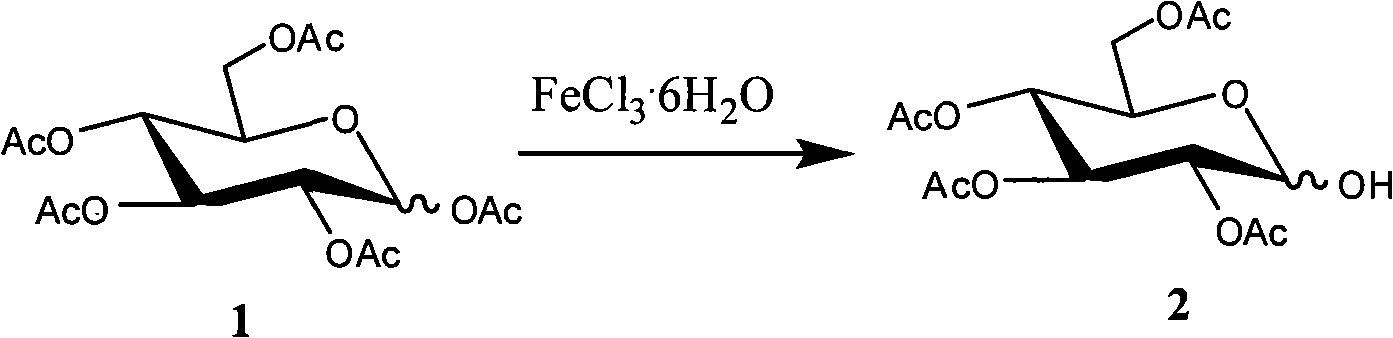
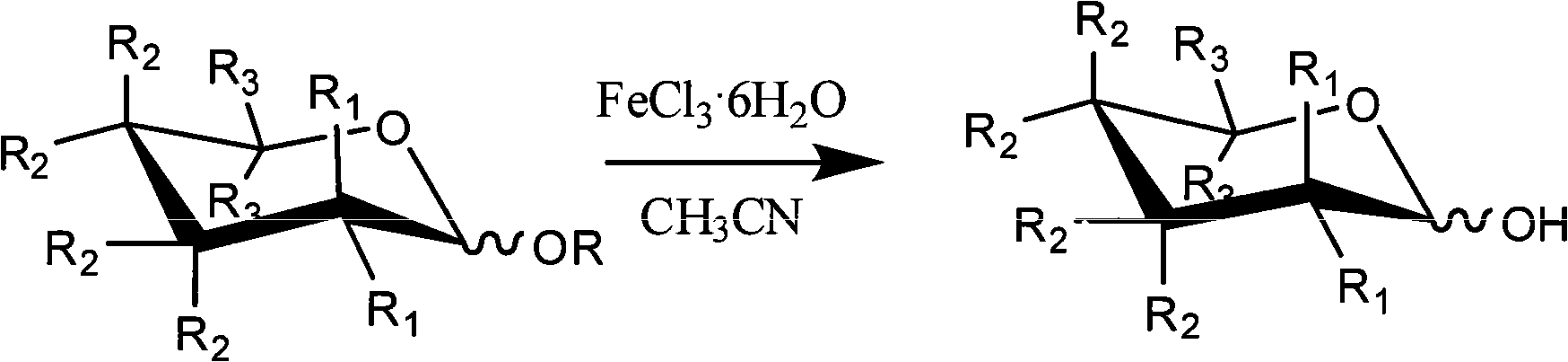Method for selectively removing carbohydrate compound anomer carbonyl by FeCl3.6H2O
A technique for anomeric carbonyl compounds, which is applied in the field of selective removal of anomeric carbonyl groups from sugar compounds by ferric trichloride hexahydrate (FeCl3 6H2O), to achieve the effects of low price, less pollution, and simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Dissolve carbohydrate compound 1 (1.0 mmol) in CH 3 CN, then FeCl was added to the reaction solution 3 ·6H 2 O (1.0mmol), heated to reflux for about 1 hour. The progress of the reaction was monitored by thin layer chromatography. After the reaction, add ethyl acetate to dilute, and then use saturated NaHCO 3 The aqueous solution was neutralized, the organic phase was collected, dried, concentrated, and separated by column chromatography to obtain Compound 2 (0.9 mmol, 90%). The molecular weight of Compound 2 was 348 according to mass spectrometry.
Embodiment 2
[0018]
[0019] The carbohydrate compound 3 (1.0 mmol) was dissolved in CH 3 CN, then FeCl was added to the reaction solution 3 ·6H 2 O (1.0mmol), heated to reflux for about 0.2 hours. The progress of the reaction was monitored by thin layer chromatography. After the reaction, add ethyl acetate to dilute, and then use saturated NaHCO 3 The aqueous solution was neutralized, the organic phase was collected, dried, concentrated, and separated by column chromatography to obtain compound 4 (0.95 mmol, 95%), and the molecular weight of compound 4 was 290 according to mass spectrometry.
Embodiment 3
[0021]
[0022] The carbohydrate compound 5 (1.0 mmol) was dissolved in CH 3 CN, then FeCl was added to the reaction solution 3 ·6H 2 O (2.0mmol), heated to reflux for about 2 hours. The progress of the reaction was monitored by thin layer chromatography. After the reaction, add ethyl acetate to dilute, and then use saturated NaHCO 3 The aqueous solution was neutralized, the organic phase was collected, dried, concentrated, and separated by column chromatography to obtain compound 6 (0.80 mmol, 80%), and the molecular weight of compound 6 was 636 according to mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com