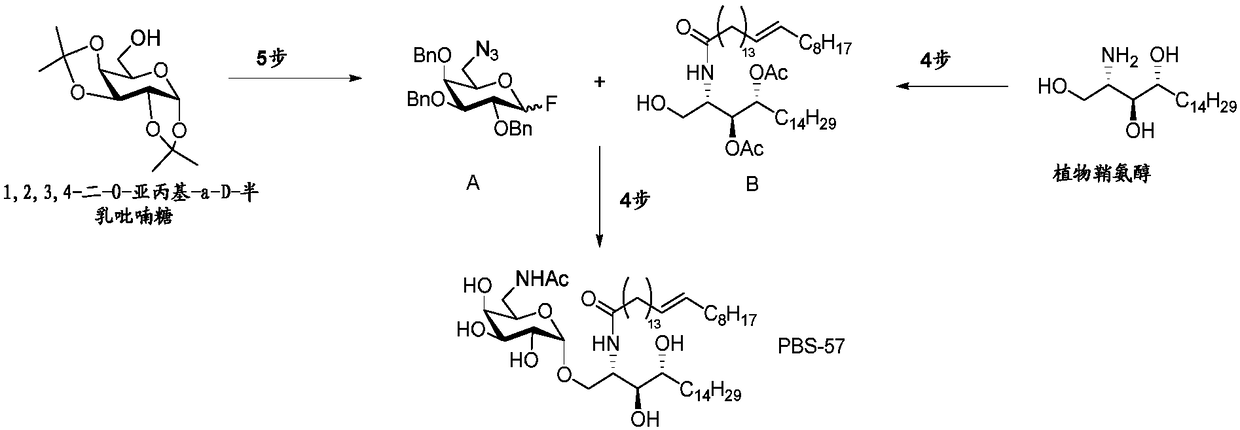
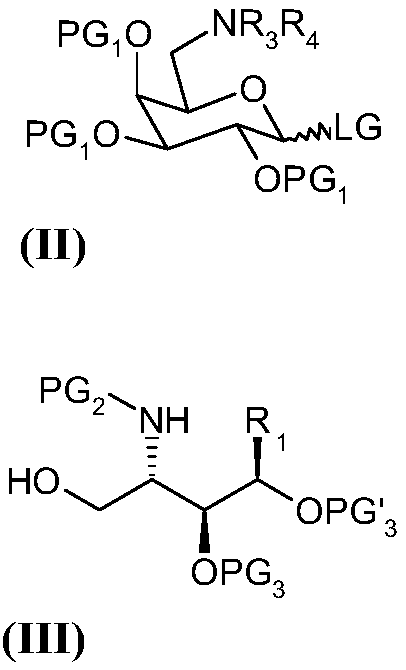
Method of preparation of alpha-galactosyl ceramides compounds
A kind of compound and alkyl technology, applied in the field of preparing a class of 6"-deoxy-6-amino α-galactosylceramide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0292] Process for preparing compound (Ia)
[0293] Batch (Ia) was prepared according to Good Manufacturing Practice for clinical trials. A detailed description of the synthetic steps follows.
[0294] Preparation of compound 1
[0295] After 7 synthetic steps, methyl 6-acetylamino-2,3,4-tri-O-benzyl-α-D-galactopyranoside (1) was prepared from commercially available (Indofine Chemical Company) 1,2,3,4-Di-O-isopropylidene-α-D-galactopyranose (XII):
[0296]
[0297] - activation of the alcohol function to a tosyl group from commercially available (XII),
[0298] - partial substitution of the tosyl group by phthalimide,
[0299] - cleavage of the alcohol protecting the isopropylidene group, methylation of the anomer position and purification on silica gel,
[0300] - cleaves the phthalimide ring to provide the free amine,
[0301] - acetalization of alcohols and amino groups,
[0302] - cleavage of acetyl esters, and
[0303] - Benzylation of the hydroxyl group and fina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com