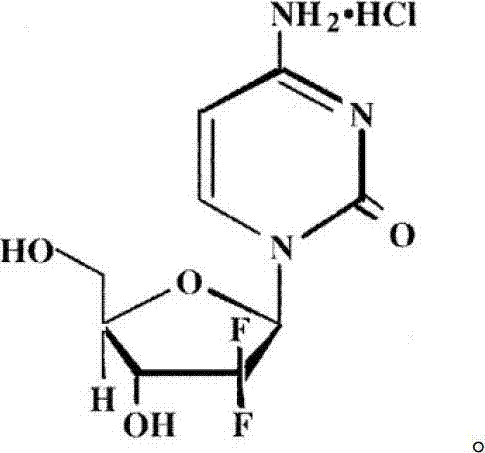
Gemcitabine hydrochloride injection and preparation method thereof
A technology of gemcitabine hydrochloride and injection, applied in the field of gemcitabine hydrochloride injection and its preparation, can solve the problems of poor stability of gemcitabine hydrochloride, difficult to store, inconvenient to use, etc., and achieve the effects of high clinical application value, good stability and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: (1000 bottles)
[0021]
[0022] According to the prescription amount, add gemcitabine hydrochloride to 1000ml of 30% ethanol solution, dissolve and stir evenly, then add sodium chloride and hydroxypropyl-β-cyclodextrin, heat to dissolve, add sodium dihydrogen phosphate to adjust the pH to 6. Dilute to volume with water for injection. The solution was sent to a sterile room, filtered through a 0.22 μm microporous membrane until clarified, filled into 25 ml vials with a volume of 6 ml per bottle, and plugged with a butyl rubber stopper.
Embodiment 2
[0023] Embodiment 2: (1000 bottles)
[0024]
[0025] Add gemcitabine hydrochloride to 1000ml of water for injection according to the prescription amount, dissolve and stir evenly, then add sodium chloride and hydroxypropyl-β-cyclodextrin, heat to dissolve, add sodium dihydrogen phosphate to adjust the pH to 6, Dilute to volume with water for injection. The solution was sent to a sterile room, filtered through a 0.22 μm microporous membrane until clarified, filled into 25 ml vials with a volume of 6 ml per bottle, and plugged with a butyl rubber stopper.
Embodiment 3
[0026] Embodiment 3: (1000 bottles)
[0027]
[0028] According to the prescription amount, add gemcitabine hydrochloride to 1000ml of 30% ethanol solution, dissolve and stir evenly, then add sodium chloride and hydroxypropyl-β-cyclodextrin, heat to dissolve, add sodium dihydrogen phosphate to adjust the pH to 6. Dilute to volume with water for injection. The solution was sent to a sterile room, filtered through a 0.22 μm microporous membrane until clarified, filled into 25 ml vials with a volume of 6 ml per bottle, and plugged with a butyl rubber stopper.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com