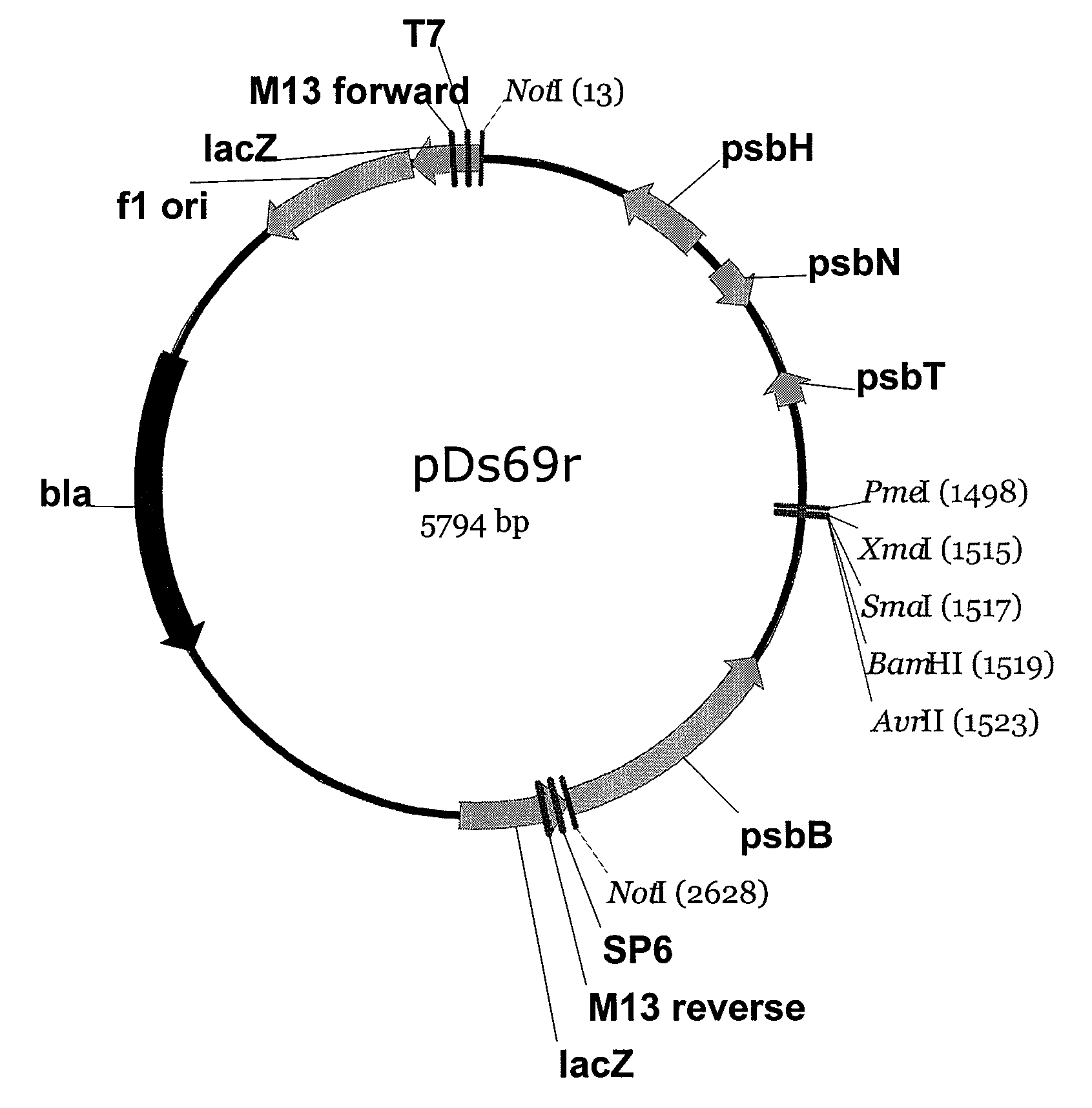
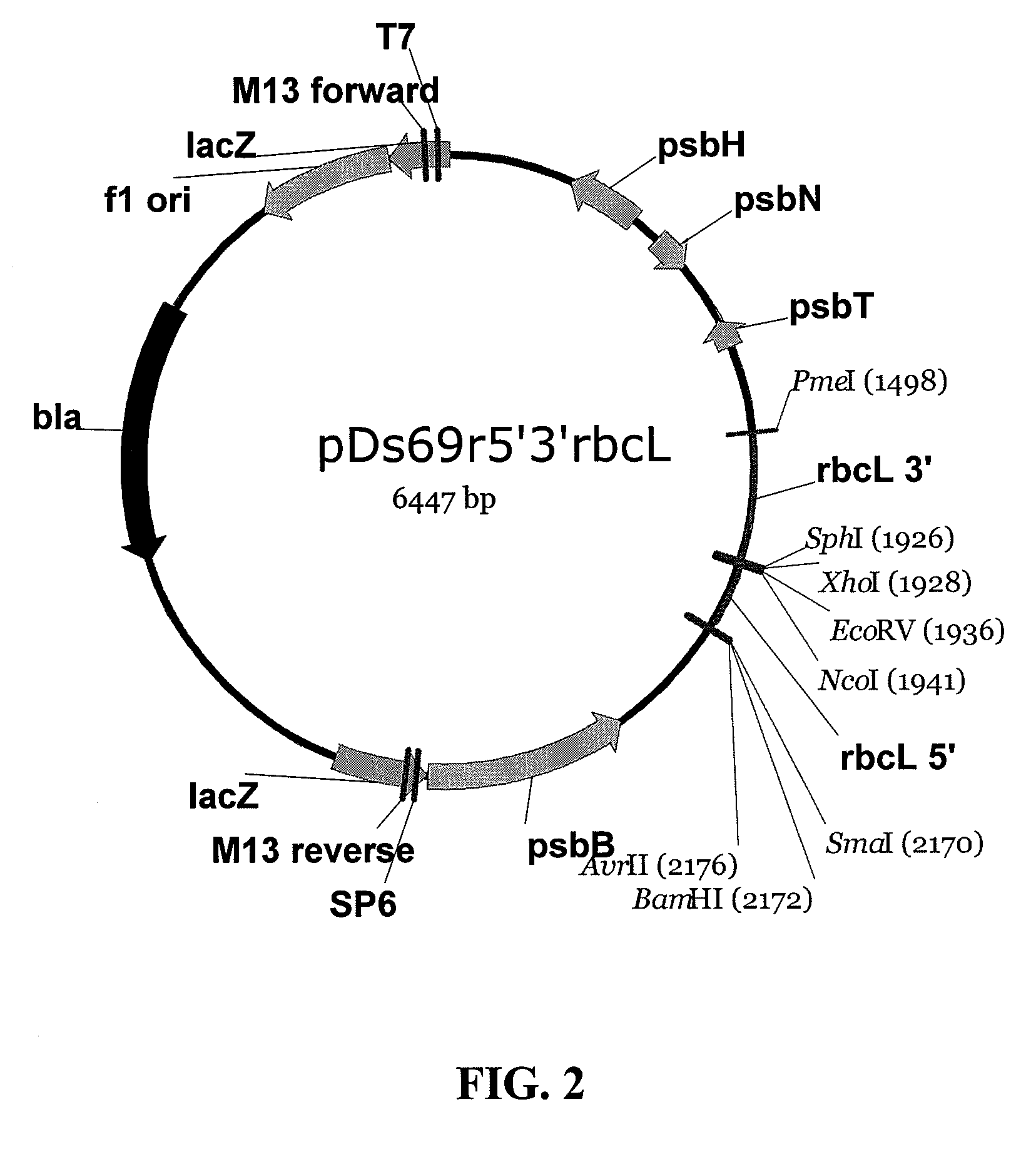
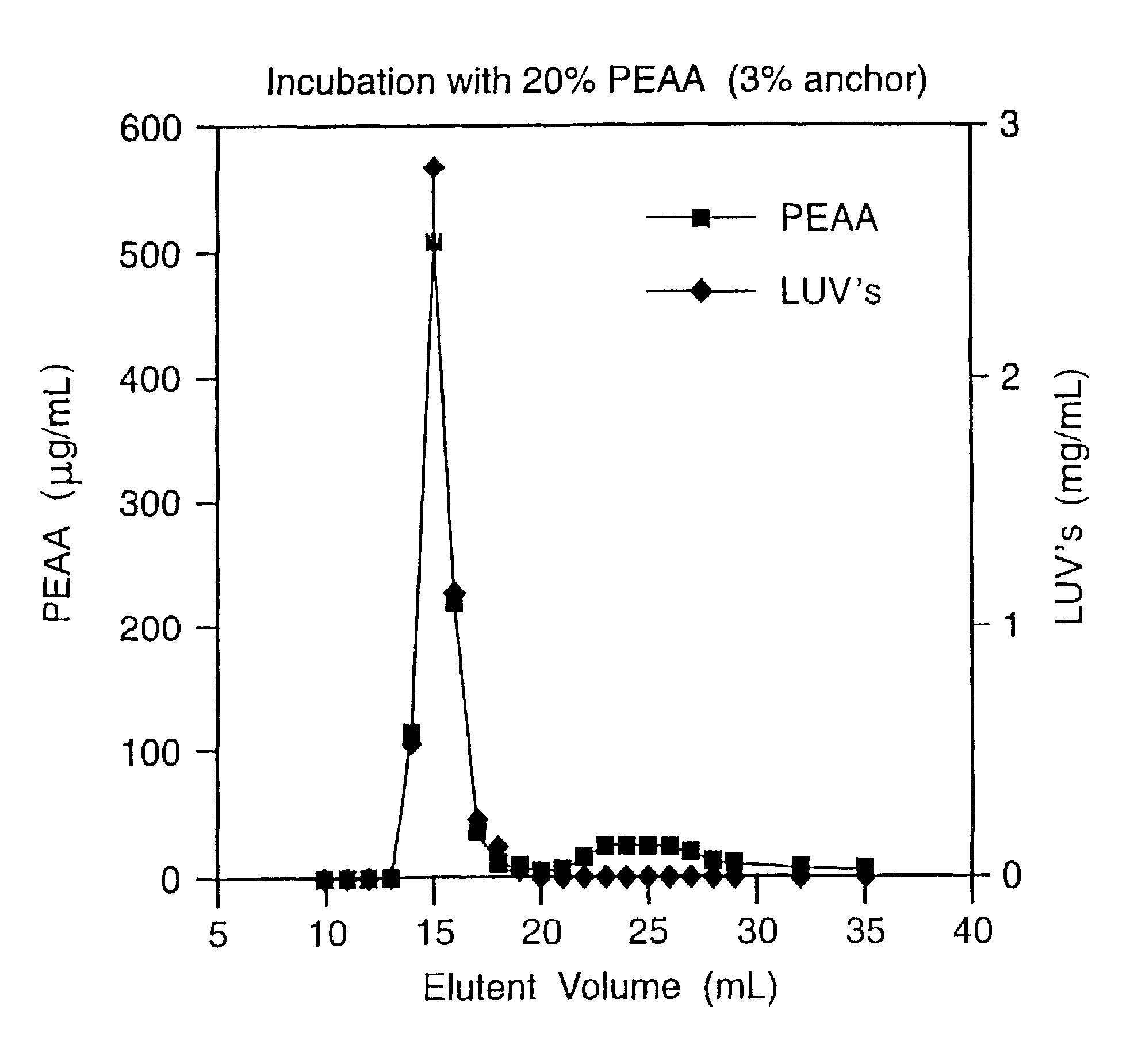
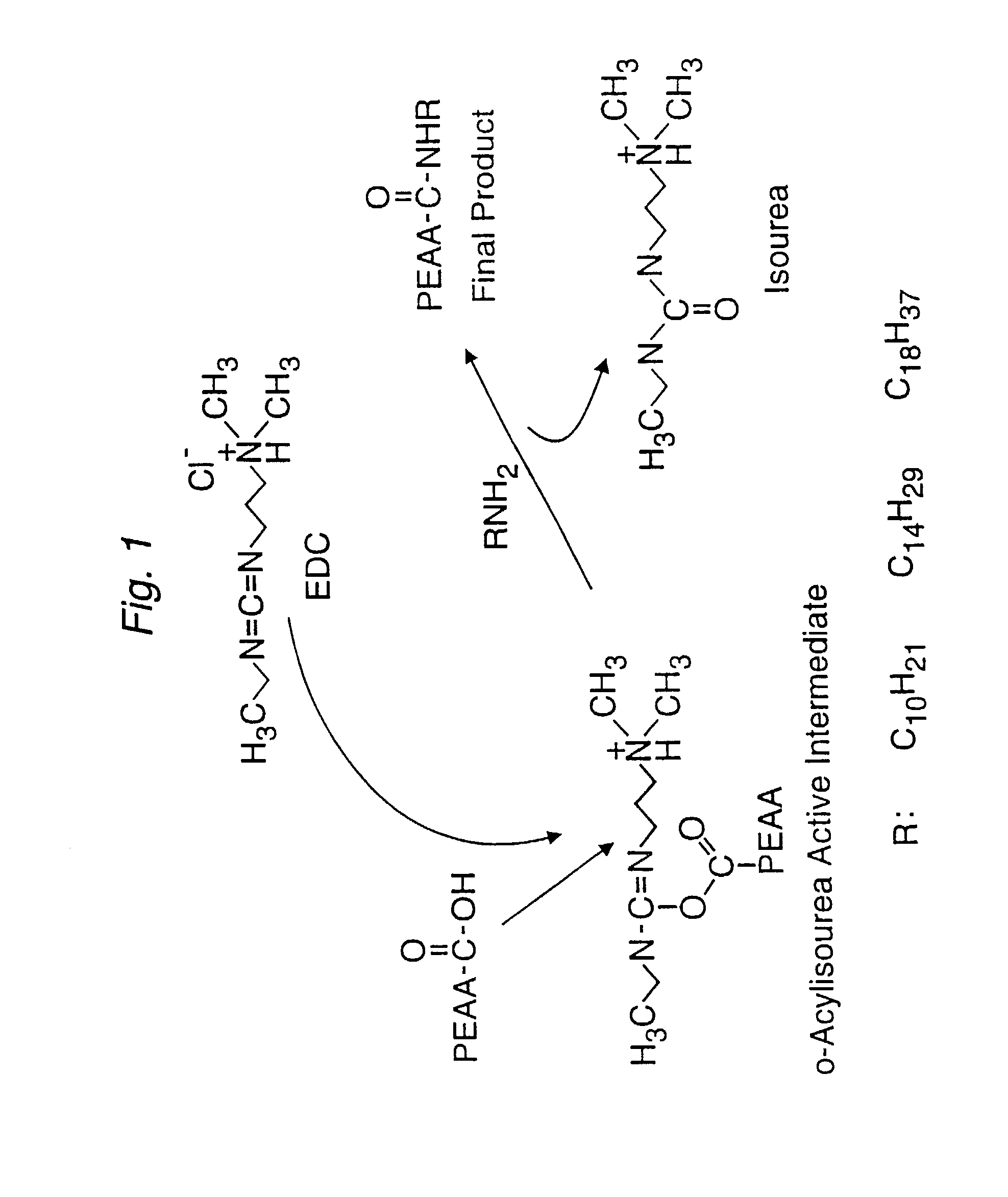
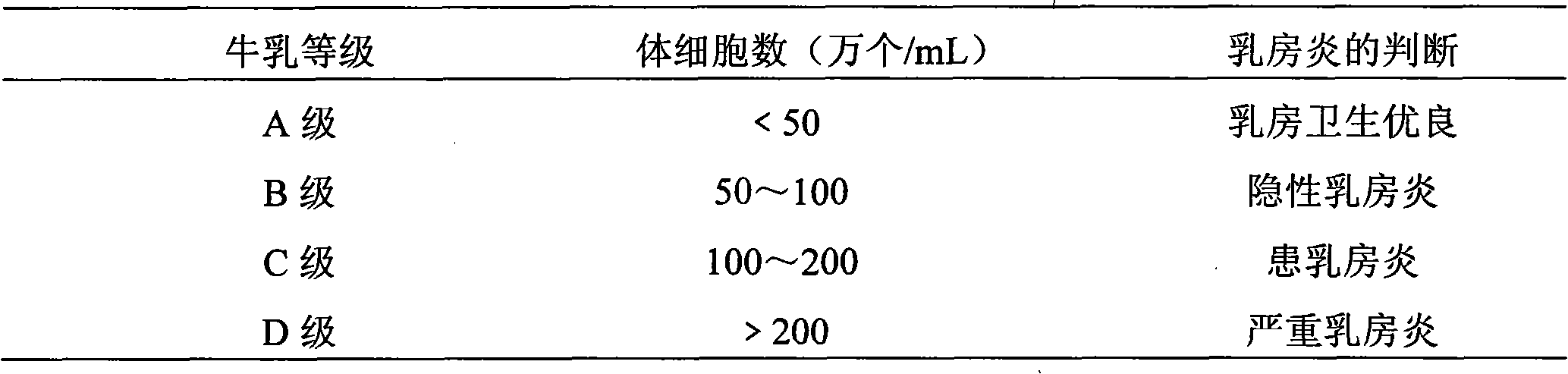
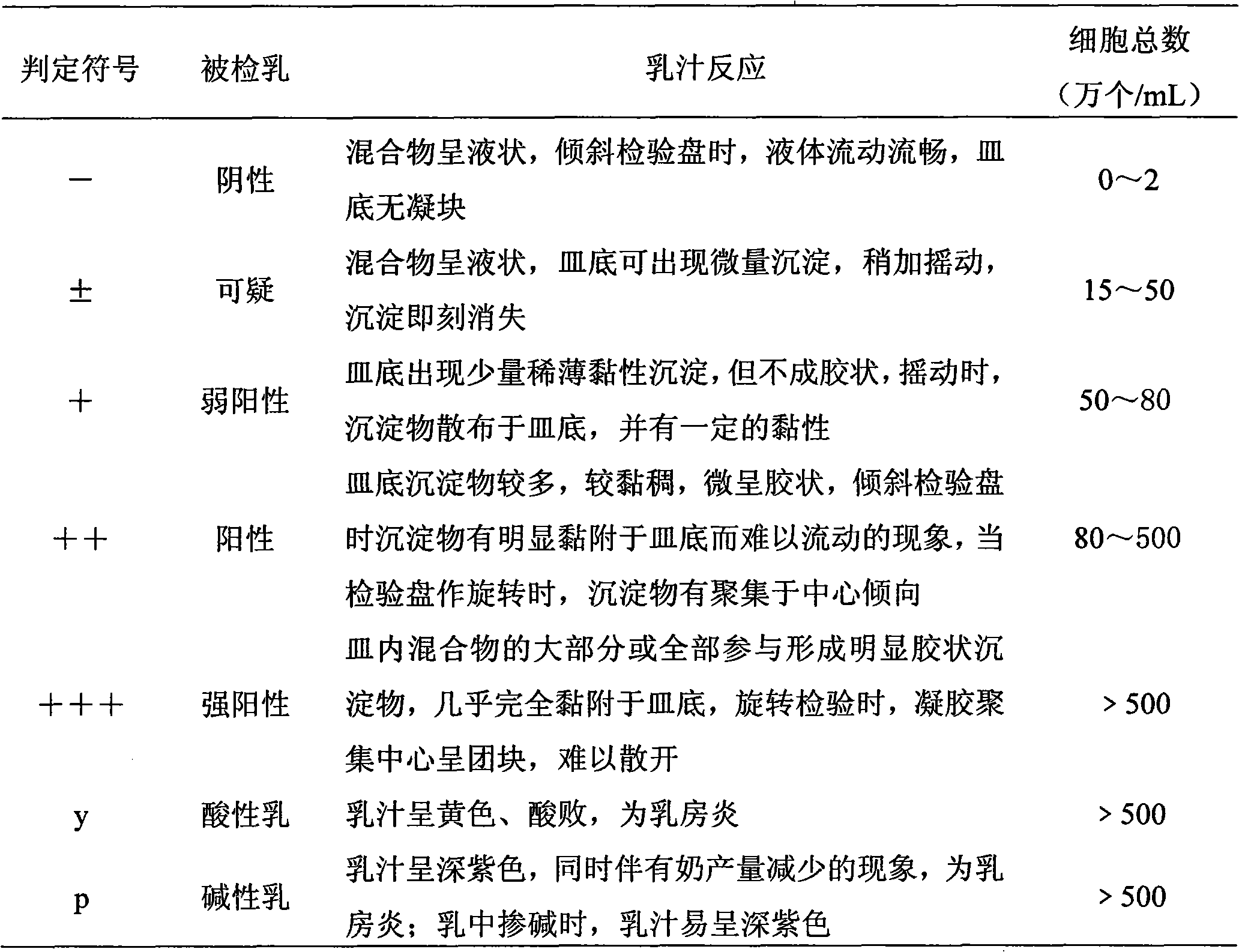
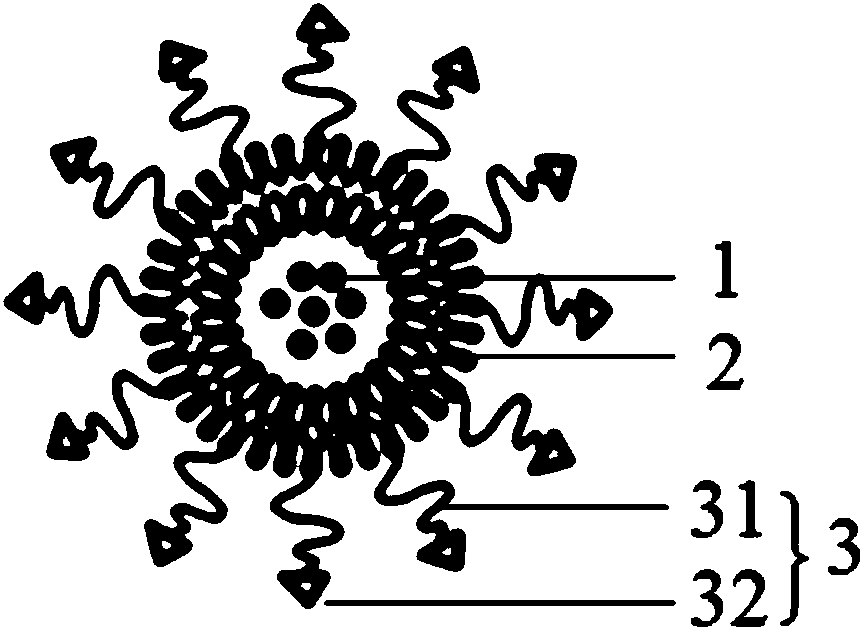
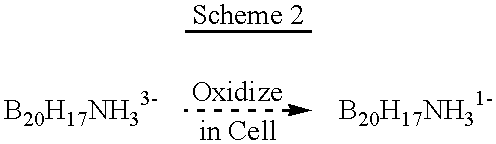Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
213 results about "Etioplasts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
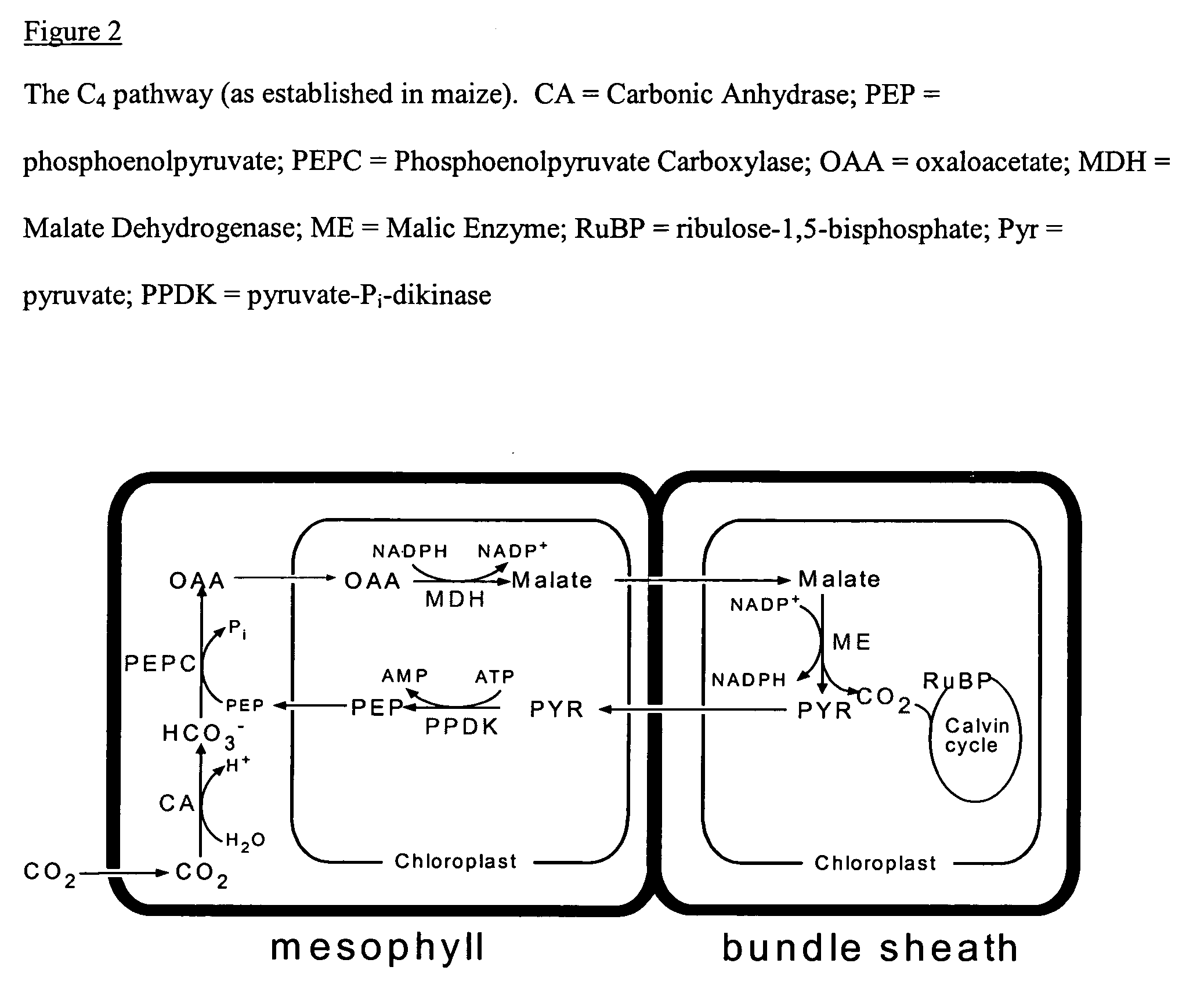
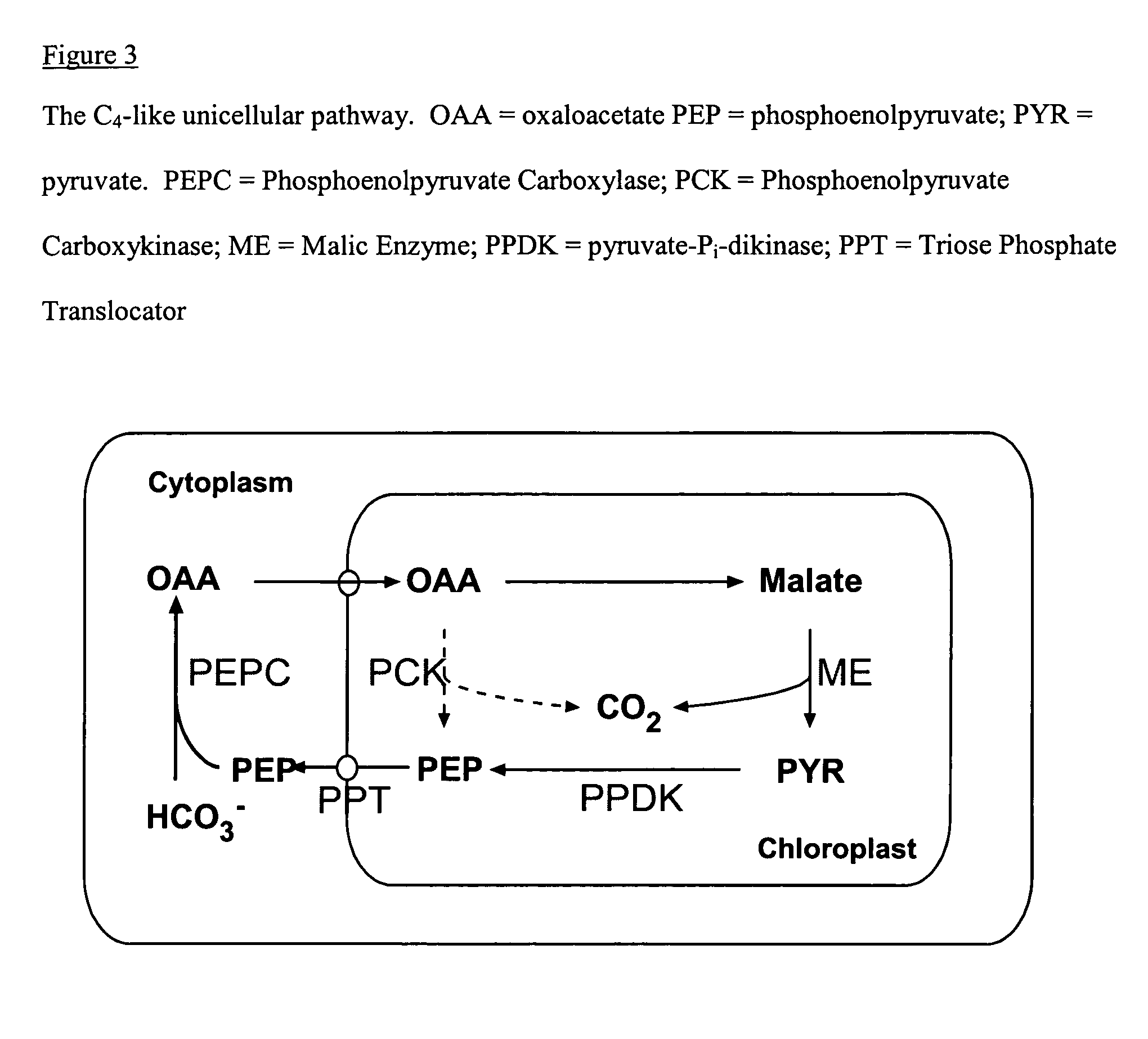
Plastids that can develop into CHLOROPLASTS.
Methods for transforming plants to express Cry2Ab delta-endotoxins targeted to the plastids
InactiveUS6489542B1Reduce in quantityHigh expressionSugar derivativesClimate change adaptationDelta endotoxinBacillus e
Disclosed is a means of controlling plant pests by a novel method of expressing Cry2Ab B. thuringiensis delta-endotoxins in plants, targeted to the plastids. The invention comprises novel nucleic acid segments encoding proteins comprising Cry2Ab B. thuringiensis delta-endotoxins. The nucleic acid segments are disclosed, as are transformation vectors containing the nucleic acid segments, plants transformed with the claimed segments, methods for transforming plants, and methods of controlling plant infestation by pests.
Owner:MONSANTO CO (MONSANTO CY)
Amphoteric liposomes and their use
InactiveUS7371404B2Produced simply and inexpensivelyBinding can be restoredOrganic active ingredientsPeptide/protein ingredientsEtioplastsCharge carrier
Owner:BIONTECH DELIVERY TECH GMBH
Expression of nucleic acid sequences for production of biofuels and other products in algae and cyanobacteria
Various embodiments provide, for example, vectors, expression cassettes, and cells useful for transgenic expression of nucleic acid sequences. In various embodiments, vectors can contain plastid-based sequences of unicellular photosynthetic bioprocess organisms for the production of food- and feed-stuffs, oils, biofuels, pharmaceuticals or fine chemicals.
Owner:KUEHNLE AGROSYST
Polyanionic polymers which enhance fusogenicity
InactiveUS6986902B1Ultrasonic/sonic/infrasonic diagnosticsIn-vivo radioactive preparationsPolyelectrolyteEtioplasts
The present invention relates generally to the amphiphilic polyelectrolyte, poly(2-ethylacrylic acid) and covalently bonded lipids to generate Lipo-PEAA. These Lipo-PEAA are then used to make pH-sensitive liposomes which become unstable, permeable or fusogenic with certain pH changes. In addition, this invention generally describes methods for delivering therapeutic compounds and drugs to target cells by administering to a host the pH-sensitive liposomes of the present invention.
Owner:TEKMIRA PHARMA CORP
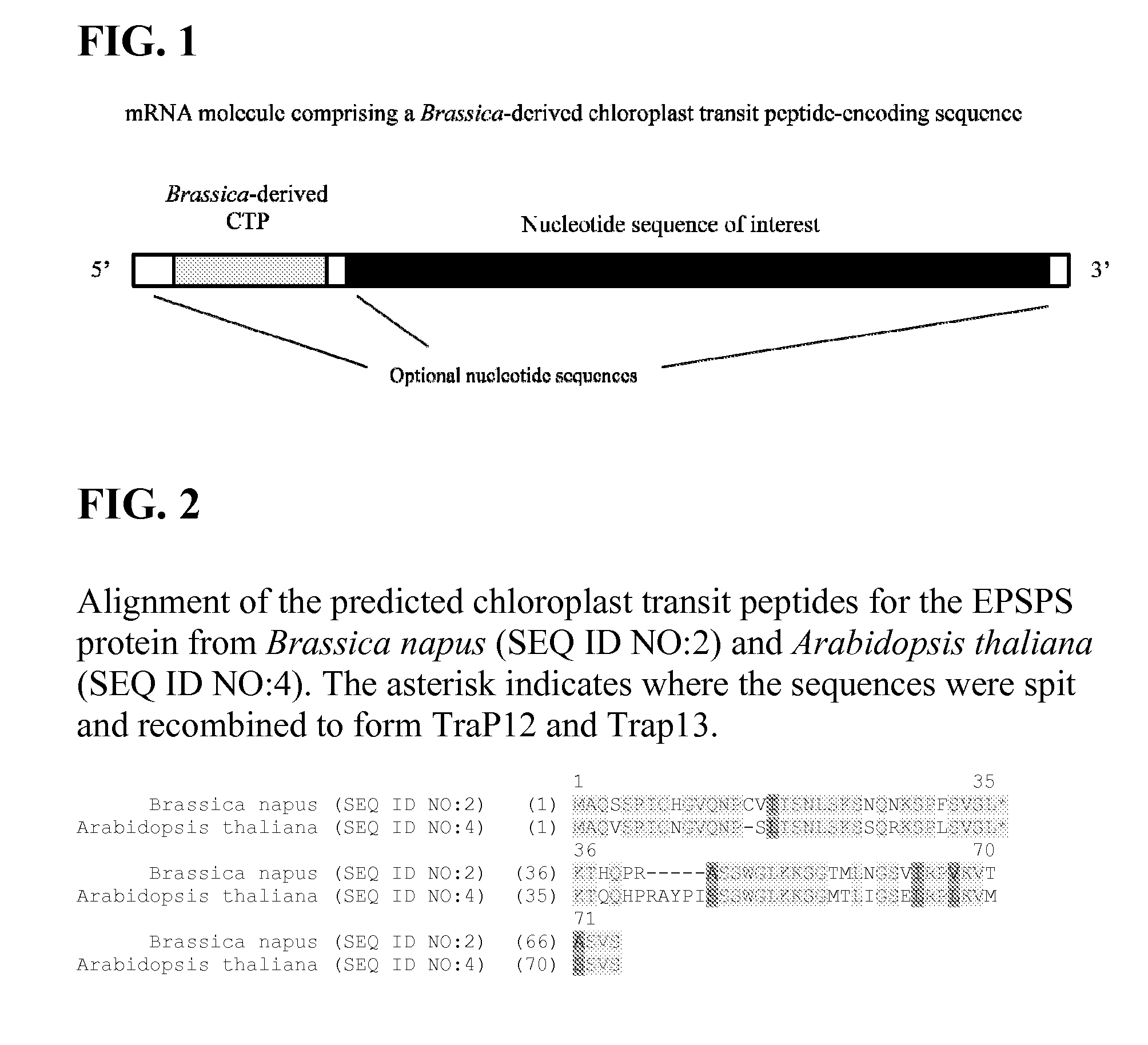
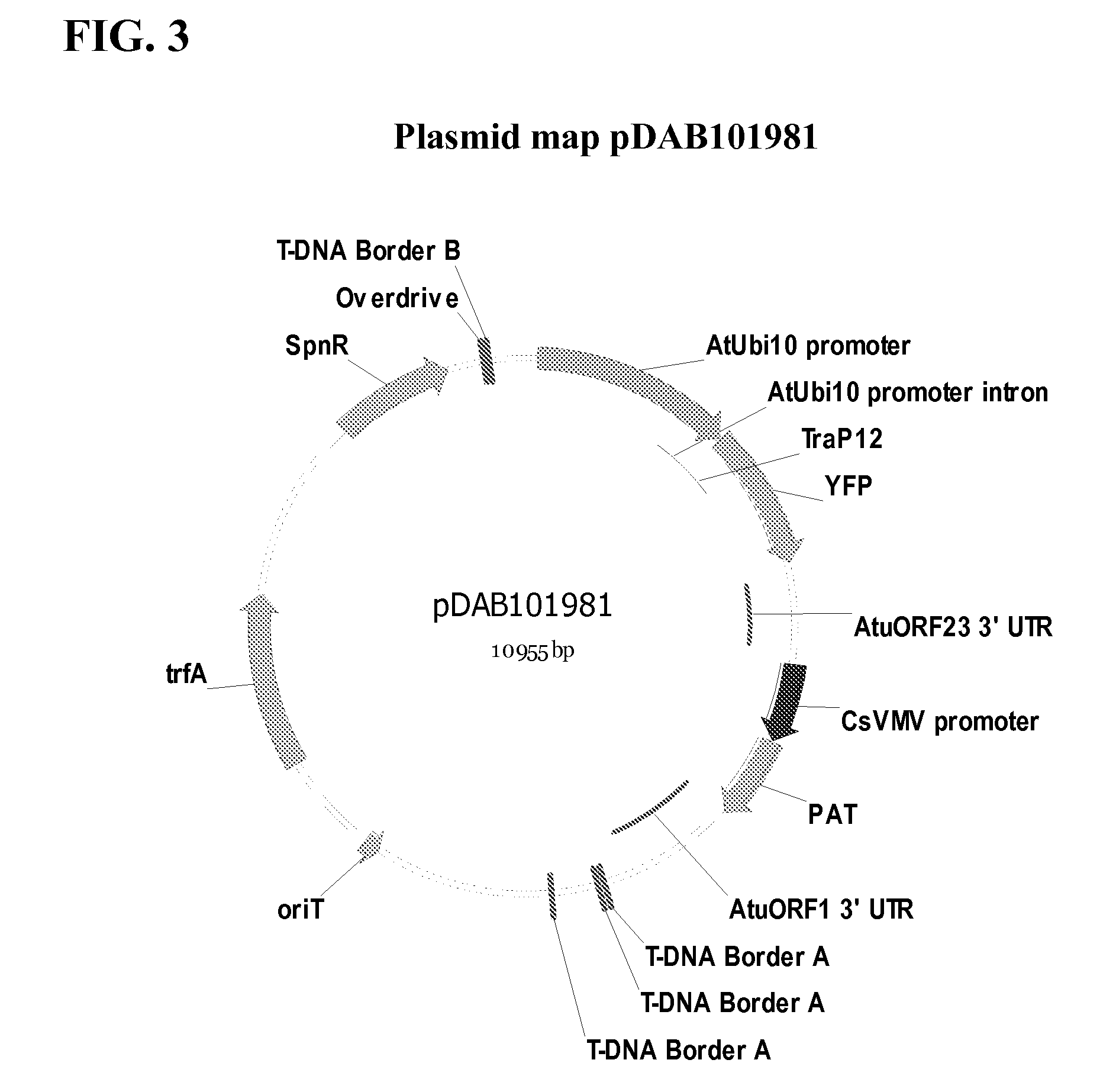
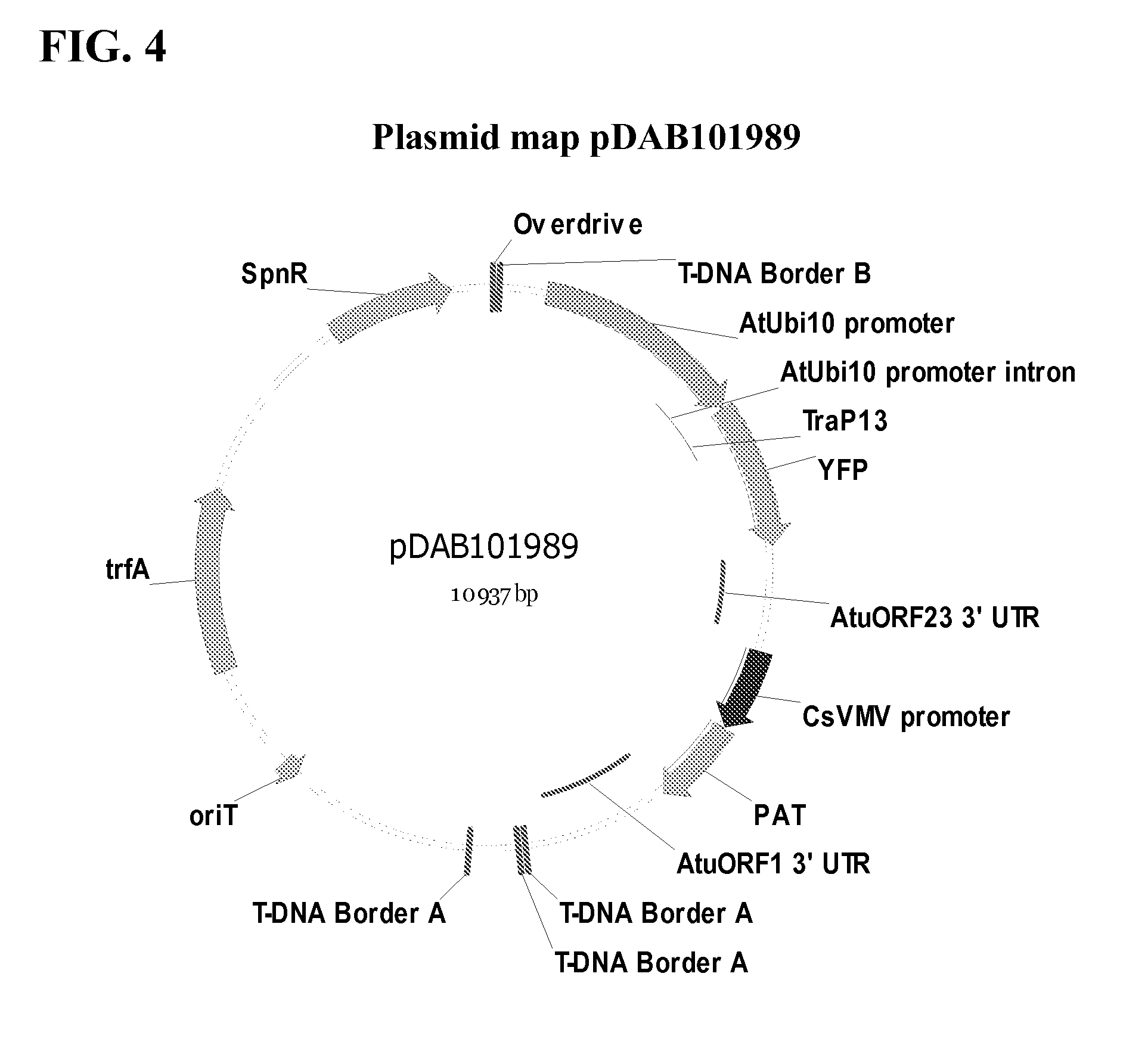
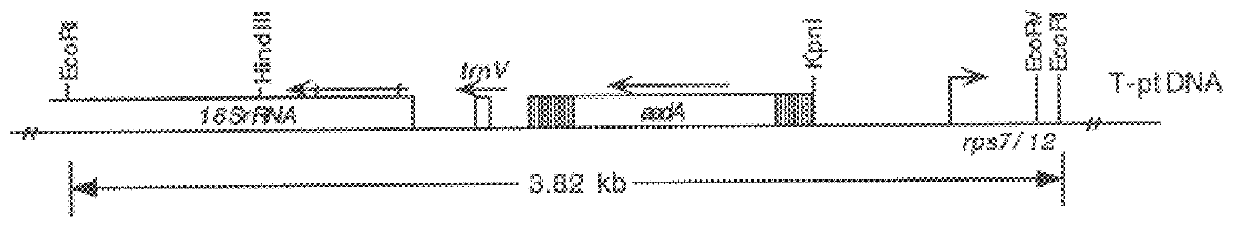
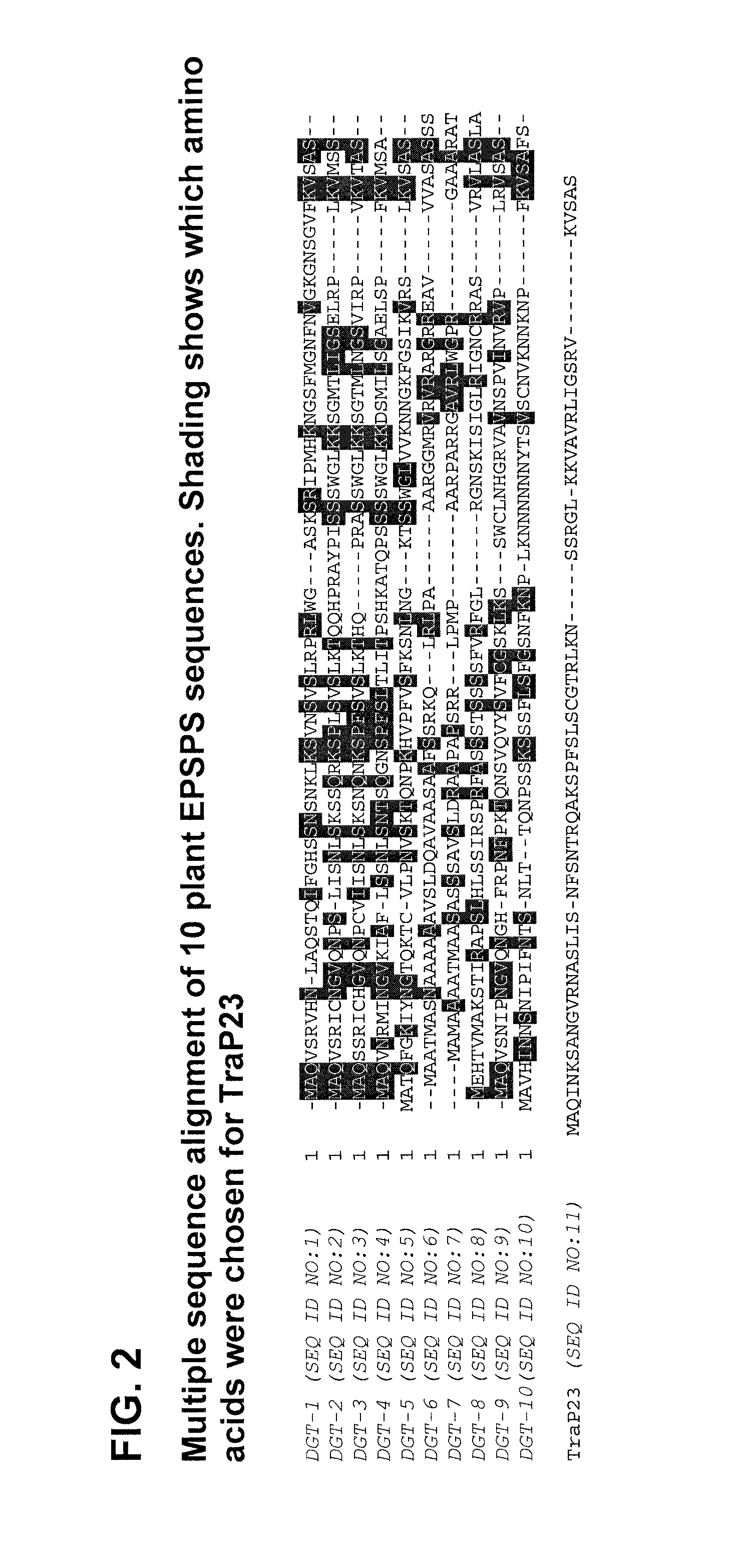
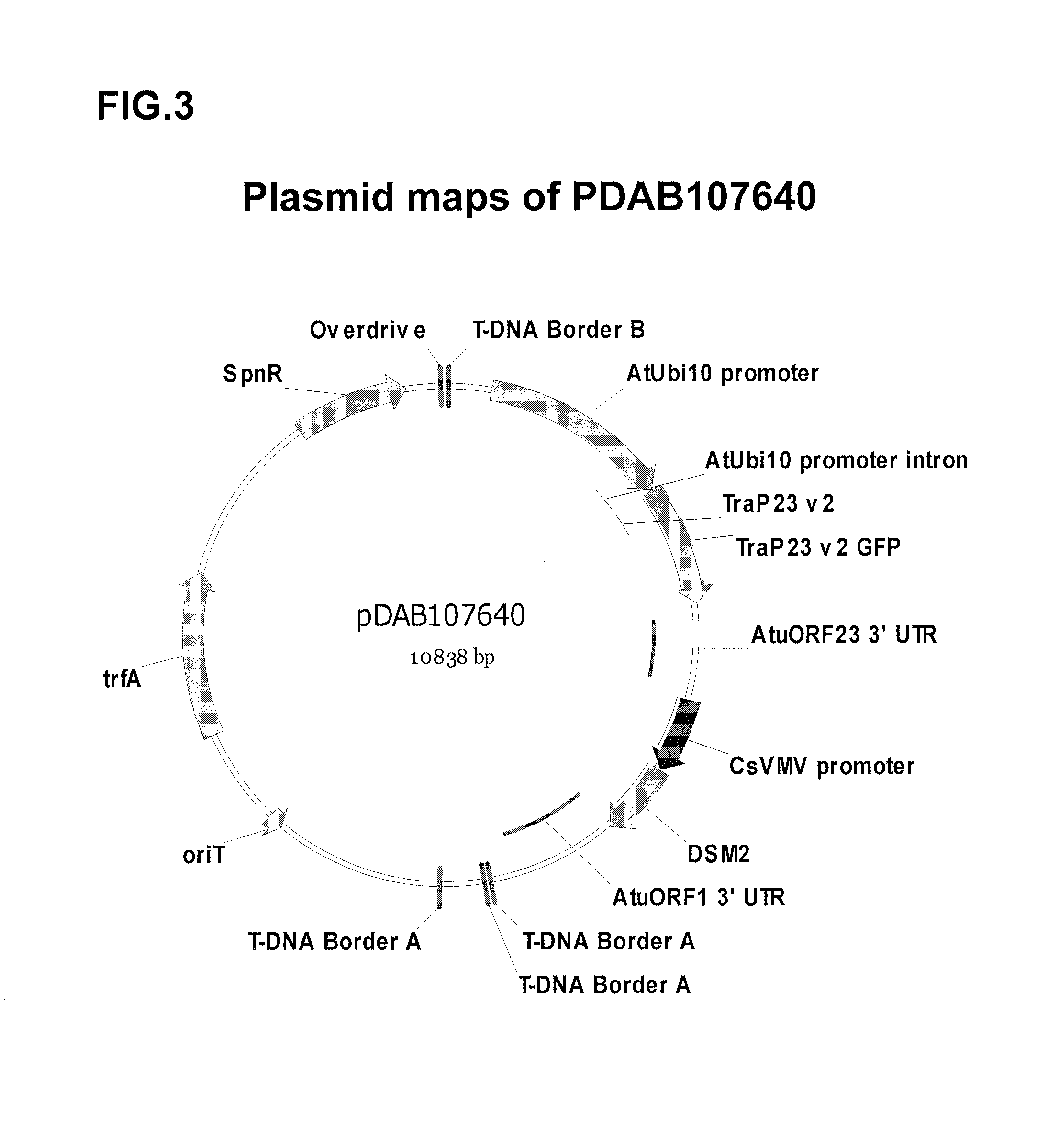
Synthetic brassica-derived chloroplast transit peptides
This disclosure concerns compositions and methods for targeting peptides, polypeptides, and proteins to plastids of plastid-containing cells. In some embodiments, the disclosure concerns chloroplast transit peptides that may direct a polypeptide to a plastid, and nucleic acid molecules encoding the same. In some embodiments, the disclosure concerns methods for producing a transgenic plant material (e.g., a transgenic plant) comprising a chloroplast transit peptide, as well as plant materials produced by such methods, and plant commodity products produced therefrom.
Owner:CORTEVA AGRISCIENCE LLC
A kind of preparation method and application of multivesicular liposome
ActiveCN102274183AHigh encapsulation efficiencyGood sustained release effectOrganic active ingredientsSaccharide peptide ingredientsHydroxyethyl starchArginine
The invention relates to a multi-vesicular liposome, a blank multi-vesicular liposome and a preparation method and application thereof. The multi-vesicular liposome contains the following components in part by weight: 1 part of liposome, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and medicinal active ingredients; the medicament-to-lipid ratio of the multi-vesicular liposome is 1:(0.1-1):200; the lipid contains of a specific amount of neutral phospholipid, cholesterol and triglyceride; and the auxiliary emulsifier is selected from dextran, polyvinyl pyrrolidone, hydroxyethyl starch, gelatin, albumin, arginine and hydroxymethyl starch. The blank multi-vesicular liposome contains the following components in part by weight: 1 part of lipid, 0.01 to 20 parts of auxiliary emulsifier, 1 to 50 parts of osmotic pressure regulator and 0.1 to 50 parts of ion gradient regulator; the osmotic pressure of the in vivo water phase of the multi-vesicular liposome is equal to the osmotic pressure of human plasma; and the auxiliary emulsifier is as previously mentioned. The multi-vesicular liposome has high entrapment rate, and can achieve good sustained-release effect on in vivo and in vitro experiments.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Plastid transformation in Arabidopsis thaliana
InactiveUS6376744B1High expressionImprove stabilityOther foreign material introduction processesFermentationBiotechnologyEtioplasts
The invention provides methods and compositions for obtaining transplastomic Arabidopsis plants. Specifically, the method provides culturing protocols and compositions that facilitate the regeneration of transformed plants following delivery of exogenous DNA molecules.
Owner:RUTGERS THE STATE UNIV
Synthetic chloroplast transit peptides
This disclosure concerns compositions and methods for targeting peptides, polypeptides, and proteins to plastids of plastid-containing cells. In some embodiments, the disclosure concerns chloroplast transit peptides that may direct a polypeptide to a plastid, and nucleic acid molecules encoding the same. In some embodiments, the disclosure concerns methods for producing a transgenic plant material (e.g., a transgenic plant) comprising a chloroplast transit peptide, as well as plant materials produced by such methods, and plant commodity products produced therefrom.
Owner:CORTEVA AGRISCIENCE LLC
Bdellovibrio strain for preventing and treating mastitis and applications thereof
The invention discloses a Bdellovibrio strain for preventing and treating mastitis and applications thereof. A Bdellovibrio BDS01 is obtained by separation, and is unicellular, arced and 0.9-0.25 micrometer in size; the end of the Bdellovibrio BDS01 has flagellum which is 4 micrometers long. When cultured by a two-layer plating method at 28 DEG C for four days, the Bdellovibrio BDS01 can form transparent round plaques with the diameter of 1-2 mm. The microbial preparation prepared by using the Bdellovibrio BDS01 plastid has favorable effects for preventing cow mastitis. Compared with the telotroch, the plastid has the characteristics of convenient preparation and storage, strong environmental resistance, slightly slow sterilization action, longer sterilization action time, and the like, and does not have toxic or side effect on cows. Therefore, the microbial preparation containing the Bdellovibrio BDS01 plastid is suitable for large-size factory culture of cows, and a novel method forgreen processing and production of milk and for preventing and treating mastitis can be provided.
Owner:SOUTH CHINA UNIV OF TECH
Indocyanine green nanometer targeted liposome as well as preparation method and application thereof
ActiveCN103690486AImprove stabilityAvoid decompositionEnergy modified materialsPharmaceutical non-active ingredientsMass ratioEtioplasts
The invention provides indocyanine green nanometer targeted liposome. The indocyanine green nanometer targeted liposome comprises indocyanine green, a phospholipid layer and a folic acid-modified polyethylene glycol derived phospholipid layer, wherein the indocyanine green is surrounded by the phospholipid layer; the folic acid-modified polyethylene glycol derived phospholipid layer comprises polyethylene glycol derived phospholipid and folic acid connected with the polyethylene glycol derived phospholipid through an amido bond; phospholipid in the polyethylene glycol derived phospholipid is inserted in the phospholipid layer; the folic acid is exposed outside the phospholipid layer; the mass ratio of the indocyanine green to the phospholipid to the folic acid-modified polyethylene glycol derived phospholipid is (1-5): (1-3): (1-3). The invention further provides a preparation method of the indocyanine green nanometer targeted liposome and photo-thermal treating application thereof; when the indocyanine green nanometer targeted liposome is applied, the indocyanine green is difficultly gathered, and has excellent stability and a targeting property.
Owner:珠海中科先进技术研究院有限公司
Method for performing transient expression by introducing foreign gene into poplar bioplast
InactiveCN102373235AEasy to operateEasy to implementVector-based foreign material introductionAngiosperms/flowering plantsEtioplastsPolyethylene glycol
The invention discloses a method for performing transient expression by introducing a foreign gene into a poplar bioplast. The method comprises the following steps of: introducing a transient expression vector containing a foreign gene into a poplar bioplast by using PEG (Polyethylene Glycol)-Ca<2+>; culturing; and expressing the foreign gene in the bioplast. A poplar bioplast transient expression system constructed with the method is easy to operate and realize, a place with broad prospects is opened up for the research of functional genes of a poplar, and the transformation efficiency of the method can be up to 70 percent.
Owner:NANJING FORESTRY UNIV
Chloroplast transit peptide
This disclosure concerns compositions and methods for targeting peptides, polypeptides, and proteins to plastids of plastid-containing cells. In some embodiments, the disclosure concerns chloroplast transit peptides that may direct a polypeptide to a plastid, and nucleic acid molecules encoding the same. In some embodiments, the disclosure concerns methods for producing a transgenic plant material (e.g., a transgenic plant) comprising a chloroplast transit peptide, as well as plant materials produced by such methods, and plant commodity products produced therefrom.
Owner:CORTEVA AGRISCIENCE LLC
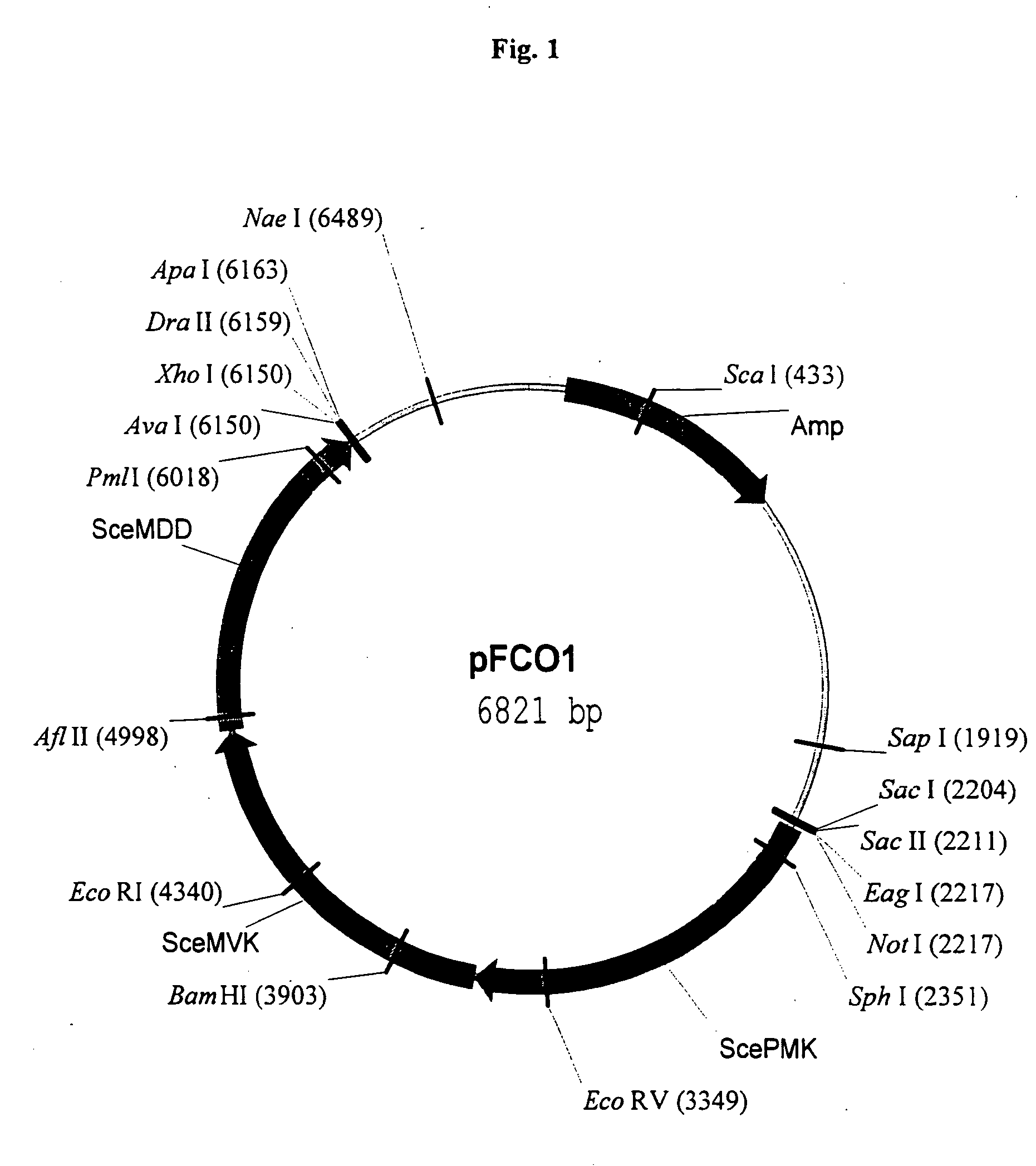
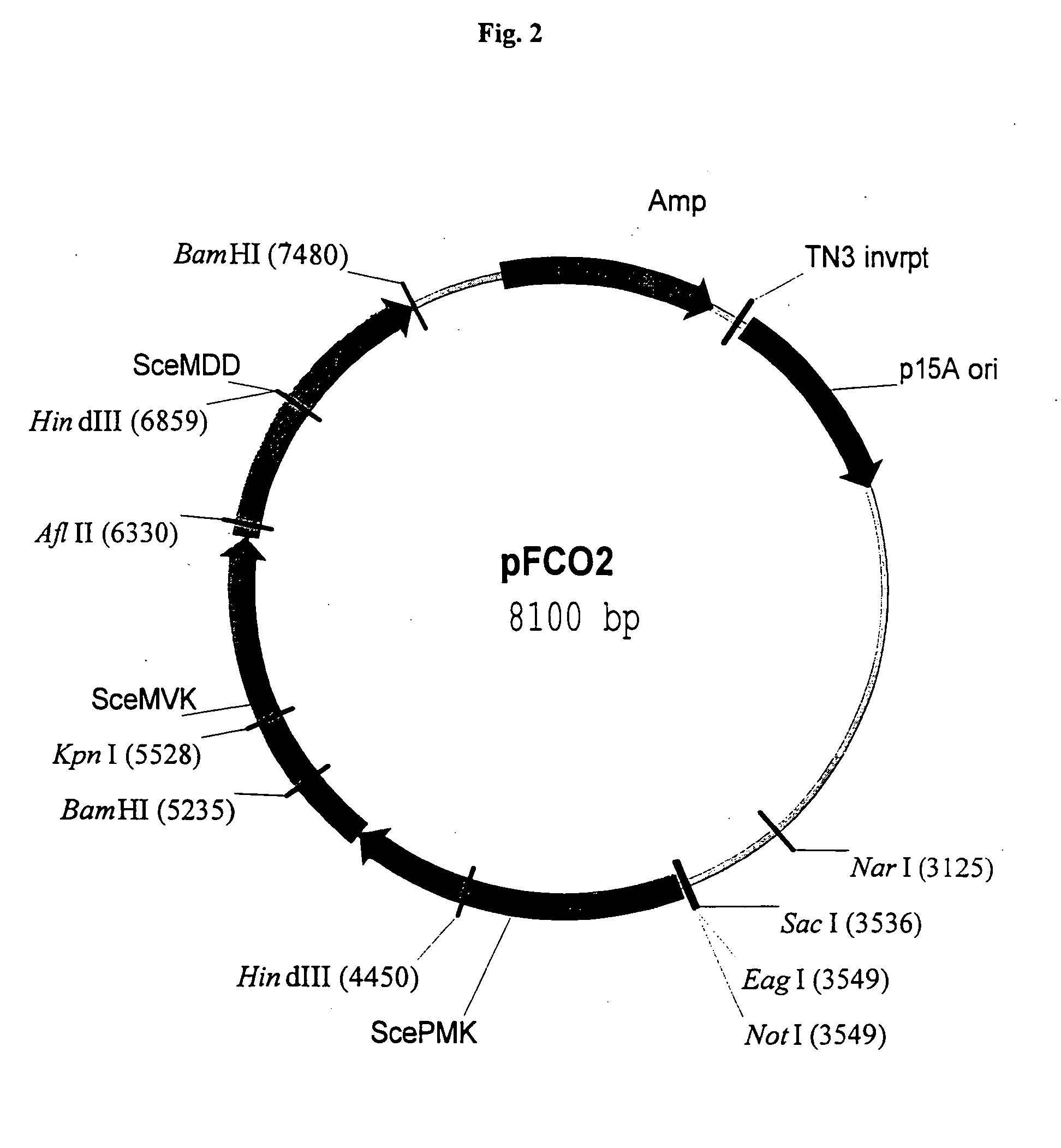
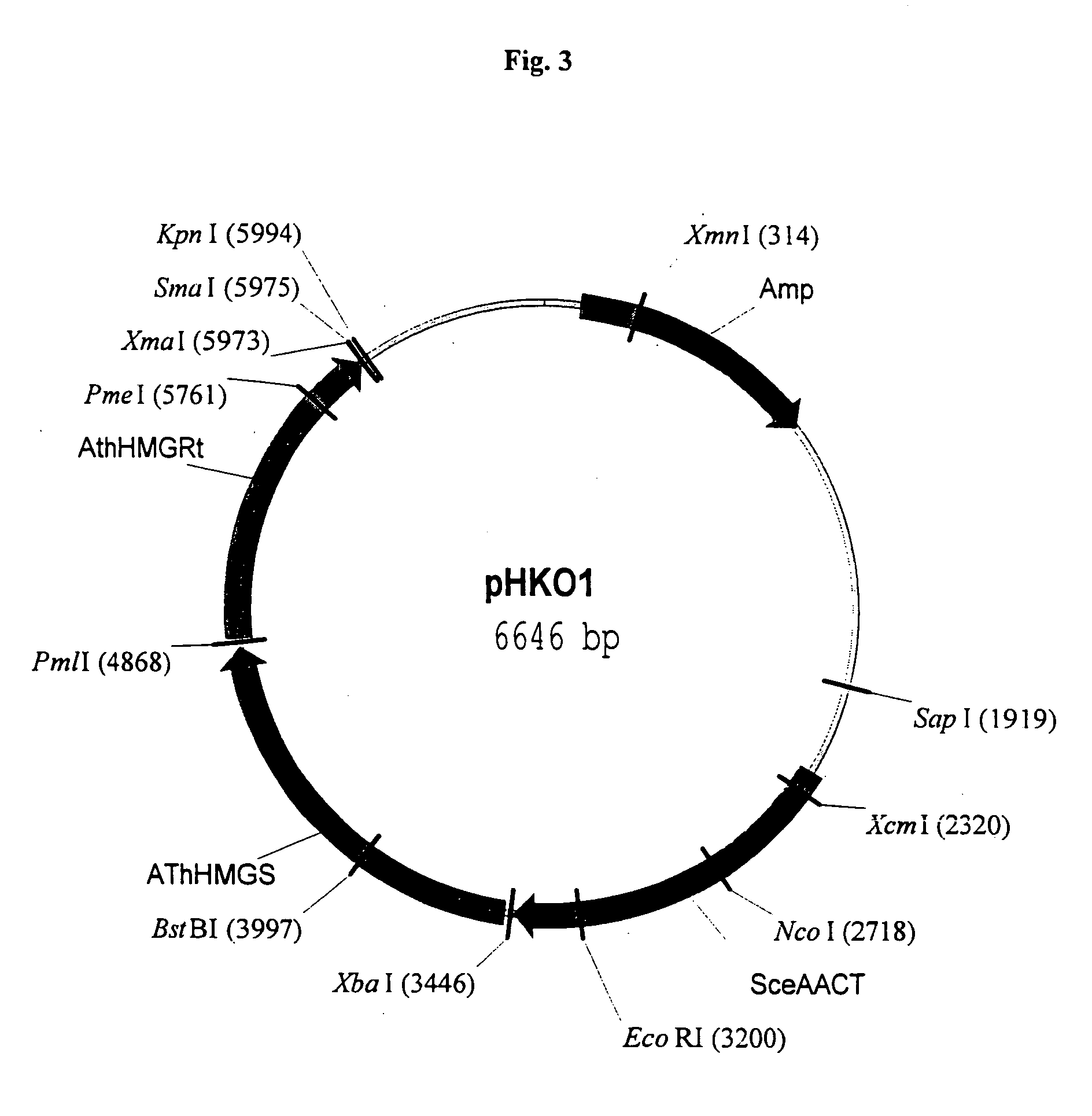
Manipulation of genes of the mevalonate and isoprenoid pathways to create novel traits in transgenic organisms
InactiveUS20050241017A1Other foreign material introduction processesIsomerasesOpen reading frameNucleotide
Disclosed are the uses of specific genes of the mevalonate and isoprenoid biosynthetic pathways, and of inactive gene sites (the pseudogene) to (1) enhance biosynthesis of isopentenyl diphosphate, dimethylallyl diphosphate and isoprenoid pathway derived products in the plastids of transgenic plants and microalgae, (2) create novel antibiotic resistant transgenic plants and microalgae, and (3) create a novel selection system and / or targeting sites for mediating the insertion of genetic material into plant and microalgae plastids. The specific polynucleotides to be used, solely or in any combination thereof, are publicly available from GeneBank and contain open reading frames having sequences that upon expression will produce active proteins with the following enzyme activities: (a) acetoacetyl CoA thiolase (EC 2.3.1.9), (b) 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) synthase (EC 4.1.3.5), (c) HMG-CoA reductase (EC 1.1.1.34), (d) mevalonate kinase (EC 2.7.1.36), (e) phosphomevalonate kinase (EC 2.7.4.2), (f) mevalonate diphosphate decarboxylase (EC 4.1.1.33), (g) isopentenyl diphosphate (IPP) isomerase (EC 5.3.3.2), and (h) phytoene synthase (EC 2.5.1.32).
Owner:UNIV OF HAWAII +1
Thermo-sensitive liposome as well as preparation method and application thereof
ActiveCN103908429AHighly permeableDistribution real-time monitoringOrganic active ingredientsHeavy metal active ingredientsFluorescencePolyethylene glycol
The invention provides thermo-sensitive liposome. The thermo-sensitive liposome comprises a chemotherapeutic drug, a photosensitizer, phosphatidylcholine and polyethylene glycol-derived phospholipid, wherein the photosensitizer is indocyanine green or chlorins e6; the grain size of the thermo-sensitive liposome is 40-60 nm; by virtue of living-body fluorescence imaging, a distribution process of the thermo-sensitive liposome can be observed in real time; under a near-infrared light irradiation condition, the chemotherapeutic drug can be quickly and effectively released, so that the chemotherapeutic drug can be enriched at a target part very well; the obtained thermo-sensitive liposome particle has smaller particle diameter less than 100 nm, and good biocompatibility. The preparation method of the thermo-sensitive liposome is simple and easy to operate.
Owner:珠海中科先进技术研究院有限公司
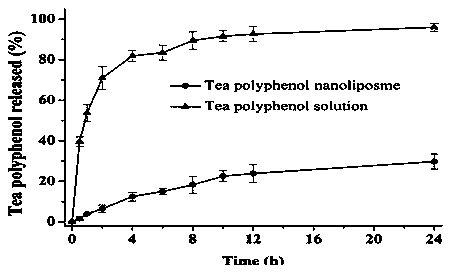
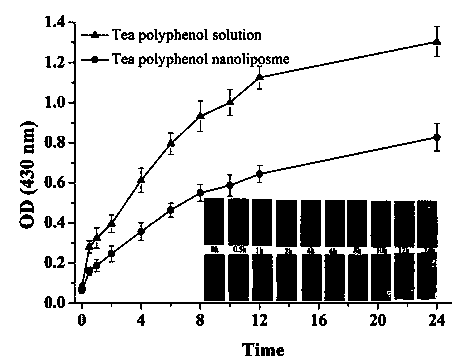
Preparation method of tea polyphenol nano-liposomes by ethanol injection-dynamic high-pressure microfluidization-enzymolysis
InactiveCN103637989AHigh encapsulation efficiencyEvenly distributedCosmetic preparationsOrganic active ingredientsZeta potentialEthanol Injection
The invention discloses a preparation method of tea polyphenol nano-liposomes by combination of an ethanol injection method and dynamic high-pressure microfluidization-enzymolysis. Tea polyphenol, lecithin, tween-80 and a phosphate buffer solution are adopted as raw materials. The tea polyphenol nano-liposomes are prepared by utilization of an ethanol injection-dynamic high-pressure microfluidization-enzymolysis method. According to the prepared tea polyphenol nano-liposomes, the average particle size is 67.4 nm plus or minus 3.0 nm, the zeta potential is -6.07 mV plus or minus 0.59 mV, the distribution coefficient is 0.220 plus or minus 0.010, and the encapsulation efficiency is 79.7% plus or minus 5.4%. The prepared tea polyphenol nano-liposomes show good slow-release property. Only 30.3% plus or minus 2.9% of the tea polyphenol is released after 24 h. In a small-intestine simulation environment where pH is 7.4, the tea polyphenol nano-liposomes show good stability.
Owner:NANCHANG UNIV
Method for preparing and regenerating phomopasis asparagi protoplast
InactiveCN103451110AHigh number of preparationsHigh regeneration rateFungiMicroorganism based processesMyceliumEtioplasts
The invention discloses a method for preparing and regenerating a phomopasis asparagi protoplast, which relates to the technical field of fungus protoplast preparation and regeneration in cell engineering. The method comprises the following detailed operation steps of: (1) preparing a phomopasis asparagi conidium suspension; (2) preparing a fresh mycelium; (3) performing enzymolysis on mycelium cell walls; (4) separating the protoplast; and (5) regenerating the protoplast. The method is easy to operate, low in requirements on equipment and short in enzymolysis time and regeneration period, and effectively enhances the efficiency of preparation and regeneration of the protoplast.
Owner:VEGETABLE & FLOWER INST JIANGXI ACADEMY OF AGRI SCI
DC cell membrane bionic liposome drug carrier as well as preparation method and application thereof
InactiveCN106309369ARaise the ratioImprove toughnessAntibacterial agentsAntiviralsAntigenCell membrane
The invention relates to a targeting personalized DC cell membrane bionic liposome drug carrier as well as a preparation method and application thereof. The DC cell membrane bionic liposome drug carrier is characterized in that (1) the liposome drug carrier has a cell membrane protein component; (2) the liposome drug carrier can carry a drug in vitro and is used for cell targeting fused release; and (3) the cell membrane protein component of the liposome comes from immortalized somatic cells. The invention has the following advantages: the drug carrier can be applied to the targeting delivery to in-vivo DC cells of a drug or antigen, the antigen presentation ability of the DC cells is enhanced, and the specific immunity is improved.
Owner:李因传
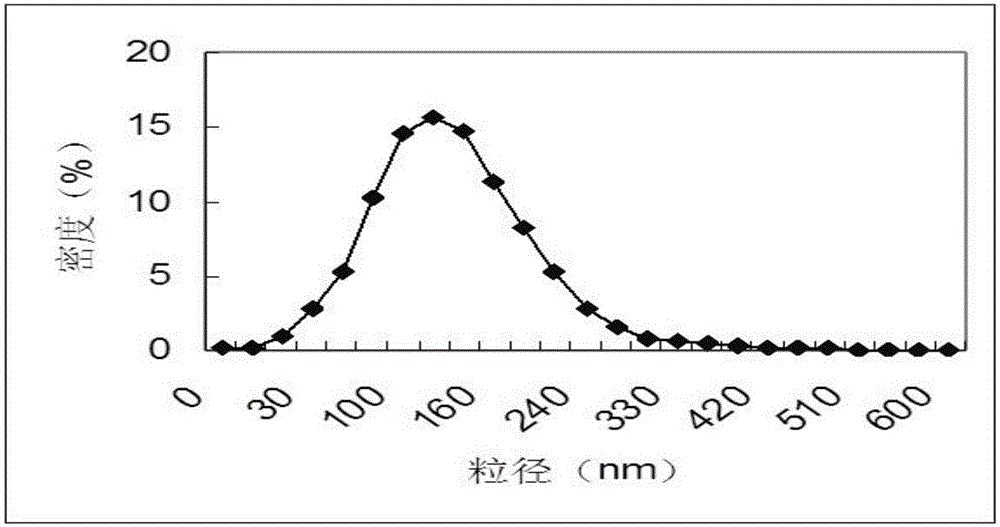
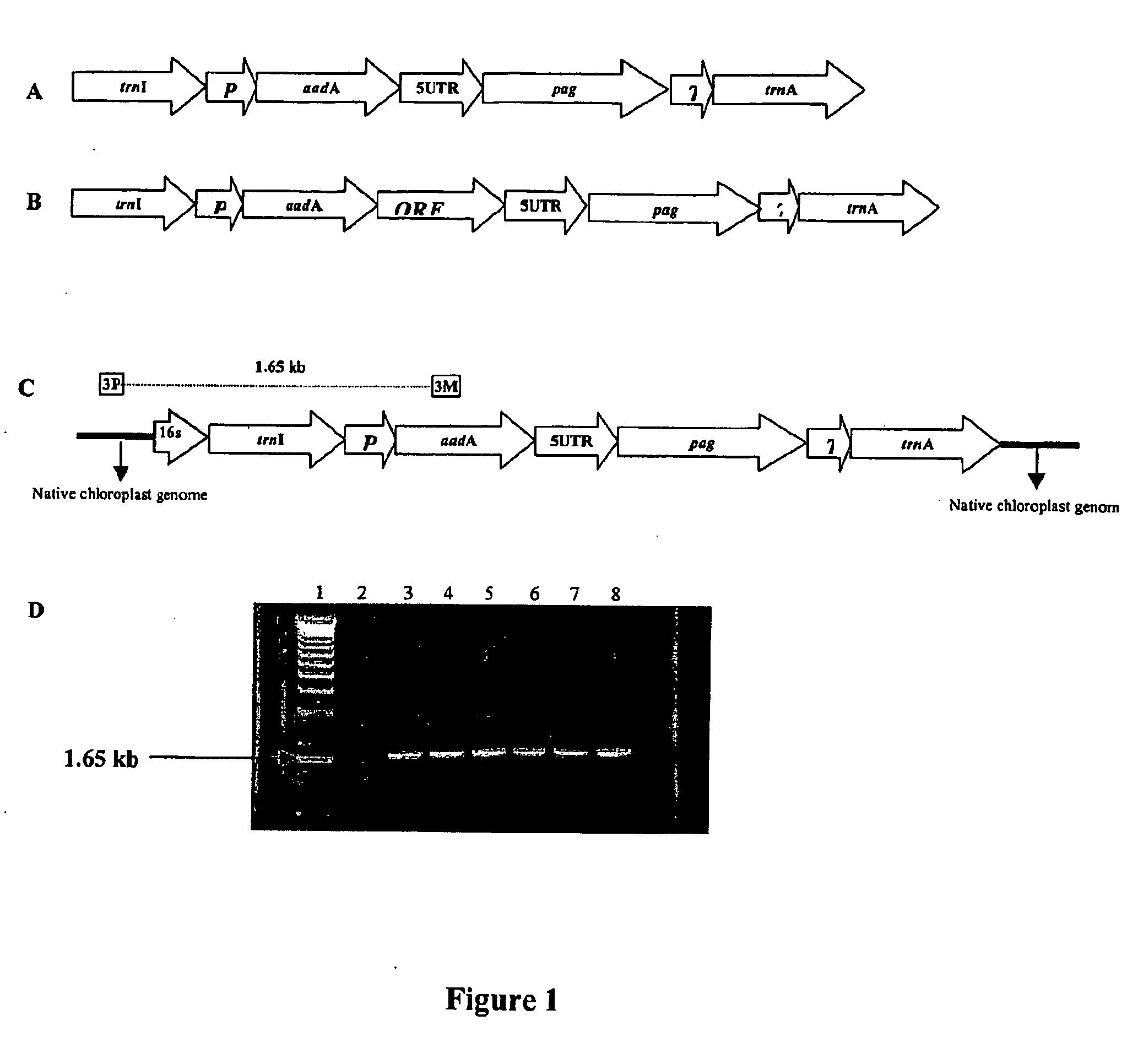
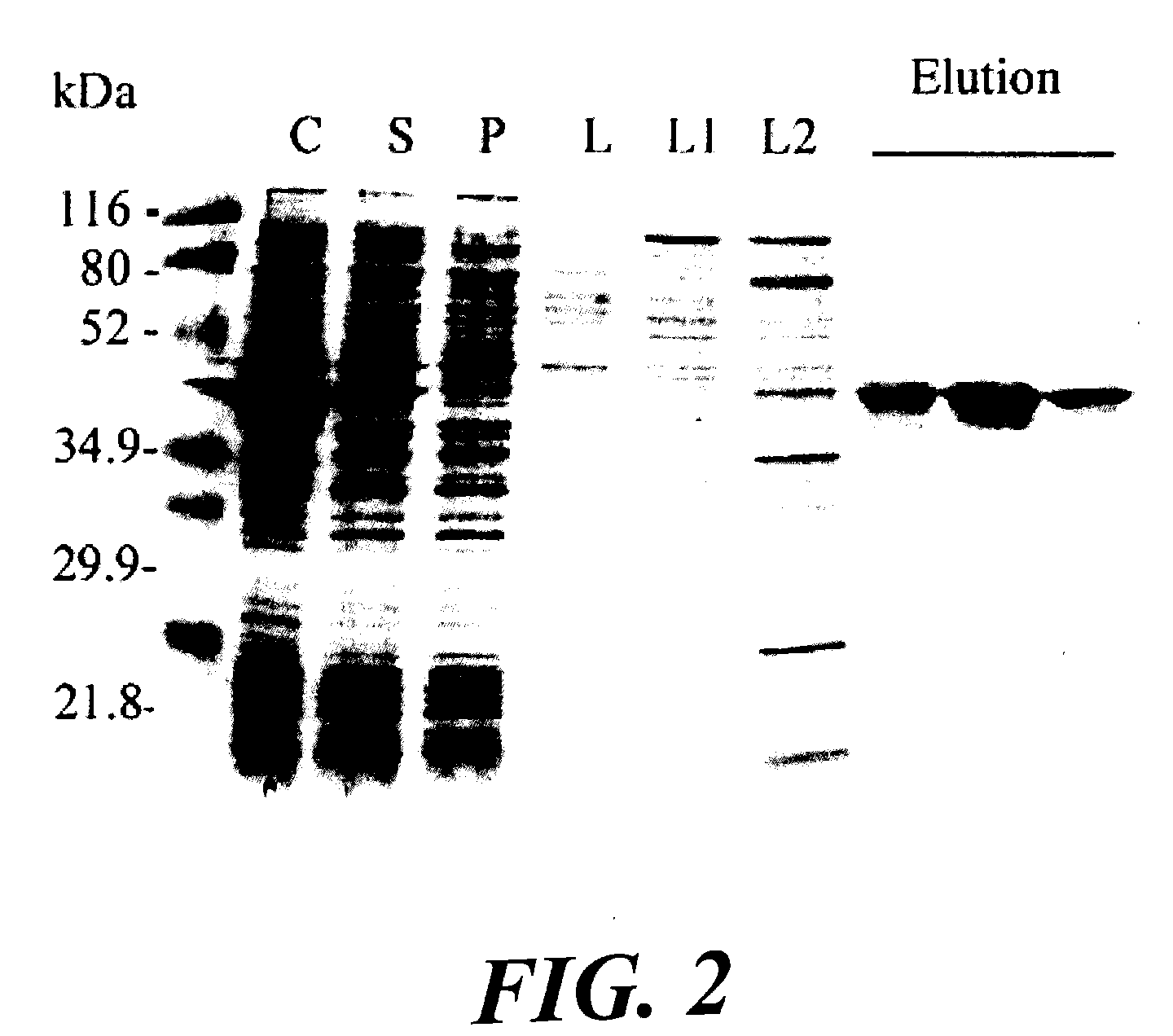
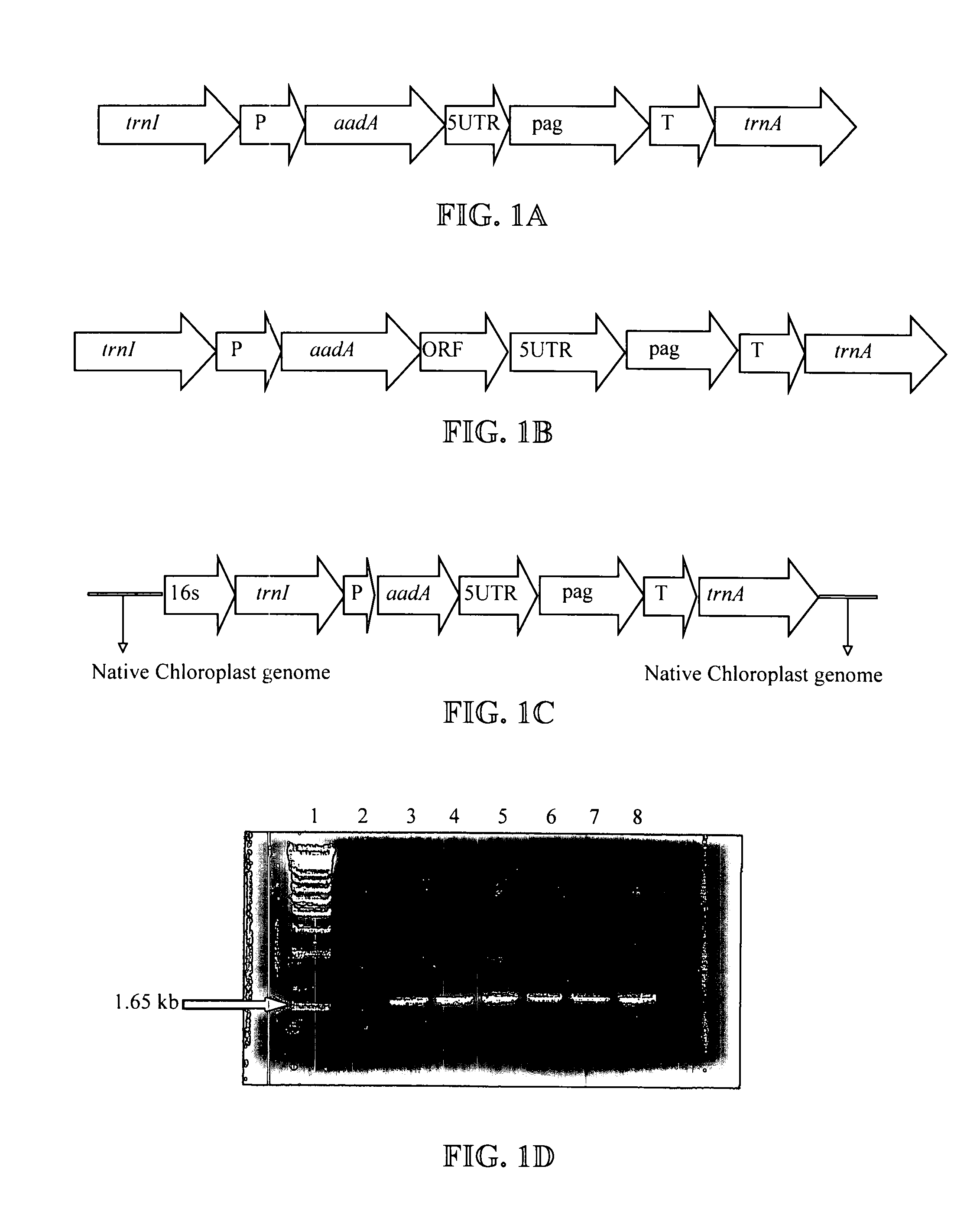
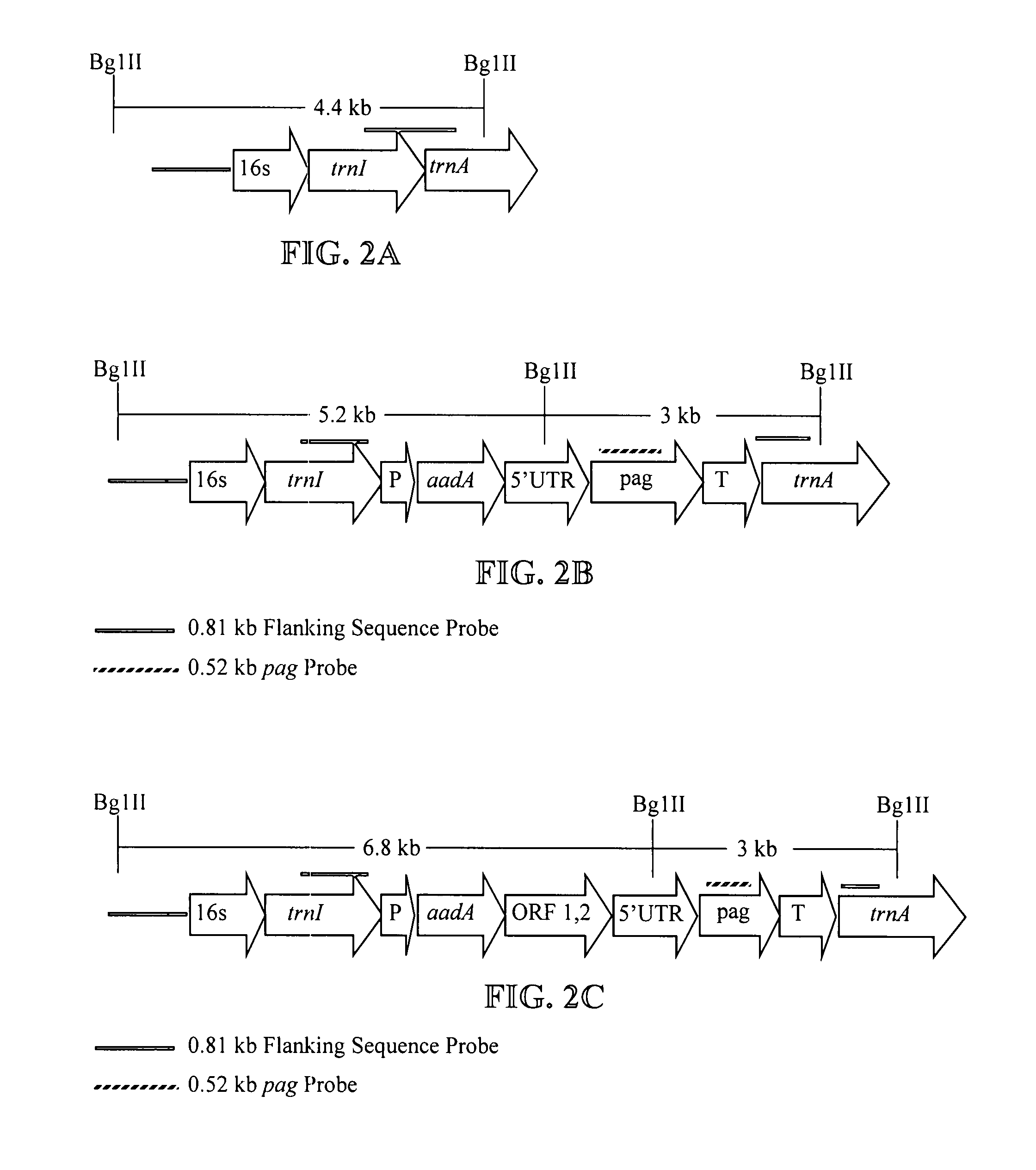
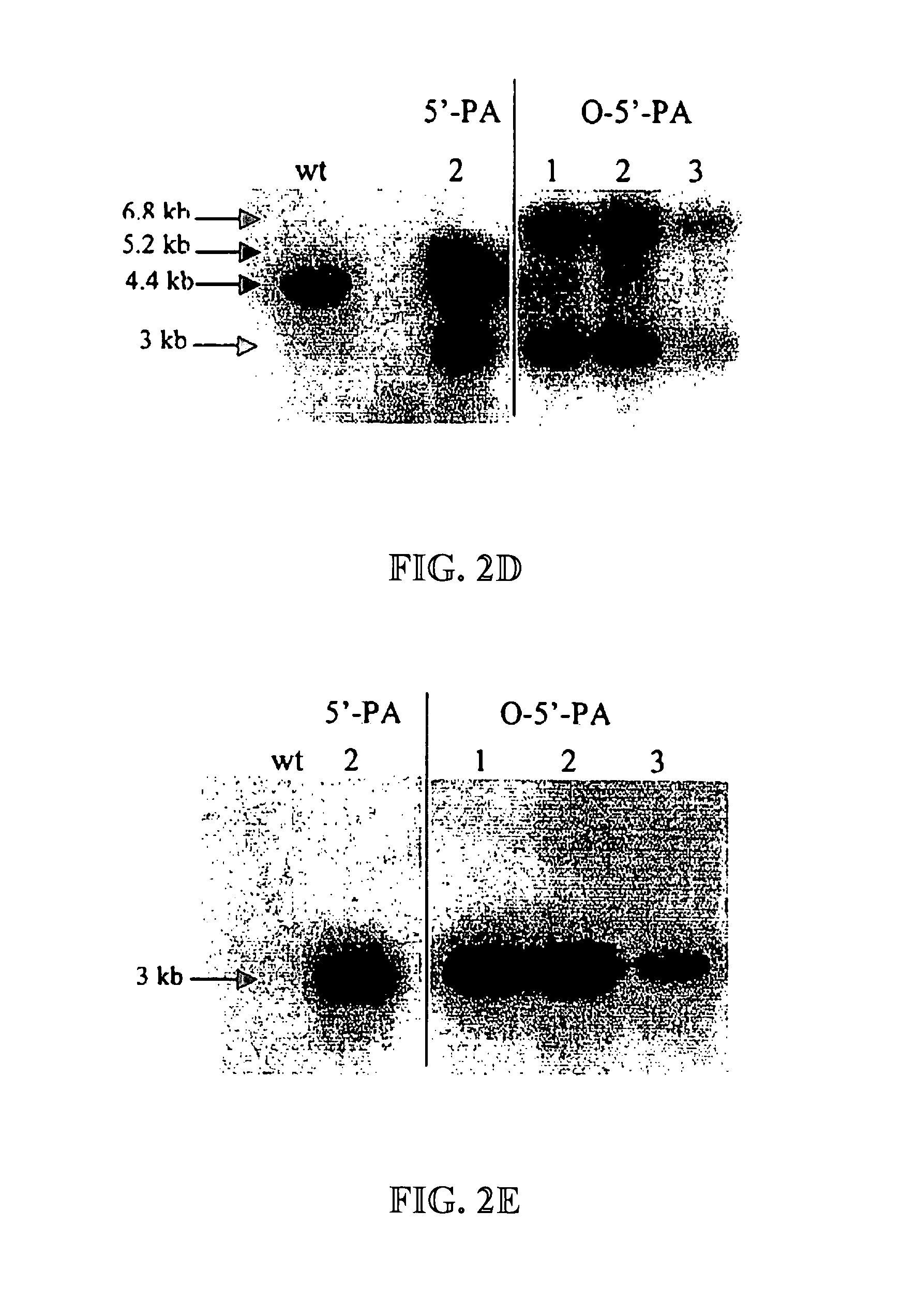
Expression of protective antigens in transgenic chloroplasts and the production of improved vaccines
InactiveUS20050108792A1Bacterial antigen ingredientsAntibody mimetics/scaffoldsAntigenDevelopmental stage
Vaccines for conferring immunity in mammals to infective pathogens are provided, as well as vectors and methods for plastid transformation of plants to produce protective antigens and vaccines for oral delivery. The invention further provides transformed plastids having the ability to survive selection in both the light and the dark, at different developmental stages by using genes coding for two different enzymes capable of detoxifying the same selectable marker, driven by regulatory signals that are functional in proplastids as well as in mature chloroplasts. The invention utilizes antibiotic-free selectable markers to provide edible vaccines for conferring immunity to a mammal against Bacillus anthracis, as well as Yersina pestis. The vaccines are operative by parenteral administration as well. The invention also extends to the transformed plants, plant parts, and seeds and progeny thereof. The invention is applicable to monocot and dicot plants.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Ginkgolide B nanometric liposomes medicine and the preparing method thereof
InactiveCN101036642AImprove solubilityPhysiological activity unchangedPowder deliveryOrganic active ingredientsCholesterolPolyethylene glycol
The invention relates to a ginkgolide B nanoliposomes drug and a method for preparing the same. The invention provides the ginkgolide B nanoliposomes drug including the following materials and the following radios: 8-12 mol compositions of cephalin and distearoyl choline with mole ratio of 1:3-6; 6-8 mol compositions of sojasterol and cholesterin with mole ratio of 1:2-4; 4-6 mol compositions of gamma-cyclodextrin / 2-hychoxypropyl-beta-cyclodextrin and alpha-cyclodextrin with mole ratio of 3:1; 0.2-0.4 mol methoxy polyethylene glycol 2000-hydrogenated soybean phosphatidylethanolamine; 2 mol ginkgolide B; and 0.1-0.5 mol vitamine E. A method for preparing the ginkgolide B nanoliposomes drug is also provided in the invention to produce the ginkgolide B nanoliposomes for target treating blocking of vessel or endothelial cell thereof, with a better healing effect than ordinary preparations of ginkgolide B.
Owner:江苏仲德医药科技有限公司
Method for separating and converting rape protoplast
InactiveCN107488675AReduce the ratioShort experiment cycleVector-based foreign material introductionPlant cellsSaccharumMannitol
The invention discloses a method for separating and converting rape protoplast. The method comprises the following steps: 1, selecting rape cotyledons or true leaves, and removing lower epidermis; 2, putting into enzymolysis liquid for carrying out enzymolysis, wherein the enzymolysis liquid is prepared from the following formula components: 1wt% of cellulase, 0.3wt% of macerozyme, 0.5M of mannitol, 20mM of KCl, 20nM of MES with a pH value of 5.7, 10mM of CaCl2 and 0.1wt% of BSA; 3, adding an isovolumetric W5 solution into a mixed solution obtained by means of enzymolysis, carrying out low speed centrifugation for removing supernatant, adding W5 for washing and resuspending, adding resuspended liquid into 20wt% of a sucrose solution for purifying, horizontally centrifuging, and taking supernatant green protoplast liquid; 4, centrifuging for removing the W5 solution, and resuspending protoplast precipitate by using an MMG solution; 5, converting the protoplast. The protoplast separating and converting method is established in rape for the first time; the rape protoplast is used as a receptor for converting, so that yellow fluorescent protein (YFP), luciferase (Luc) and renilla reniformis luciferase (Ren) are successfully expressed.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
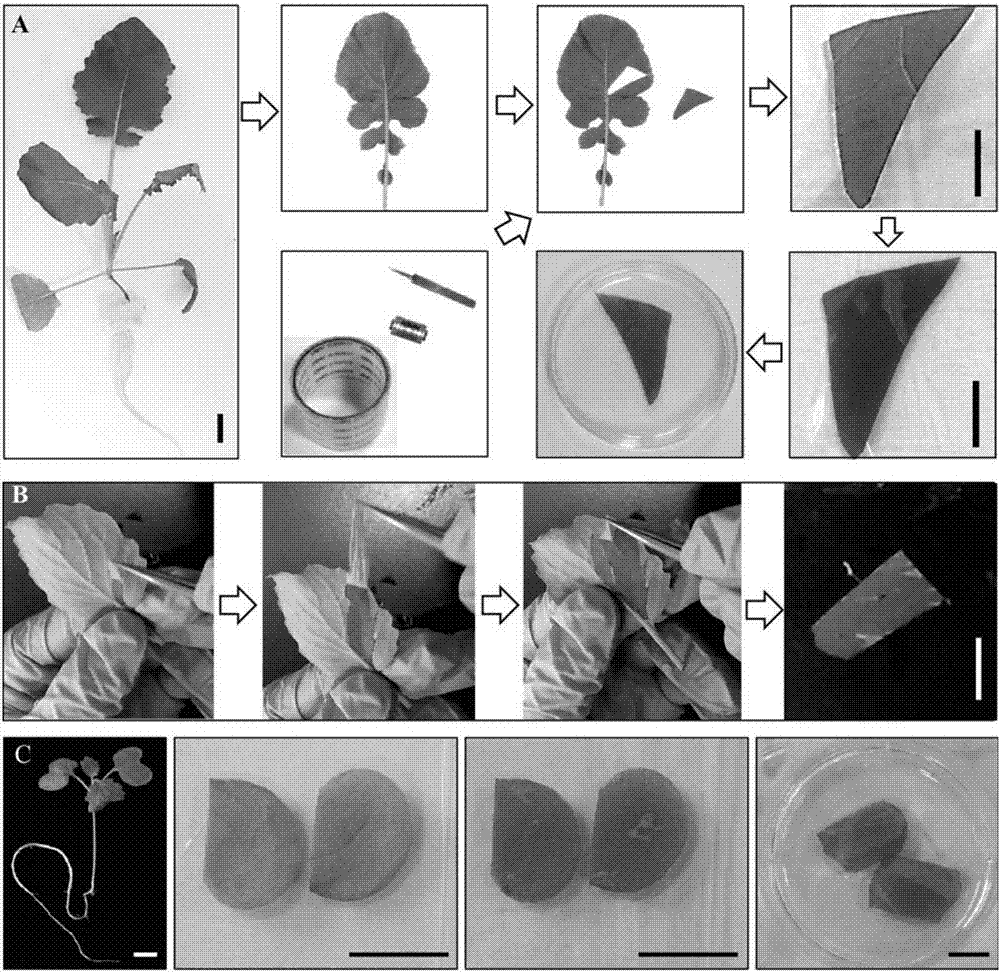
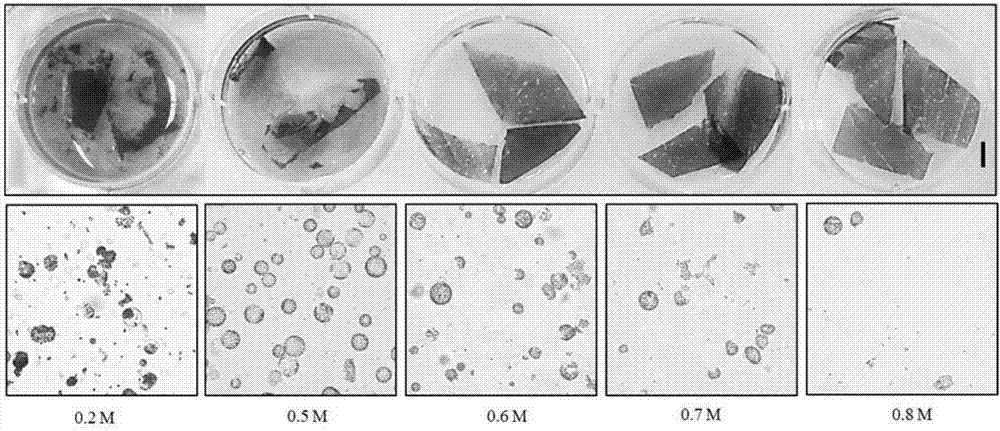
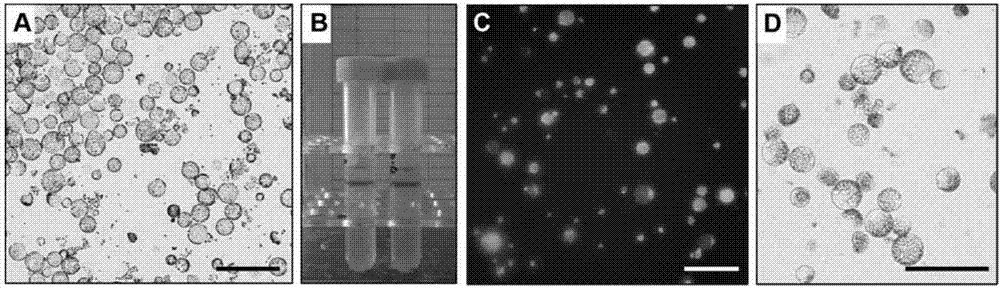
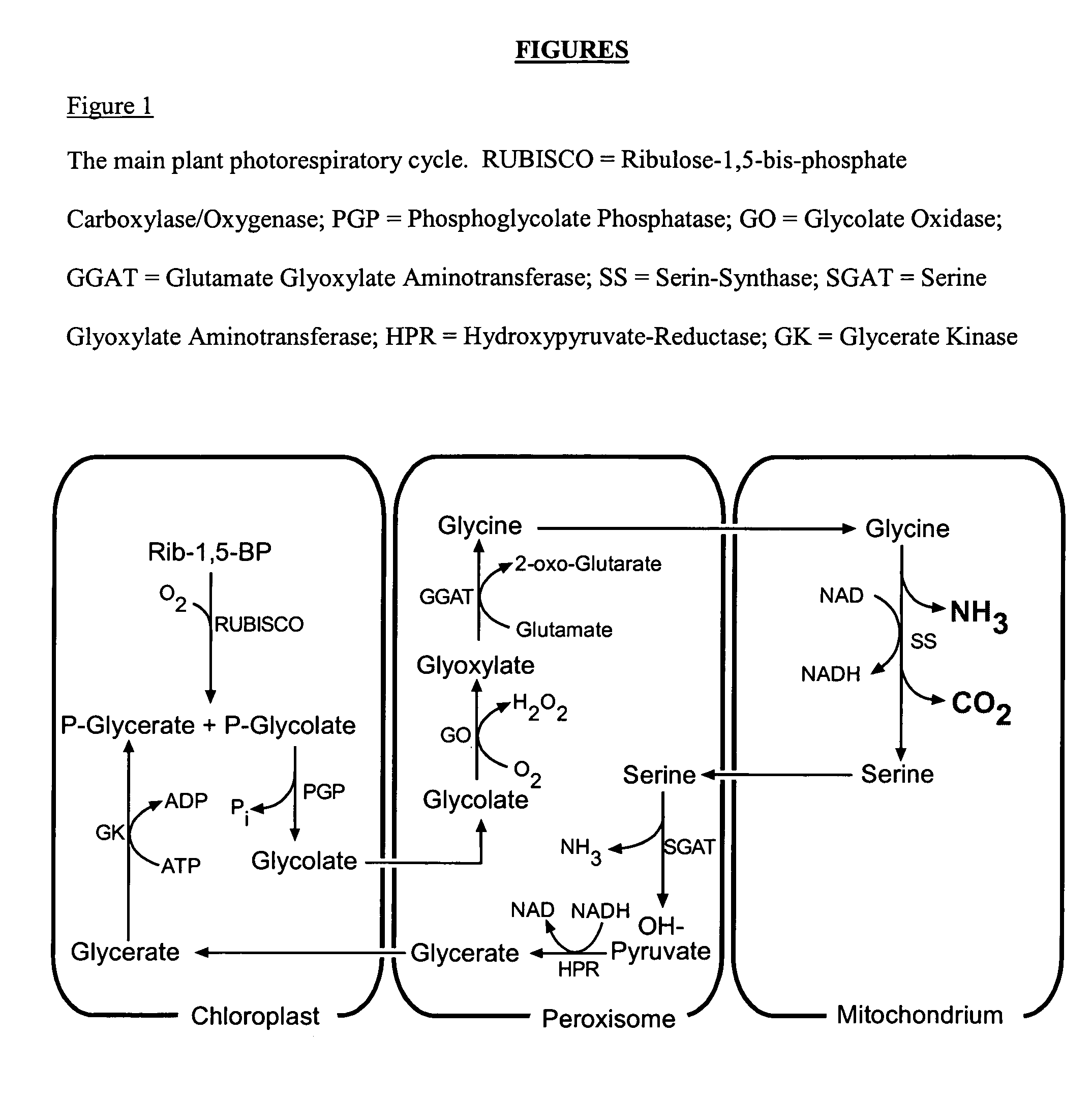
Method for producing plants with suppressed photorespiration and improved CO2 fixation
InactiveUS7208318B2Improve productivity and yieldInhibition of photorespirationSugar derivativesOther foreign material introduction processesChloroplastAlgae
The invention relates to a method for the production of plants with suppressed photo-respiration and improved CO2 fixation. In particular, the invention relates to a re-use of phosphoglycolate produced in photorespiration. The reaction product will be converted to a component that may be reintegrated into the plant assimilatory metabolism inside the chloroplast. This is accomplished by the transfer of genes derived from glycolate-utilizing pathways from bacteria, algae, plants and / or animals including humans into the plant nuclear and / or plastidial genome. The method of the invention leads to a reduction of photorespiration in C3 plants and by this will be of great benefit for food production especially but not exclusively under non-favourable growth conditions.
Owner:BASF SE
Plastidial targeting peptide
The invention relates to a non-cleavable, plastidial targeting polypeptide derived from a protein from the inner membrane of plant chloroplasts. Said peptide is particularly suitable for importing proteins of interest in plants.
Owner:GENOPLANTE VALOR
Expression of protective antigens in transgenic chloroplasts
InactiveUS7354760B2Bacterial antigen ingredientsAntibody mimetics/scaffoldsAntigenProtective antigen
Vectors and methods for plastid transformation of plants to produce protective antigens and vaccines for oral delivery are provided. The invention provides edible vaccines for conferring immunity to a mammal against Bacillus anthracis, as well as Yersina pestis.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
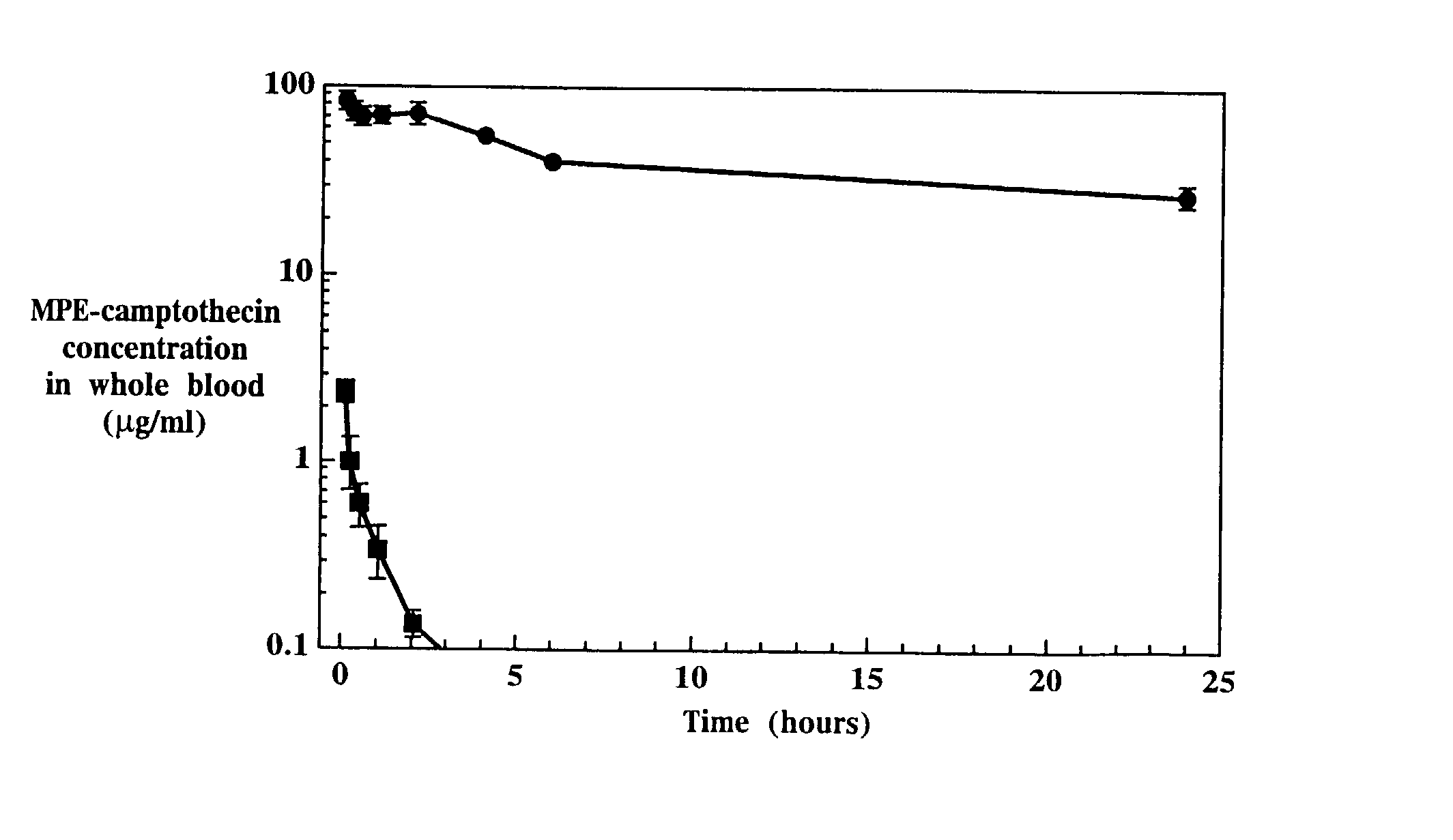
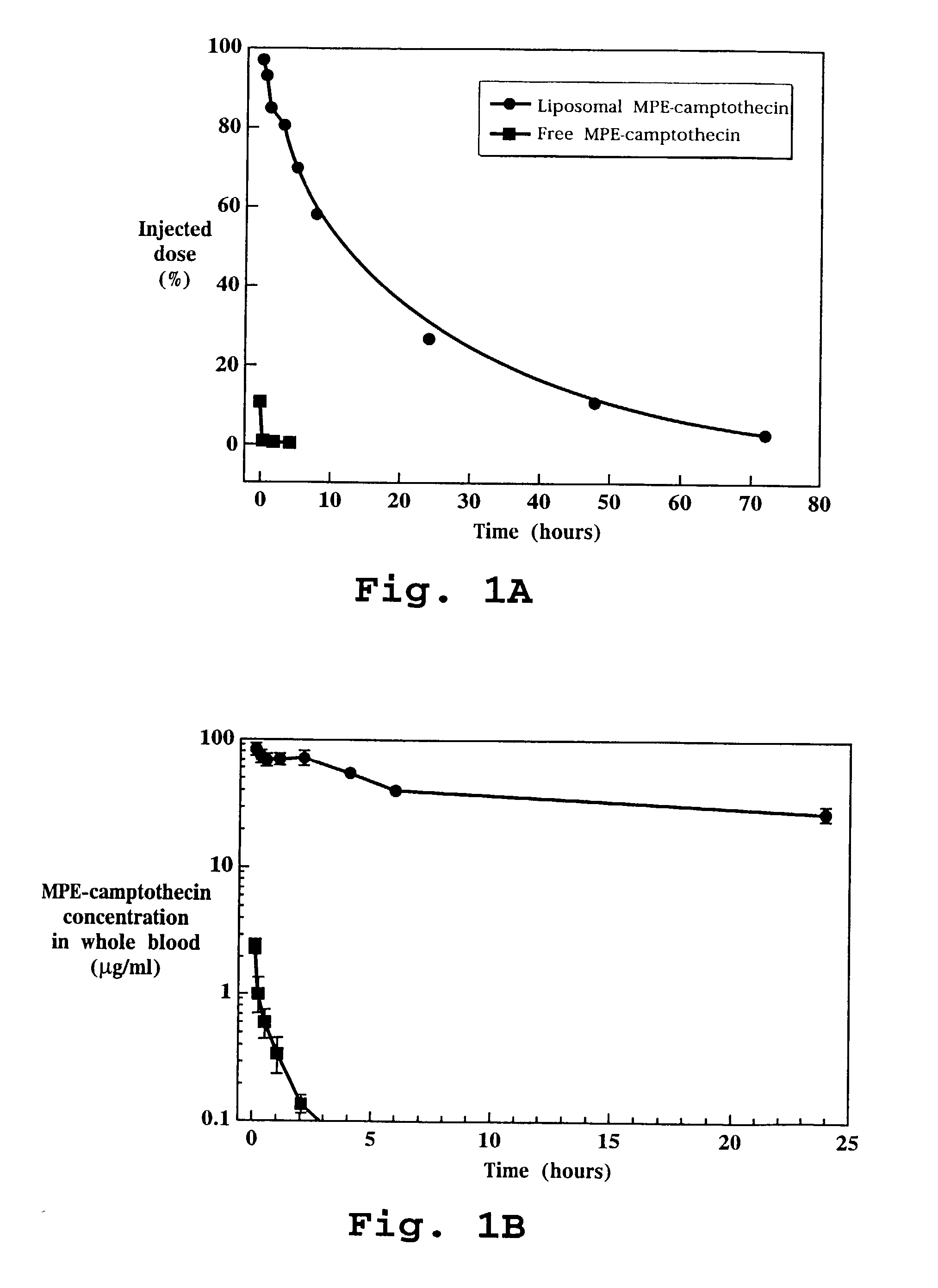
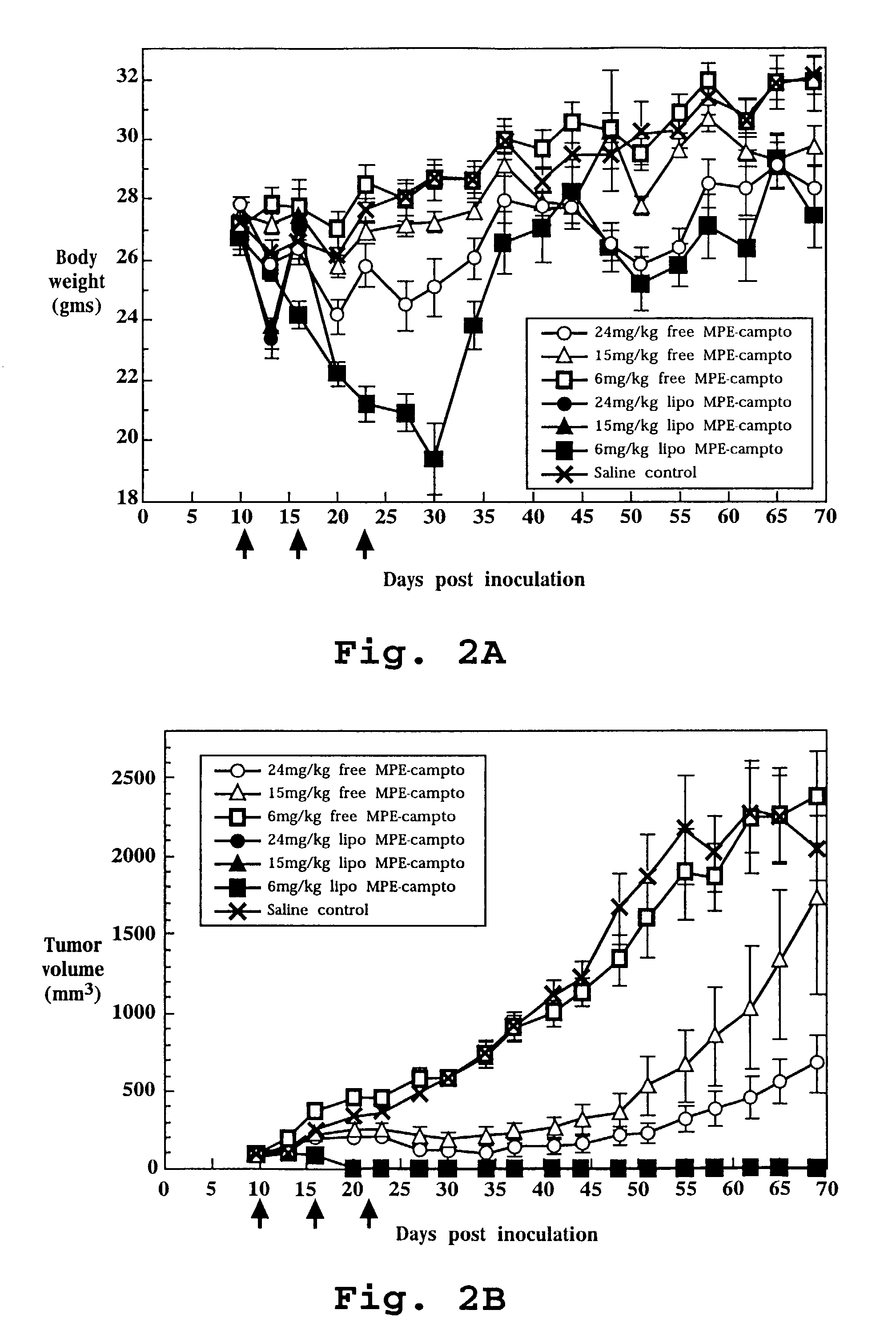
Liposome-entrapped topoisomerase inhibitors
A composition for administration of a therapeutically effective dose of a topoisomerase inhibitor I or topoisomerase I / II inhibitor is described. The composition includes liposomes having an outer surface and an inner surface defining an aqueous liposome compartment, and being composed of a vesicle-forming lipid and of a vesicle-forming lipid derivatized with a hydrophilic polymer to form a coating of hydrophilic polymer chains on both the inner and outer surfaces of the liposomes. Entrapped in the liposomes is the topoisomerase inhibitor at a concentration of at least about 0.10 μmole drug per μmole lipid.
Owner:ALZA CORP
Flurbiprofen liposome and preparation method thereof
InactiveCN101732255AReduce lossesGood biocompatibilityOrganic active ingredientsAntipyreticYolkWater baths
The invention relates to a flurbiprofen liposome and a preparation method thereof. The flurbiprofen liposome is prepared by weighting the following materials in part by weight: 1 to 10 parts of cholesterol, 5 to 40 parts of yolk lecithin, 2 to 50 parts of flurbiprofen and 1 to 10 parts of vitamin C; dissolving the materials with chloroform and methanol in a mass ratio of 4:1; uniformly mixing the materials to obtain mixed solution; putting the solution into a rotary evaporator; distilling the solution under reduced pressure for removing the chloroform and the methanol to obtain a lipid membrane; adding phosphate buffer with pH between 6 and 8 into the lipid membrane; rotating for 1 to 4 h in warm bath at the temperature of between 20 and 60 DEG C on the rotary evaporator; and carrying out water bath ultrasound for 5 to 30 min, and then filtering the mixture with a 0.22 to 0.45 mu m filter membrane to obtain the flurbiprofen liposome. The flurbiprofen liposome prepared by the invention has good stability, targeting property and slow-releasing property, can reduce administration dosage and reduce toxic and side effect of medicaments, is suitable for percutaneous administration and oral administration, and can be widely used for preparing oral liquid, aerosol, spray, and ophthalmic and external liposome administration formulations.
Owner:TONGJI UNIV
Construction method of recombination double expressions cultivated silkworm polyhedrosis baculovirus containing SOD gene
A method for constructing a recombinant double-expression silkworm polyhedral baculiform virus which contains the SOD gene comprises that the general RNA of the silkworm is extracted and an MnSOD primer is designed; a single-stranded cDNA is synthesized and an MnSOD gene is synthesized; the MnSOD gene is connected with a p FastBacHTa to transform XL-Blue cells; and the recombinant donor plasmid p FastBacHTa / MnSOD can be extracted; the baculiform virus DNA of the silkworm is taken as the template to synthesize the silkworm polyhedral gene and the gene is cloned to the p FastBac Dual plasmid; the MnSOD gene is inserted under the P10 promoter of the double-expression plasmid to construct the double-expression vector: Bm polyhedrin pFB dual / MnSOD; after the transinfection of the Bm polyhedrin pFB dual / MnSOD, the double-expression virus DNA which contains the MnSOD gene and the polyhedral gene is distilled; the DNA is transduced into the silkworm culture cell of transinfection with the mediation of transinfection reagent lipidosome to obtain the recombinant virus. Virus particles which can feed and infect the silkworm is provided and the object gene MnSOD is expressed by the silkworm and large-scale and industrial production is realized.
Owner:ZHEJIANG UNIV
Composition of cationic phospholipid nanoparticles for effective delivery of nucleic acids
InactiveUS20100203112A1Reduce deliveryLow cytotoxicityOrganic active ingredientsGenetic material ingredientsNanoparticleCholesterol
The present invention provides a cationic phospholipid liposome composition comprising 1,2-dioleoyl-sn-glycero-S-ethylphosphocholine (EDOPC), 3β-[N—(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-cholesterol) and 1,2-diphytanoyl-sn-glycero-3-phosphoethanolamine (DPhPE), a liposome-nucleic acid complex which is capable of forming a complex therewith, and a pharmaceutical composition comprising the same. The cationic phospholipid liposome of the present invention is highly effective for intracellular delivery of nucleic acids and reduction of cytotoxicity, as compared to conventional liposome products. Therefore, the present invention can be useful for gene therapy via intracellular delivery of a desired material to target cells.
Owner:KOREA UNIV IND & ACADEMIC CALLABORATION FOUND +1
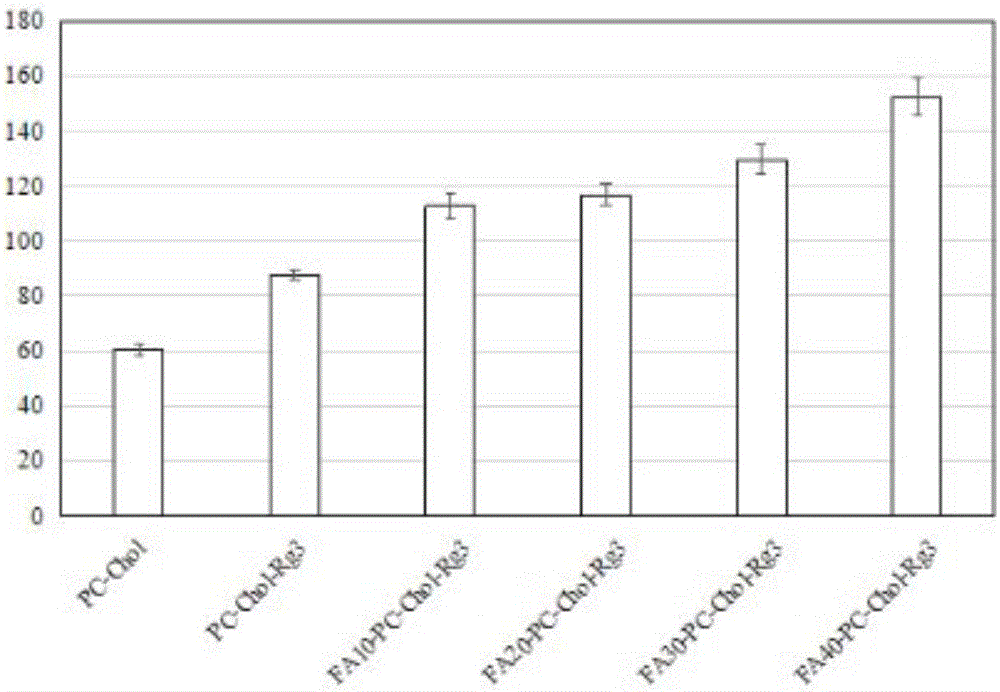
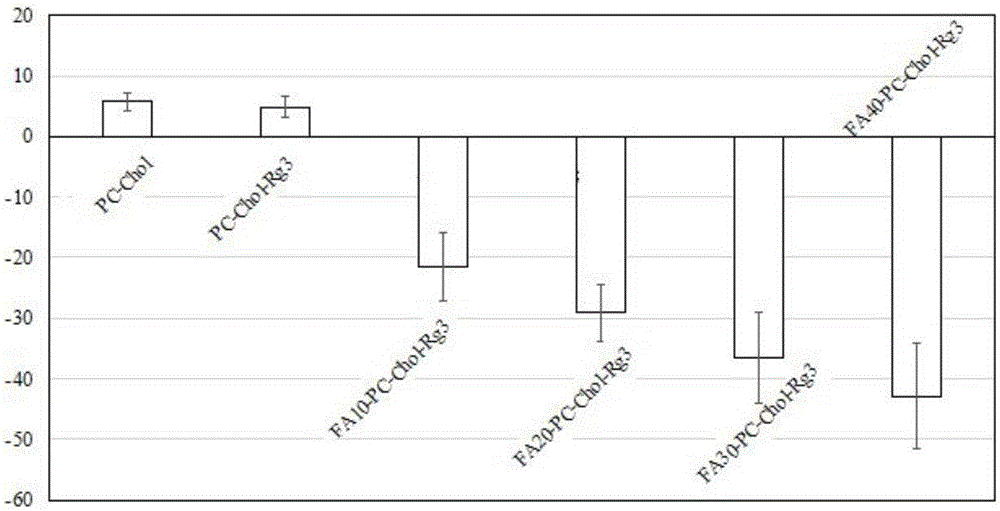
Preparation method and application of 20 (R)-ginsenoside Rg3/soya bean lecithin/cholesterol/folic acid lipidosome medicine
InactiveCN105708801AHigh encapsulation efficiencyEasy to prepareOrganic active ingredientsPharmaceutical non-active ingredientsCancer cellCholesterol
The invention relates to a preparation method and application of a 20 (R)-ginsenoside Rg3 / soya bean lecithin / cholesterol / folic acid lipidosome medicine and belongs to the field of medicine health care products.By optimizing the ratio of soya bean lecithin, cholesterol and an encapsulation medicine to obtain 20 (R)-ginsenoside Rg3 lipidosome, the grain diameters of the 20 (R)-ginsenoside Rg3 lipidosome are reduced, and meanwhile the stability of the lipidosome medicine is enhanced; folic acid is used for conducting target modification on the encapsulated 20 (R)-ginsenoside Rg3 medicine lipidosome, and then target medicine delivery of cancer cells is achieved.Experimental results show that the encapsulation rate of the 20 (R)-ginsenoside Rg3 lipidosome can be up to 90% or above.The preparation method of the composite lipidosome medicine is simple and efficient.The prepared composite lipidosome is low in cytotoxicity, has the slow release feature and is capable of prolonging the curative effect, safe, efficient and high in targeting, good evaluating effects are obtained in physical representation and extracorporeal biological study, and a new approach is provided for research and development of anti-cancer medicines.
Owner:DALIAN NATIONALITIES UNIVERSITY
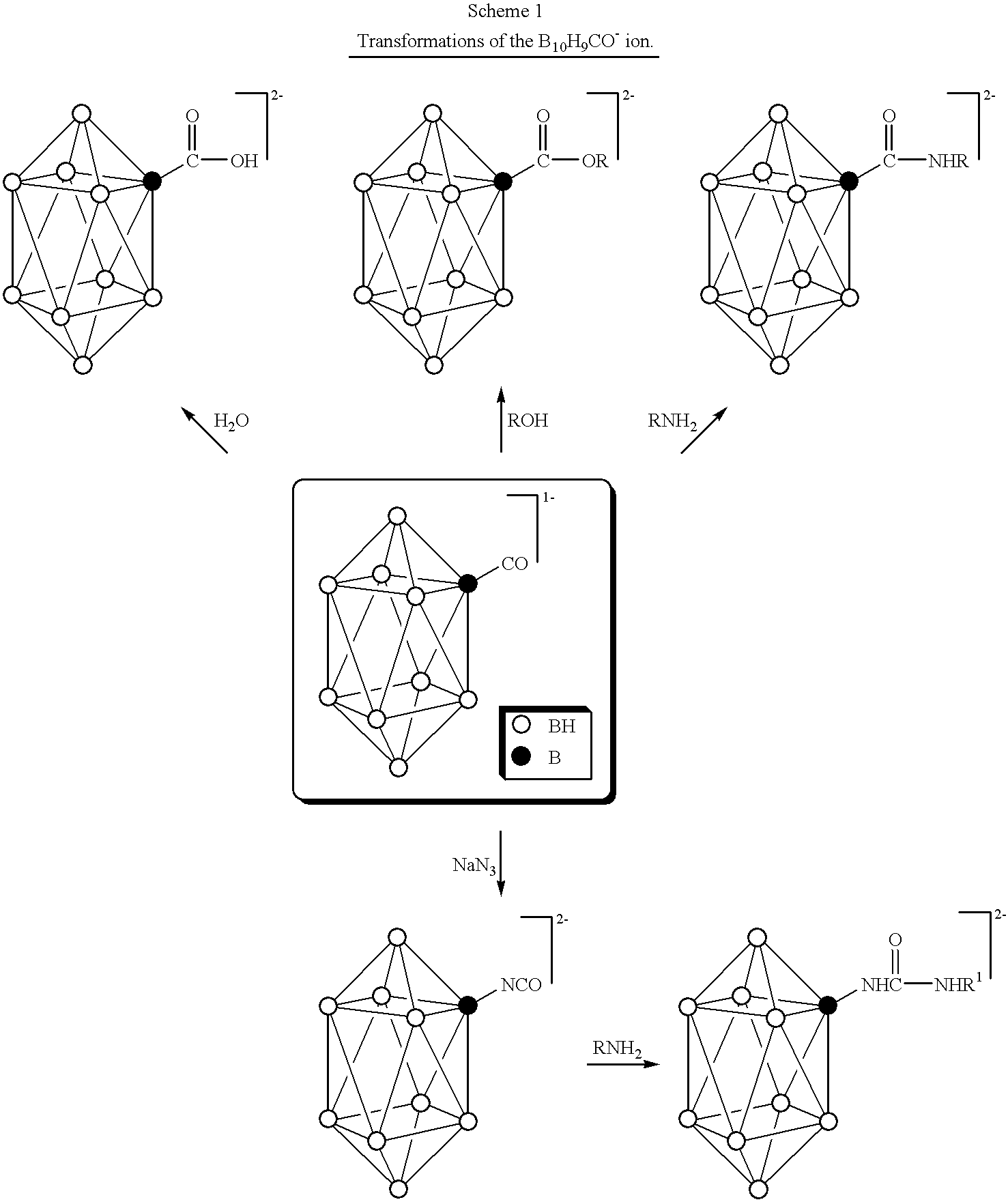
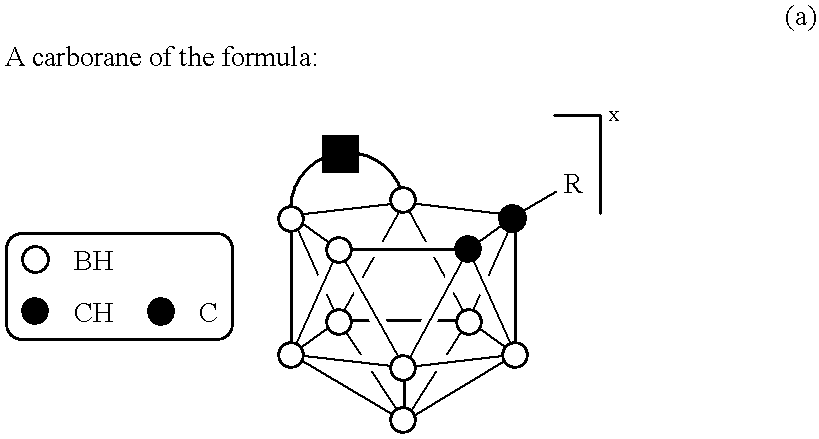
Compositions for boron delivery to mammalian tissue
InactiveUS6274116B1Minimize concentrationMinimise concentrationIn-vivo radioactive preparationsEnergy modified materialsAbnormal tissue growthElectron donor
Boron neutron capture therapy can utilize XyB20H17L where X is an alkali metal, y is 1 to 4, and L is a two electron donor such as NH3, and Na2B10H9NCO, among others. These borane salts may be used free or encapsulated in liposomes. Liposomes may also have embedded within their bilayers carboranes to increase the amount of delivered 10B and / or to increase the tumor specificity of the liposome.
Owner:UNIVERSITY OF MISSOURI
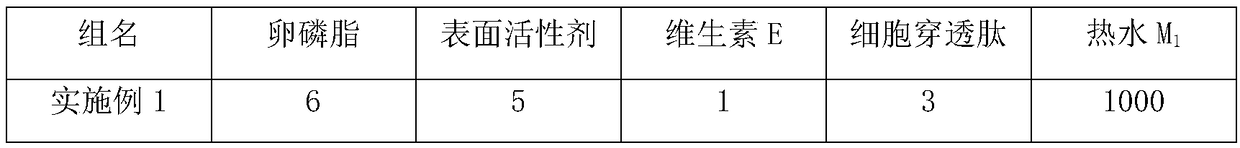
Nano-liposome containing cell penetrating peptide and preparation method and application thereof
ActiveCN109392900ACompact structureEvenly dispersedBiocideAnimal repellantsTherapeutic effectSurface-active agents
The invention discloses a nano-liposome containing cell penetrating peptide. The nano-liposome is prepared from lecithin, a surface active agent, vitamin E and the cell penetrating peptide in the weight ratio of (10-60) to (10-30) to (0.1-10) to (0.2-20); and the cell penetrating peptide is selected from at least one of biological source cell penetrating peptide, chimeric cell penetrating peptideand artificially synthesized cell penetrating peptide. According to a preparation method of the nano-liposome, a brand-new high pressure homogenization dispersion method is utilized, the stability ofthe nano-liposome is greatly improved, and the nano-liposome can be applied to a tank-mix assistant or a fly prevention assistant agriculturally. The nano-liposome containing the cell penetrating peptide has the high stability, can be stored for a long time at room temperature, is not sensitive to changes of pH value and ionic strength, and has good compatibility with pesticides and / or fertilizers. No organic solvents are needed in the preparation process, and no pollution is generated. The nano-liposome can be transmitted fast in vivo after being applied to crops, and reaches the minimum effective drug concentration rapidly, prolongs the persistence time, improves the prevention and treatment effect and delays occurrence of drug resistance.
Owner:武汉康科植保技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com