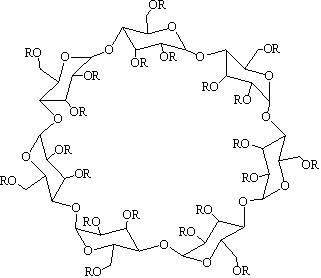
Method for preparing hydroxypropyl-beta-cyclodextrin
A cyclodextrin and hydroxypropyl technology, applied in the field of medicine, can solve the problems of inappropriate use of pharmaceutical excipients, difficult control of drug quality, increase in impurity content, etc., and achieve a narrow range of product substitution, shorten drying time, batch effects with small differences in properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Etherification reaction: In a 5000mL round bottom flask, add 2160mL of water for injection and 216g of sodium hydroxide, stir to dissolve, cool down to 5-10°C, add 681g of β-cyclodextrin (after drying), and stir until the solid is completely After dissolving, slowly add 315mL of 1,2-propylene oxide dropwise. During the dropping process, the temperature is controlled at 5-8°C, and the dropping time is 3.5 hours. After the dropwise addition is completed, continue to stir at 5-10°C for 3 hours. ;
[0026] (2) Neutralization: Add concentrated hydrochloric acid dropwise to the reaction solution obtained in step (1) to adjust the pH value to 7.1, and the obtained solution is set aside;
[0027] (3) Decolorization: Add 20 g of activated carbon to the solution obtained in step (2), control the temperature at 20°C to 25°C, stir for 30 minutes, filter to remove carbon, and use the filtrate for later use;
[0028] (4) Desalting and purification: perform nanofiltration on the ...
Embodiment 2
[0032] (1) Etherification reaction: In a 5000mL round bottom flask, add 1800mL of water for injection, 216g of sodium hydroxide, stir to dissolve, cool down to 5-10°C, add 681g of β-cyclodextrin (after drying), and stir until the solid is completely After dissolving, slowly add 336mL of 1,2-propylene oxide dropwise. During the dropping process, the temperature is controlled at 8-12°C, and the dropping time is 3.5 hours. After the dropwise addition is completed, continue to stir at 5-10°C for 3 hours. The obtained reaction solution is used for later use. ;
[0033] (2) Neutralization: Add concentrated hydrochloric acid dropwise to the reaction solution obtained in step (1) to adjust the pH value to 7.2, and the obtained solution is set aside;
[0034] (3) Decolorization: Add 20 g of activated carbon to the solution obtained in step (2), control the temperature at 20°C to 25°C, stir for 30 minutes, filter to remove carbon, and use the filtrate for later use;
[0035] (4) Desalt...
Embodiment 3
[0039](1) Etherification reaction: In a 5000mL round bottom flask, add 2160mL of water for injection and 216g of sodium hydroxide, stir to dissolve, cool down to 5-10°C, add 681g of β-cyclodextrin (after drying), and stir until the solid is completely After dissolving, slowly add 357mL of 1,2-propylene oxide dropwise. During the dropping process, the temperature is controlled at 8-12°C, and the dropping time is 3.5 hours. After the dropwise addition is completed, continue to stir at 5-10°C for 3 hours. The obtained reaction solution is used for later use. ;
[0040] (2) Neutralization: Add concentrated hydrochloric acid dropwise to the reaction solution obtained in step (1) to adjust the pH value to 7.2, and the obtained solution is set aside;
[0041] (3) Decolorization: Add 20 g of activated carbon to the solution obtained in step (2), control the temperature at 20°C to 25°C, stir for 30 minutes, filter to remove carbon, and use the filtrate for later use;
[0042] (4) Desa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com