New isomer of α-galactosylceramide and its synthesis method
A technology of galactosylceramide and synthesis method, which is applied in the direction of chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of high reactivity and instability of iodized sugar, reduce the difficulty of operation, and reduce the synthesis cost Reduced, highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Construction of 4,5-cis sphingosine (see image 3 )
[0024] The raw material uses galactose with a relatively cheap structure as a chiral source. In the first step, acetal is formed to protect the 4 and 6 hydroxyl groups of galactose; in the second step, an aldehyde group is formed at the 3 hydroxyl, and the carbons at the 1 and 2 positions of the sugar are removed; in the third step, the Wittg reaction is used to react with Pre-synthesized tetradecylphosphorus ylide reaction to obtain 4,5-cis-alkene, which is a relatively critical step; the fourth step is to azidize the original 5-position hydroxyl on the carbon, and a carbon configuration occurs at this position Invert to obtain the desired configuration; the fifth step is deketalization reaction to obtain (2S,3R,4Z)-2-azido-1,3-diol derivatives.
[0025] The reported compounds were identified by mass spectrum or hydrogen spectrum; the new compounds were determined by nuclear magnetic resonance hydrogen spectrum, c...
Embodiment 2
[0039] Synthesis of new isomers of α-galactosylceramide (see Figure 4 )
[0040] First, sugar donors are synthesized, and full TMS galactose is synthesized from galactose. After simple treatment, TMS iodine sugar is obtained after reacting with iodine reagent. Without post-treatment, it immediately reacts with sugar acceptor-sphingosine under the action of a catalyst to obtain A single α-glycosyl isomer; then remove TMS on galactose under mild and simple conditions; the azide on the obtained compound is converted into an amino group, and because the compound is very active, it is directly put into the next step; the final amide reaction adopts The carboxylic acid is firstly derivatized into an active ester, and then reacted with an amino group to obtain a new isomer of α-galactosylceramide with mild conditions and a reasonable yield. The new compounds were determined by H-NMR and C-NMR spectra, and the final identification of the main compounds was by high-resolution mass sp...
Embodiment 3
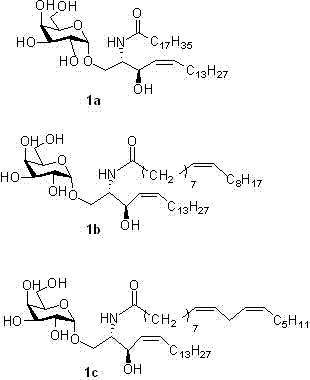
[0054] Synthesis of (α-D-galactopyranose)-(1→1)-(2S,3R,4Z)-2-octadecylamido-4-octadecene-l,3-diol (1b)
[0055] The synthesis method was the same as 1a, except that compound 13b was substituted for compound 13a to obtain a white solid with a yield of 67%. 1 H NMR (400 MHz, MeOD: CDCl 3 = 1:3) δ 7.43 (1 H, s, CONH) 5.52 (1 H, dt, J =11.1, 7.5Hz, H-5’), 5.33 (dd, 3 H, J =20.6, 8.1 Hz, H-4’, H- 9”, H-10”), 4.86 (1 H, d, J =3.5 Hz, H-1), 4.41 (d, 1 H, J =7.9 Hz, H-3'), 3.97-3.86 (m, 2 H, H-2', H-3), 3.79-3.66 (m, 7 H, H-1', H-2, H-4 , H-5, H-6), 2.15 (t, 2 H, J =7.5 Hz, H-6’), 2.00 (dd, 4 H, H-8”, H-11”), 1.56 (s, 1H, H-7’), 1.25 (m, 43 H, CH 2 ),0.84 (t, 6 H, J =6.5 Hz, 2×CH 3 ). 13 C NMR (101 MHz, MeOD: CDCl 3 = 1:3) δ 174.47(CONH), 133.70 (CH, CH=CH), 129.57 (CH, CH=CH), 129.31 (CH, CH=CH), 128.55(CH, CH=CH), 99.67 (CH, americ C), 70.35 , 69.92, 69.40, 68.69, 67.25 (C-2,C-3, C-4, C-5, C-6), 66.99(C-3'), 61.42(C-1'), 53.66(CH, C-2'), 48.89(CH 2, C-6’), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com