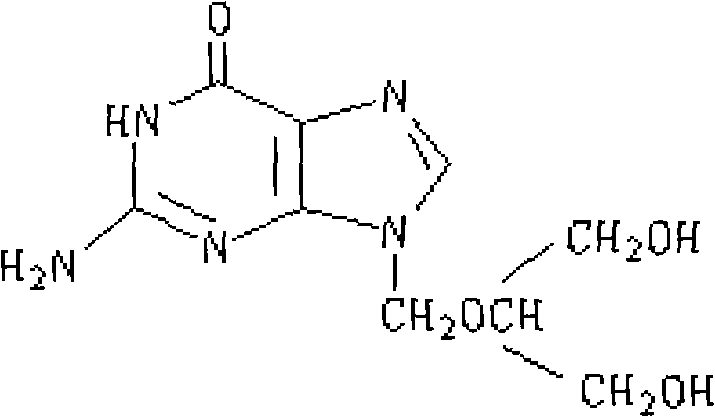
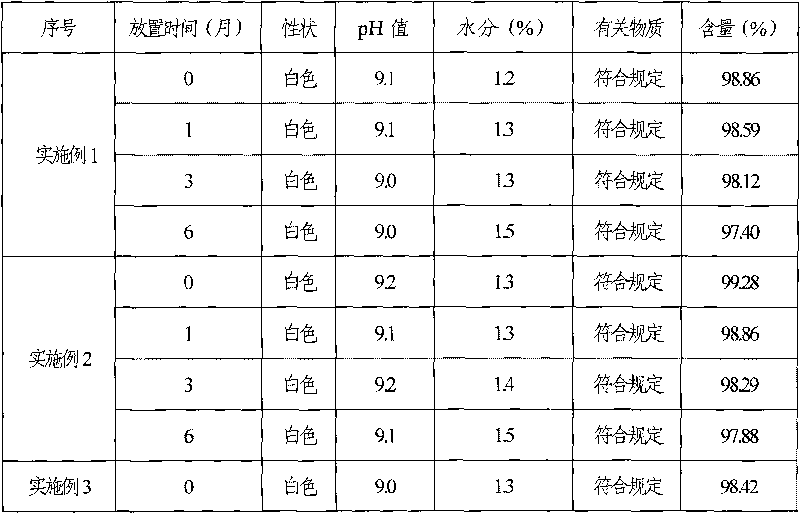
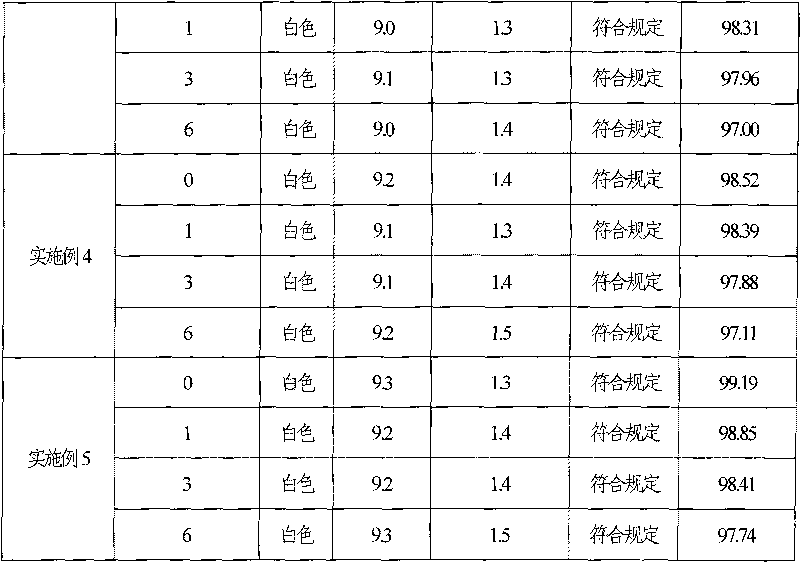
Ganciclovir freeze-dry preparation for injection and preparation method thereof
A technology for ganciclovir and freeze-dried preparations, which is applied in the field of ganciclovir freeze-dried preparations for injection and its preparation, can solve the problems of low solubility of ganciclovir, unstable product quality, and high production costs, and achieve The effects of shortening freeze-drying time, increasing hardware investment, and increasing production capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]The ganciclovir freeze-dried preparation for injection of the present invention is prepared from the following components: 50 g of ganciclovir; 100 g of hydroxypropyl-β-cyclodextrin; and 2600 ml of water for injection.
[0045] Its preparation method is as follows:
[0046] 1) Weigh the prescribed amount of hydroxypropyl-β-cyclodextrin, add about 1300ml of water for injection, stir until completely dissolved, add activated carbon with a prepared volume of 0.2%, stir for 30 minutes, filter and decarbonize to obtain the filtrate ①.
[0047] 2) Measure about 1000ml of water for injection, adjust the pH value to 9.0-9.5, add the prescribed amount of ganciclovir, stir well, add the filtrate ①stir quickly to dissolve it completely, add water for injection to the full amount, and adjust the pH value to 8.5 ~9.5, filtered through a sterilizing filter to the storage tank, and after the semi-finished product is qualified, it is divided into 10ml control bottles according to 2.6ml ...
Embodiment 2
[0055] The ganciclovir freeze-dried preparation for injection of the present invention is prepared from the following components: 250 g of ganciclovir; 200 g of hydroxypropyl-β-cyclodextrin; and 2600 ml of water for injection.
[0056] Its preparation method is as follows:
[0057] 1) Weigh the prescribed amount of hydroxypropyl-β-cyclodextrin, add about 1300ml of water for injection, stir until completely dissolved, add activated carbon with a prepared volume of 0.2%, stir for 30 minutes, filter and decarbonize to obtain the filtrate ①.
[0058] 2) Measure about 1000ml of water for injection, adjust the pH value to 9.0-9.5, add the prescribed amount of ganciclovir, stir well, add the filtrate ①stir quickly to dissolve it completely, add water for injection to the full amount, and adjust the pH value to 8.5 ~9.5, filtered through a sterilizing filter to the storage tank, and after the semi-finished product is qualified, it is divided into 10ml control bottles according to 2.6m...
Embodiment 3
[0066] The ganciclovir freeze-dried preparation for injection of the present invention is prepared from the following components: 50 g of ganciclovir; 100 g of hydroxypropyl-β-cyclodextrin; and 2600 ml of water for injection.
[0067] Its preparation method is as follows:
[0068] 1) Weigh the prescribed amount of hydroxypropyl-β-cyclodextrin, add about 1300ml of water for injection, stir until completely dissolved, add activated carbon with a prepared volume of 0.2%, stir for 30 minutes, filter and decarbonize to obtain the filtrate ①.
[0069] 2) Measure about 1000ml of water for injection, adjust the pH value to 9.0-9.5, add the prescribed amount of ganciclovir, stir well, add the filtrate ①stir quickly to dissolve it completely, add water for injection to the full amount, and adjust the pH value to 8.5 ~9.5, filtered through a sterilizing filter to the storage tank, and after the semi-finished product is qualified, it is divided into 10ml control bottles according to 2.6ml...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com