Chemical synthesis method of valnemulin hydrochloride
A technology of vonimulin hydrochloride and chemical synthesis, applied in the directions of organic chemistry, thioether preparation, etc., can solve the problems of difficult recovery, high price of palladium carbon, increase cost, etc., and achieve the effect of easy reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
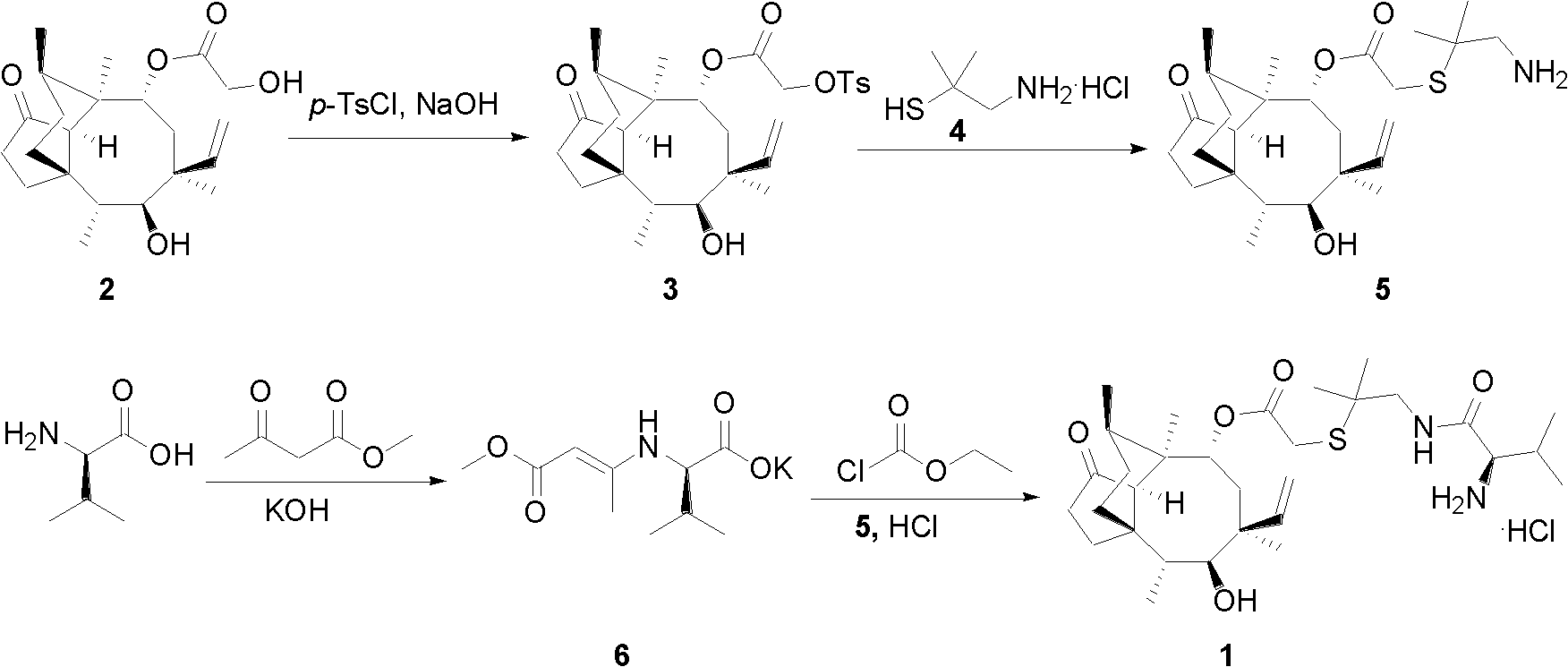
[0019] A kind of chemical synthesis method of warnemulin hydrochloride, the steps are as follows:
[0020] (1) Refining of pleuromutilin
[0021] First, dissolve 250 g of pleuromutilin in 2000 mL of methyl tert-butyl ether, and stir to dissolve. Then add 50 g of activated carbon, stir at room temperature for 3 hours to decolorize, filter out the activated carbon, and when the filtrate is heated and concentrated to about 500 mL, a large amount of crystals precipitate out, stop heating, cool to room temperature, and then continue to stir for 4 hours. After the crystals were centrifuged, they were dried to obtain 220 g of fine pleuromutilin (yield 88%).
[0022] (2) Synthesis of p-toluenesulfonyl pleuromutilin (3)
[0023] Dissolve 20 g (0.105 mol) of p-toluenesulfonyl chloride in 300 mL of methyl tert-butyl ether, stir to dissolve, then add 40 g (0.105 mol) of pleuromutilin fine product prepared in step (1), and add dropwise 30% NaOH The solution was 15 mL (0.11 mol), and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com