an important role in carbohydrate medicine synthesis.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Highly efficient synthesis of alpha-O-galactosyl ceramides
A method for the production of a-O-galactosyl ceramide precursor is demonstrated. The method involves the reaction of galactosyl iodide with a sphingosine derivative or phytosphingosine derivative in the presence of a quaternary ammonium iodide salt to prepare the a-O-galactosyl ceramide precursor in the a-anomer form. The a-O-galactosyl ceramide is then prepared by reaction with a suitable fatty acid or fatty acid derivative.
Owner:RGT UNIV OF CALIFORNIA
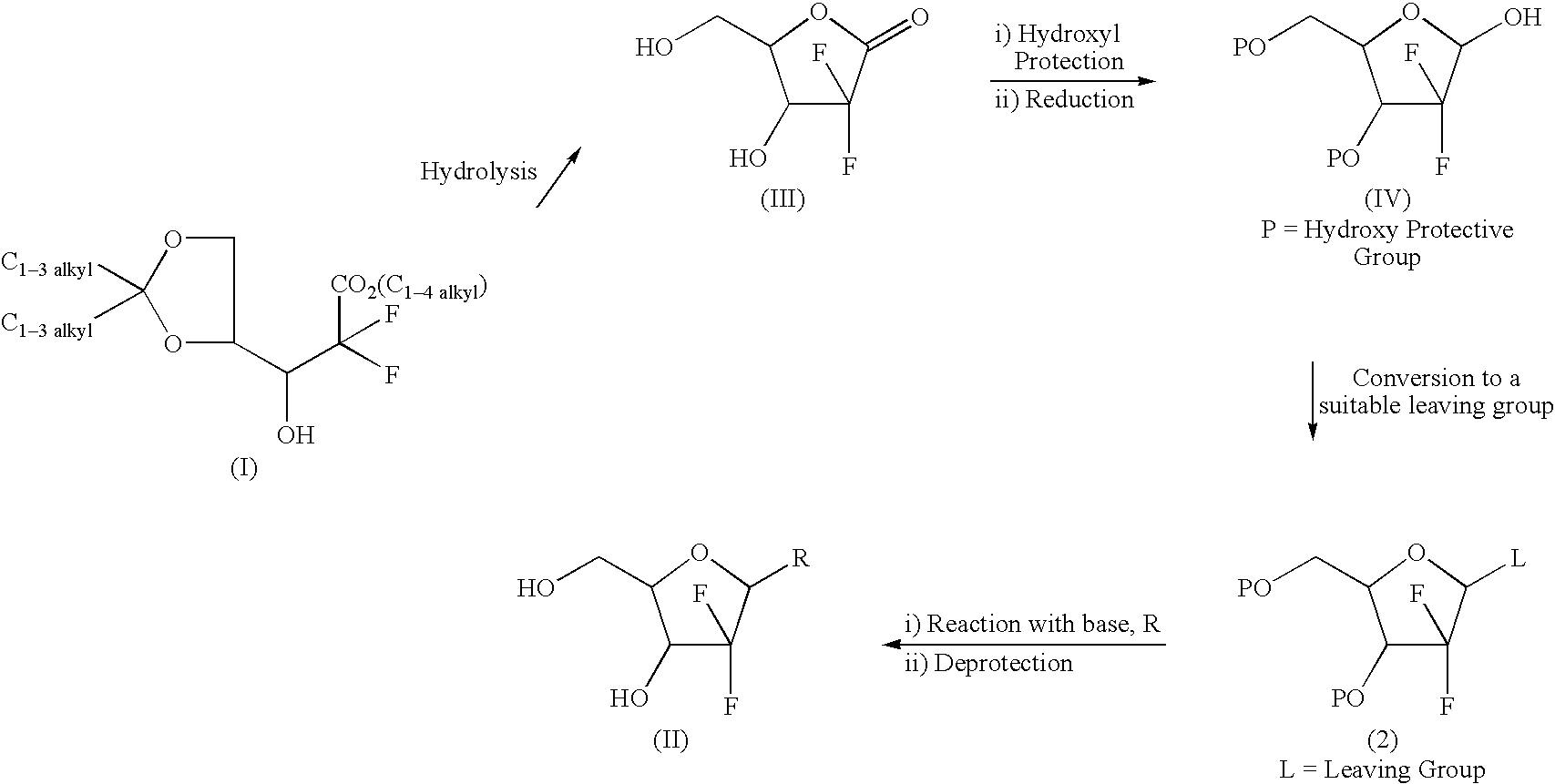
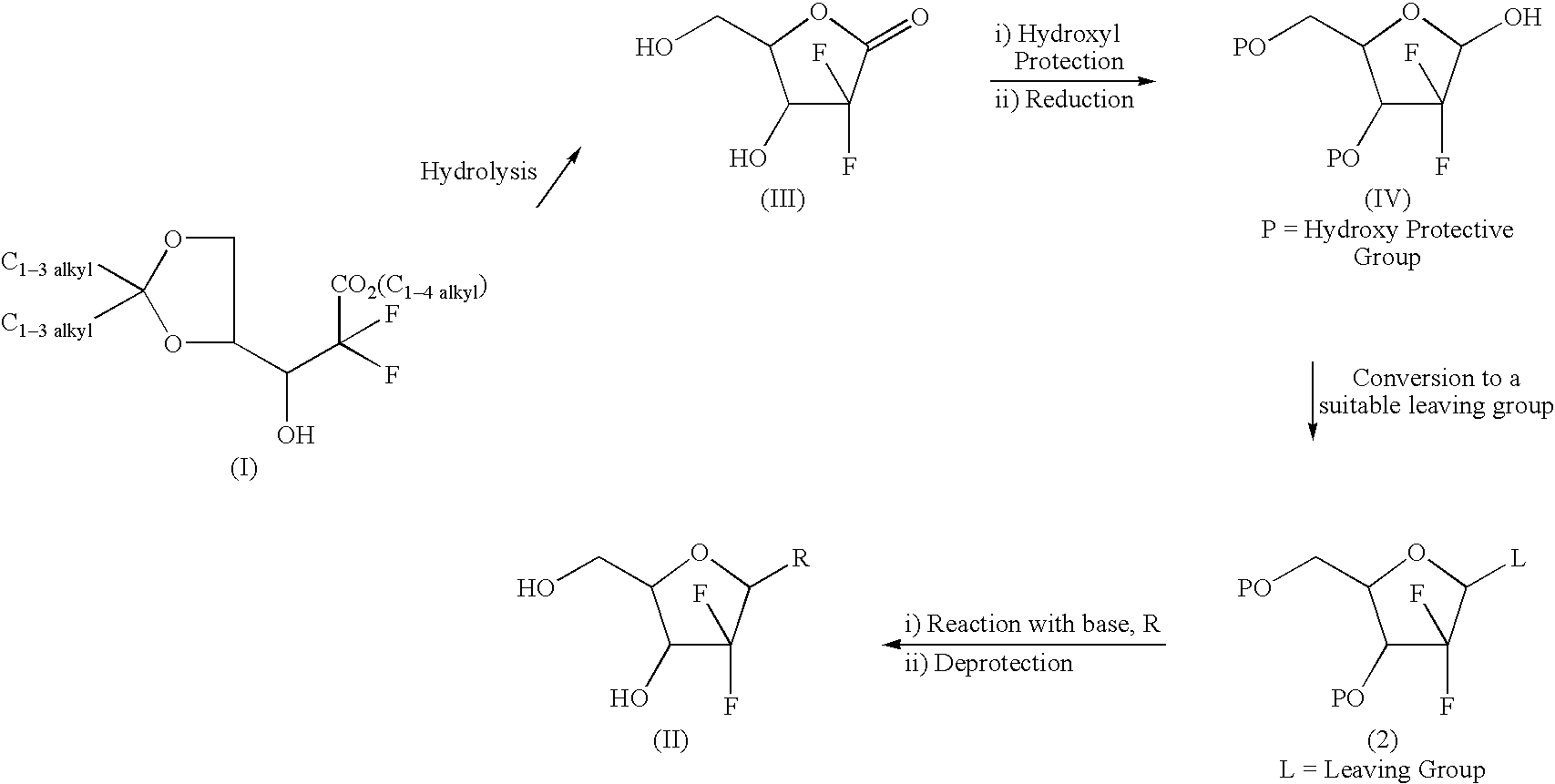
A manufacturing process of 2',2'-difluoronucleoside and intermediate
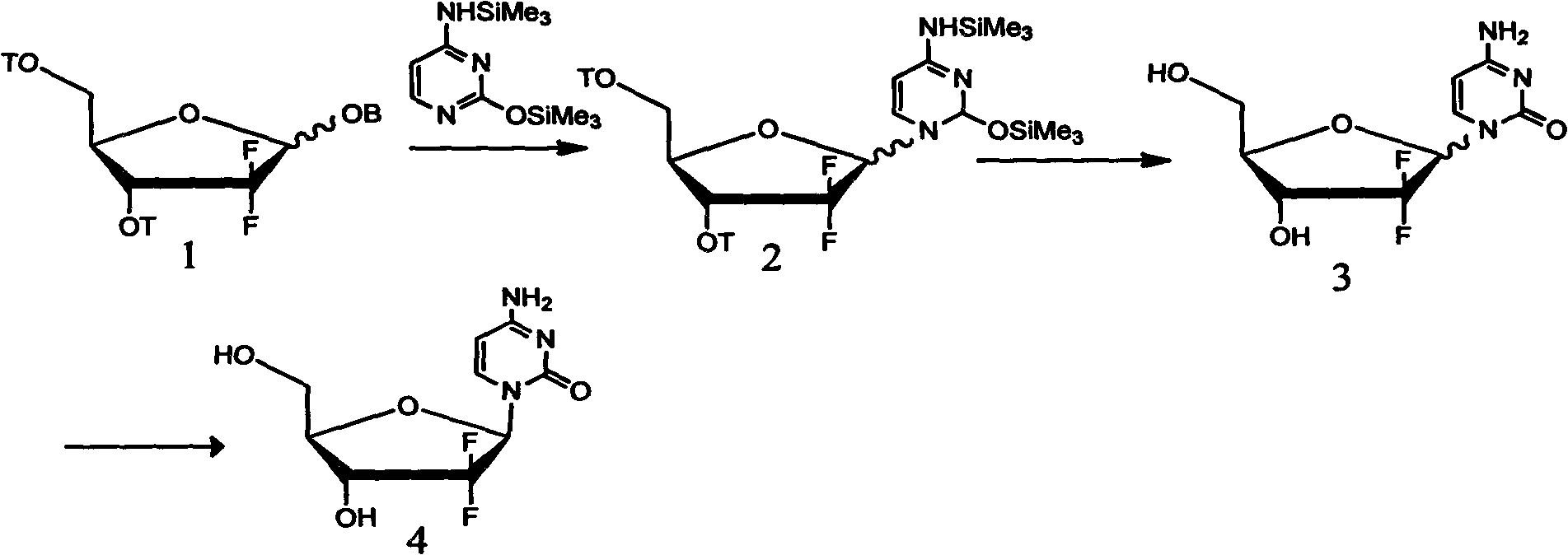
The present invention relates to more improved process for preparing 2'-deoxy-2',2'-difluoronucleoside and its intermediate. The present invention provide a process for preparing an erythro enantiomer in greater than 98% purity, comprising forming a lactone ring by hydrolyzing ethyl (3RS )-2,2-difluoro-3-hydroxy-3-(2,2-dimethyloxolan-4-yl)propionate is hydrolyzed in the presence of hydrolysis reagents selected from acetic acid or chloroacetic acid, water and a mixture of organic solvents selected from the group comprising acetonitrile, dioxane, tetrahydrofuran or toluene, in not troducing a substituted benzoyl protecting group at the 3-position and 5-position, and recrys- tallizing said erythro enantiomer. Further, the present invention provides a process for selectively preparing, in greater than 99% purity, a beta-anomer 2'-deoxy-2',2'-difluoronucleoside at the 3'-position and 5'-position that are protected by a substituted benzoyl in a 2:3 alpha / beta anomeric ratio.
Owner:DONG A PHARMA
Methods for selective N-9 glycosylation of purines
A process for providing regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs is described. The introduction of the sugar moiety on to 6-(azolyl)-substituted purine bases is performed so that highly stereoselective formation of the β anomers of only the 9 position regioisomers of the purine nucleoside analogs (either D or L enantiomers) is obtained. This regiospecific and stereoselective introduction of the sugar moiety allows the synthesis of nucleoside analogs, and in particular 2′-deoxy, 3′-deoxy, 2′-deoxy-2′-halo-arabino and 2′,3′-dideoxy-2′-halo-threo purine nucleoside analogs, in high yields without formation of the 7-positional regioisomers. Processes for providing novel 6-(azolyl)purines for the regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs are described. The compounds are drugs or intermediates to drugs.
Owner:BRIGHAM YOUNG UNIV
Method for producing furanose derivative
An object of the present invention is to provide a industrially appropriate method for producing the β-anomers of ribofuranose derivatives in a highly selective manner at a high yield. The present invention provides a method for producing ribofuranose derivatives wherein β-anomers is precipitated from among the generated furanose derivatives by controlling the amount of a reaction reagent used and / or using a poor solvent in the acetolysis reactions of 2,3,5-tri-O-acyl-1-O-alkyl-ribofuranose and 2,3-di-O-acyl-1-O-alkyl-5-deoxy-ribofuranose.
Owner:API CORP (JP)
Method for preparing gemcitabine hydrochloride
ActiveCN102603838BSugar derivativesSugar derivatives preparationThree levelGemcitabine Hydrochloride
Owner:江苏八巨药业有限公司
A method for producing activated sugar chain derivative and activated sugar chain derivative produced therefrom
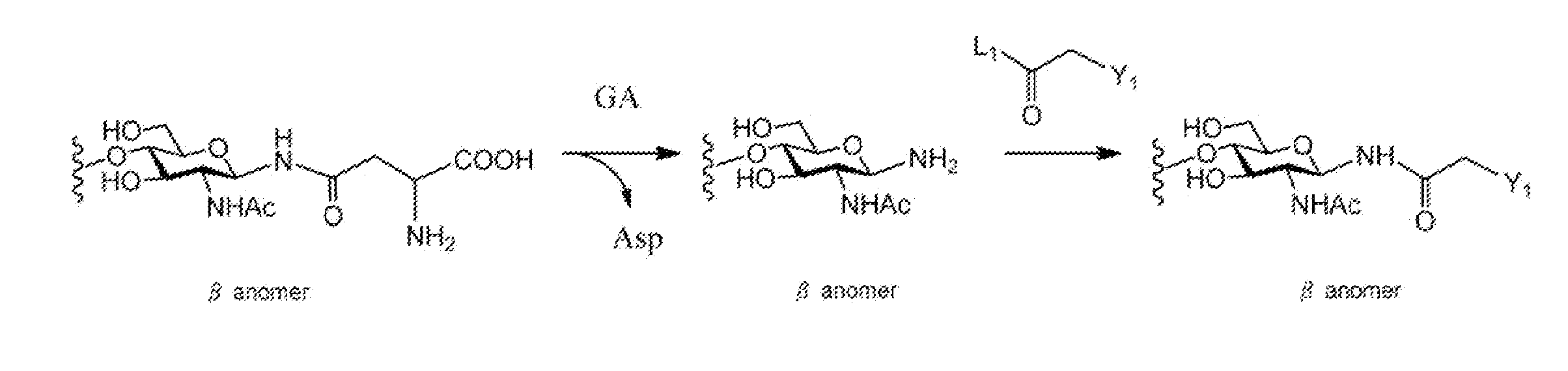
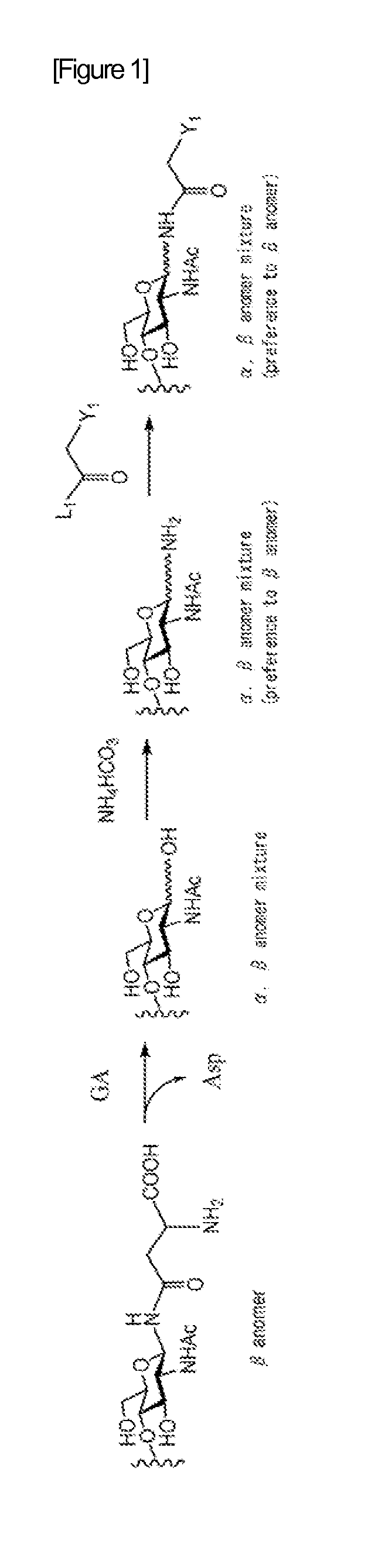
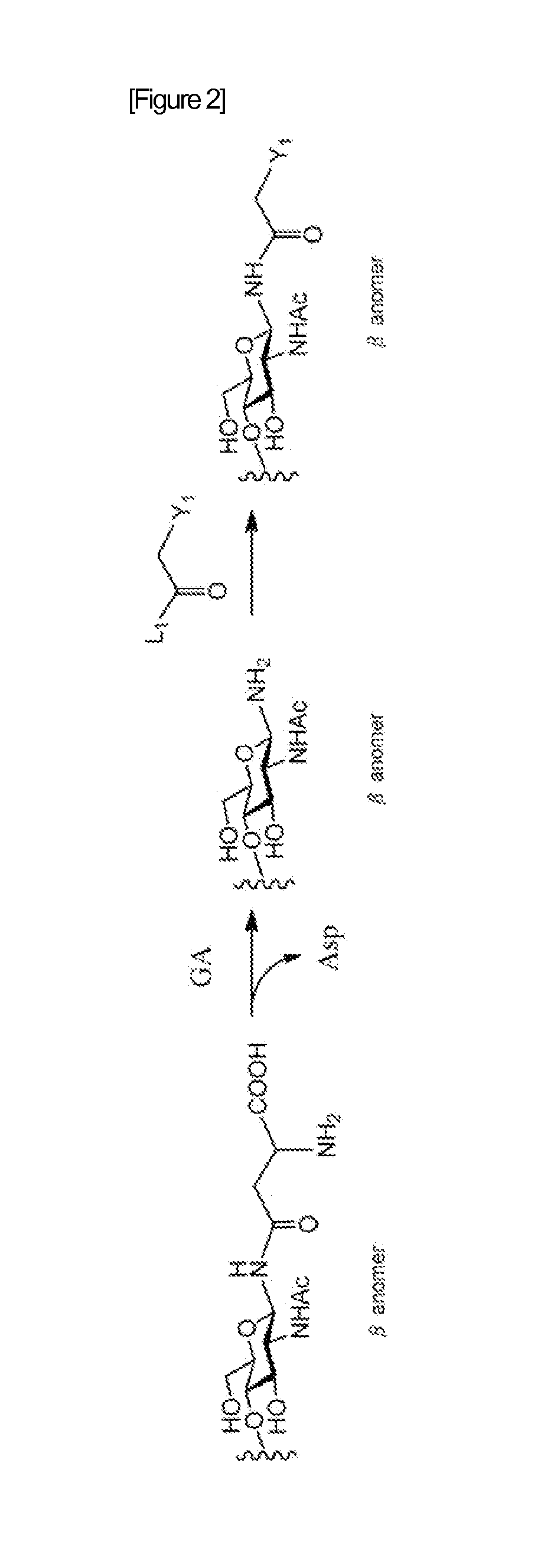
When manufacturing a sugar chain compound having an activating group, there was a problem with the ammonium carbonate method that is the conventional technology that during the process of introducing an amino group at the reducing terminal of the sugar chain, the unit sugar located at the reducing terminal of the sugar chain is subjected to ring-opening, thus causing a mixture of α and β anomers to be produced. The present invention provides a method for manufacturing a sugar chain compound having an activating group with high β selectivity using a compound having a sugar chain asparagine structure as the source material by cleaving the sugar chain from the sugar chain peptide so that the reducing terminal of the sugar chain and the nitrogen atom derived from the asparagine side chain will remain in a bound state, and introducing an activating group to said nitrogen atom while retaining not only the covalent bond between the reducing terminal of said sugar chain and said nitrogen atom but also the β configuration. A novel sugar chain compound which is a β anomer and has an activating group is also provided as a compound of the present invention.
Owner:GLYTECH
Fluorescent dye-labeled glucose bioprobe, synthesis method and usage thereof
ActiveUS8679854B2Improve performanceUltrasonic/sonic/infrasonic diagnosticsSugar derivativesFluorescenceSynthesis methods
The present invention relates to a fluorescent dye-labeled glucose analog, and a synthesis method and usage of the same, and more particularly, to novel glucose α and β anomers in which a fluorescent dye is labeled by O-1-glycosylation, an asymmetric synthesis method of the anomers, a molecular bioimaging method of the anomers, and a screening method of curing or preventing drugs for diseases related to glucose metabolism.
Owner:SEOUL NAT UNIV R&DB FOUND
Method for producing furanose derivative
An object of the present invention is to provide a industrially appropriate method for producing the β-anomers of ribofuranose derivatives in a highly selective manner at a high yield. The present invention provides a method for producing ribofuranose derivatives wherein β-anomers is precipitated from among the generated furanose derivatives by controlling the amount of a reaction reagent used and / or using a poor solvent in the acetolysis reactions of 2,3,5-tri-O-acyl-1-O-alkyl-ribofuranose and 2,3-di-O-acyl-1-O-alkyl-5-deoxy-ribofuranose.
Owner:API CORP (JP)
Cosmetic compositions
InactiveUS20020076386A1Excellent propertyLow visible depositCosmetic preparationsHair cosmeticsHydrogenSoft solids
A cosmetic composition, preferably an antiperspirant composition, in solid or soft-solid form has a continuous phase which contains a water-immiscible liquid carrier and also contains a structurant which is partially or fully esterified maltose of the formulae: which is the beta-anomer, and optionally which is the alpha-anomer; wherein each Z is independently hydrogen or an acyl group of the formula: where R denotes a hydrocarbyl group containing from 8 to 31 carbon atoms, with the proviso that not more than half of the Z groups are hydrogen, and the ratio of beta-anomer to alpha-anomer is from 65:35 to 100:0.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Process for selectively producing 1-phosphorylated sugar derivative anomer and process for producing nucleoside
InactiveUS7038039B2Improve conversion rateLow costBiocideTransferasesPhosphorylationNucleoside phosphorylase
A desired isomer is selectively prepared by phosphorolyzing and isomerizing an anomer mixture of a 1-phosphorylated saccharide derivative while crystallizing one of the isomers to displace the equilibrium. Furthermore, using the action of a nucleoside phosphorylase, a nucleoside is prepared from the 1-phosphorylated saccharide derivative obtained and a base with improved stereoselectivity and a higher yield. This process is an anomer-selective process for preparing a 1-phosphorylated saccharide derivative and a nucleoside.
Owner:MITSUI CHEM INC
Separating Agent for Chromatography, Chromatography Column, and Method for Separation by Chromatography
InactiveUS20180361353A1Accelerate anomer conversionAvoid separationComponent separationOther chemical processesChromatographic separationPorous substrate
The present invention provides a chromatographic separating agent which exhibits excellent performances in separation of organic compounds, such as reducing sugars without formation of Schiff bases and anomer separation in analysis of saccharides. The chromatographic separating agent includes a porous substrate surface-modified with silane functional groups represented by the formula:wherein R1 represents an alkylene group or oxyalkylene group having a carbon number of 1 to 10. R2 and R3 independently represent an alkylene group or alkyleneoxyalkylene group having a carbon number of 1 to 6.
Owner:SHINWA CHEM INDS
Application of alpha-lactose in preparation of drugs for treatment of sepsis
InactiveCN106581014AConvenient for clinical operationMild side effectsOrganic active ingredientsAntiinfectivesInflammatory factorsSide effect
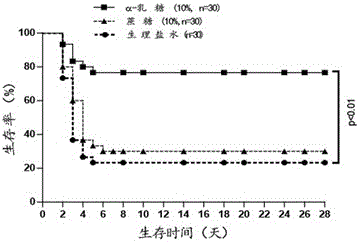
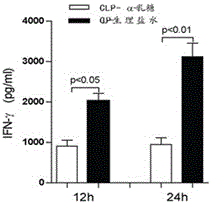
The invention discloses a method for treatment of sepsis. Specifically, multiple aspects of the invention relate to application of alpha-lactose in preparation of drugs for treatment of sepsis. Alpha-lactose is a disaccharide, and is an anomer. Research shows that subcutaneous injection of an alpha-lactose solution can significantly improve the 28d survival rate of a sepsis model mouse, can significantly improve release of inflammatory factors in a waterfall mode after sepsis and immune dysfunction caused by heavy immune cell apoptosis, and can alleviate liver injury induced by sepsis. Alpha-lactose is a pharmaceutic adjuvant included in the "Pharmacopoeia of the People's Republic of China", is widely used as a flavoring agent or filler, and has the advantages of low price, clear physicochemical properties and toxicity, easy dissolution in water, intravenous injection, and slight side effect. Under the current situation of high incidence and high mortality of sepsis patients, and lack of effective therapeutic drugs, alpha-lactose has enormous application value and prospects in preparation of drugs for treatment of sepsis.
Owner:唐朝晖
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com