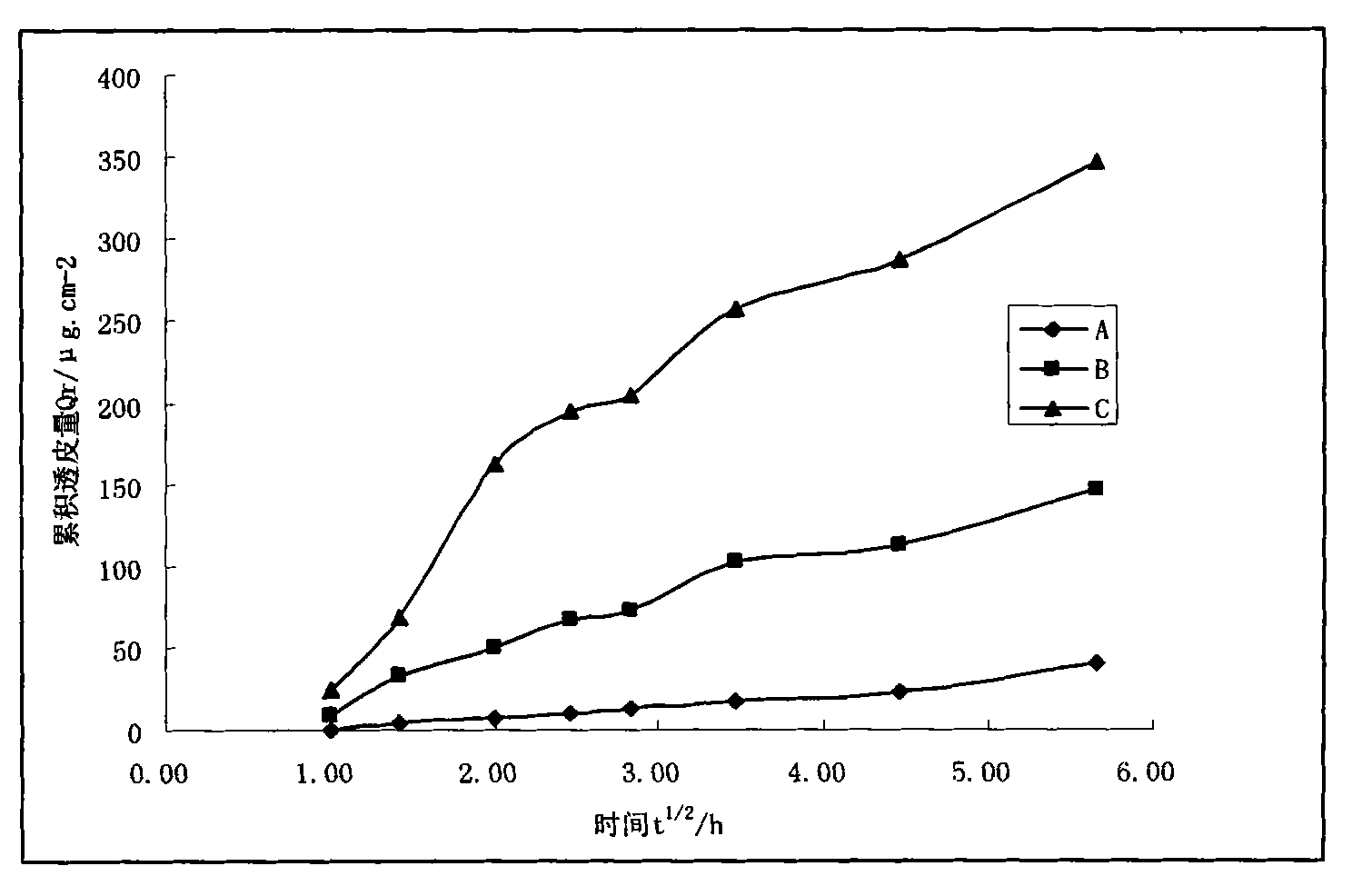
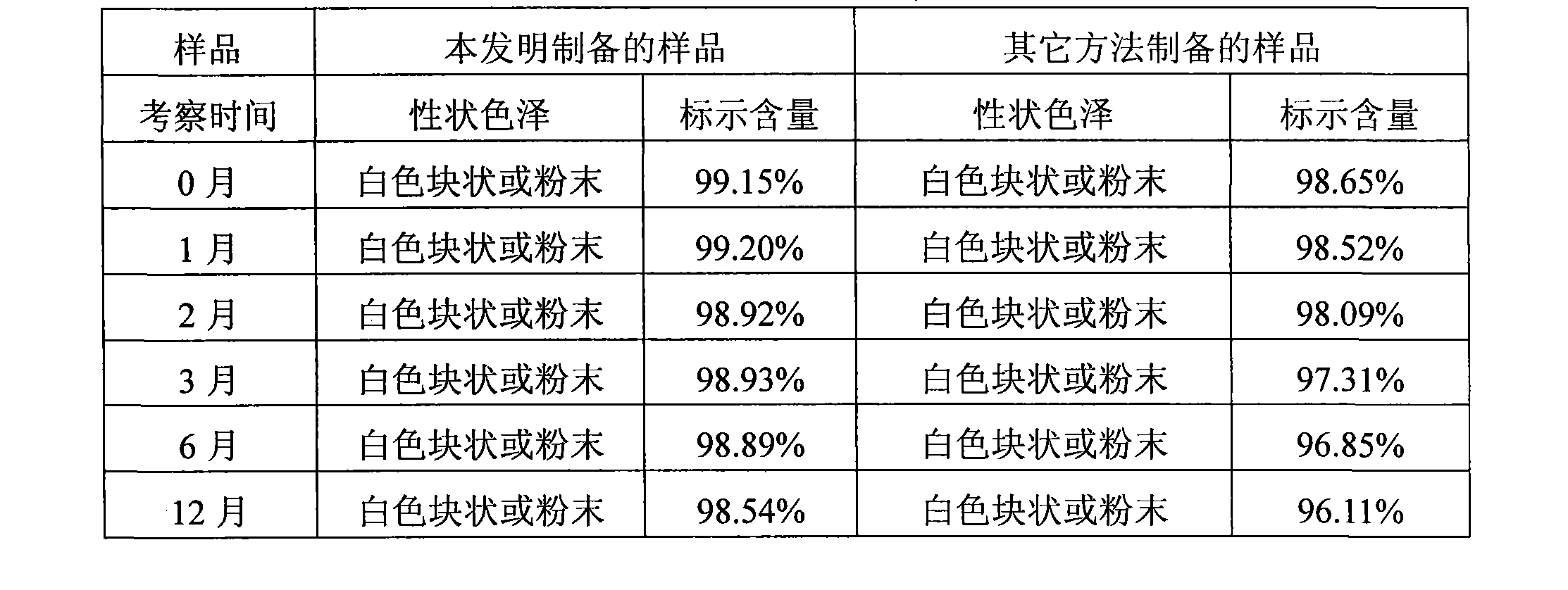
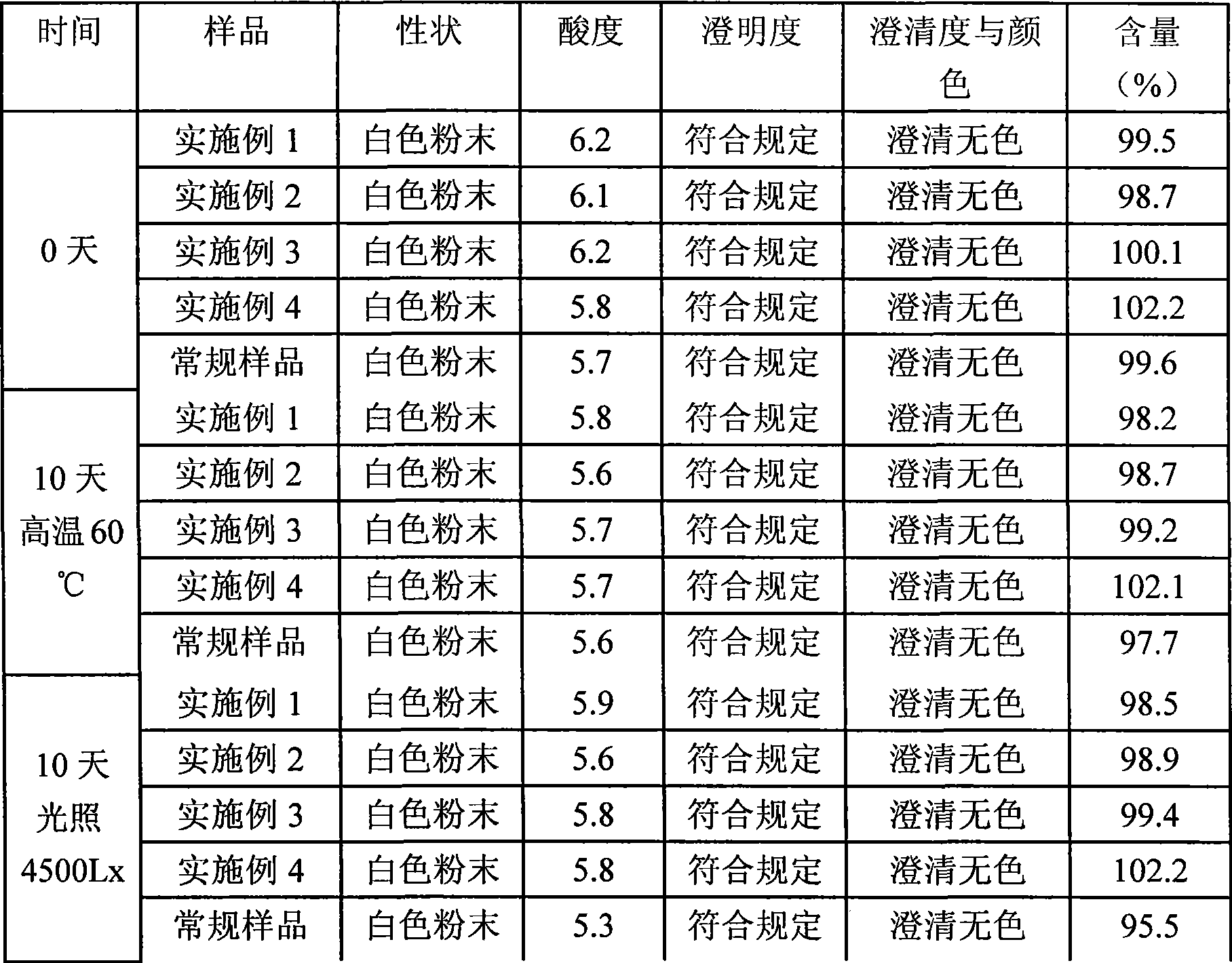
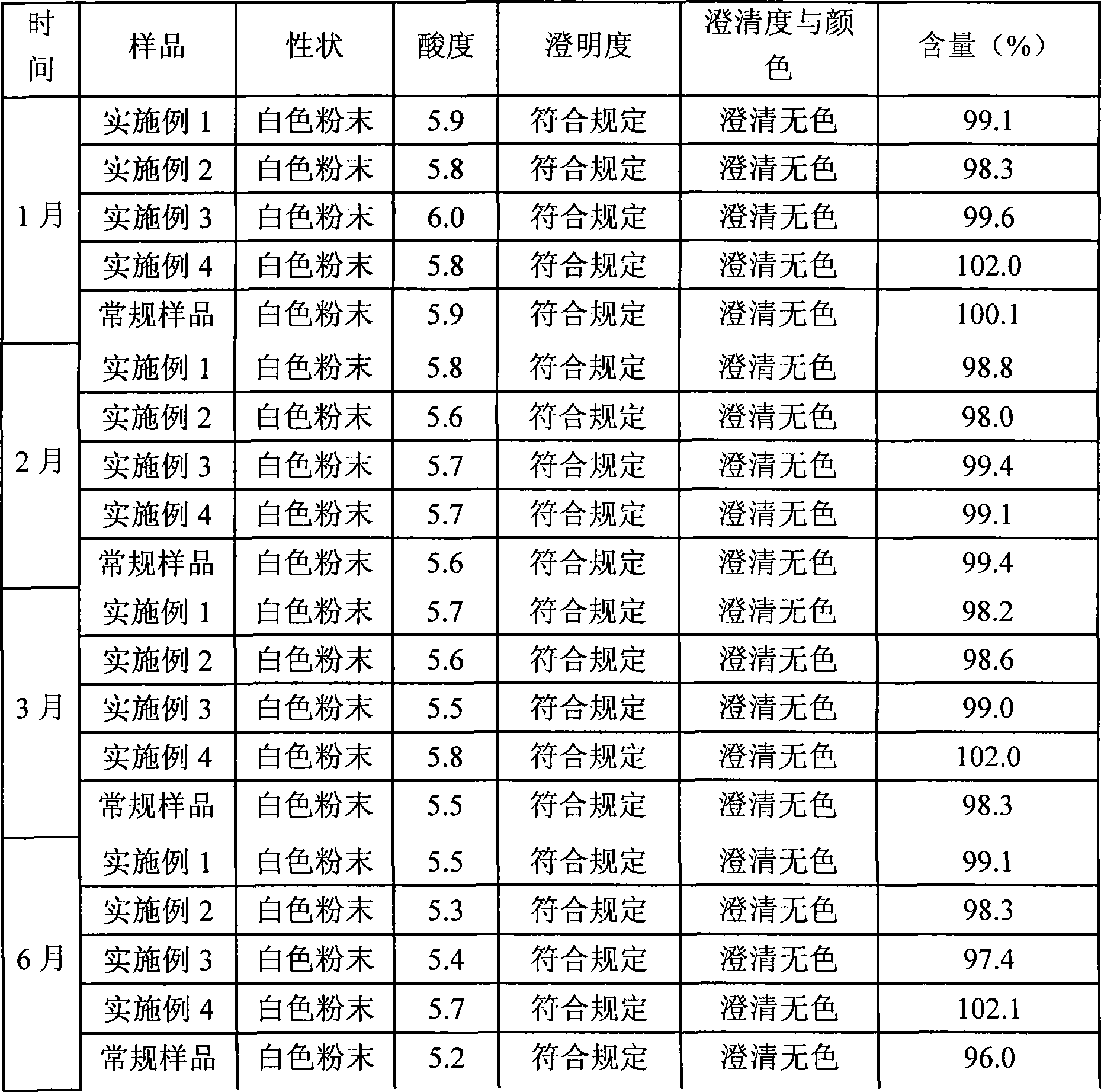
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
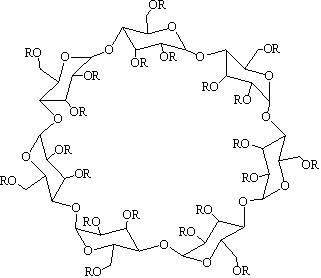
652 results about "Hydroxypropyl-beta-cyclodextrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
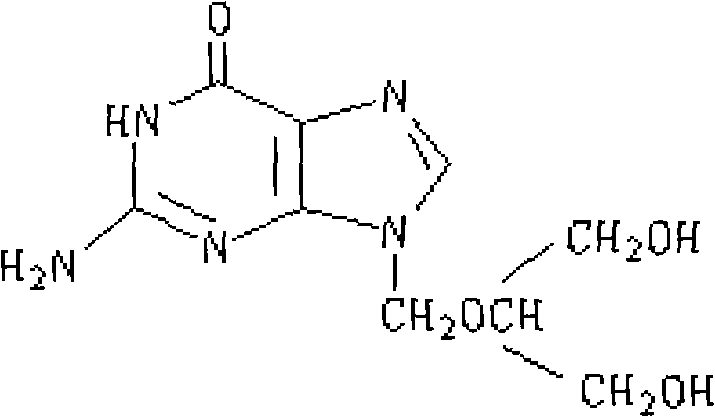
Hydroxypropyl beta cyclodextrin (HPbCD) is a drug used to slow neurological progression in Niemann Pick Type C Disease. Because this substance does not pass the blood-brain barrier, it is injected directly into the central nervous system.
Gingko lactone powder injection and its preparation method
ActiveCN1557297AGood curative effectReduce lossesOrganic active ingredientsPowder deliveryCurative effectBilobalides
The present invention discloses one kind of bilobalide powder for injection and features its composition including bilobalide, meglumine and hydroxypropyl-beta-cyclodextrin in the weight ratio of 1 to 0.2-0.5 to 6-9. The present invention also discloses the preparation process of the bilobalide powder for injection. The bilobalide powder for injection has supplementary material capable of dissolving bilobalide completely without destroying its structure, and bilobalide as the main material is slightly soluble in water and easily soluble in acetone. The key point of the present invention is to select hydroxypropyl-beta-cyclodextrin as the solubilkizing supplementary material to raise the solubility of bilobalide greatly, and this raises the bioavailability of bilobalide and the curative effect of the injection greatly.
Owner:JIANGSU KANION PHARMA CO LTD
Method for preparing hydroxypropyl-beta-cyclodextrin
ActiveCN102040675AWill not cause decompositionLow impurity contentMacromolecular non-active ingredientsNanofiltrationPharmaceutic Adjuvant
The invention discloses a method for preparing hydroxypropyl-beta-cyclodextrin, which comprises the steps of etherification, neutralization, decoloring, nanofiltration, resin purification, and spray drying. The method of the invention is small in pollution, and the yield in weight is more than 80 percent; meanwhile, the product prepared by using the method of the invention is narrow in substituted ratio range and low in impurity content, and can be used as a pharmaceutic adjuvant.
Owner:石药集团中诺药业(石家庄)有限公司
Voriconazole freeze-drying powder injection and its preparation process
The present invention relates to one kind of freeze dried voriconazole composition including voriconazole in effectively treating amount and hydroxypropyl-beta-cyclodextrin. Each of the preparation unit contains voriconazole in 50-400 mg, pregreably 200 mg and hydroxypropyl-beta-cyclodextrin of 5-40 time, preferably 15-25 times, weight of voriconazole.
Owner:大道隆达(北京)医药科技发展有限公司
Match of organic medicine and beta-cyclodextrin derivative and its preparing process
InactiveCN1379047AComposition is stableFast dissolutionBiocideNervous disorderWater solubleBeta-Cyclodextrins
A mating member of organic mecicine and beta-cyclodextrin derivative is prepared from beta-CD (hydroxypropyl beta-cyclodextrin-HP-beta-CD) and organic medicinal materials or other organic molecules through recognizing and assembling in the existance of water to obtain water-soluble coordination compound. Its advantages are easy dissolving in water, high dissolving speed and infinite diluting stability.
Owner:刘 云清 +3
Furan resin self-hardening sand and preparation method thereof
The invention discloses furan resin self-hardening sand and a preparation method thereof. The self-hardening sand is prepared from the following raw materials: silica sand, furan resin, epoxy resin, glycerin water solution, a silane coupling agent, titanium dioxide, boron carbide, hydroxypropyl-beta-cyclodextrin and pyralene. The preparation method comprises the following steps of mixing silica sand with the size of larger than 200 meshes with pyralene, sequentially feeding the silane coupling agent, titanium dioxide, boron carbide, hydroxypropyl-beta-cyclodextrin, glycerin water solution, epoxy resin and furan resin, and rapidly stirring until the raw materials are fully mixed, wherein the stirring speed is 120r / min. The preparation method is simple; all the raw materials are matched and act; the silica sand can achieve good bonding property and curing effect by virtue of furan resin, epoxy resin, silane coupling agent and pyralene, and glycerin water solution, titanium dioxide, boron carbide and hydroxypropyl-beta-cyclodextrin are capable of improving the humidity resistance, the fluidity and the compressive strength of the silica sand.
Owner:WUJIANG HYDRAULIC COMPONENTS FOUNDRY
Solid nano pharmaceutical formulation and preparation method thereof
A method of preparing low water-soluble medicine into solid nanometer pharmaceutical formulation is disclosed. According to the characters of molecular aggregates such as supramolecular chemical micelles and vesicles, the formulation, which based on the hydroxypropyl-beta-cyclodextrin and phospholipid, is prepared under the condition of hyperthermia sterilization and decompression. Such nanometer formulation is sterile particle or powder with loose porosity. For directly intravenous use, the formulation has targeting activity, sustained release and long circulating characters. While as a solid oral product, it is fast-release, fast-effective, and improved bioavailability characters, and is readily melted in mouth. The formulation utilizes secure accessories, traditional equipments and methods, thus, it is suited to be used and manufactured widely. Also disclosed is intravenous formulation of anticancer paclitaxel, which characterized that there has no polyoxyethylenated castor oil in it. Such intravenous formulation is nonallergic so that it has higher security and efficiency compared to present commercially available paclitaxel formulations.
Owner:LIU YUNGING +3
Voriconazole eye drops and preparation method thereof
InactiveCN101849905AImprove comfortGood for antifungal effectOrganic active ingredientsSenses disorderMedicineBromine
The invention discloses voriconazole eye drops. Every 1000 milliliters of eye drops contain 3-50g of voriconazole powder, 20-500g of hydroxypropyl-beta-cyclodextrin, 0.1-0.3g of bacteriostat, 0.5-2.5g of sodium hyaluronate and the balance of water for injection, wherein the bacteriostat is benzalkonium chloride, benzalkonium bromine or ethylparaben. The invention also discloses a preparation method of the voriconazole eye drops. The invention greatly increases the solubility of voriconazole in water to 0.3-5%, so that the medicine can exist in water solution in the form of molecules, thereby providing convenience for exerting the antifungal action of the medicine. Meanwhile, when being applied to eyes, the invention provides convenience for exerting the antifungal action of the medicine through cornea. The preparation method can be carried out at room temperature without affecting the medicine stability, and has the advantages of simple and easy realization and low cost.
Owner:河南省眼科研究所
Water soluble coenzyme Q10 hydroxyl-beta-cyclodextrin inclusion compound and its preparation method
ActiveCN101053556AGood water solubilityDissolution rate is fastPowder deliveryOrganic active ingredientsDissolutionTherapeutic effect
The invention relates to a water-solubility coenzyme Q10 hydroxypropyl-beta-cyclodextrin clathrate and its producing method belonging to medicine and health product field, which has a good treatment effect to the cardiovascular diseases, hepatitis, weak immunity. It contains following material with a weight ratio of coenzyme Q10: hydroxypropyl-beta-cyclodextrin=1:0.5-100 and containing the producing steps of: blending in the water medium by high-speed scattering machine or shears, then in a high pressure homogeneous machine, finally spary drying or freeze-out to prepare the coenzyme Q10 clathrate. The clathrate can be produced to the oral liquid and injection type, which has a high solubility and dissolution, a small wettability, a good stability, and a high relative bioavailability.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES +1
Electrodynamic remediation device and method for soil polluted by heavy metal or organic matters
ActiveCN103316909AImprove conductivityFacilitate desorptionContaminated soil reclamationDesorptionElectrochemistry
The invention belongs to the technical field of environmental management, and particularly relates to an electrodynamic remediation device and a method for soil polluted by heavy metal or organic matters. The device is composed of a direct-current power supply, an anode chamber, a gas collecting device, a soil chamber, an anode electrode and a cathode electrode. The device provided by the invention has the advantages that a soil solution which is near the electrode has electrochemistry element reaction under the power-driven pressure, the physicochemical properties such as redox potential and pH value in the coil are changed, the low-permeability soil can have high conductivity through hydroxypropyl-beta-cyclodextrin, and the desorption of the heavy metal or organic matters in a soil solid is accelerated.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Method for preparing benzaldehyde by taking hydroxypropyl-beta-cyclodextrin as accelerating agent
ActiveCN101648853ARapid responseImprove solubilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsBenzaldehydePotassium hydroxide
The invention discloses a method for preparing benzaldehyde by taking hydroxypropyl-beta-cyclodextrin as an accelerating agent. The method comprises the following steps: taking cinnamon oil or cinnamaldehyde as raw material, hydroxypropyl-beta-cyclodextrin as an accelerating agent and alkaline water which formed by one or more mixtures of sodium hydroxide, potassium hydroxide, sodium carbonate, potassium carbonate, ethanolamine, diethanol amine, triethanolamine and triethylamine and is used as a solvent react; after reaction, carrying out extraction concentration by using ester solvent, methanol, acetone or acetonitrile as an organic solvent to obtain the benzaldehyde. The method has the advantages of simple process, low energy consumption, low / no pollution, environmental protection, highbenzaldehyde yield and natural degree, and the like.
Owner:SUN YAT SEN UNIV
Process for extracting ginkgolide, ginkgolide injection and process for preparing same
ActiveCN1594319AEasy to operateReduce pollutionOrganic active ingredientsOrganic chemistrySodium acetateGinkgolide
The invention discloses a process for extracting ginkgolide, ginkgolide injection and process for preparing same, wherein the extracting process consists of disintegrating the ginkgo leaves, leaching by diluted acetone solution, recovering acetone, removing impurities, extracting and purifying with acetic ether, removing impurities with sodium acetate again, reclaiming acetic ether, recrystallizing in ethanol or methanol, filtering, low temperature drying, solubilizing the bilobalide with hydroxypropyl-beta-cyclodextrin to obtain the bilobalide injection.
Owner:HEILONGJIANG ZBD PHARMA
Ganciclovir freeze-dry preparation for injection and preparation method thereof
ActiveCN101711746AGuarantee product qualityGood inclusion effectPowder deliveryPharmaceutical product form changeMedicineFreeze-drying
The invention relates to a Ganciclovir freeze-dry preparation for injection, comprising 50-250 parts of Ganciclovir and 100-200 parts of hydroxypropyl-beta-cyclodextrin. The invention also relates to a preparation method of the Ganciclovir freeze-dry preparation for injection. In the preparation process of the Ganciclovir freeze-dry preparation, Ganciclovir freeze-dry preparation can be dissolved into solvent when the pH value is almost neutral to improve the compliance for clinical application; freeze-dry time is shortened to be 22-26 hours, thus improving product quality, saving energy and lowering consumption.
Owner:MAANSHAN BBCA PHARMA
Method for improving stability and water solubility of resveratrol and application thereof
InactiveCN101927148AImprove stabilityEasy to operateMicroballoon preparationFood preparationFruit wineWhite powder
The invention discloses a method for improving the stability and the water solubility of resveratrol and an application thereof, belonging to the technical field of food processing. The method comprises the following steps of: including the resveratrol molecule with hydroxypropyl-beta-cyclodextrin according to the mole ratio of 1:1-2 under 20-30DEG C; and preparing a resveratrol microcapsule by means of cooling and drying. The product has the appearance of white powder, is odourless, is easy to dissolve in water (the solubility of the resveratrol under the normal temperature is not less than 7mg / ml), obviously improves the stability thereof, and is good for widening the application range of the resveratrol in the field of health food, pharmacy and the like. The resveratrol microcapsule is added into the juice beverage or the fruit wine according to a certain proportion and is evenly mixed to prepare the juice beverage or the fruit wine with the health-protecting functions such as angiocardiopathy resistance, oxidation resistance and the like, wherein each 100ml of the juice beverage or the fruit contains 1-200mg of the resveratrol. The invention is simple in implementation and operation, is good for popularization, and has wide market prospect.
Owner:HEBEI AGRICULTURAL UNIV.
Serum-free culture medium without animal origin components for culturing Vero cell micro-carrier
InactiveCN101864393APromote growthSame densityVertebrate cellsArtificial cell constructsSerotoninVitamin C
The invention discloses a serum-free culture medium without animal origin components for culturing a Vero cell micro-carrier. The serum-free culture medium consists of a DMEM / F12 (1:1) culture medium and culture medium additive components such as epidermal growth factors, insulin, serotonin, aurin tricar-boxylic acid, biotin, vitamin C, amino acid, fructose, trehalose, trace elements, hydroxypropyl-beta-cyclodextrin and the like. The serum-free culture medium has the following advantages that: (1) the attachment growth of Vero cells in a tissue culture vessel and on the surface of the micro-carrier can be supported, and the cells can be transferred from a serum culture medium to the serum-free culture medium without a course of adaptation; (2) the serum-free culture medium contains no animal origin components, and has basically definite chemical components, and a low cost; and (3) the cells grow well, and the cellular morphology, the density and the vitality are basically the same as those of the serum culture medium.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
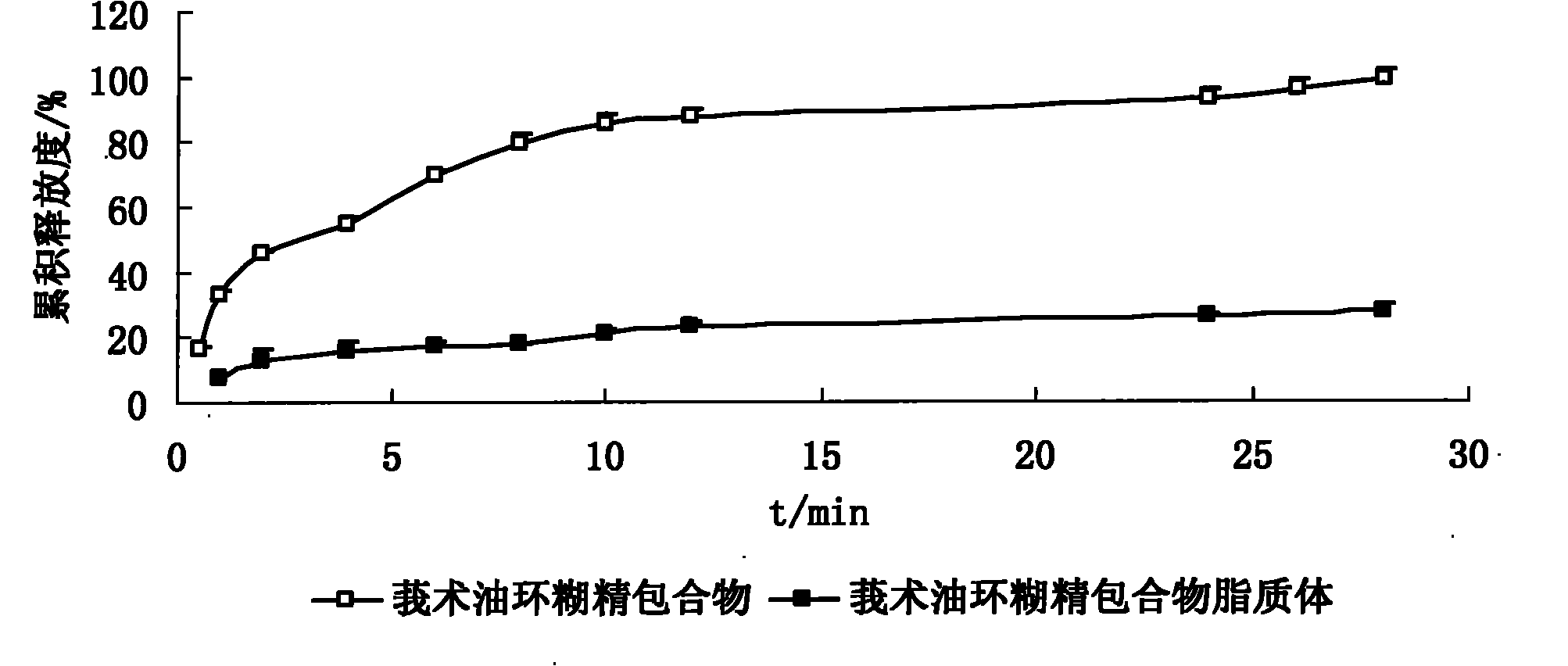
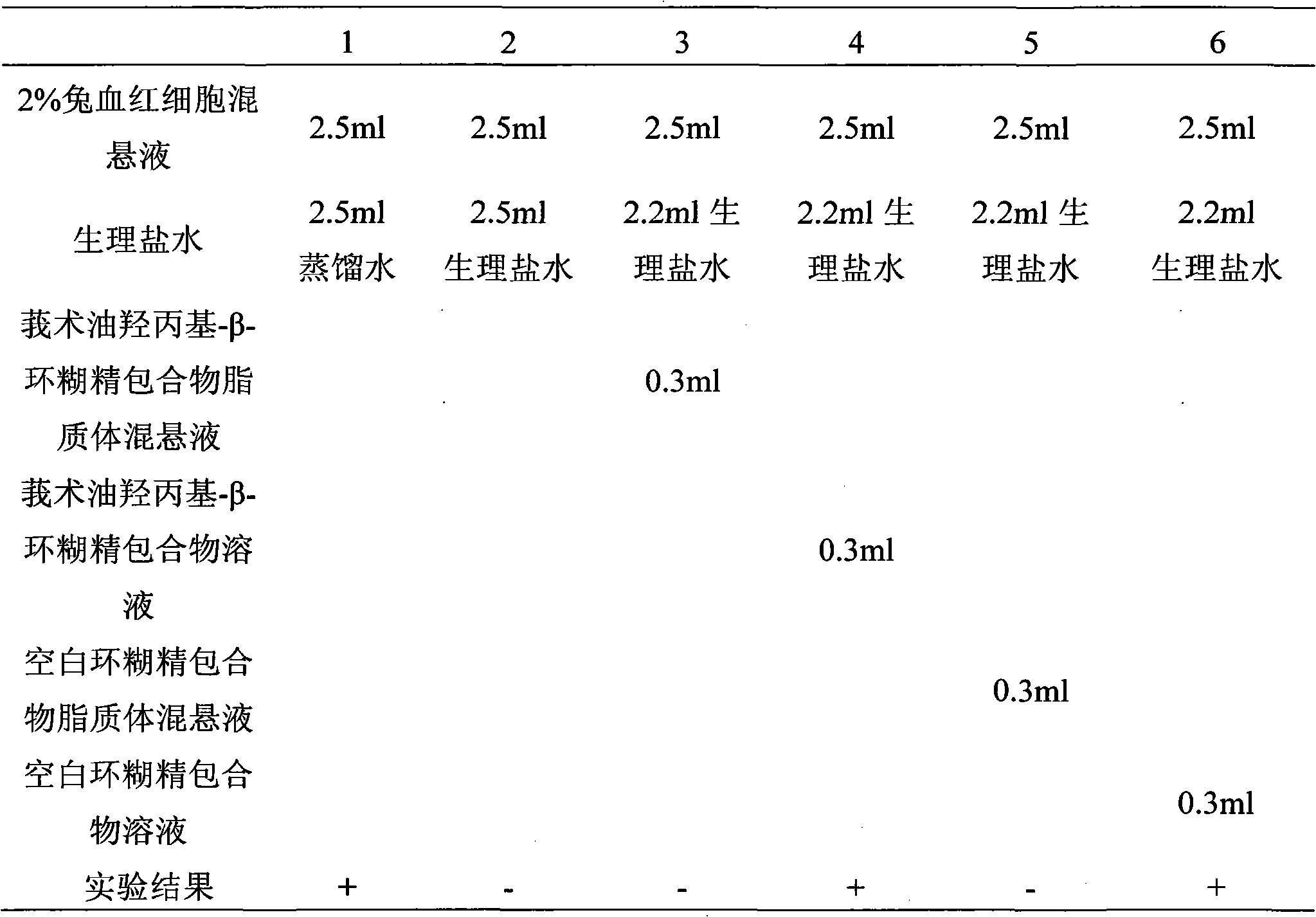
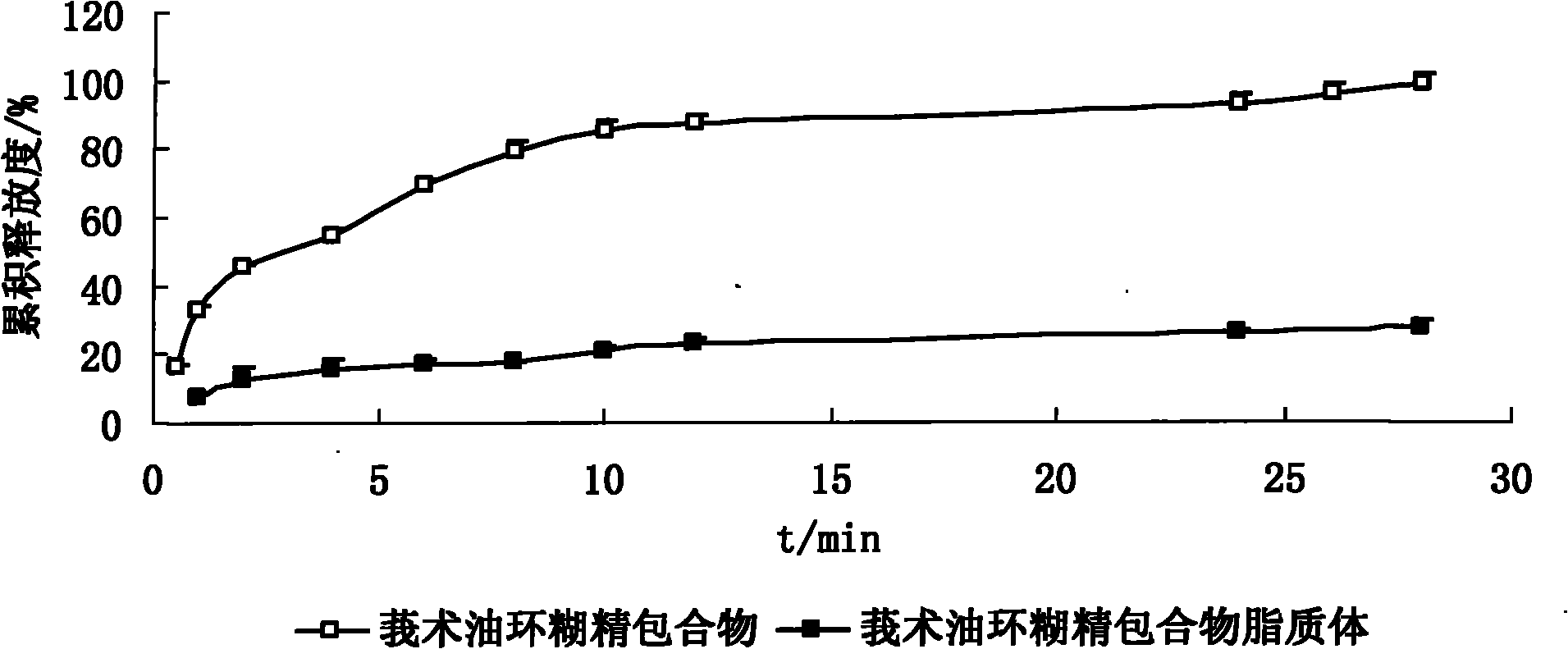
Hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and preparation method thereof
InactiveCN101926962ANo hemolytic toxicityObvious slow-release and long-actingPharmaceutical non-active ingredientsAntineoplastic agentsHemolysisEthanol Injection
The invention discloses a hydroxypropyl-beta-cyclodextrin inclusion liposome of zedoary turmeric oil and a preparation method thereof. The liposome is prepared by the following steps of: preparing a hydroxypropyl-beta-cyclodextrin inclusion of the zedoary turmeric oil from the zedoary turmeric oil and hydroxypropyl-beta-cyclodextrin through an inclusion process; and preparing the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil from the hydroxypropyl-beta-cyclodextrin inclusion, phospholipid and cholesterol through an ethanol injection method. Experimental results show that: the hydroxypropyl-beta-cyclodextrin inclusion liposome of the zedoary turmeric oil has the advantages of good sustained release and long action, high loading rate, particularly no untoward effect such as hemolysis, and higher safety.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Method for preparing naringenin/hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process
ActiveCN106265596AImprove solubilityHigh dissolution rateOrganic active ingredientsAntinoxious agentsSupercritical anti solventOrganic solvent
The invention discloses a method for preparing naringenin / hydroxypropyl-Beta-cyclodextrin microcapsules by supercritical anti-solvent process, comprising the steps of S1, dissolving naringenin and hydroxypropyl-Beta-cyclodextrin in an organic solvent to obtain a sample solution; S2, introducing CO2 into a crystallizer, and adjusting temperature and pressure in the crystallizer; S3, continually introducing CO2 at a flow speed, maintaining the temperature and pressure in the crystallizer constant, and introducing the sample solution of step S1 into the crystallizer; S4, after introduction of the sample solution, continuously introducing CO2, maintaining the temperature and pressure in the crystallizer constant, and relieving the pressure over a period of time; when the pressure in the crystallizer drops to atmospheric pressure, opening the crystallizer to collect the naringenin / hydroxypropyl-Beta-cyclodextrin microcapsules. The method provided herein wraps naringenin in HP-Beta-CD by using supercritical anti-solvent process, dissolving property of the naringenin in an aqueous solution is greatly improved, dissolvability is significantly improved, and bioavailability of the naringenin can be improved.
Owner:CHINA PHARM UNIV
An additive cigarette production technology
InactiveCN1543884AImprove stabilityNo effect on tasteTobacco treatmentCigar manufactureNitrosoNitroso Compounds
The invention relates to an addition agent for lowering the content of N-nitroso compound in cigarette and the cigarette production process, wherein the addition agent is a plant polyphenol microcapsule with plant polyphenol as the core, and alpha-cyclodextrin or beta-cyclodextrin or hydroxypropyl beta-cyclodextrin is used as molecular embedding agent for charging plant polyphenol microcapsule into the molecular embedding agent, forming stabilized colloid, by dissolving the colloidal solution and adding into the shredded tobacco, the content of N-nitroso compound in cigarette can be substantially reduced.
Owner:李波
Synthetic method for hydroxypropyl-beta-cyclodextrin
The invention relates to a synthetic method for hydroxypropyl-beta-cyclodextrin. The synthetic method is characterized by comprising the following steps: (1) charging beta-cyclodextrin, propylene oxide, basic catalyst and deionized water in a molar ratio of 1:2.5-10.5:0.6-3.4:62.9-126 into a closed stainless steel high-pressure autoclave, and carrying out etherification reaction on the materials at a temperature of between 50 and 90 DEG C and a gauge pressure of between 0 and 0.6MPa; (2) neutralizing and filtering the mixture after the reaction; (3) washing the mixture with ethanol; (4) extracting with acetone; and (5) obtaining the hydroxypropyl-beta-cyclodextrin after dialysis and other processes. The synthetic method for the hydroxypropyl-beta-cyclodextrin has higher product yield which can be more than 70 percent (calculated by inventory rating of the beta-cyclodextrin), and the obtained product quality index is in accordance with the 5.0 quality standard of European pharmacopoeia. The synthetic method has the characteristics of simple process, short etherification reaction time, moderate reaction conditions, low impurity content, and the like.
Owner:NANJING WELL CHEM
Solid nano-medicine and preparing method thereof
A method of preparing low water-soluble medicine into solid nanometer pharmaceutical formulation is disclosed. According to the characters of molecular aggregates such as supramolecular chemical micelles and vesicles, the formulation, which based on the hydroxypropyl-beta-cyclodextrin and phospholipid, is prepared under the condition of hyperthermia sterilization and decompression. Such nanometer formulation is sterile particle or powder with loose porosity. For directly intravenous use, the formulation has targeting activity, sustained release and long circulating characters. While as a solid oral product, it is fast-release, fast-effective, and improved bioavailability characters, and is readily melted in mouth. The formulation utilizes secure accessories, traditional equipments and methods, thus, it is suited to be used and manufactured widely. Also disclosed is intravenous formulation of anticancer paclitaxel, which characterized that there has no polyoxyethylenated castor oil in it. Such intravenous formulation is nonallergic so that it has higher security and efficiency compared to present commercially available paclitaxel formulations.
Owner:刘 云清 +3
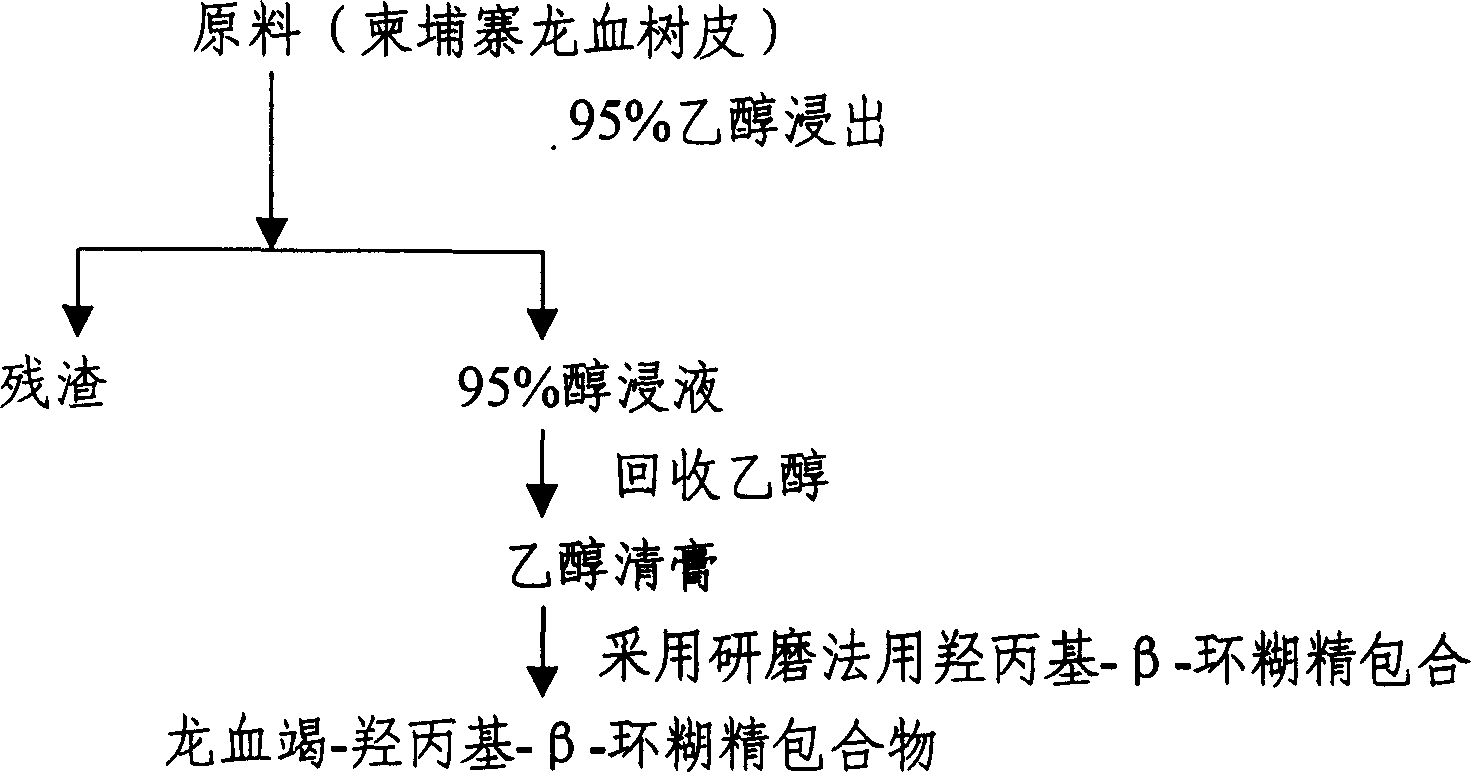
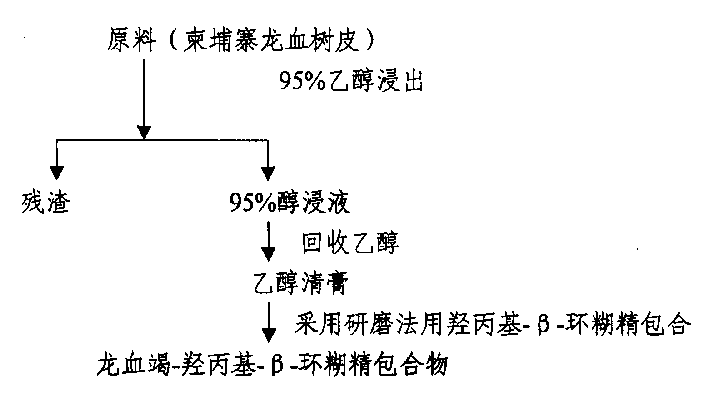
Preparation of water soluble dragon's blood as medicine
InactiveCN1552406APromote absorptionSimple processPowder deliveryUnknown materialsAlcoholWater soluble
A process for preparing the water-soluble medicine 'Longxuejie' includes such steps as immersing Draeaena cam bodiama in alcohol, recovering alcohol to obtain its extract, mixing water or alcohol with hydroxypropyl-beta-cyclodextrin, grinding, adding said extract, grinding and spray (or freeze) drying to obtain powder. Its advantages are high medical effect and easy absorption.
Owner:陈才义
Compound for hormonous dermatitis, preparation, preparation method and application
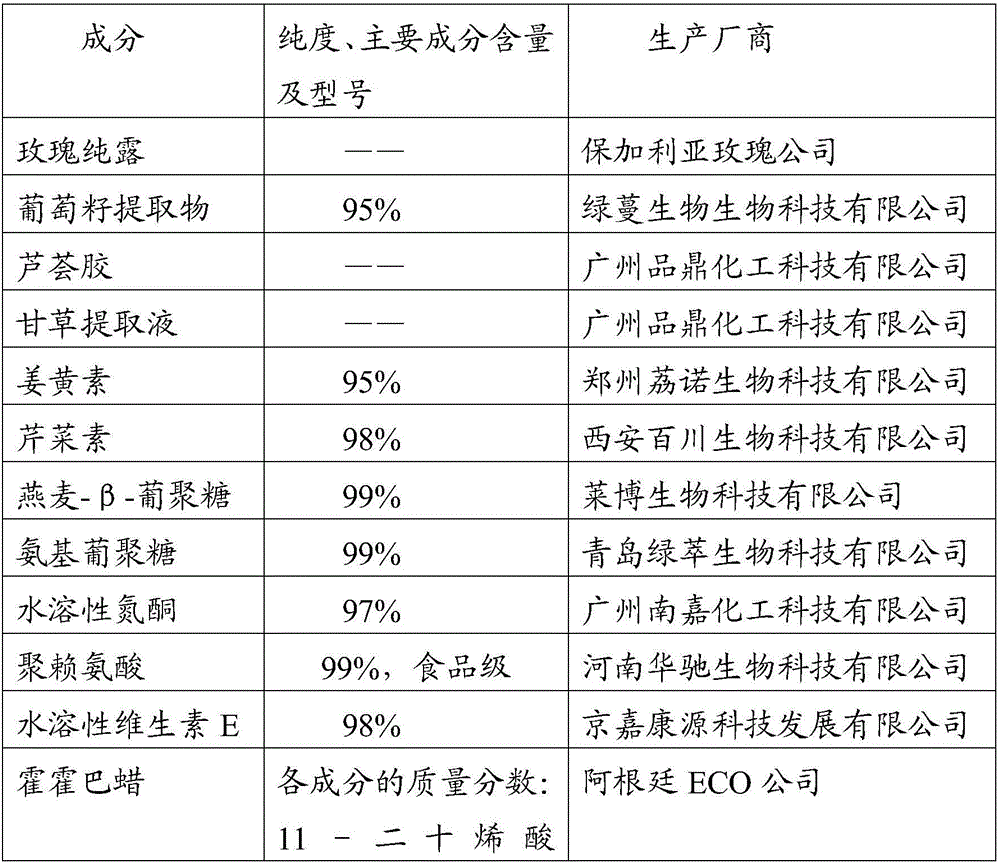
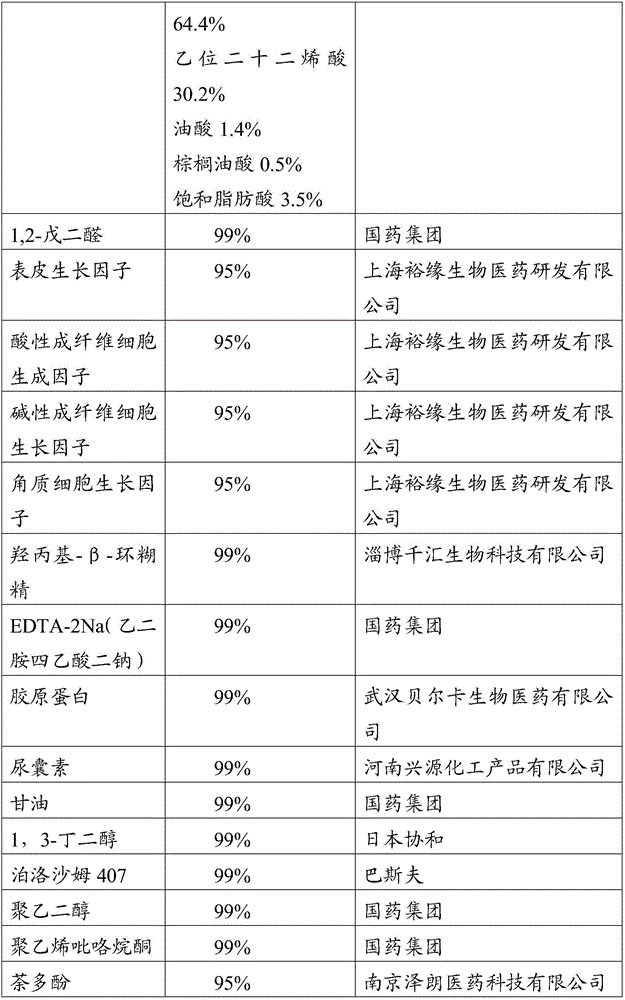
InactiveCN106075399ABroad spectrum antibacterialGrowth inhibitionCosmetic preparationsPeptide/protein ingredientsAnaphylaxisCuticle
The invention relates to the field of biological medicine and specifically relates to a compound for hormonous dermatitis, a preparation, a preparation method and an application thereof. The compound comprises the following components: rose hydrosol, grape seed extract, aloe vera gel, liquorice extracting solution, curcumin, apigenin, oat-beta-glucan, amidogen glucan, water-soluble azone, polylysine, water soluble vitamin E, buxus chinensis, 1,2-glutaraldehyde, compound growth factor, hydroxypropyl-beta-cyclodextrin, EDTA-2Na, collagen, humectants, auxiliary material and natural preservatives. The components in the compound have mild action to skin and are free from irritation; the components are collocated with each other, can prevent inflammation and anaphylaxis caused by hormonous dermatitis on the basis of supplying moisture preservation and can restrain bacteria to infect the damaged skin. The added compound growth factor can promote metabolism and repairing of cells of corium layer, epidermis and especially cuticle and can promote the forming of intercellular substance, so as to repair the damaged skin and achieve the effects of protection and curing.
Owner:简婷婷
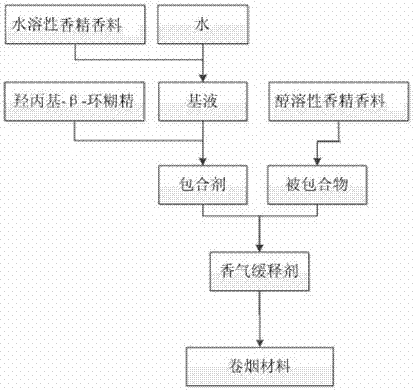
Aroma sustained-release agent for cigarettes, and preparation method and application thereof
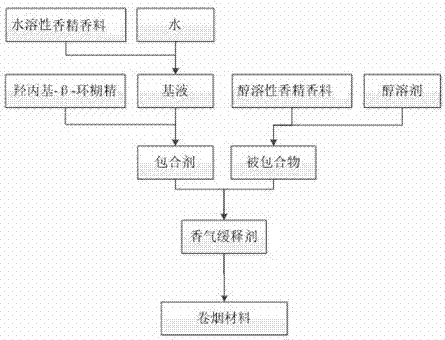
InactiveCN107365628ASolve the problem of volatile lossAchieve mutual complementarityTobacco preparationEssential-oils/perfumesFlavorAlcohol
The invention relates to an aroma sustained-release agent for cigarettes and a preparation method and application thereof, belonging to the technical field of tobacco. The aroma sustained-release agent for cigarettes comprises an inclusion agent and an included compound, wherein the inclusion agent comprises hydroxypropyl-beta-cyclodextrin and a base fluid in a weight ratio of 1: 1 to 1: 10, the base fluid is water and water-soluble essence and flavor, and the weight percentage of the water-soluble essence and flavor is 0.25% to 50%; and the included compound comprises an alcohol-soluble essence and flavor for cigarettes and an alcohol solvent in a weight ratio of 100: 0 to 30: 70. The aroma sustained-release agent for cigarettes in the invention realizes coexistence of alcohol-soluble and water-soluble essence and flavor in a same system, and aroma substances can be uniformly and stably released when cigarettes are smoked.
Owner:CHINA TOBACCO YUNAN NEW MATERIAL
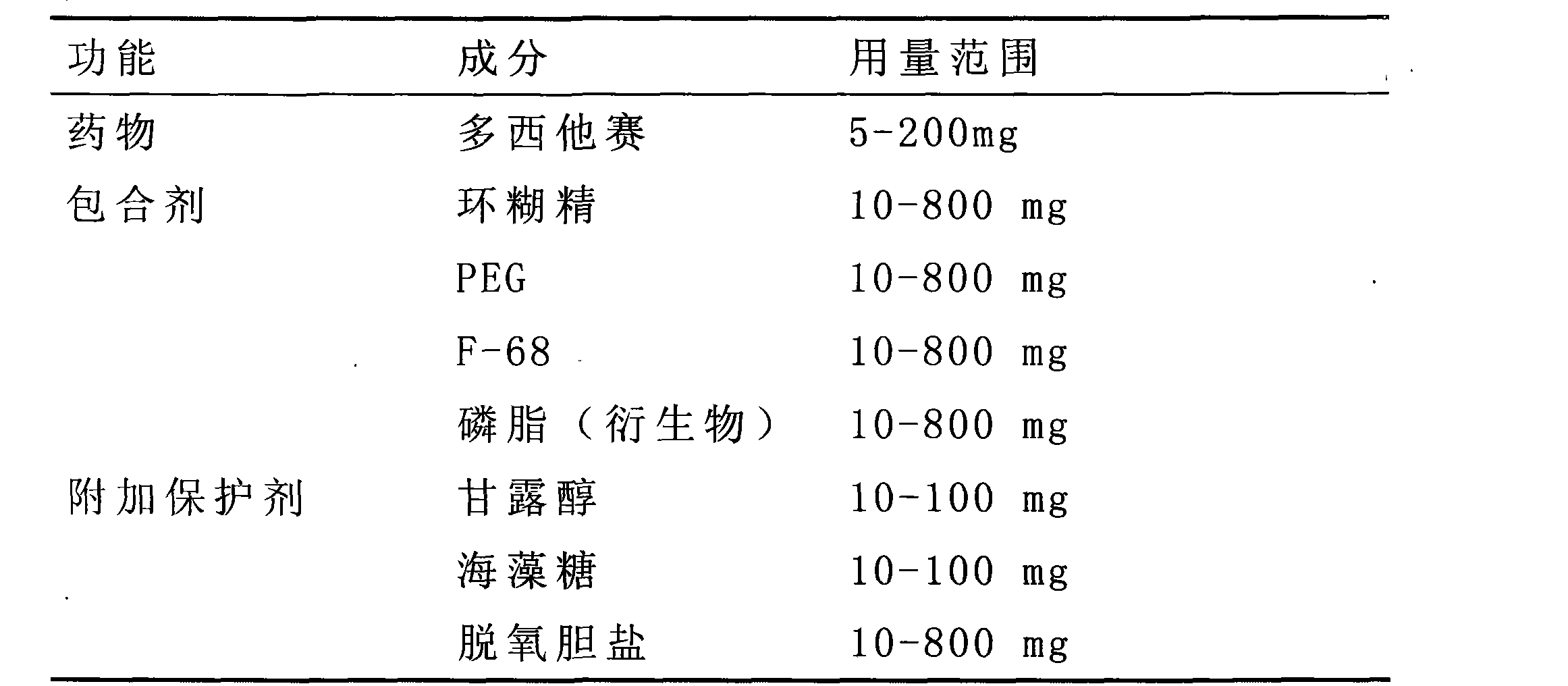
Method for preparing polymer aqueous solution formulation of taxane anti-tumor medicament
InactiveCN101439017AImprove complianceMedication convenienceOrganic active ingredientsPharmaceutical delivery mechanismDocetaxelMedicine
The invention relates to a developed injection preparation product. A preparation method for a polymer aqueous solution preparation for insoluble medicines of a taxanes antitumor medicine is used to prepare and produce a solution preparation product of the insoluble medicines, thereby the requirements for injection medicine are satisfied. The preparation method adopts technologies in multiple aspects such as surfactant solubilization, hydroxypropyl-beta-cyclodextrin inclusion and PEG cosolvent, PEG-modified phospholipid, additional protective agents, and the like. By adopting the technological method, the solubility of the insoluble medicines in aqueous solution can be dramatically enhanced such as paclitaxel and docetaxel, the dissolving ability of the insoluble medicines in the aqueous solution is enhanced from 2 mg / ml reported in literature to or exceeding 6 mg / ml.
Owner:石茂光
Cleaning solution for metal surface oil and rust removal
The invention relates to a cleaning solution for metal surface oil and rust removal and relates to the technical field of metal surface treatment. The cleaning solution is prepared from the following components in parts by weight: 75-85 parts of deionized water, 4-6 parts of sophocarpidine, 4-6 parts of alkaloid of tea leaves, 4-6 parts of tartaric acid, 4-6 parts of hydroxyacetic acid, 4-6 parts of urea, 4-6 parts of hydroxypropyl-beta-cyclodextrin, 4-6 parts of octadecanol, 4-6 parts of graphene and 4-6 parts of a modifier. The cleaning solution is prepared by the following steps: feeding the components together into a reaction container; and stirring for 30 minutes at 80-90 DEG C. The cleaning solution provided by the invention is low in cost, simple in preparation method and safe and environment-friendly and has a good cleaning effect to metal pieces with much greasy dirt and rusts, and the product quality can be guaranteed.
Owner:SHANDONG TOGET BRAKE SYST CO LTD
Voriconazole freeze-dried powder injection for injection and preparation method thereof
InactiveCN103251565AImprove solubilityImprove stabilityPowder deliveryOrganic active ingredientsFiltrationFreeze-drying
Belonging to the technical field of drug preparation, the invention relates to a voriconazole freeze-dried powder injection for injection and a preparation method thereof. The invention provides a pharmaceutical preparation that treats invasive fungal infections and has good solubility and high stability for clinical practice. According to a prescription, the voriconazole freeze-dried powder injection for injection provided in the invention is composed of voriconazole, hydroxypropyl-beta-cyclodextrin, mannitol, hydrochloric acid, sodium hydroxide, and water for injection. The preparation process includes: subjecting an aqueous solution of hydroxypropyl-beta-cyclodextrin and an acid solution of voriconazole to clathration, adding mannitol, then conducting stirring dissolving, using a sodium hydroxide solution to adjust the pH value, carrying out activated carbon adsorption, decarbonization, fine filtration by a microfiltration membrane and semi-finished product inspection, if the semi-finished product is qualified, performing subpackaging, freeze-drying, and conducting complete inspection. The cruxes of the preparation method are proportioning of voriconazole and hydroxypropyl-beta-cyclodextrin in the prescription, control of clathration temperature and time, charging sequence and other technical parameters. At the same time, the invention also provides a method for determination of the inclusion encapsulation efficiency.
Owner:ZHUHAI EBANG PHARMA +1
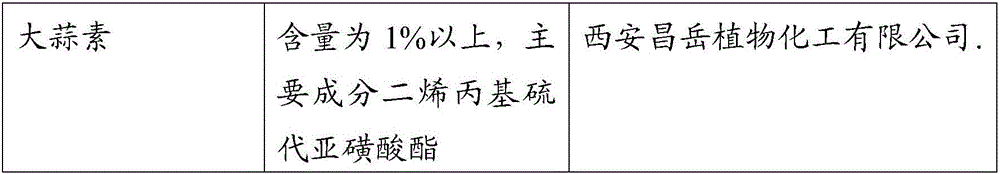
Allicin cyclodextrin clathrate compound, formulation and its preparation method
InactiveCN1565430ASolve the problem of water solubilityResolve irritationOrganic active ingredientsDigestive systemCurative effectWater soluble
The invention discloses a garlicin cyclodextrin clathrate compound, preparation and the process for preparing same, wherein the allicin, cyclodextrin or their derivatives (such as beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin) can be used for preparing garlicin cyclodextrin clathrate compound, which can be used for preparing various preparations of different oral preparation, suppositorium, spray, and injection.
Owner:毛友昌
A kind of gel patch containing curcumin and bletilla striata gum and preparation method thereof
ActiveCN102284012AGuaranteed stabilityLess irritatingSkeletal disorderKetone active ingredientsPolyvinyl alcoholAdhesive
The invention provides a gel plaster containing curcumin and bletilla hyacinthina gum, which comprises the following components in parts by weight: 0.2-0.5 part of curcumin, 1.0-2.5 parts of bletilla hyacinthina gum, 2.0-5.0 parts of polyacrylic acid, 0.4-1.4 parts of adhesive, 25.0-65.0 parts of humectant, 0.1-0.2 part of aluminum hydroxide, 0-5.0 parts of solid dispersion material, 0-1.5 parts of permeation promoter and 35.0-58.0 parts of water. The adhesive is selected from polyvinylpyrrolidone, hydroxypropyl methylcellulose, polyvinyl alcohol and the like; the humectant is selected from glycerol, propylene glycol, polyethylene glycol and the like; the solid dispersion material is selected from polyvinylpyrrolidone, mannitol, polyethylene glycol, poloxamer and the like; and the permeation promoter is selected from hydroxypropyl-beta-cyclodextrin, borneol, pennyroyal, olive oil and the like. The curcumin in the gel plaster can be transdermally absorbed and slowly and continuously released, and can stably be taken for a long time; and the bletilla hyacinthina gum can protect the skin, and reduce the irritation of the drugs to the skin.
Owner:山东福阳科技发展有限公司
Garlicin injection preparation
InactiveCN1456150AGood water solubilityNon-irritatingAntibacterial agentsAntimycoticsSpray dryingChemistry
A garlicin injection with greatly improved water solubility is prepared through adding hydroxypropyl beta-cyclodextrin in the water for injection, stirring, adding garlicin, stirring, grinding or ultrasonic pulverizing to obtain the garlicin inclusion compound solution, preparing injection or drying and preparing injection. Its advantages are high safety and stability and less irritation.
Owner:王景成
Tropisetron preparation for injection and preparation method thereof
InactiveCN101444508AFlat surfaceNot crackedDigestive systemMacromolecular non-active ingredientsMANNITOL/SORBITOLActive component
The invention provides a tropisetron preparation for injection. The main active components of the tropisetron preparation are tropisetron, beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin and mannitol. The method comprises the following steps: dissolving tropisetron hydrochloride and the beta-cyclodextrin or the hydroxypropyl-beta-cyclodextrin, then adding the mannitol for dissolving, adjusting the pH value with citric acid-disodium hydrogen phosphate buffer solution to obtain a liquid medicine, quickly prefreezing the liquid medicine after filling, and then lyophilizing to obtain the tropisetron preparation. The tropisetron preparation for injection has flat surface, is fine and smooth and uniform, and is free from crack, breakage and sticking to bottles. The tropisetron preparation is a white loose block which is well formed and very easily dissolved, has good redissolution performance, clean and transparent solution, stable product quality, and practicability.
Owner:海南瑞基药物研究有限公司
Nano particles of taxane cyclodextrin inclusion compound and preparation method thereof
ActiveCN1879612AOrganic active ingredientsMacromolecular non-active ingredientsMedicinePharmaceutical formulation
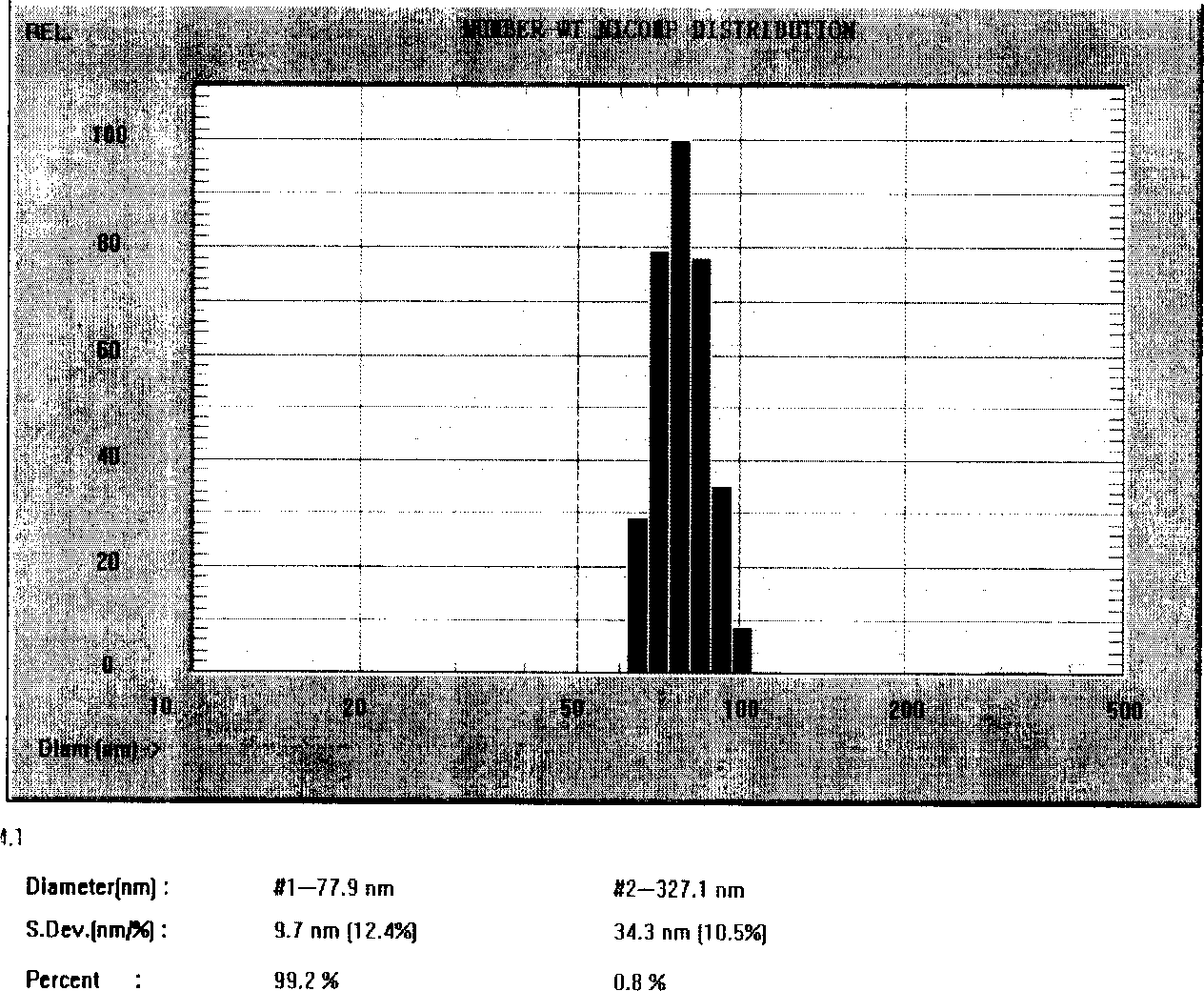
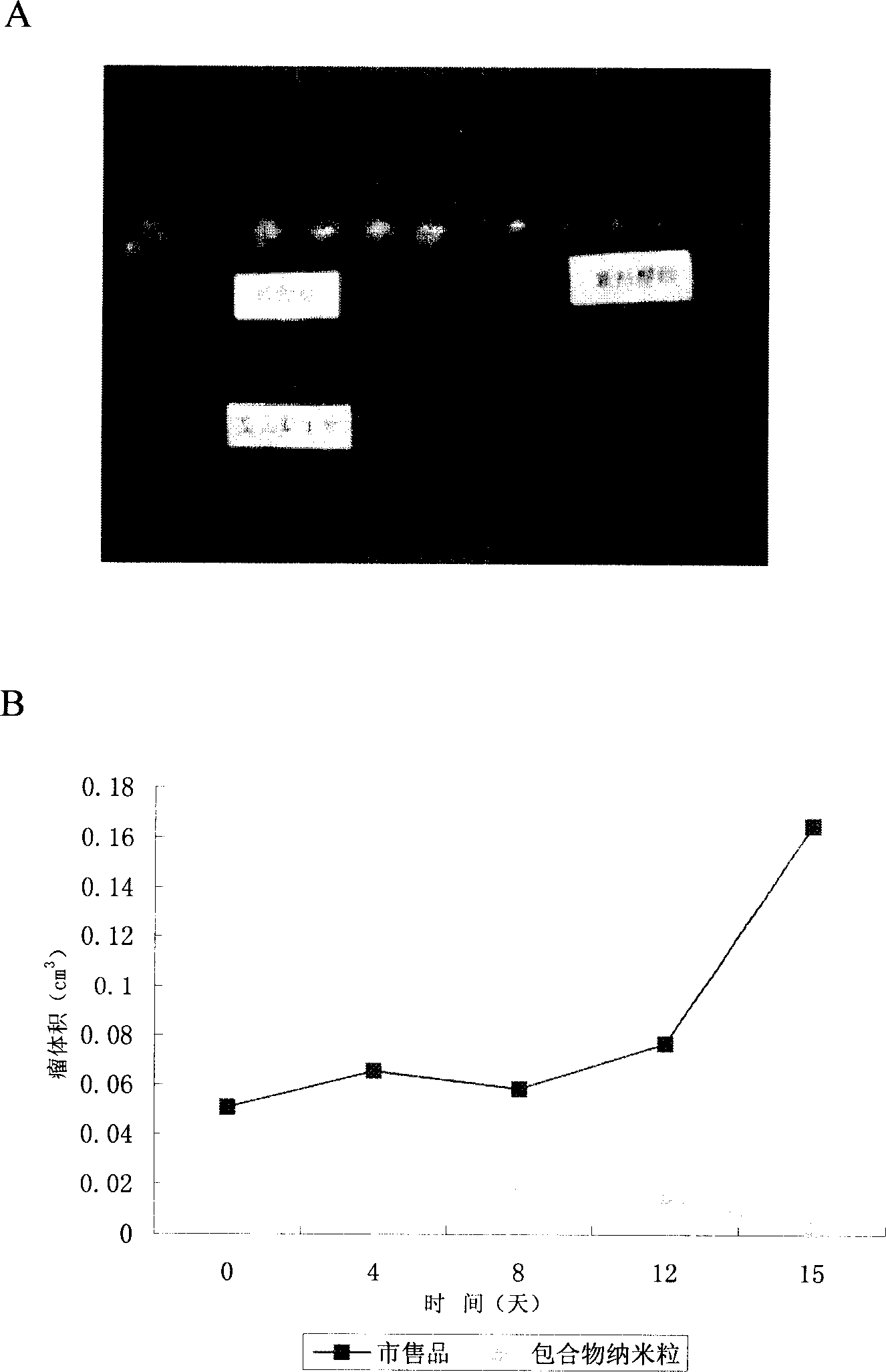
The invention relates to a Paclitaxel cyclodextrin clathrate compound nanometer grain and relative medicine agent, wherein it uses hydroxypropyl-beta-cyclodextrin as carrier and PVP as stabilizer to prepare Paclitaxel clathrate compound nanometer grain, whose diameter is 40-200nm, to improve the dissolvability of Paclitaxel as 100-50times. The animal experiment has proven that: compared with present Paclitaxel, its resistant agent is improved 2.67tims with better cancer resistant effect. The invention uses general material and simple condition, with stable quality and the application to be processed into oral agent.
Owner:SHANGHAI ALLIST PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com