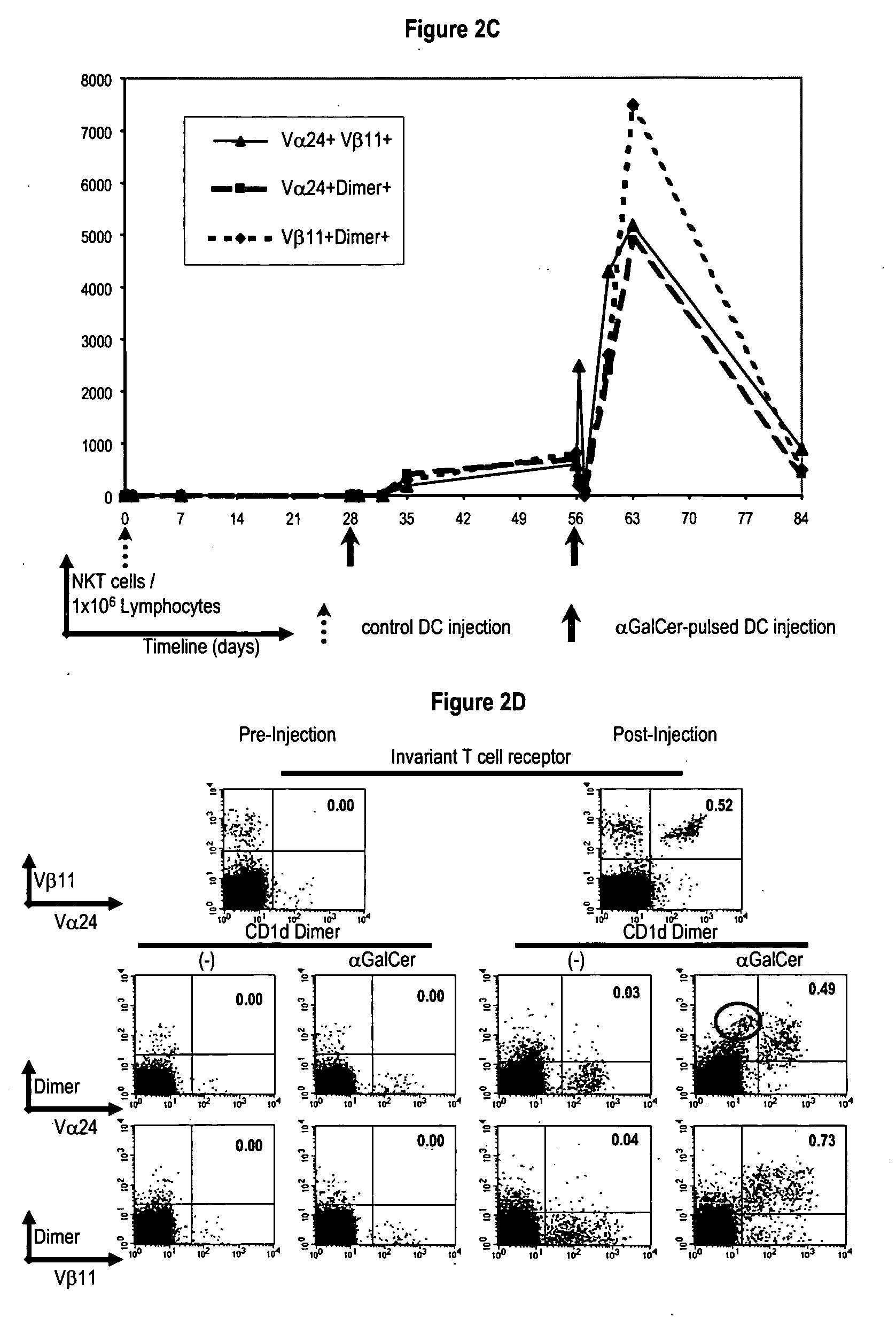
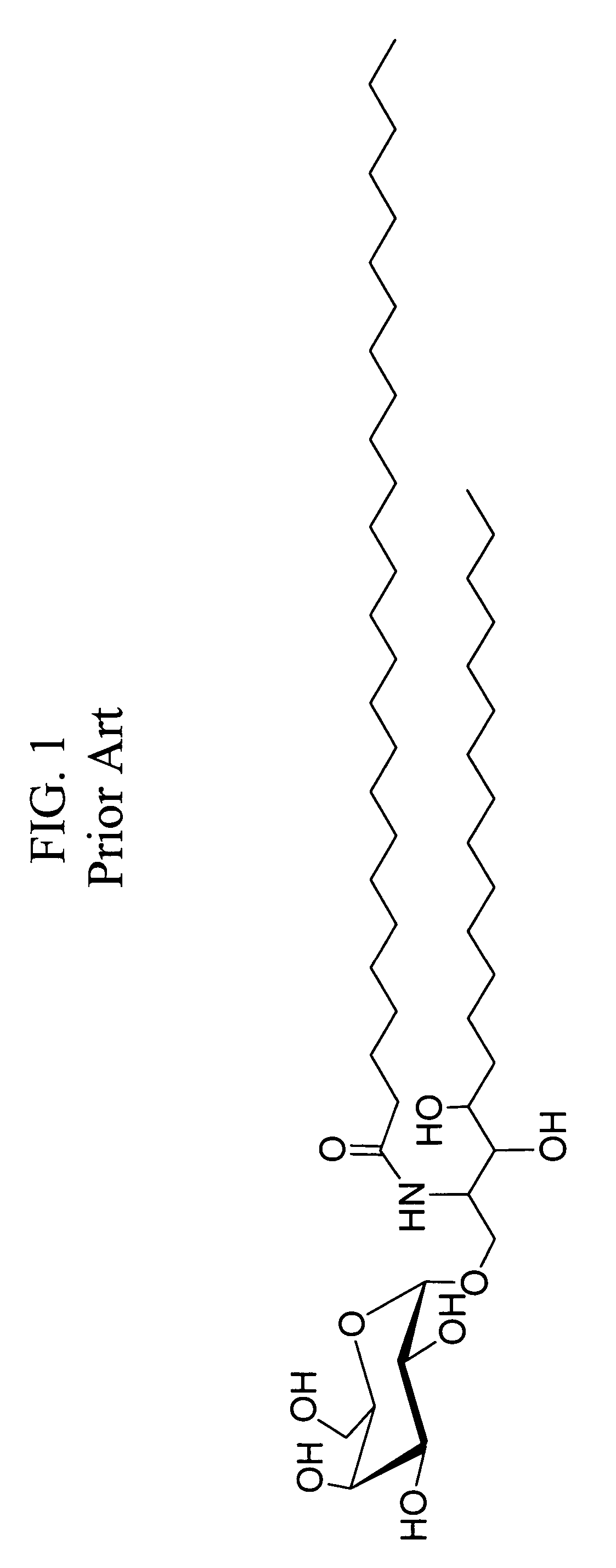
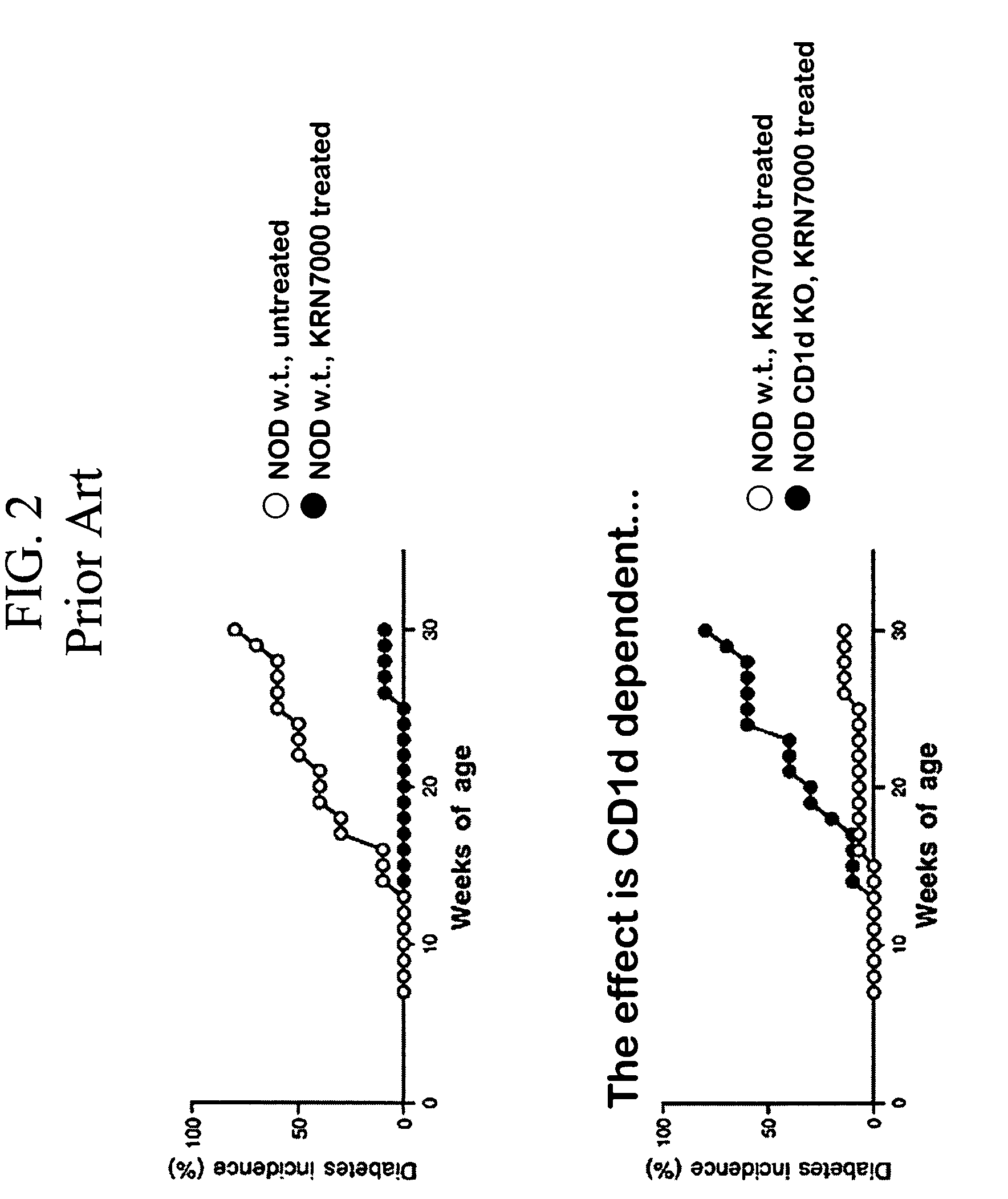
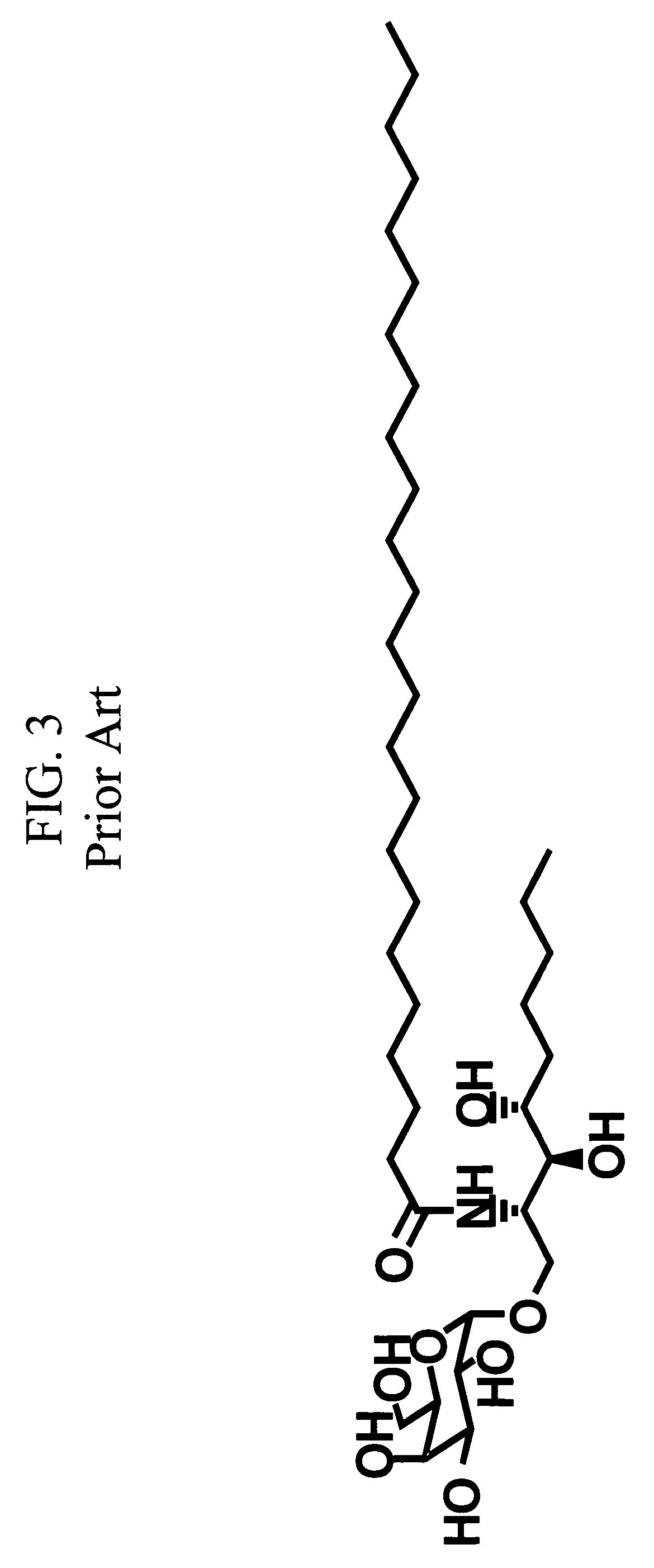
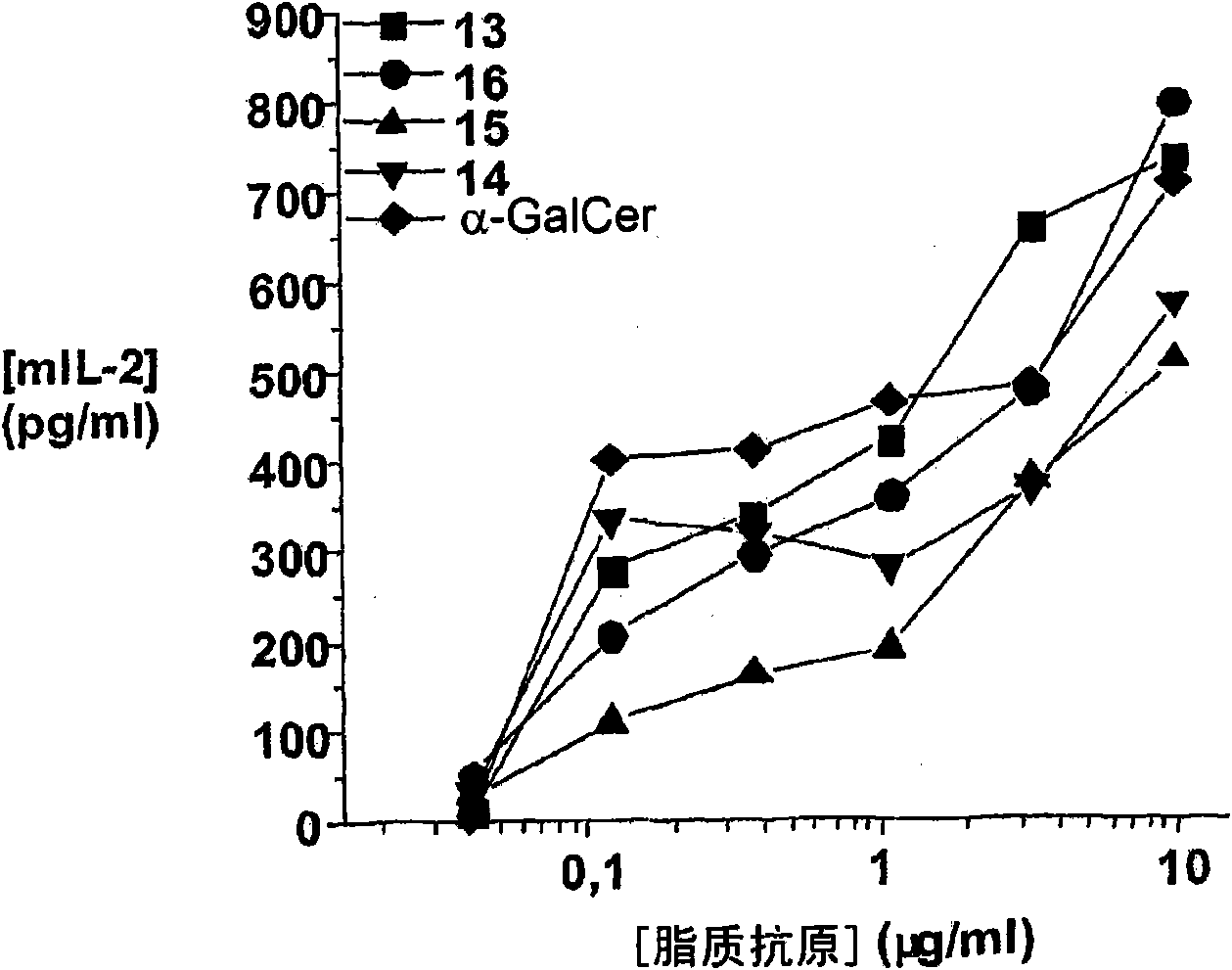
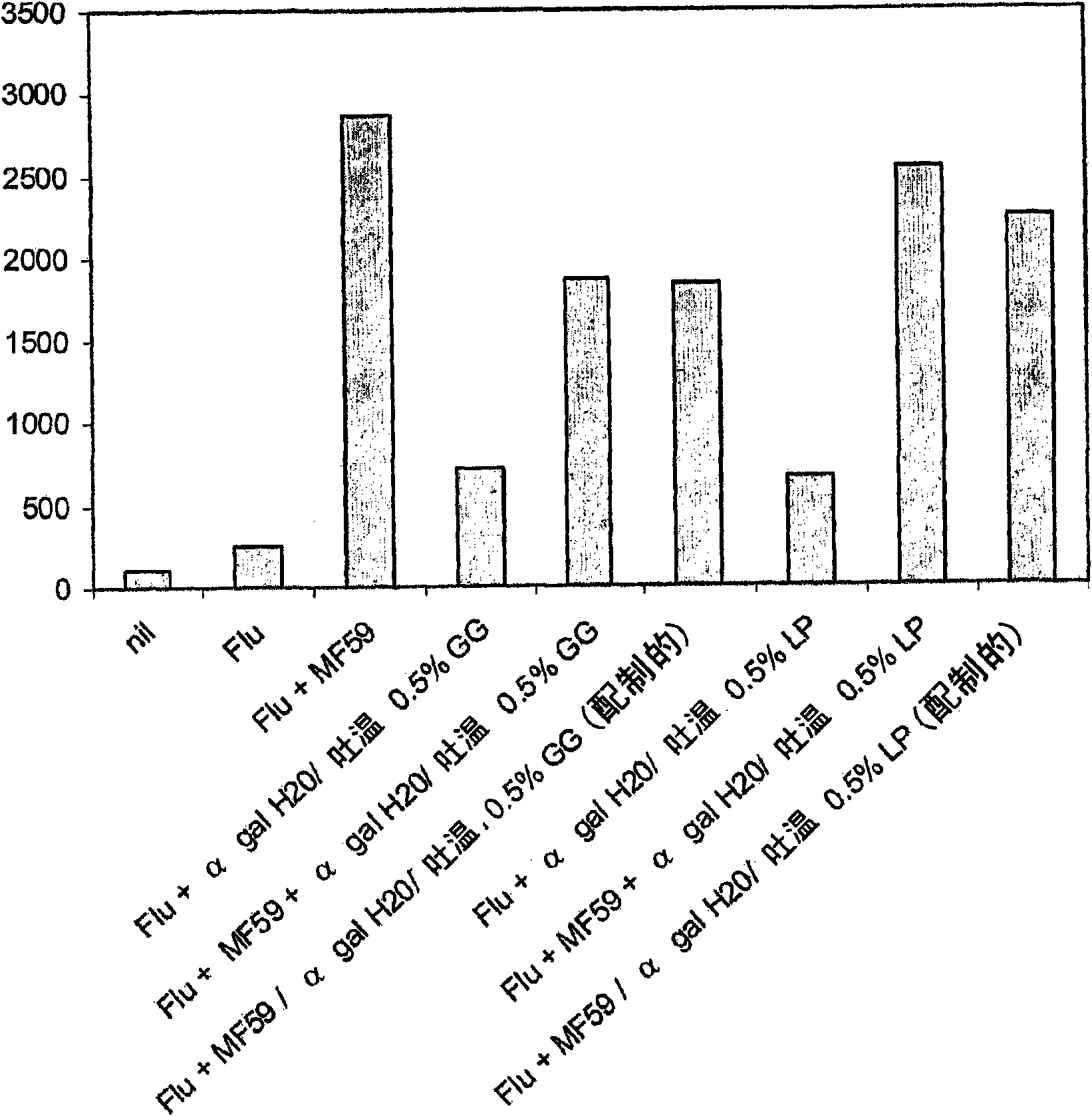
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Galactosyl ceramide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
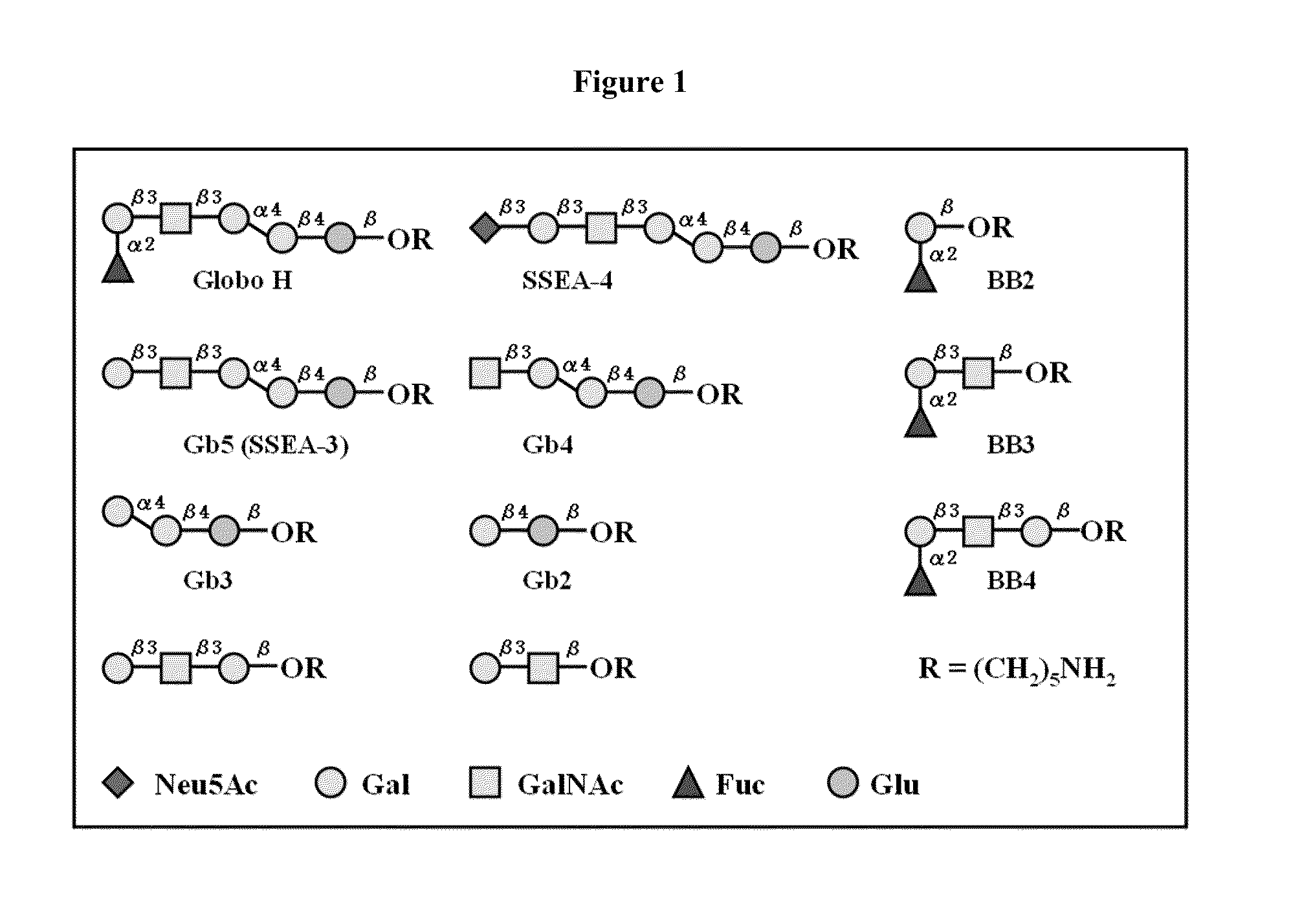
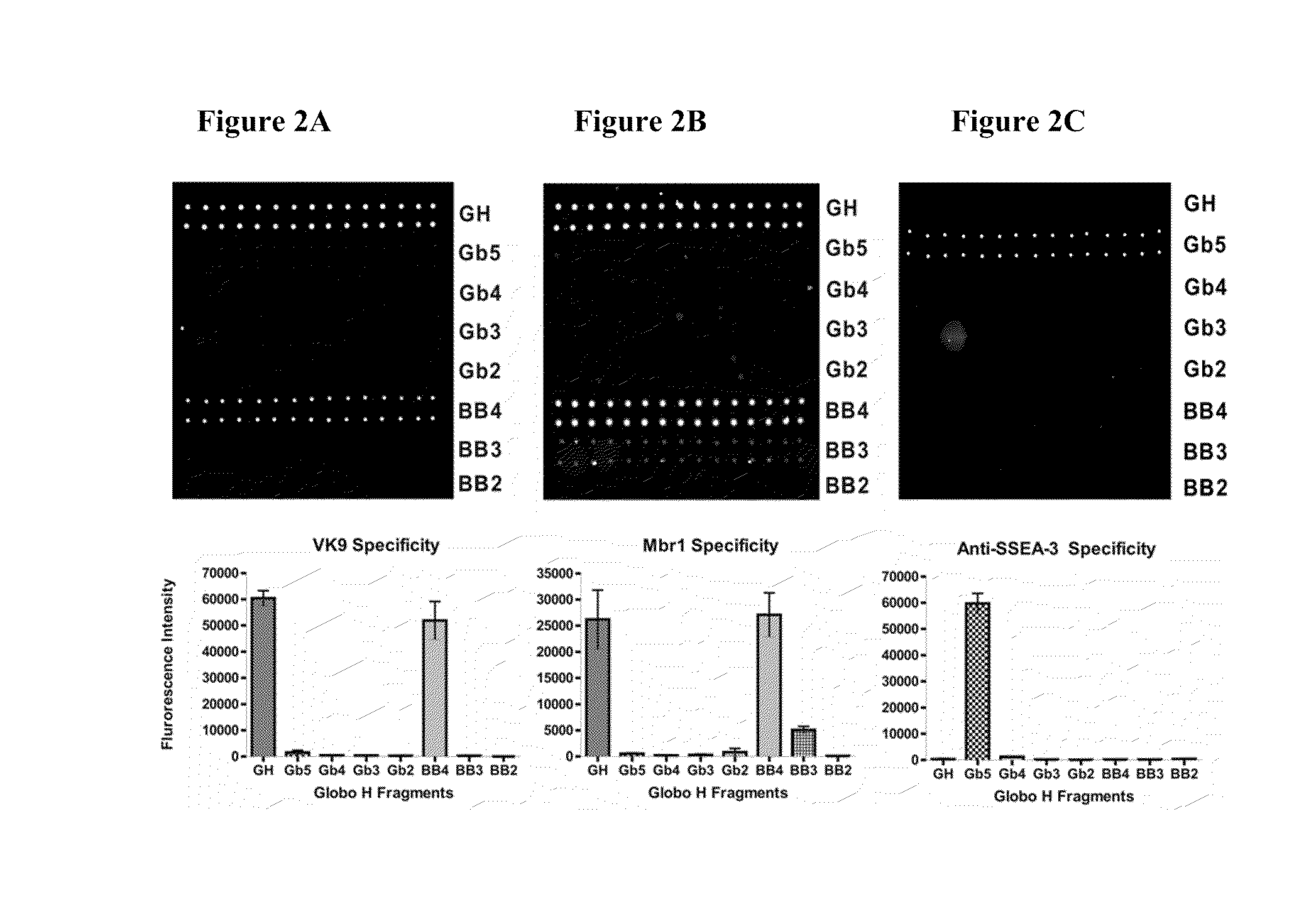
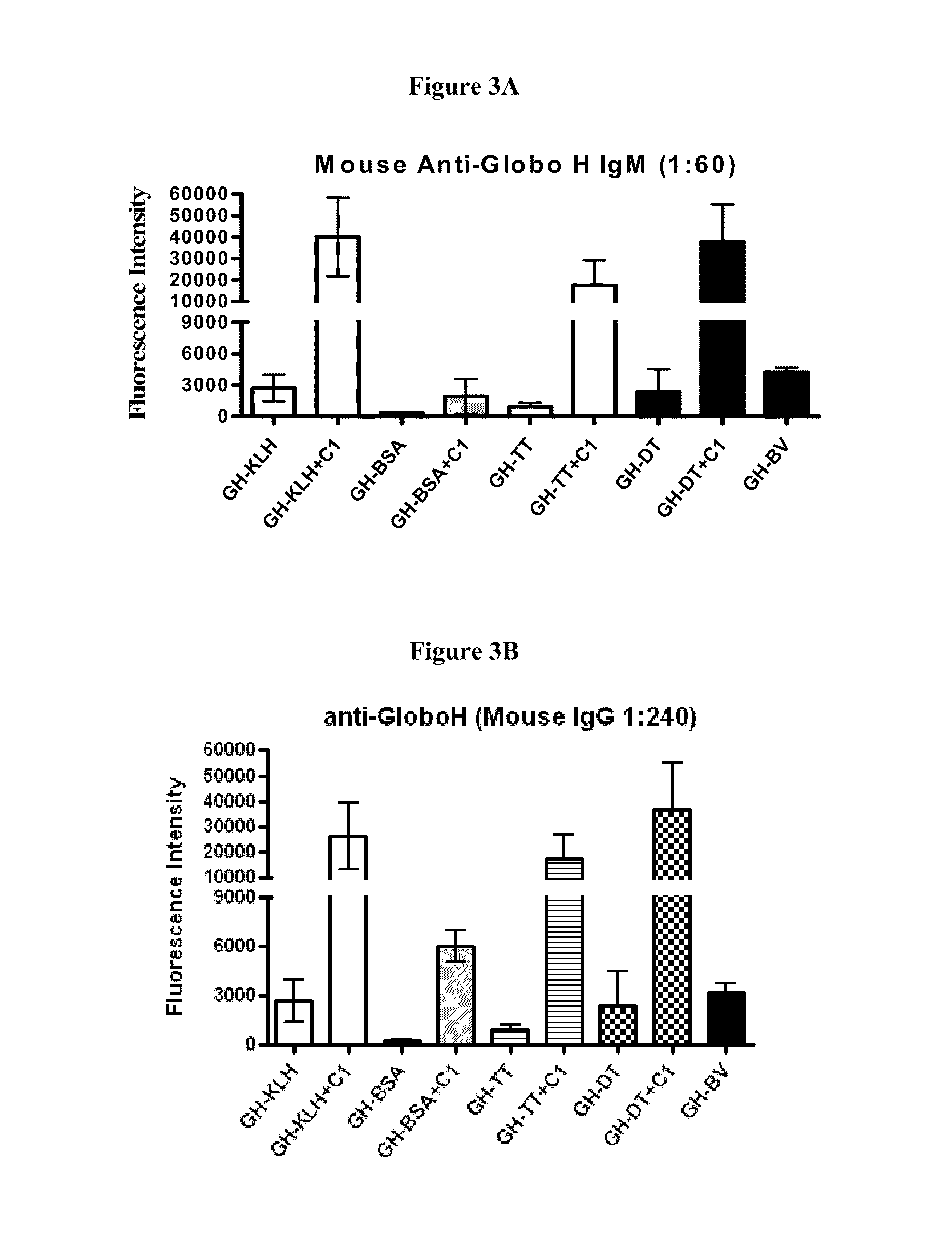
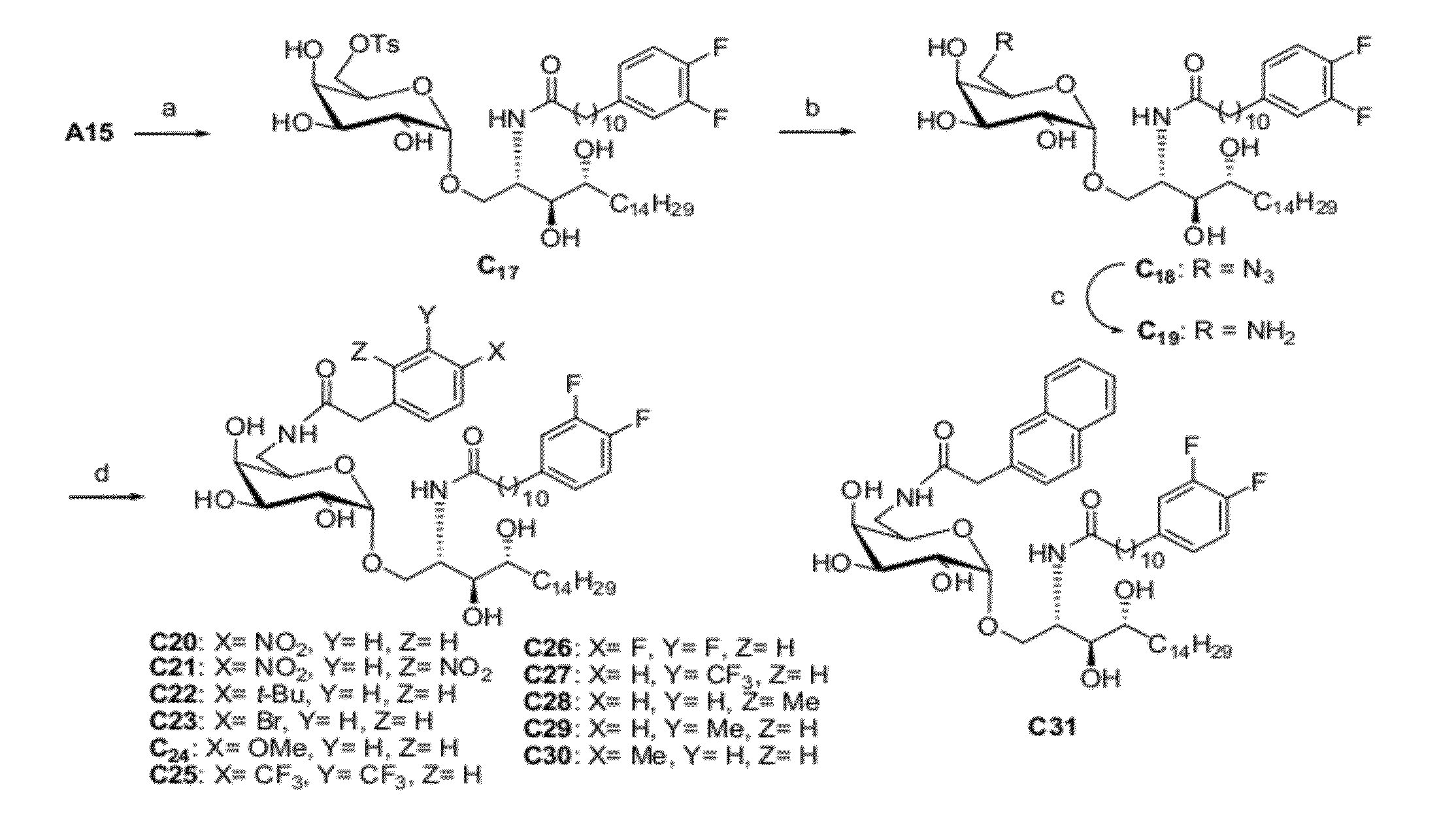
Globo h and related Anti-cancer vaccines with novel glycolipid adjuvants
ActiveUS20100136042A1Shrink tumorInhibit tumor growthOrganic active ingredientsSugar derivativesDendritic cellAdjuvant
Owner:ACAD SINIC
Methods for preparation of glycosphingolipids and uses thereof
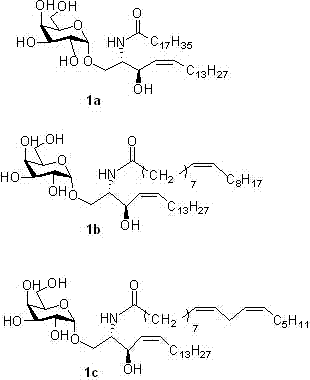
Methods for synthesis and preparation of alpha-glycosphingolipids are provided. Methods for synthesis of α-galactosyl ceramide, and pharmaceutically active analogs and variants thereof are provided. Novel alpha-glycosphingolipids are provided, wherein the compounds are immunogenic compounds which serve as ligands for NKT (natural killer T) cells.
Owner:LIANG PI HUI
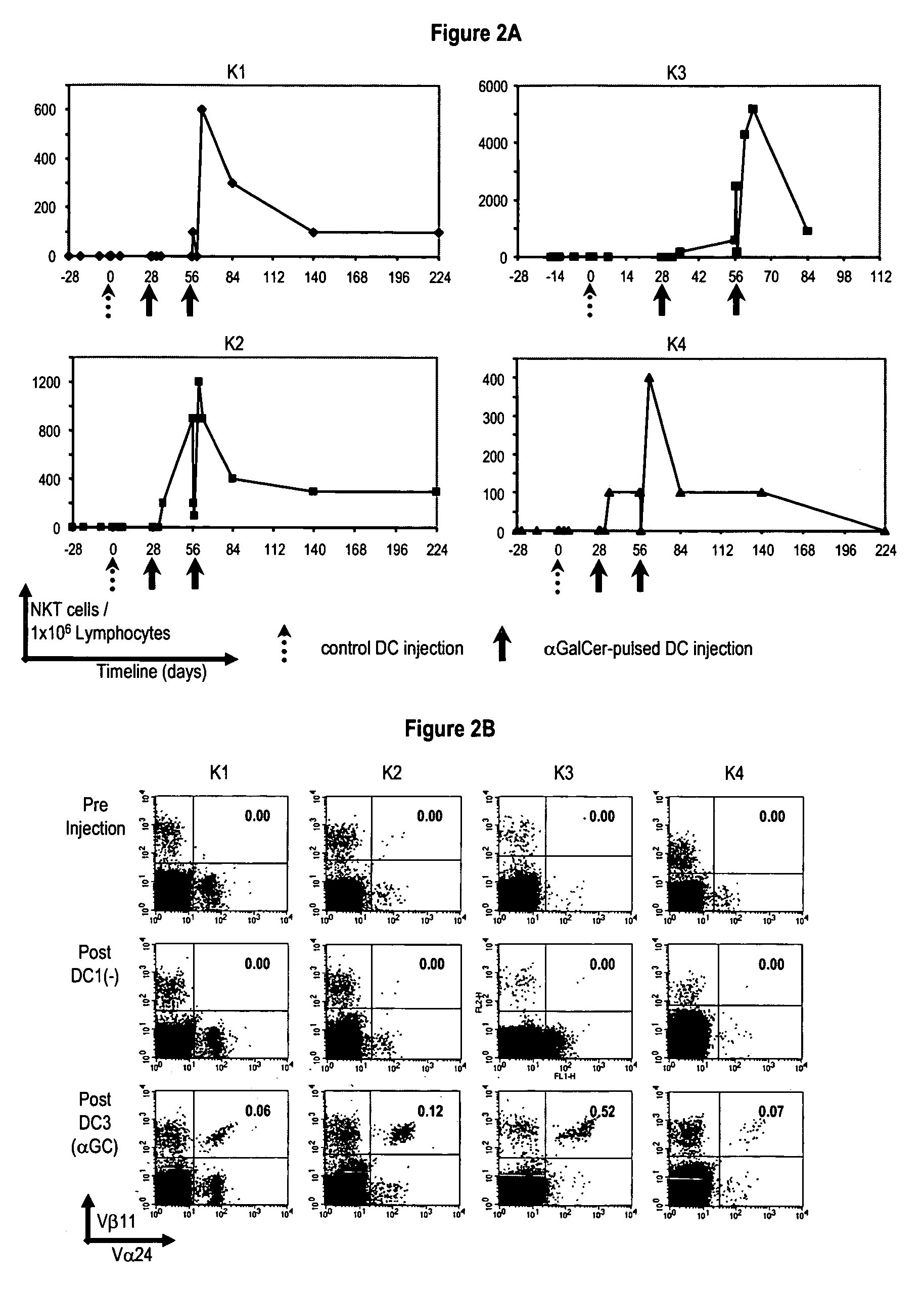
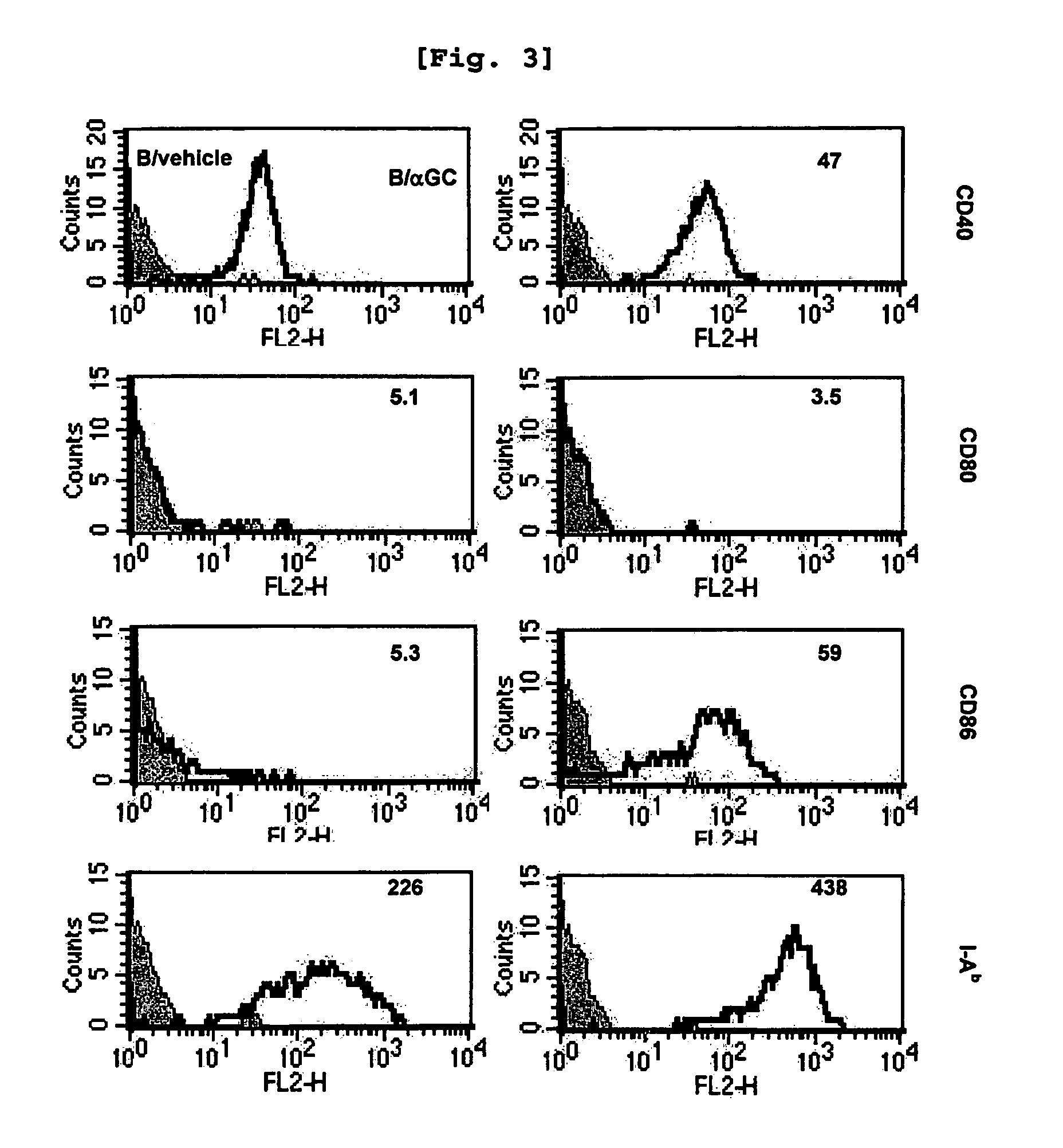
In vivo expanded NKT cells and methods of use thereof
This invention relates to the in vivo expansion of NKT cells by their exposure to mature dendritic cells expressing α-galactosyl ceramide and to methods of use thereof in modulating immune responses, such as anti-cancer responses, and enhancing memory responses.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Ceramide derivatives as modulators of immunity and autoimmunity
α-Galactosylceramides and glycosylceramides (“ceramide-like glycolipids”) that modulate NK T cells. The ceramide-like glycolipids vary in the cytokines induced in NK T cells and vary in the antigen-presenting cells that are capable of efficiently presenting the compounds to NK T cells. Pharmaceutical compositions of the ceramide-like glycolipids are provided, as are pharmaceutical compositions of the ceramide-like glycolipids combined with dendritic cells. Methods utilizing the ceramide-like glycolipids in vaccines, to activate NK T cells, to stimulate the immune system, and to treat mammals are also provided. The invention also provides methods of evaluating a compound for its ability to activate an NK T cell in the presence of a cell expressing a CD1d protein.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
NKT CELL-DERIVED iPS CELLS AND NKT CELLS DERIVED THEREFROM
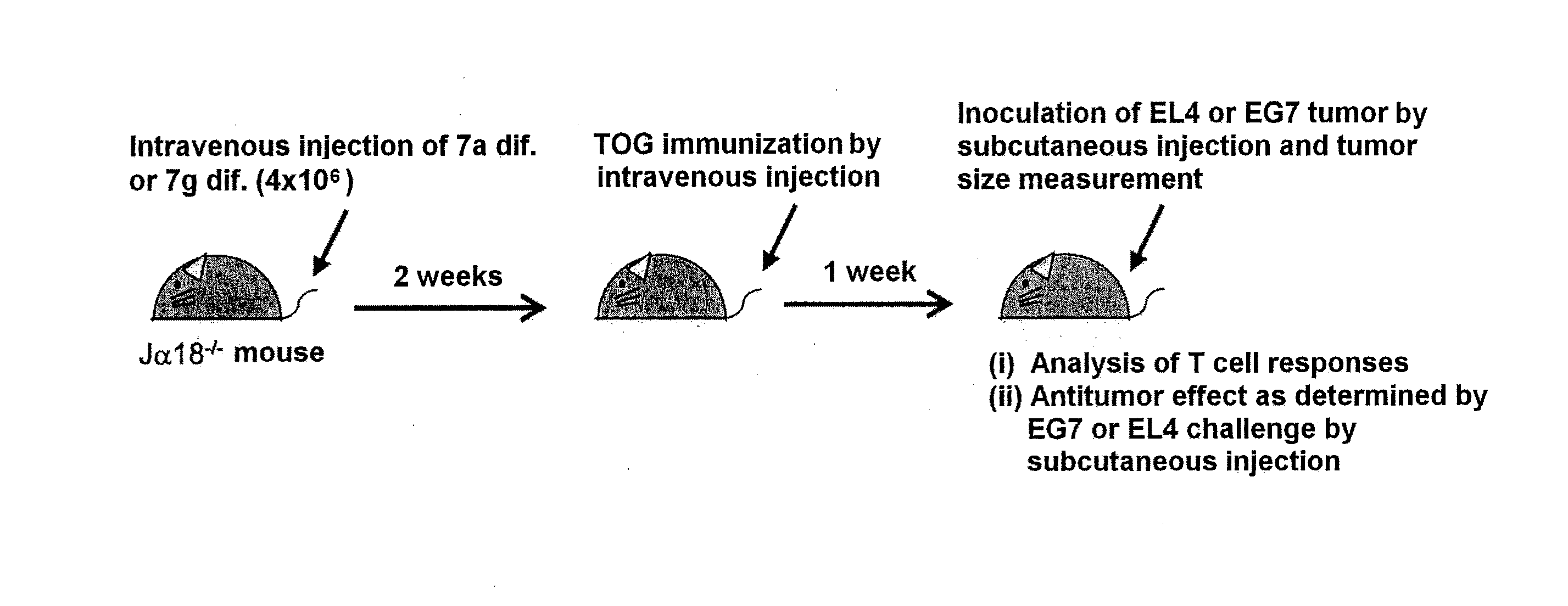
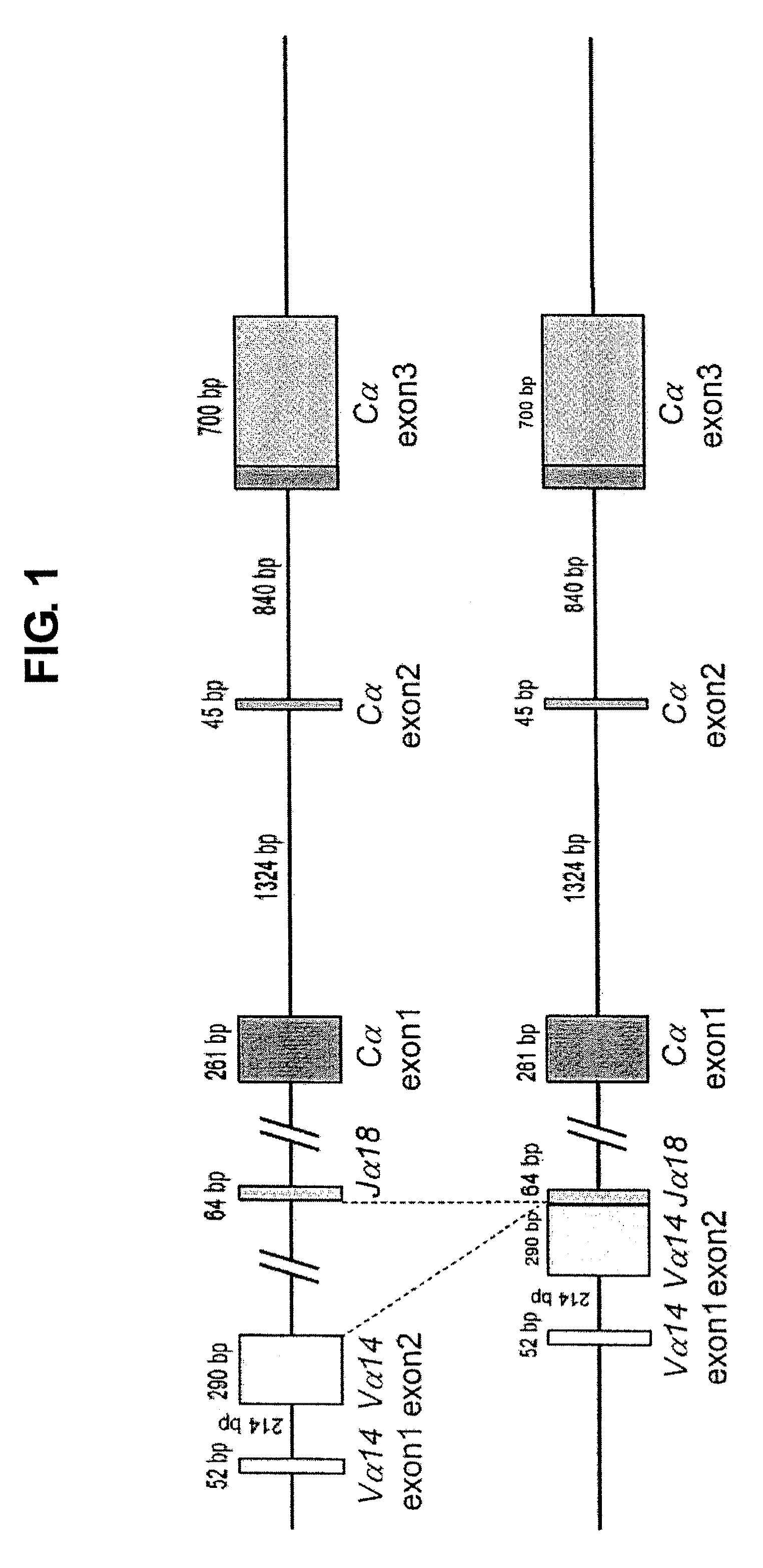
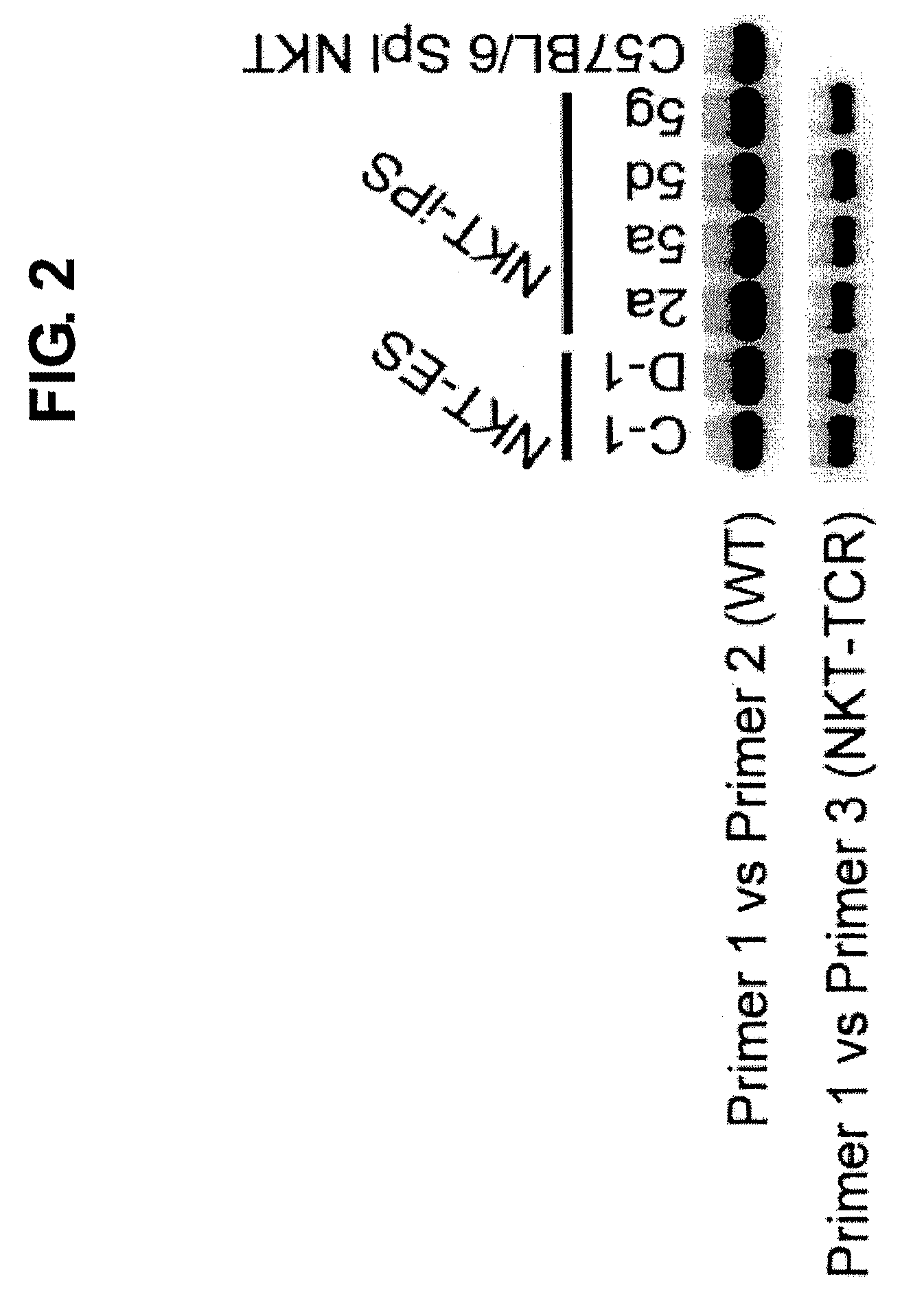
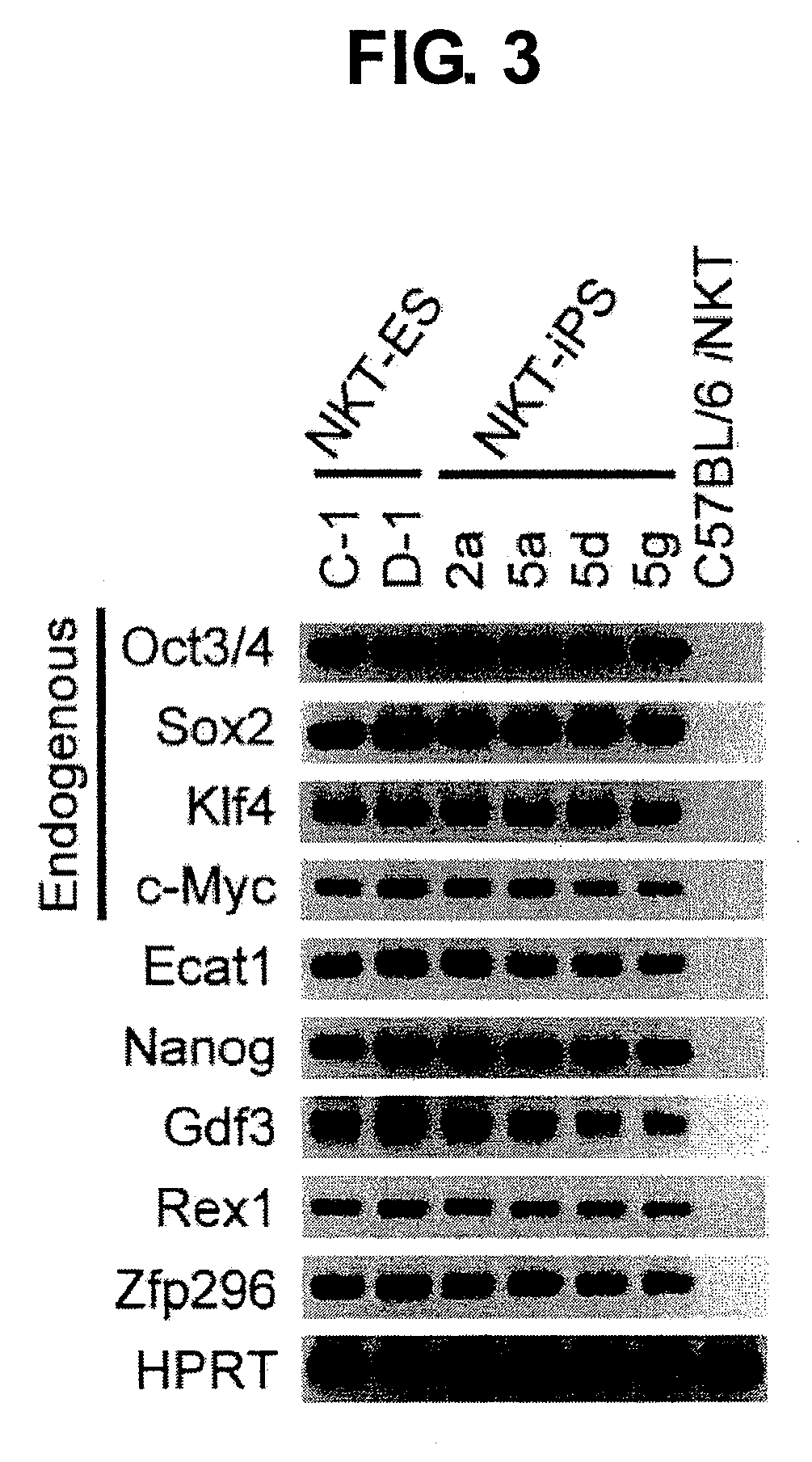
Provided are an iPS cell derived from a somatic cell such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, NKT cells differentiated from the iPS cell, a method of creating the same, and an immune cell therapy agent prepared using cells differentiated from the iPS cell. Also provided are an iPS cell having TCRα rearranged to NKT-TCR (NKT-iPS cell), obtained by contacting a somatic cell, such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, with nuclear reprogramming factors, isolated NKT cells obtained by differentiating the iPS cell ex vivo (iPS-NKT cell), a method of generating CD4 / CD8-double positive NKT cells (DP-NKT cells) and mature NKT cells from NKT-iPS cells by altering the combination of feeder cells and / or cytokines, a method of expanding the iPS-NKT cells, and an NKT cell cytotherapy agent comprising NKT cells activated with α-galactosyl ceramide (α-GalCer), or iPS-NKT cells, and α-GalCer in combination.
Owner:RIKEN
Globo h and related anti-cancer vaccines with novel glycolipid adjuvants
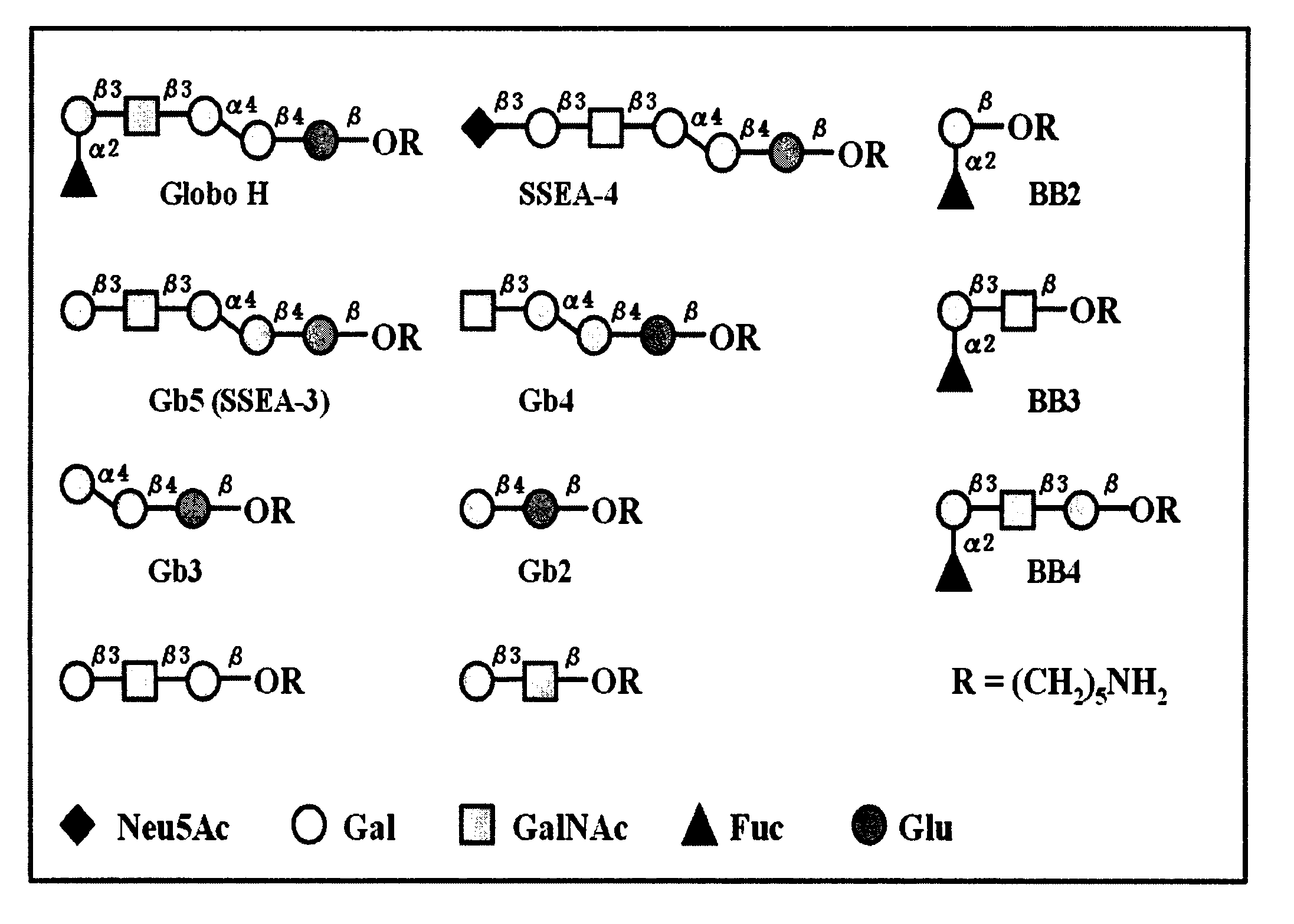
Immunogenic compositions, cancer vaccines and methods for treating cancer are provided. Compositions comprising: (a) a glycan such as Globo H or an immunogenic fragment thereof, wherein the glycan is conjugated with a carrier protein by a linker such as para-nitrophenyl; and (b) an adjuvant comprising glycolipid capable of binding CDId on a dendritic cell, such as an a-galactosyl-ceramide derivative, wherein the immunogenic composition induces an immune response that induces a higher relative level of IgG isotype antibodies as compared to IgM isotype antibodies, are provided. Immunogenic compositions comprising the carrier protein diphtheria toxin cross-reacting material 197 (DT-CRM 197) and the adjuvant C34 are provided. Antibodies generated by immunogenic compositions disclosed herein further neutralize at least one of the antigens Globo H, stage-specific embryonic antigen-3 (SSEA-3) and stage-specific embryonic antigen-4 (SSEA-4). Therapeutics against breast cancer stem cells comprising immunogenic compositions comprising Globo H, SSEA-3 or SSEA-4 conjugated with DT-CRM 197.
Owner:ACAD SINIC
Analogs of Alpha Galactosyceramide and Uses thereof
There are disclosed compound of formula I, in which R1 represents a hydrophobic moiety adapted to occupy the C′ channel of human CDId, R2 represents a hydrophobic moiety adapted to occupy the A′ channel of human CDId, such that R1 fills at least at least 30% of the occupied volume of the C′ channel compared to the volume occupied by the terminal nC14H29 of the sphingosine chain of α-galactosylceramide when bound to human CDId and R2 fills at least 30% of the occupied volume of the A′ channel compared to the volume occupied by the terminal nC25H51 of the acyl chain of α-galactosylceramide when bound to human CDId R3 represents hydrogen or OH, Ra and Rb each represent hydrogen and in addition, when R3 represents hydrogen, Ra and Rb together may form a single bond, X represents or —CHA(CHOH)nY or —P(═0)(0−)0CH2(CH0H)mY, in which Y represents CHB1B2, n represents an integer from 1 to 4, m represents 0 or 1, A årepresents hydrogen, one of B1 and B2 represents H, OH or phenyl, and the other represents hydrogen or one of B1 and B2 represents hydroxyl and the other represents phenyl, in addition, when n represents 4, then A together with one of B1 and B2 together forms a single bond and the other of B1 and B2 represents H, OH or OSO3H and pharmaceutically acceptable salts thereof; the compounds of formula I are indicted for use in the treatment of a virus, microbial infection, parasite, an autoimmune disease, cancer, allergy or asthma
Owner:LUDWIG INST FOR CANCER RES
Compound immunopotentiator, inactivated vaccine for poultry, and preparation method thereof
InactiveCN102793920AShorten the immune window periodHigh potencyOrganic active ingredientsViral antigen ingredientsAntigenLevamisole
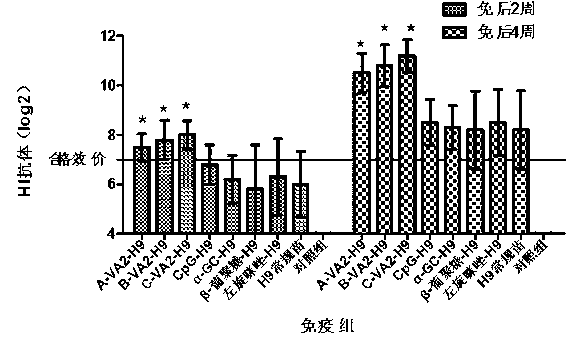
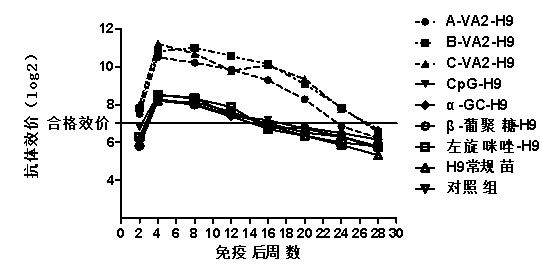
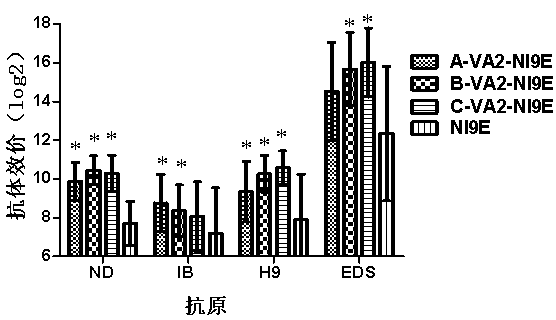
The invention relates to a compound immunopotentiator and an application in preparation of veterinary vaccines. The compound immunopotentiator contains 0.5-100 microgram / mL of nucleic acid sequence with a CpG motif, 0.5-1000 microgram / mL of alpha-galactosyl ceramide, 5-2000 microgram / mL of beta-glucan, and 5-2000 microgram / mL of levomisole, wherein the nucleic acid sequence with a CpG motif is shown in SEQ ID NO.1. When the compound immunopotentiator of the invention is mixed with an H9-subtype avian influenza inactivated vaccine, the immune window period is shortened by 2 weeks; the antibody persistent period is prolonged by 8 weeks; sterilizing immunity protection is provided for H9-subtype homotype strain attack; and the antigen content for preparing vaccines is reduced.
Owner:JIANGSU ACAD OF AGRI SCI
Β glycolipids as immuno-modulators
The invention relates to the use of β-glycolipids as immunomodulators. More particularly, the invention relates to the use of β-glycolipids, preferably, β-lactosyl-ceramide, β-glucosylceramide, β-galactosyl-ceramide, ceramid and β-lactosyl-ceramide, as well as any mixture or combination thereof for the treatment of immune related disorders. The present invention further relates to a process for the modulation of the Th1 / Th2 cell balance toward anti-inflammatory cytokine producing cells, in a subject suffering from an immune related disorder. Therapeutic compositions and method for the preparation of these compositions are also provided.
Owner:ENZO THERAPEUTICS ENZO BIOCHEM
Generating a mature NKT cell from a reprogrammed somatic cell with a T-cell antigen receptor α-chain region rearranged to uniform Va-Ja in a NKT-cell specific way
Provided are an iPS cell derived from a somatic cell such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, NKT cells differentiated from the iPS cell, a method of creating the same, and an immune cell therapy agent prepared using cells differentiated from the iPS cell. Also provided are an iPS cell having TCRα rearranged to NKT-TCR (NKT-iPS cell), obtained by contacting a somatic cell, such as an NKT cell, having the α-chain region of the T cell antigen receptor gene rearranged to uniform Vα-Jα in an NKT cell receptor-specific way, with nuclear reprogramming factors, isolated NKT cells obtained by differentiating the iPS cell ex vivo (iPS-NKT cell), a method of generating CD4 / CD8-double positive NKT cells (DP-NKT cells) and mature NKT cells from NKT-iPS cells by altering the combination of feeder cells and / or cytokines, a method of expanding the iPS-NKT cells, and an NKT cell cytotherapy agent comprising NKT cells activated with α-galactosyl ceramide (α-GalCer), or iPS-NKT cells, and α-GalCer in combination.
Owner:RIKEN
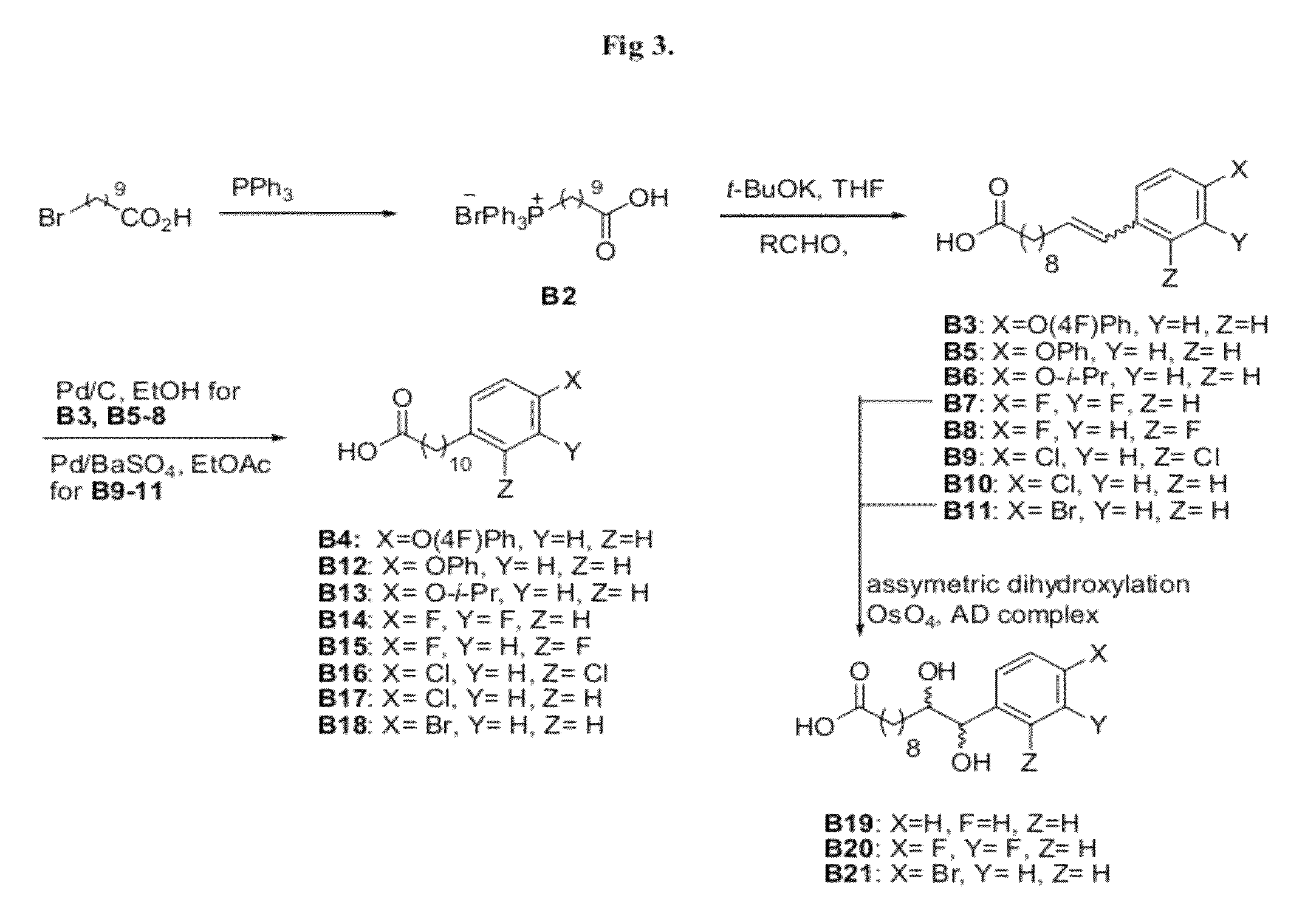
Alpha-galactosyl ceramide new isomer and synthetic method thereof
ActiveCN104497064ADoes not affect double bondsIncrease costSugar derivativesSugar derivatives preparationDouble bondCombinatorial chemistry
The invention provides an alpha-galactosyl ceramide new isomer and its synthetic method. Configuration of sphingosine chain is changed to 4,5-cis double bond sphingosine chain. According to the invention, reaction steps are shortened, yield is raised, and aftertreatment and purification steps are omitted. The synthetic method can be used for total synthesis of similar glycosyl ceramide and satisfies wide range of development, research and application of different glycosyl ceramide.
Owner:ZHEJIANG UNIV
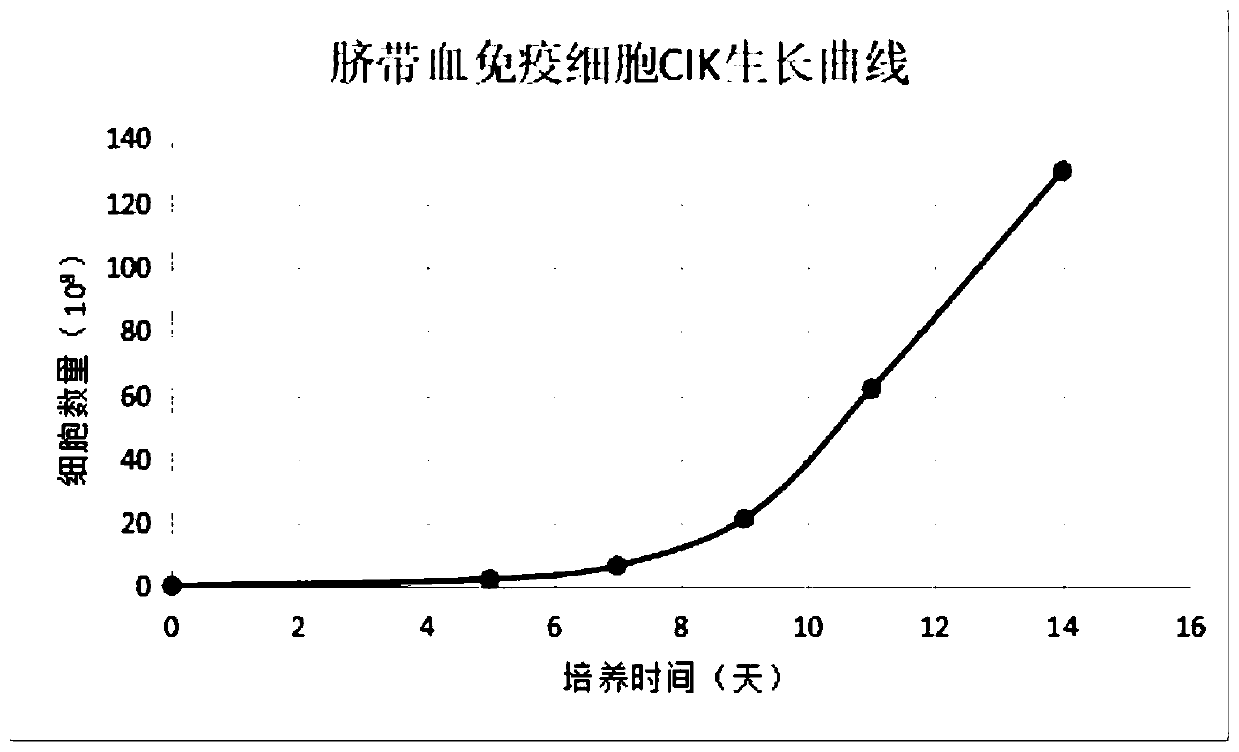
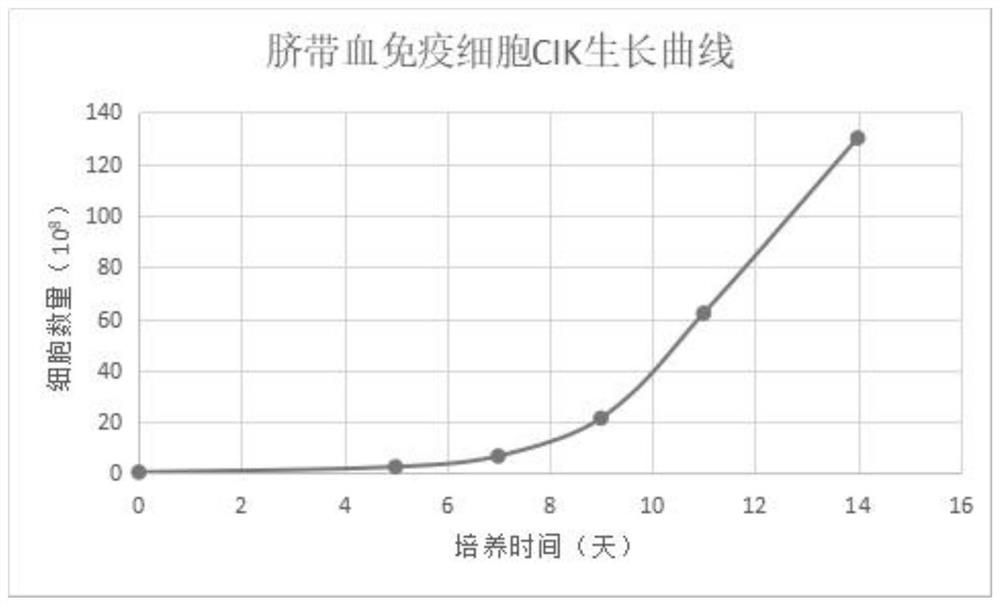
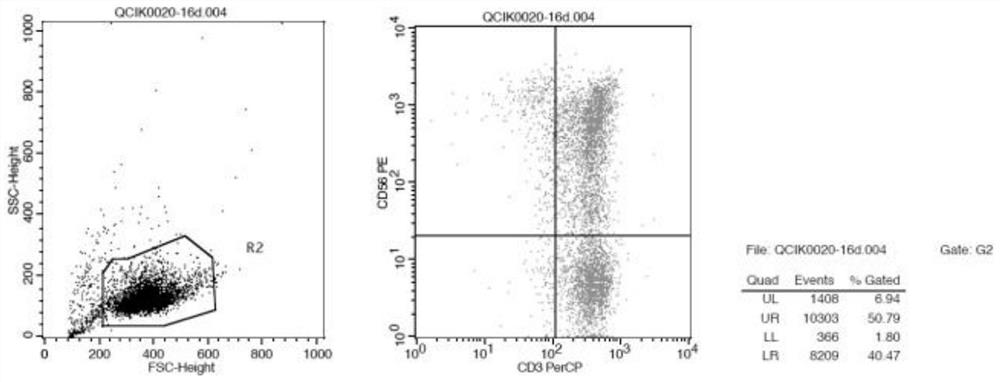
Culture method of umbilical cord blood lymphocyte CIK
ActiveCN111394308AIncrease multiplierImprove securityCulture processBlood/immune system cellsEffector cellUmbilical cord
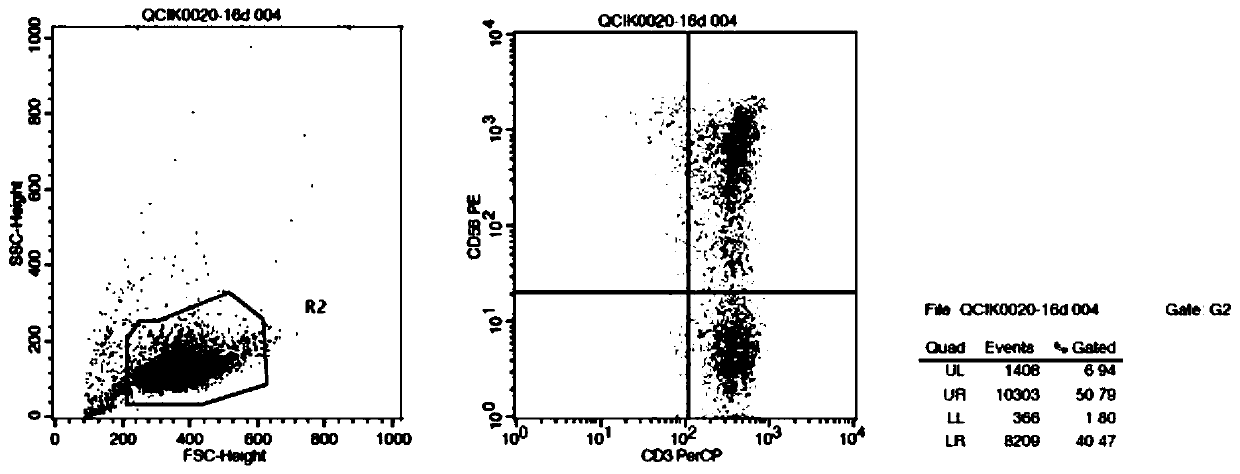
The invention belongs to the technical field of immune cell in-vitro culture, and particularly discloses a culture method of umbilical cord blood lymphocyte CIK. The method comprises the following steps: by using umbilical cord blood as a treatment object, separating the umbilical cord blood mononuclear cells, purifying and inducing the CIK cells, culturing and amplifying the CIK cells, and finally, centrifuging to collect the mature umbilical cord blood CIK cells, thereby obtaining the product. The IFN-gamma and alpha-galactose ceramide are added to activate the CIK cells, and CD3 monoclonalantibodies, IL-1 alpha, IL-2 and IL-1 beta are added in the early culture stage to perform continuous stimulation, thereby saving the coating time and enhancing the activation efficiency and amplification efficiency of effector cell groups.
Owner:GUANGDONG XIANKANGDA BIOTECH CO LTD
Biomarker for diagnosing cerebral infarction and white matter lesion and application thereof
ActiveCN113447601ADiagnosis helpsComponent separationDisease diagnosisCerebral infarctionInternal medicine
The invention provides a biomarker for diagnosing cerebral infarction and white matter lesion and application of the biomarker. The biomarker is an application of galactosylceramide in preparation of a detection reagent for diagnosing cerebral infarction and white matter lesions, and the biomarker galactosylceramide is combined with glucosylceramide, beta-allopietapentaol, platyclalene, 1, 3Z, 6Z, 9Z-nonane tetraene, p-cymene, oxalic acid, 3-(stearoyloxy)-4-(trimethyl ammonium) butyrate for diagnosing cerebral infarction and white matter lesions, so the risk of suffering from cerebral infarction and white matter lesion can be judged, and the cerebral infarction and white matter lesion can be prevented in advance.
Owner:BAO FENG BIOTECH (BEIJING) CO LTD
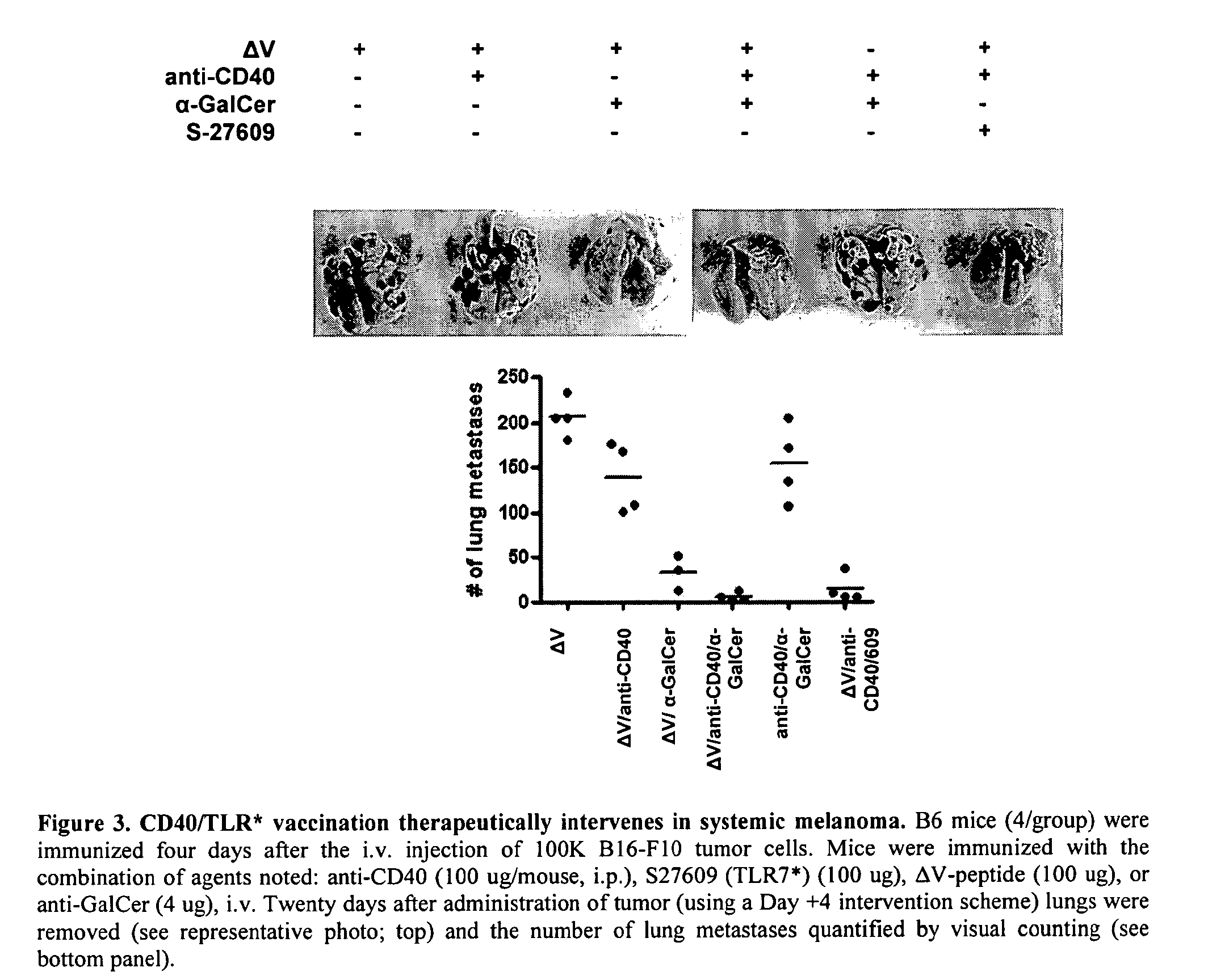
Methods of promoting antitumor immunity by administering CD40 agonists and alpha-galactosyl ceramide
Owner:IMMURX INC
Methods for preparation of glycosphingolipids and uses thereof
Methods for synthesis and preparation of alpha-glycosphingolipids are provided. Methods for synthesis of α-galactosyl ceramide, and pharmaceutically active analogs and variants thereof are provided. Novel alpha-glycosphingolipids are provided, wherein the compounds are immunogenic compounds which serve as ligands for NKT (natural killer T) cells.
Owner:LIANG PI HUI
Ceramide preparation pure dry powder, and preparation and application thereof
InactiveCN101444459AEasy to implementReduce manufacturing costCosmetic preparationsBody powdersEmulsionCholesterol
The invention relates to a ceramide preparation pure dry powder which is white without peculiar smell and is made from galactosyl ceramide, phosphatide and cholesterol which are mixed uniformly. The weights of the galactosyl ceramide, the phosphatide and the cholesterol respectively take 60 percent to 70 percent, 20 percent to 25 percent and 10 percent to 15 percent in the total weight of the dry powder. A dry pasty ceramide preparation developed by East China Normal University and ethanol are used as raw material and extracting solution respectively. The ethanol is used for extracting ceramide from the dry pasty ceramide preparation twice, the extract is frozen and dried, and the dry powder is prepared. The dry powder is used as the raw material; a small quantity of 95 percent medical ethanol is added to be heated and dissolved and injected into hot stirred distilled water by an injector; and ceramide lipidosome emulsion is prepared. The dry powder is added into cosmetic; the addition is 0.01 percent to 0.1 percent of the weight of the cosmetic; the dry powder and the cosmetic are stirred uniformly; and the cosmetic with moisturizing function is prepared.
Owner:SHANGHAI HOPE TEC BIOTECH
Drug Having Regulatory Cell Ligand Contained in Liposome
ActiveUS20100104632A1Inhibit proliferation and differentiationAvoid actionBiocideSenses disorderDiseaseBULK ACTIVE INGREDIENT
A liposome containing a regulatory cell ligand such as α-galactosyl ceramide or β-galactosyl ceramide is employed as the active ingredient of a drug for preventing or treating immune diseases etc.
Owner:RIKEN
SOD (superoxide dismutase) ethosome and preparation method thereof
InactiveCN105380905AGood transdermal effectImprove stabilityPeptide/protein ingredientsPharmaceutical non-active ingredientsAlcoholEtioplasts
The invention discloses an SOD (superoxide dismutase) ethosome and a preparation method thereof. The ethosome mainly consists of the following components: galactosyl ceramide, PEG (polyethylene glycol) modified SOD, low-molecule alcohol and water; and the preparation method mainly comprises the following steps: according to constituting proportions of the components, dissolving the galactosyl ceramide in a low-molecule alcohol mixed solution so as to obtain a solution A; dissolving the PEG modified SOD in ethanol and uniformly stirring so as to obtain a solution B; mixing the solution A with the solution B; adding a buffer solution as a uniform milk white suspension is formed; then, ultrasonically processing in an ice-bath condition; and finally, processing by virtue of a filtering membrane, so that the SOD ethosome is obtained. The SOD ethosome has the advantages of strong stability, strong transdermal penetration and simple preparation process.
Owner:AFFILIATED YONGCHUAN HOSPITAL OF CHONGQING MEDICAL UNIV
Biomarkers and their applications for the diagnosis of cerebral infarction and white matter lesions
The invention provides biomarkers for diagnosing cerebral infarction and white matter lesions and applications thereof. The biomarker is the application of galactosylceramide in the preparation of detection reagents for diagnosing cerebral infarction and white matter lesions. The biomarkers Galactosylceramide combined with glucosylceramide, β‑alpipentol, arborvitene, 1,3Z,6Z,9Z‑nonanetetraene, p-cymene, oxalic acid, 3‑(stearyloxy) ‑4‑(Trimethylammonium) butyrate can be used to judge the risk of cerebral infarction and white matter lesions, and can prevent and prevent cerebral infarction and white matter lesions in advance.
Owner:BAO FENG BIOTECH (BEIJING) CO LTD
Method for screening a modulator of a tmem16 family member
The present invention relates to a method for screening a modulator of a TMEM16 family member, which comprises the following steps:(1) treating cells expressing the TMEM16 family member with a candidate of the modulator, and(2) determining whether the candidate alters distribution of a lipid selected from phosphatidylserine, phosphatidylcholine, and galactosylceramide in plasma membrane of the cells,wherein a candidate which increases distribution of phosphatidylserine in the outer leaflet of plasma membrane compared to control is selected as a modulator enhancing a function of the TMEM16 family member, and a candidate which decreases distribution of phosphatidylserine in the outer leaflet of plasma membrane compared to control is selected as a modulator suppressing a function of the TMEM16 family member, anda candidate which increases distribution of phosphatidylcholine or galactosylceramide in the inner leaflet of plasma membrane compared to control is selected as a modulator enhancing a function of the TMEM16 family member, and a candidate which decreases distribution of phosphatidylcholine or galactosylceramide in the inner leaflet of plasma membrane compared to control is selected as a modulator suppressing a function of the TMEM16 family member.
Owner:KYOTO UNIV
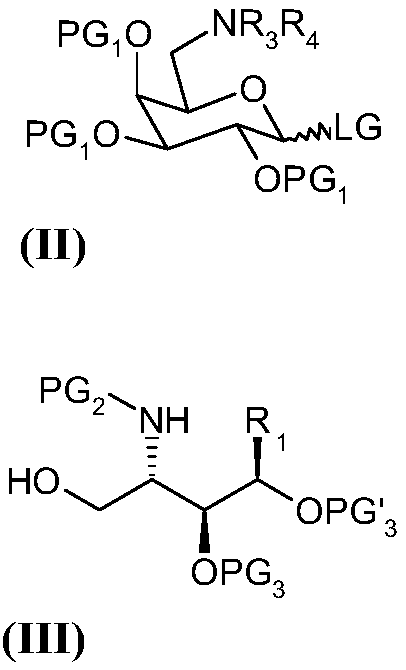
Method of preparation of alpha-galactosyl ceramides compounds
ActiveCN109503682AEsterified saccharide compoundsSugar derivativesCombinatorial chemistryGlycosylation
The invention relates to a method of preparation of alpha-galactosyl ceramides compounds of formula (I) comprising a step a) of glycosylation of a compound of formula (II) with a compound of formula (III).
Owner:ABIVAX
Analogues of glycolipids useful as immunoadjuvants
ActiveCN102066394AImprove efficiencyAntibacterial agentsOrganic active ingredientsGlycolipidGalactosyl ceramide
The invention provides analogs of alpha-galactosyl ceramide that increase the immune response elicited by various antigens. It also provides methods of using such compounds to increase the effectiveness of vaccines.
Owner:路易吉·潘扎
Biomarker for white matter lesion and application thereof
ActiveCN114236019ALow costEasy to realize detection purposeComponent separationHealth-index calculationBiologic markerCeramide binding
The invention provides a biomarker for white matter lesion and application thereof, and particularly relates to application of a biomarker ceramide (d18: 0 / 24: 1 (15Z)) in preparation of a detection reagent for diagnosing white matter lesion. The risk of the white matter lesion is judged by combining a biomarker lipoceramide (d18: 0 / 24: 1 (15Z)) with one or two of PEIPC, phosphatidylcholine (P-18: 1 (9Z) / 0: 0), phosphatidylcholine (O-18: 0 / 0: 0) or galactosylceramide (d18: 0 / 16: 0), and the white matter lesion can be prevented in advance.
Owner:BAO FENG BIOTECH (BEIJING) CO LTD
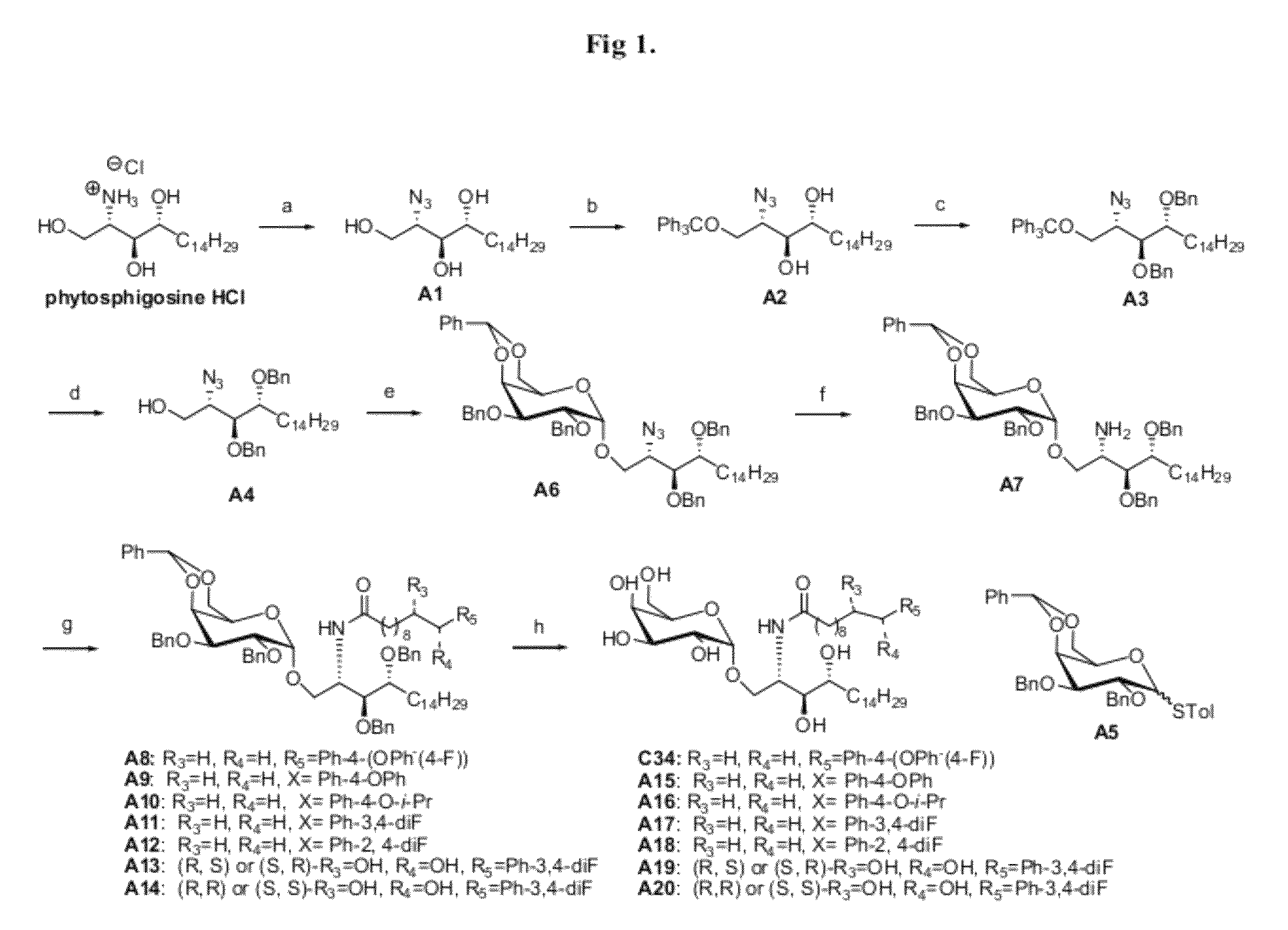
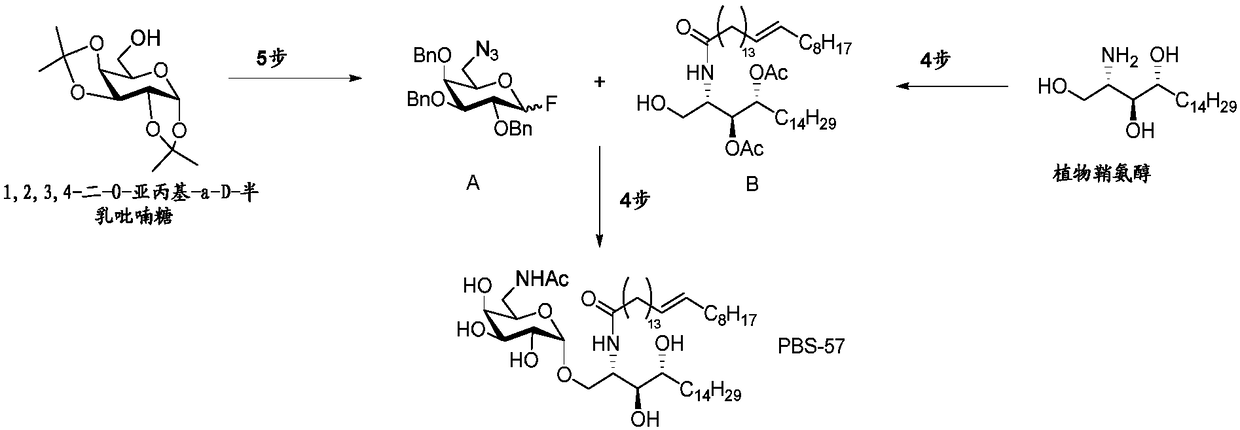
Highly efficient synthesis of alpha-O-galactosyl ceramides
A method for the production of a-O-galactosyl ceramide precursor is demonstrated. The method involves the reaction of galactosyl iodide with a sphingosine derivative or phytosphingosine derivative in the presence of a quaternary ammonium iodide salt to prepare the a-O-galactosyl ceramide precursor in the a-anomer form. The a-O-galactosyl ceramide is then prepared by reaction with a suitable fatty acid or fatty acid derivative.
Owner:RGT UNIV OF CALIFORNIA
In vivo expanded NKT cells and methods of use thereof
This invention relates to the in vivo expansion of NKT cells by their exposure to mature dendritic cells expressing α-galactosyl ceramide and to methods of use thereof in modulating immune responses, such as anti-cancer responses, and enhancing memory responses.
Owner:KYOWA HAKKO KIRIN CO LTD +1
B cell-based vaccine loaded with the ligand of natural killer T cell and antigen
The present invention relates to a B cell-based vaccine loaded with the ligand of natural killer T cell and antigen for the prevention and treatment of disease, more precisely, an immunotherapeutic and prophylactic vaccine mediated by B cells loaded with α-galactosylceramide, a kind of glycolipid which can stimulate natural killer T cells. A vaccine composition of the present invention can be effectively used as an antitumor immunotherapeutic agent. B cells therein, which are easily obtainable compared with dendritic cells, not only induce a similar level of cytotoxic T lymphocyte response to that of the conventional dendritic cell-based vaccine but also have a prophylactic and therapeutic effect on solid tumor and metastatic tumor.
Owner:CELLID
New isomer of α-galactosylceramide and its synthesis method
ActiveCN104497064BDoes not affect double bondsHigh yieldSugar derivativesSugar derivatives preparationSynthesis methodsDouble bond
The invention provides an alpha-galactosyl ceramide new isomer and its synthetic method. Configuration of sphingosine chain is changed to 4,5-cis double bond sphingosine chain. According to the invention, reaction steps are shortened, yield is raised, and aftertreatment and purification steps are omitted. The synthetic method can be used for total synthesis of similar glycosyl ceramide and satisfies wide range of development, research and application of different glycosyl ceramide.
Owner:ZHEJIANG UNIV
Preparation method and application of alpha-GalCer nanoparticles
InactiveCN111349602ALow cytotoxicityEasy to makeCulture processBlood/immune system cellsNanoparticleCombinatorial chemistry
The invention discloses a preparation method and application of alpha-GalCer nanoparticles. The preparation method comprises the following steps: respectively preparing a polyethylenimine (PEI) solution and an alpha-galactosyl ceramide (alpha-GalCer) solution; preparing an alpha-GalCer nanoparticle mixed solution by using the PEI solution and the alpha-GalCer solution through electrostatic action;and performing drying to obtain the alpha-GalCer nanoparticles. The invention provides the method for preparing the alpha-GalCer nanoparticles by combining PEI and alpha-GalCer, wherein the preparedalpha-GalCer nanoparticles reduce cytotoxicity of the PEI. The prepared alpha-GalCer nanoparticles are used for amplification of iNKT to prepare Valpha24+iNKT cells. Compared with the prior art, the preparation method has a simple preparation process and shorter preparation time.
Owner:诺莱生物医学科技有限公司
Ceramide preparation pure dry powder, and preparation and application thereof
InactiveCN101444459BEasy to implementReduce manufacturing costCosmetic preparationsBody powdersEmulsionCholesterol
The invention relates to a ceramide preparation pure dry powder which is white without peculiar smell and is made from galactosyl ceramide, phosphatide and cholesterol which are mixed uniformly. The weights of the galactosyl ceramide, the phosphatide and the cholesterol respectively take 60 percent to 70 percent, 20 percent to 25 percent and 10 percent to 15 percent in the total weight of the dry powder. A dry pasty ceramide preparation developed by East China Normal University and ethanol are used as raw material and extracting solution respectively. The ethanol is used for extracting ceramide from the dry pasty ceramide preparation twice, the extract is frozen and dried, and the dry powder is prepared. The dry powder is used as the raw material; a small quantity of 95 percent medical ethanol is added to be heated and dissolved and injected into hot stirred distilled water by an injector; and ceramide lipidosome emulsion is prepared. The dry powder is added into cosmetic; the addition is 0.01 percent to 0.1 percent of the weight of the cosmetic; the dry powder and the cosmetic are stirred uniformly; and the cosmetic with moisturizing function is prepared.
Owner:SHANGHAI HOPE TEC BIOTECH
A kind of culture method of umbilical cord blood lymphocyte cik
ActiveCN111394308BIncrease multiplierImprove securityCulture processBlood/immune system cellsCord blood lymphocyteUmbilical cord
The invention belongs to the technical field of immune cell in-vitro culture, and particularly discloses a culture method of umbilical cord blood lymphocyte CIK. The method comprises the following steps: by using umbilical cord blood as a treatment object, separating the umbilical cord blood mononuclear cells, purifying and inducing the CIK cells, culturing and amplifying the CIK cells, and finally, centrifuging to collect the mature umbilical cord blood CIK cells, thereby obtaining the product. The IFN-gamma and alpha-galactose ceramide are added to activate the CIK cells, and CD3 monoclonalantibodies, IL-1 alpha, IL-2 and IL-1 beta are added in the early culture stage to perform continuous stimulation, thereby saving the coating time and enhancing the activation efficiency and amplification efficiency of effector cell groups.
Owner:GUANGDONG XIANKANGDA BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com