A manufacturing process of 2',2'-difluoronucleoside and intermediate
An enantiomer, NO2 technology, applied in the 2' field, can solve the problems of affecting the purity and cumbersome processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
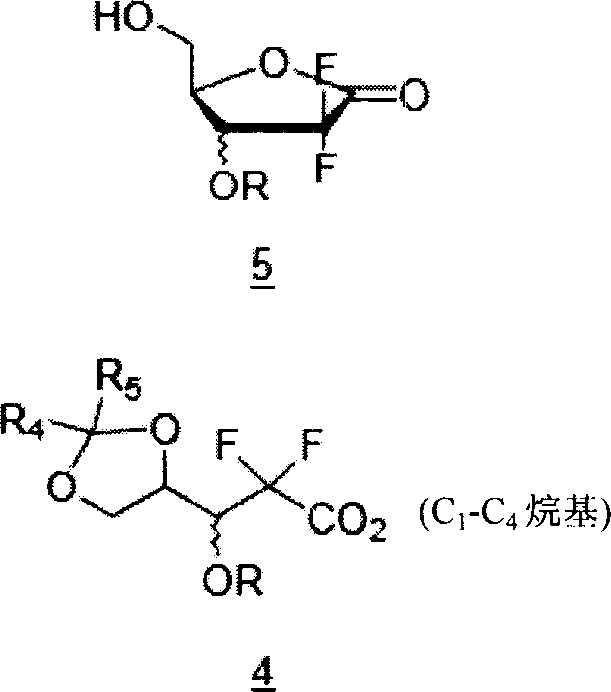
[0053] Preparation of 2-deoxy-2,2-difluoro-1-oxo-ribose
[0054] (3R, S)-2,2-difluoro-3-hydroxyl-3-(2,2-dimethyldioxolan-4-yl)propanoic acid ethyl ester (30 g, 0.118 mol) After adding acetonitrile (165 ml), acetic acid (67.6 ml) and water (11.7 ml) to dissolve, the mixture was stirred and refluxed for 4 hours. After the reaction liquid was concentrated under reduced pressure, toluene (165 ml) was added to carry out concentration under reduced pressure. Acetonitrile (165 ml) was added to the concentrate, and toluene (300 ml) was added thereto, followed by distillation and concentration under reduced pressure. Ethyl acetate (200 ml) was added to the concentrate for dilution, and activated carbon (3 g) was added thereto and stirred for 10 minutes. After the mixture was treated with anhydrous sodium sulfate, it was filtered through celite, and the obtained filtrate was concentrated under reduced pressure to obtain 2-deoxy-2,2-difluoro-1-oxoribose (20 g, 100%).
[0055] 1 H NMR...
Embodiment 2
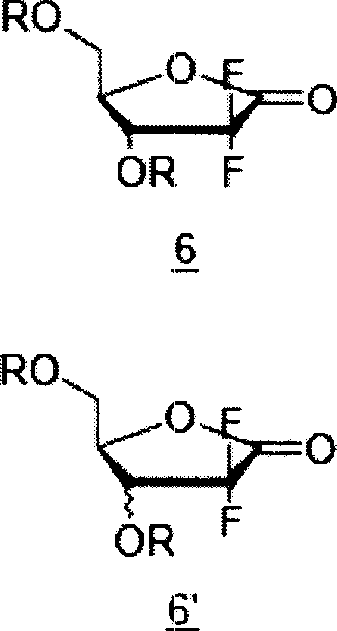
[0057] Preparation of 2-deoxy-2,2-difluoro-D-erythro-3,5-bis(3-fluorobenzoate)pentofurano-1-side
[0058] 2-deoxy-2,2-difluoro-1-oxo-ribose (20 g, 0.119 mol) was dissolved in ethyl acetate (200 ml), then 4-dimethylaminopyridine (29 g) was added, and After adding pyridine (28 g), 3-fluorobenzoyl chloride (2.5 g) was added. The reaction solution was stirred day and night at 60°C. After the reaction, it was washed with dilute hydrochloric acid aqueous solution and saturated brine, respectively. The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Ethyl acetate (23 ml) was added to the concentrate to dissolve it, and hexane (68 ml) was added thereto, followed by cooling to 0°C. After the resulting crystals were filtered, washed with a cooled mixture of ethyl acetate:hexane=1:3 (volume ratio), and dried to obtain 2-deoxy-2,2-difluoro-D-erythro-3, 5-bis(3-fluorobenzoate)pentofurano-1-side (26.7 g, 46%).
[0059] 1 H NMR (...
Embodiment 3
[0061] Preparation of 2-deoxy-2,2-difluoro-3,5-bis(3-fluorobenzoate)-D-ribofuranose
[0062] Add tetrahydrofuran (240 ml ) was dissolved, lithium tri-tert-butoxyaluminum hydride (22.2 g, 0.087 mol) was added, and stirred at room temperature for 30 minutes. After confirming the completion of the reaction, the reaction solution was diluted with ethyl acetate (960 ml), and washed with dilute aqueous hydrochloric acid, saturated aqueous sodium bicarbonate, water, and saturated brine, respectively. After drying with anhydrous sodium sulfate, filter and concentrate under reduced pressure to obtain 2-deoxy-2,2-difluoro-3,5-bis(3-fluorobenzoyl)-D-ribofuranose (24 g, 100% ).
[0063] 1 H NMR (CDCl 3 ); δ=4.4~4.75(m, 3H), 5.55(d, 1H), 5.4~5.7(m, 1H), 7.23~7.45(m, 4H), 7.70~7.89(m, 4H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com