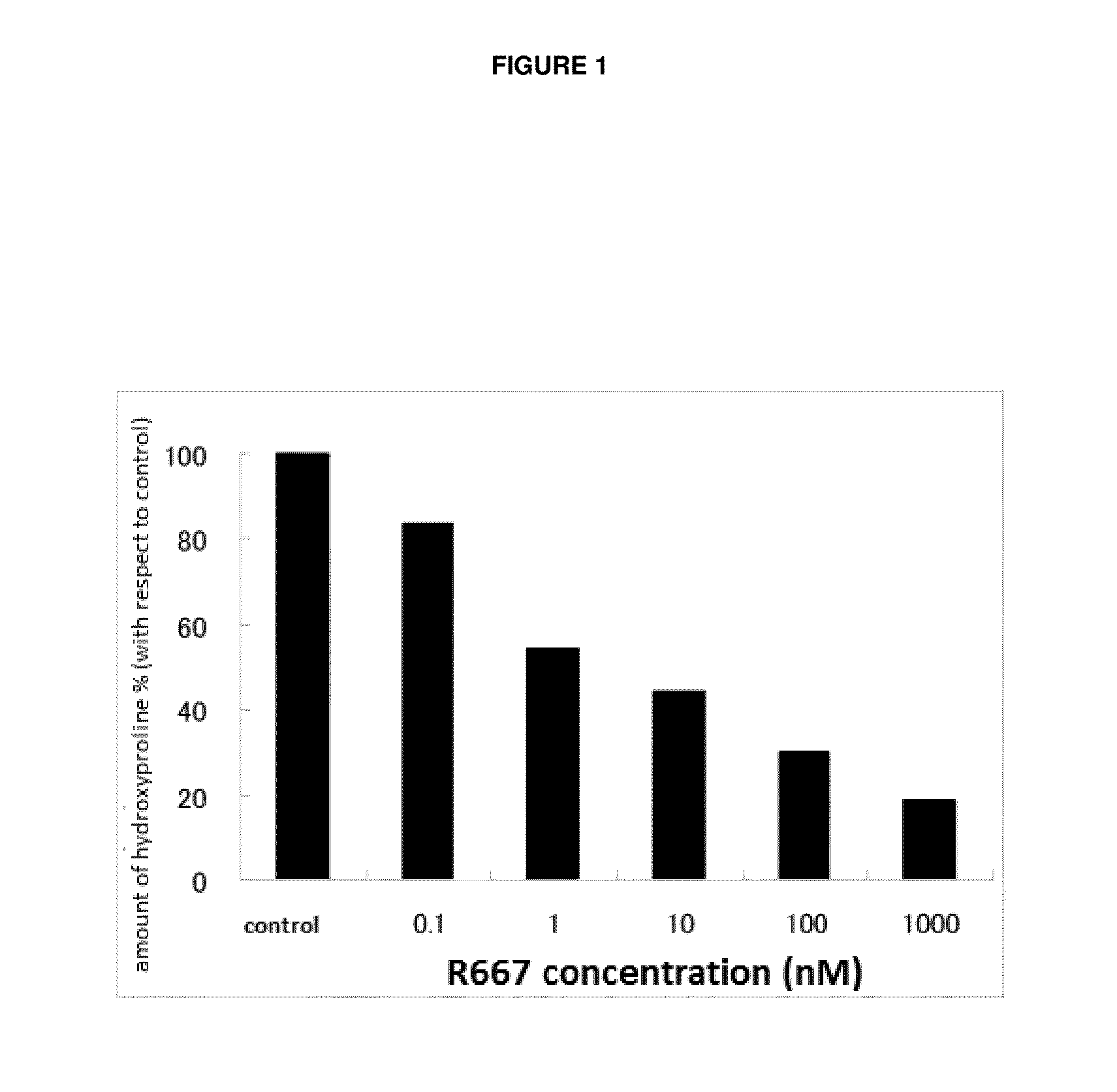
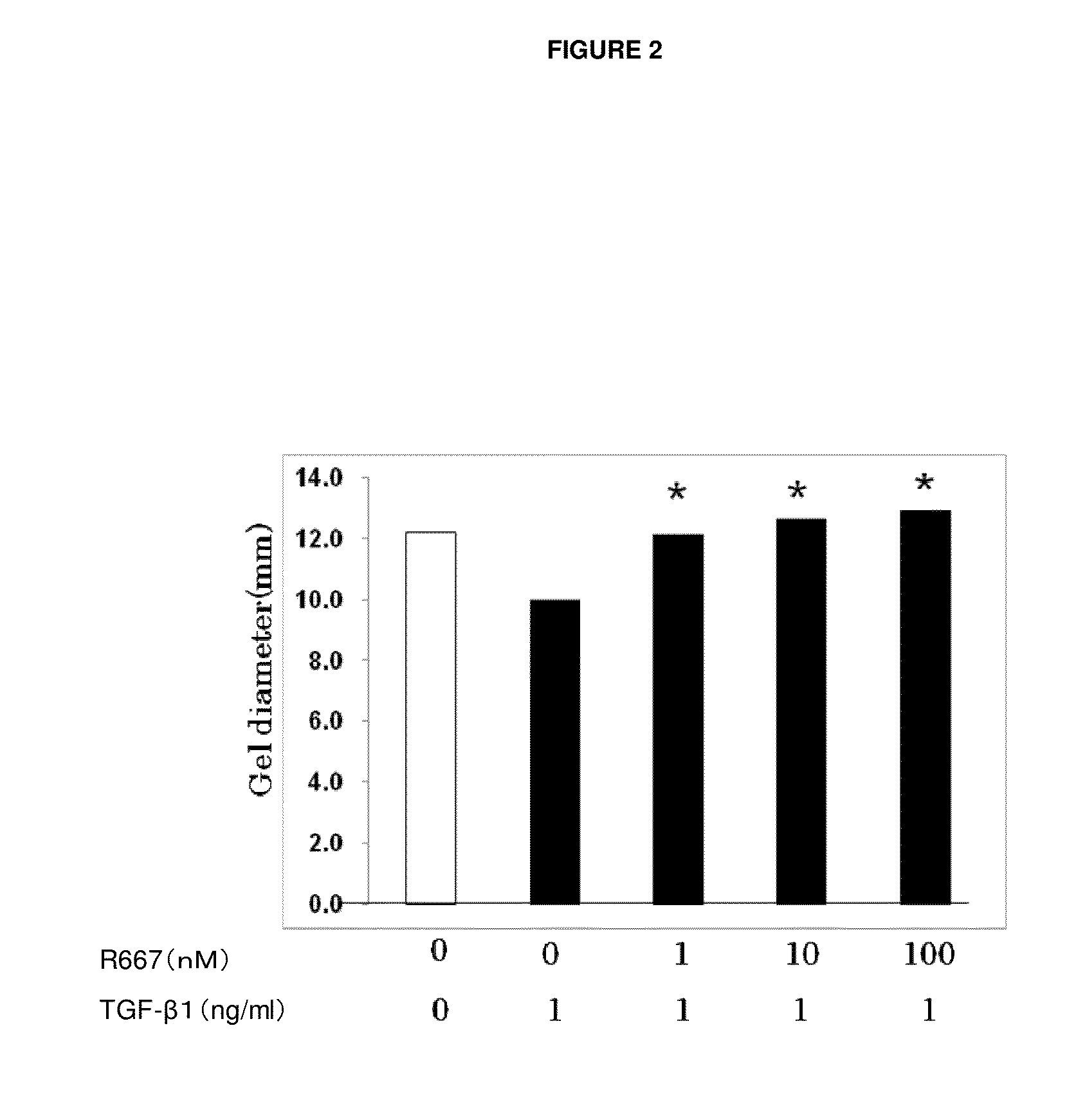
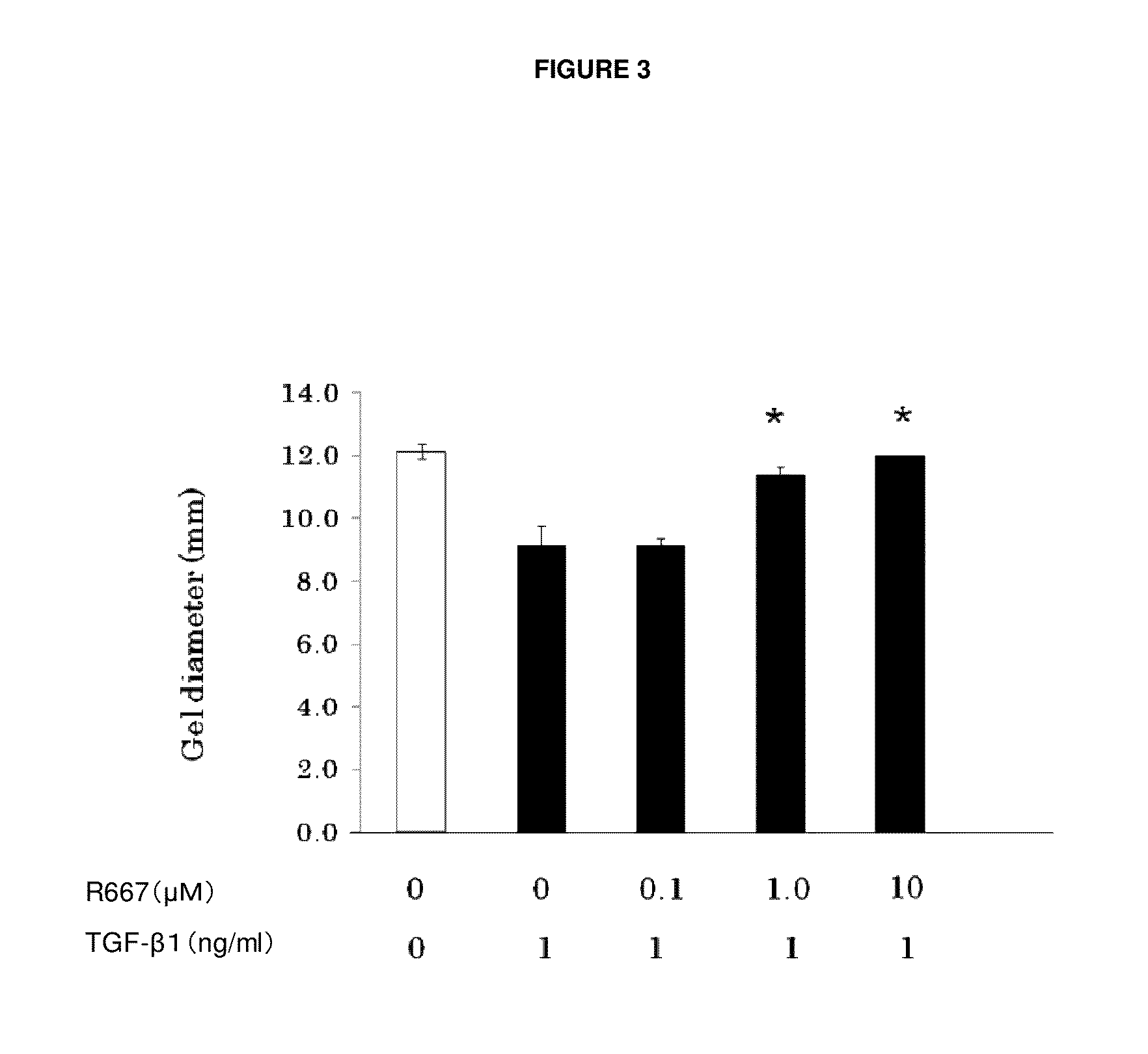
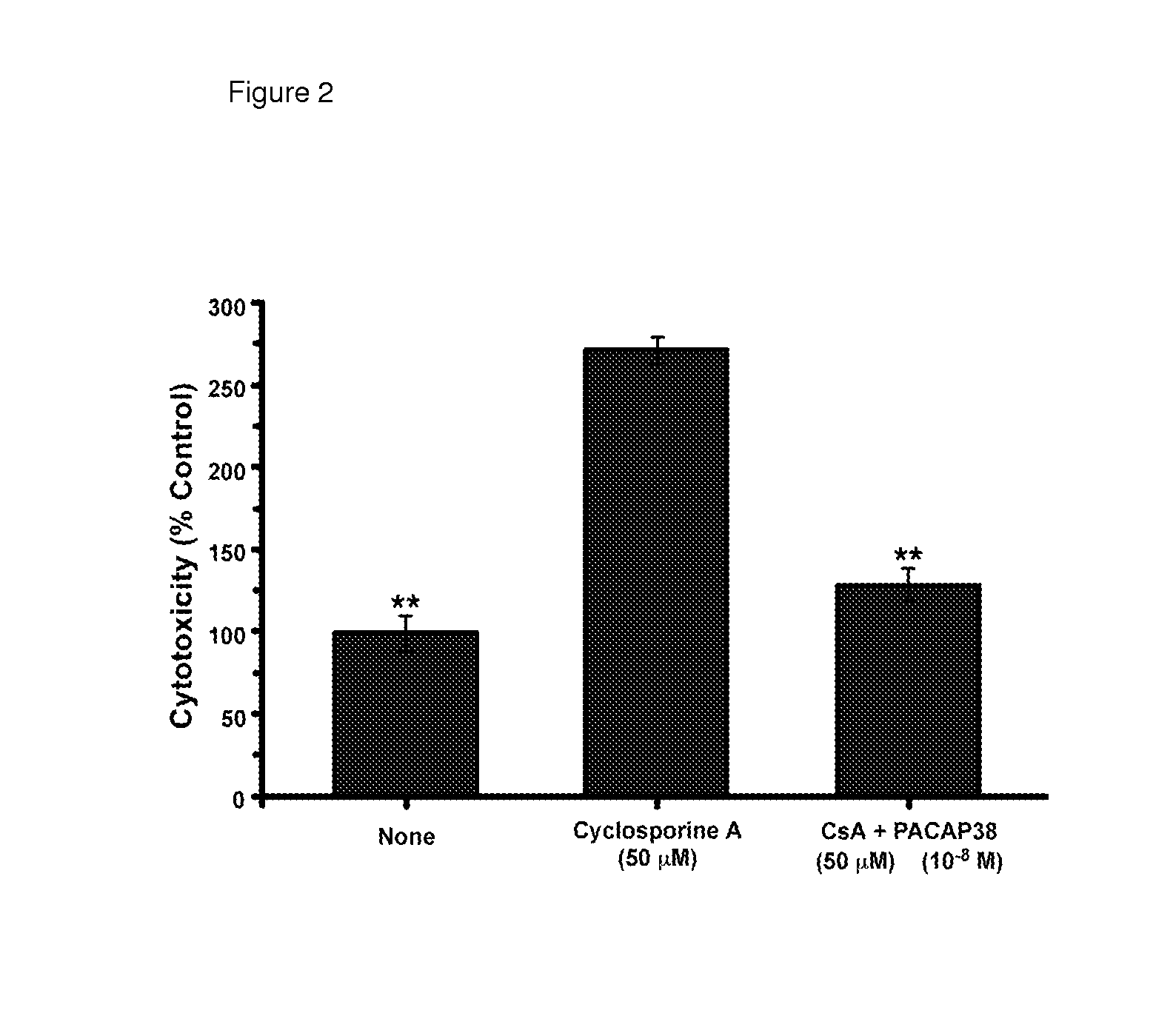
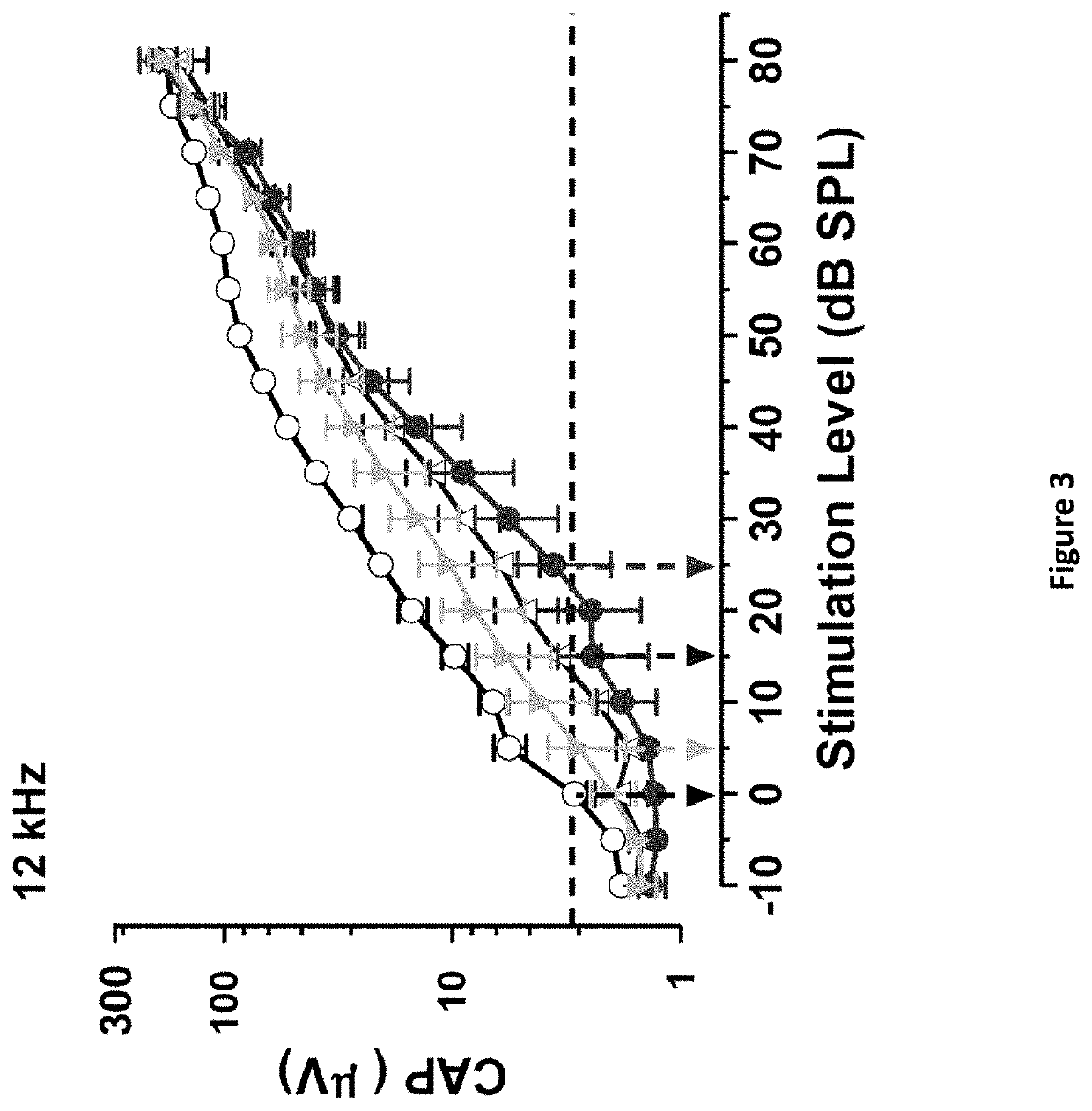
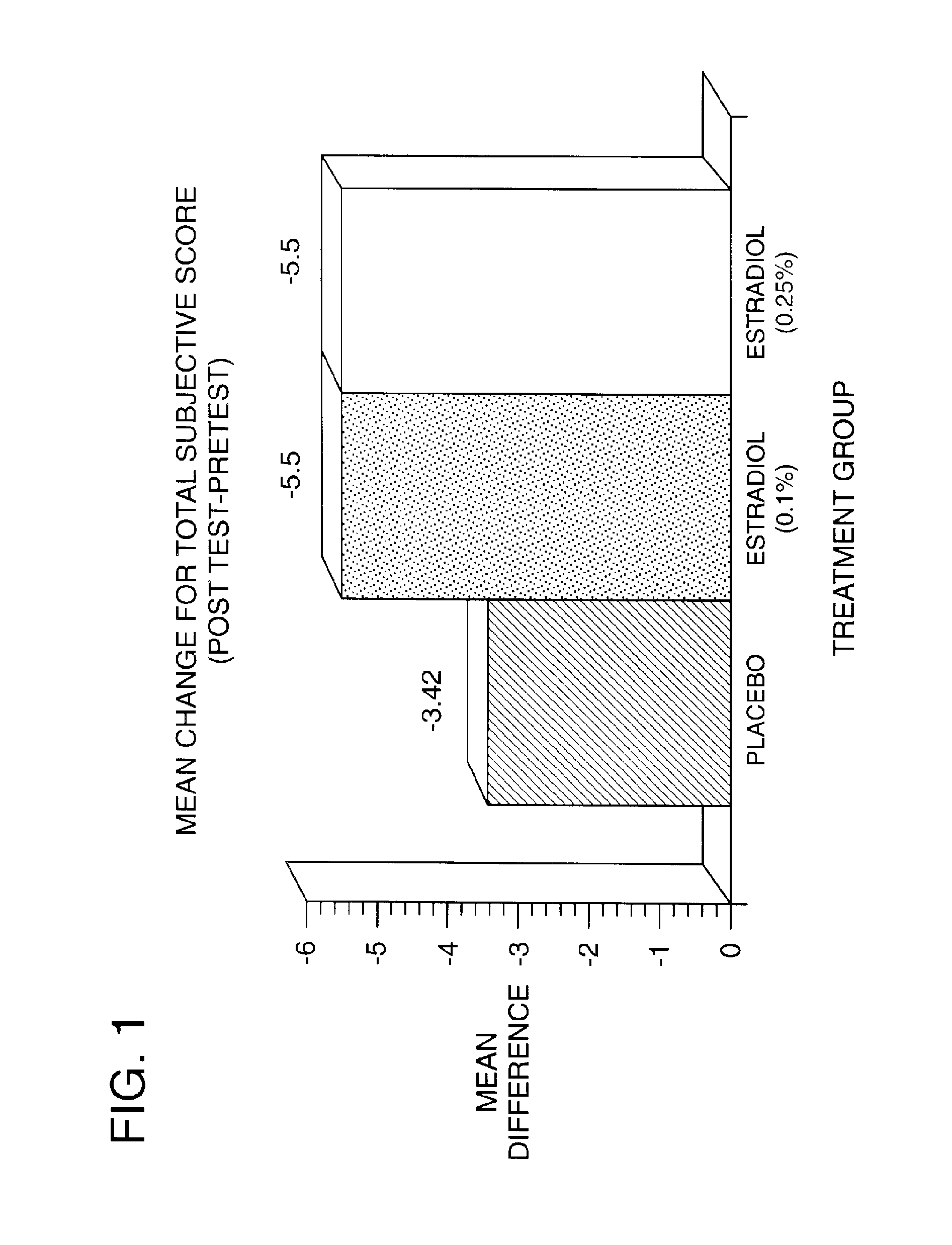
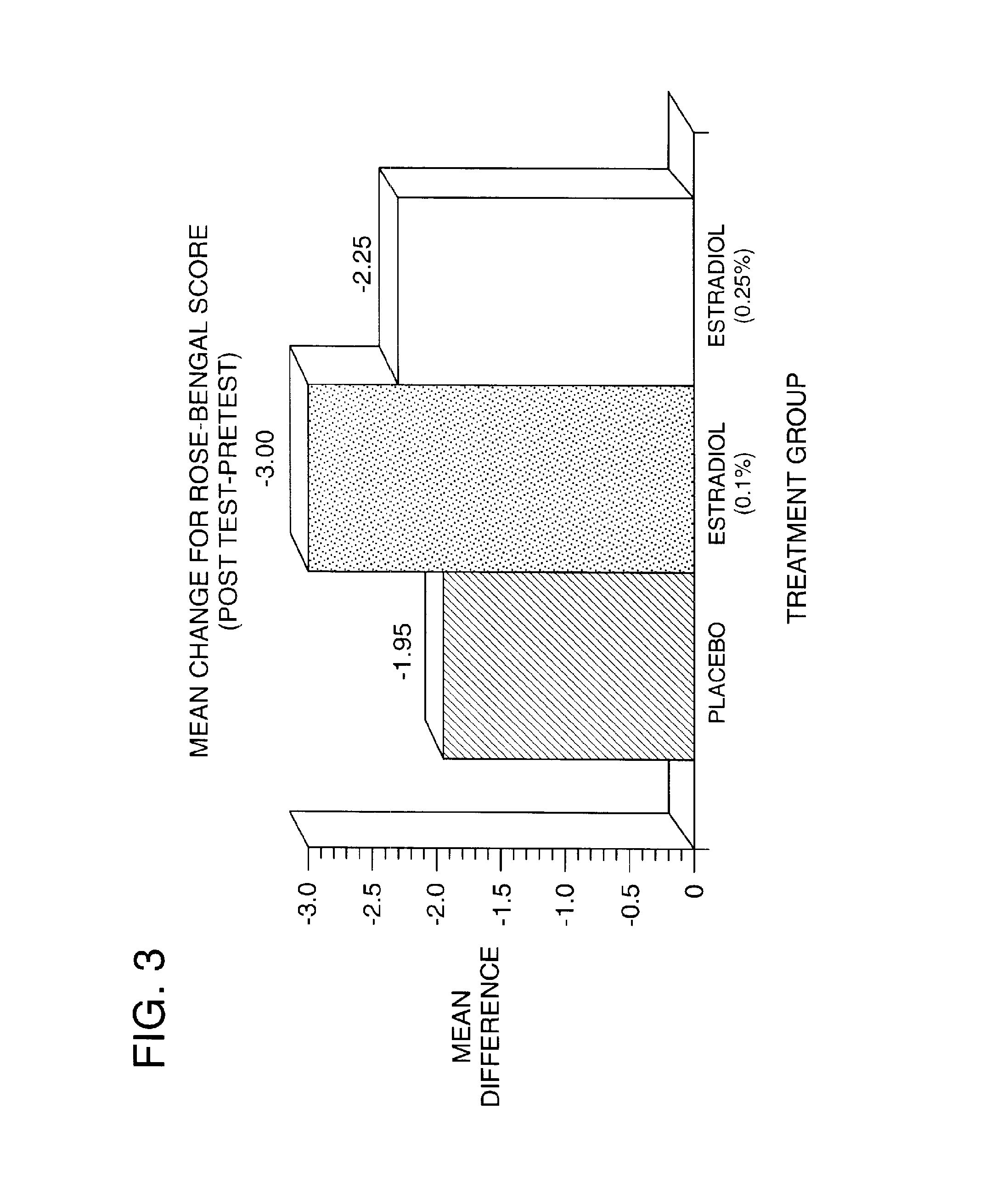
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "KCS - Keratoconjunctivitis sicca" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Keratoconjunctivitis sicca (KCS) is a condition that is also commonly referred to as "dry eye.". The medical term means inflammation of the cornea and surrounding tissues from drying. It is a common eye condition resulting from inadequate production of the aqueous portion of the tear film by the lacrimal gland and/or gland of the third eyelid gland.
Therapeutic agent for keratoconjunctival disorder
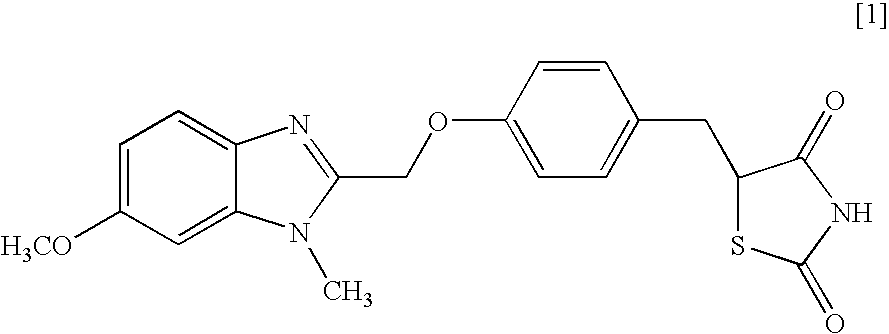
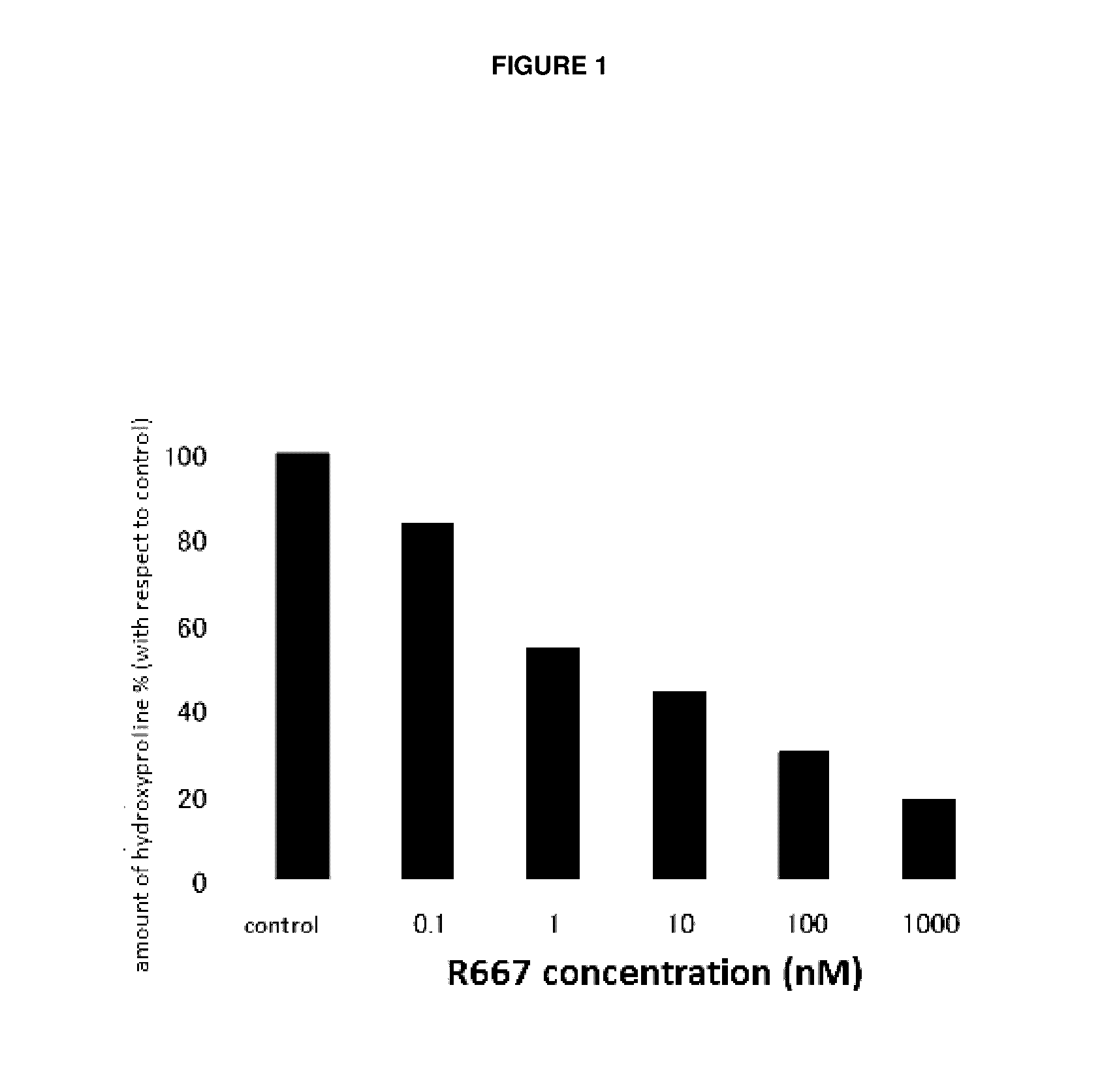
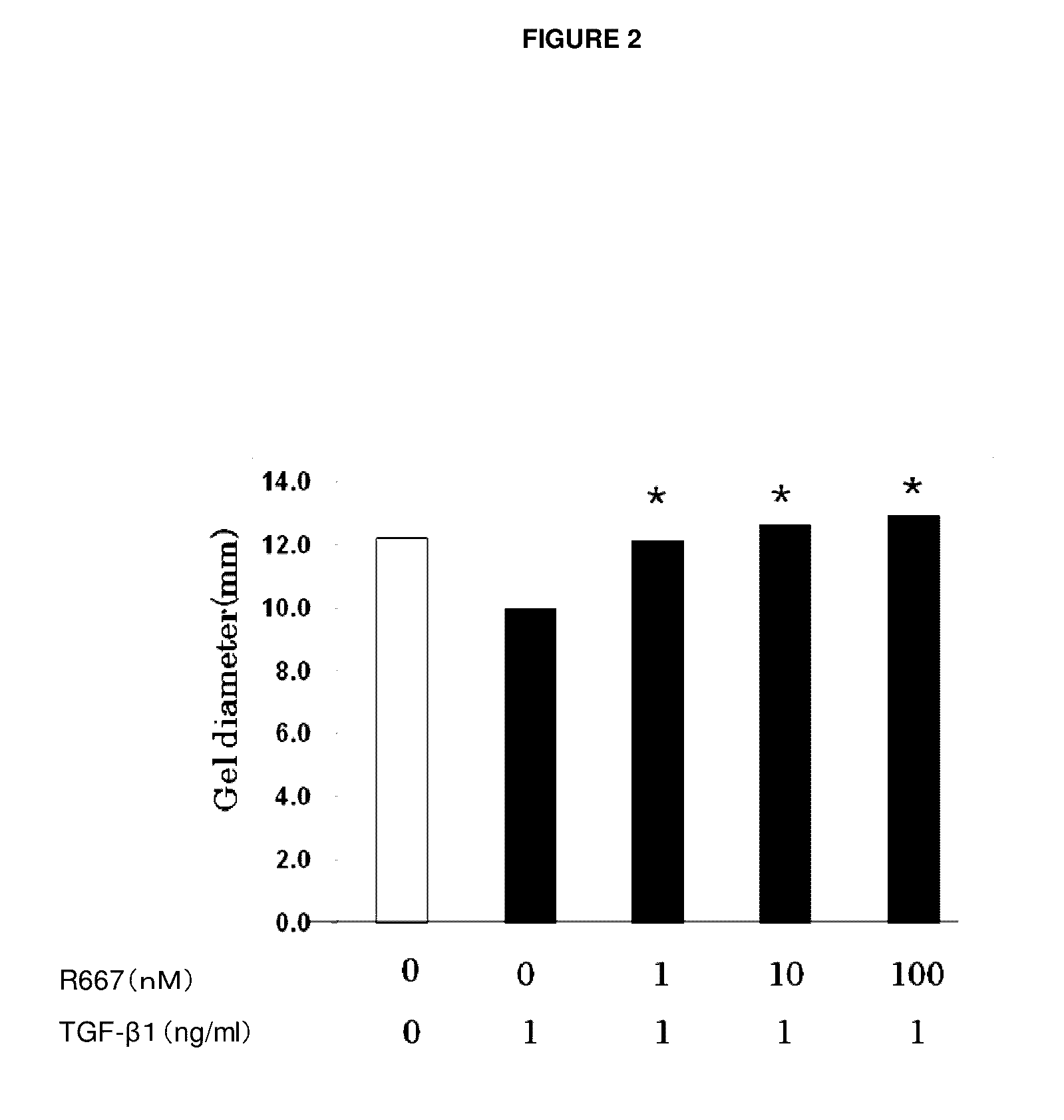
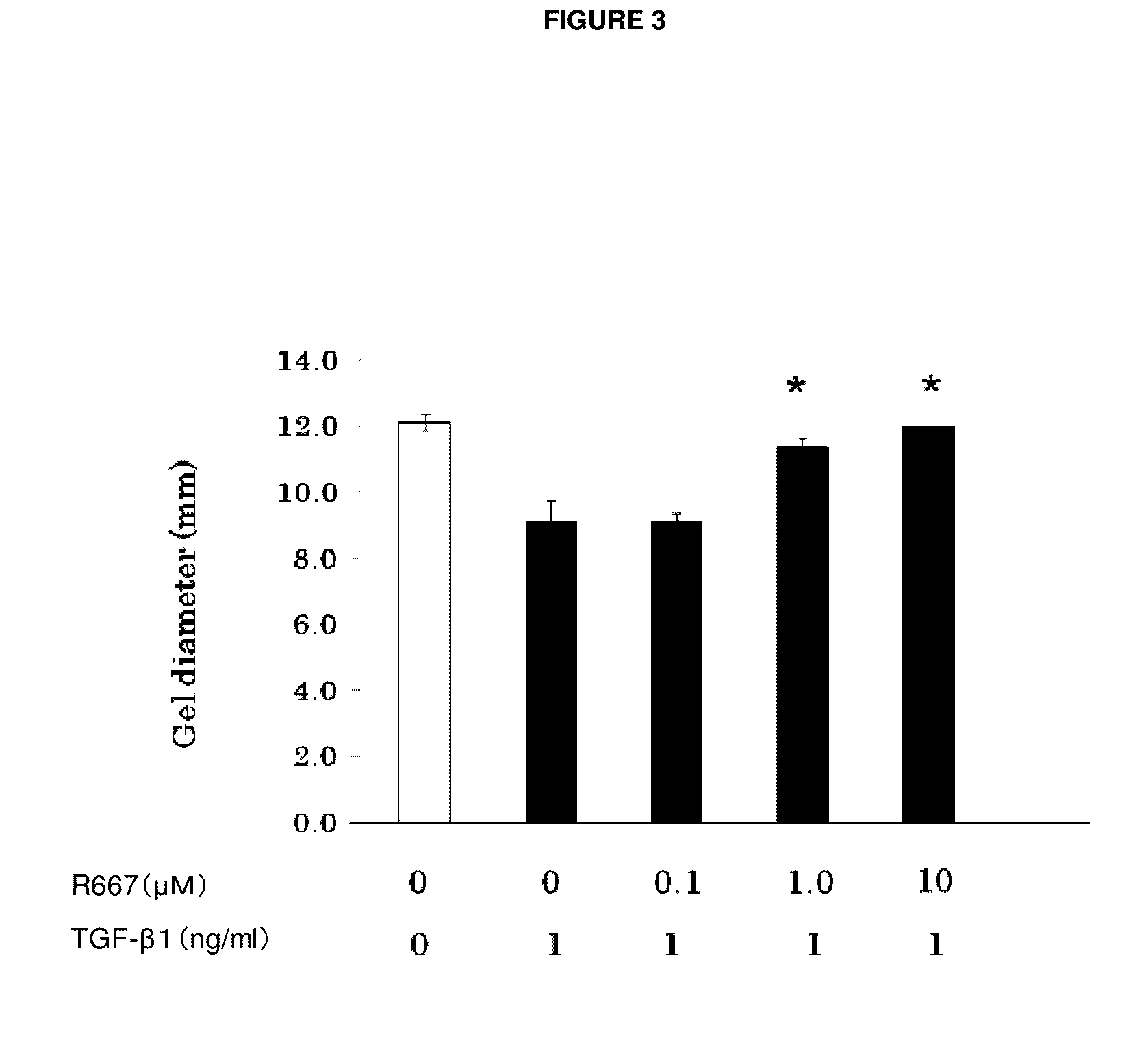
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Pharmaceutical composition for treatment of dry eye syndrome
ActiveUS8614178B2Efficient deliveryBetter tolerableSenses disorderPeptide/protein ingredientsAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel pharmaceutical compositions for the treatment of keratoconjunctivitis sicca comprising liquid vehicles which include one or more semifluorinated alkanes. The compositions incorporate an active ingredient selected from the group of macrolide immunosuppressants. They can be administered topically into the eye. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Compositions comprising mixtures of semifluorinated alkanes
ActiveUS20150224064A1Effective timeTune viscosityBiocideHalogenated hydrocarbon active ingredientsAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel compositions comprising at least two or more semifluorinated alkanes. The compositions can be used as medicines that are topically administered to an eye or ophthalmic tissue, such as for use in the treatment of keratoconjunctivitis sicca (dry eye) and / or meibomian gland dysfunction and symptoms associated therewith. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Artificial tear formulation
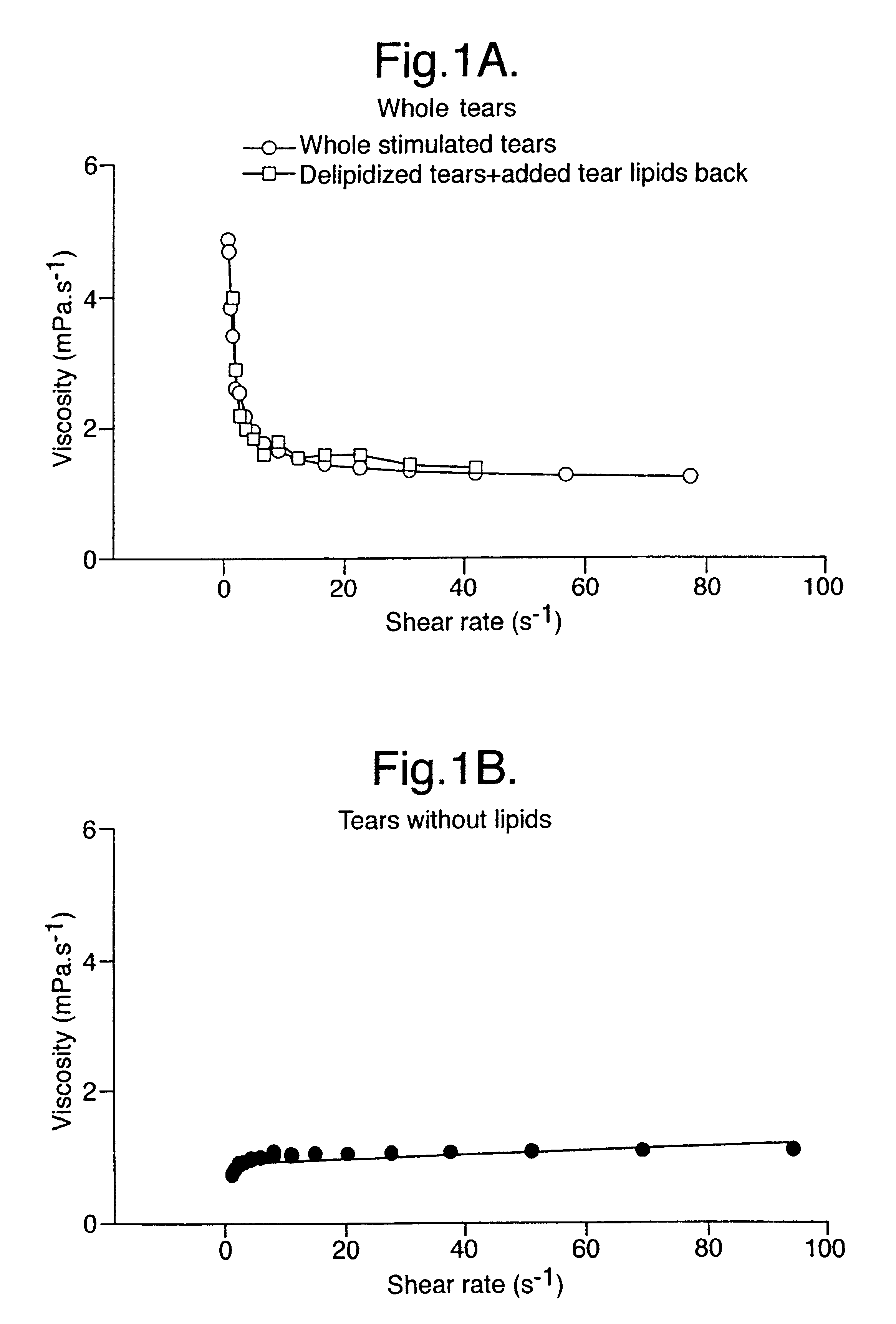
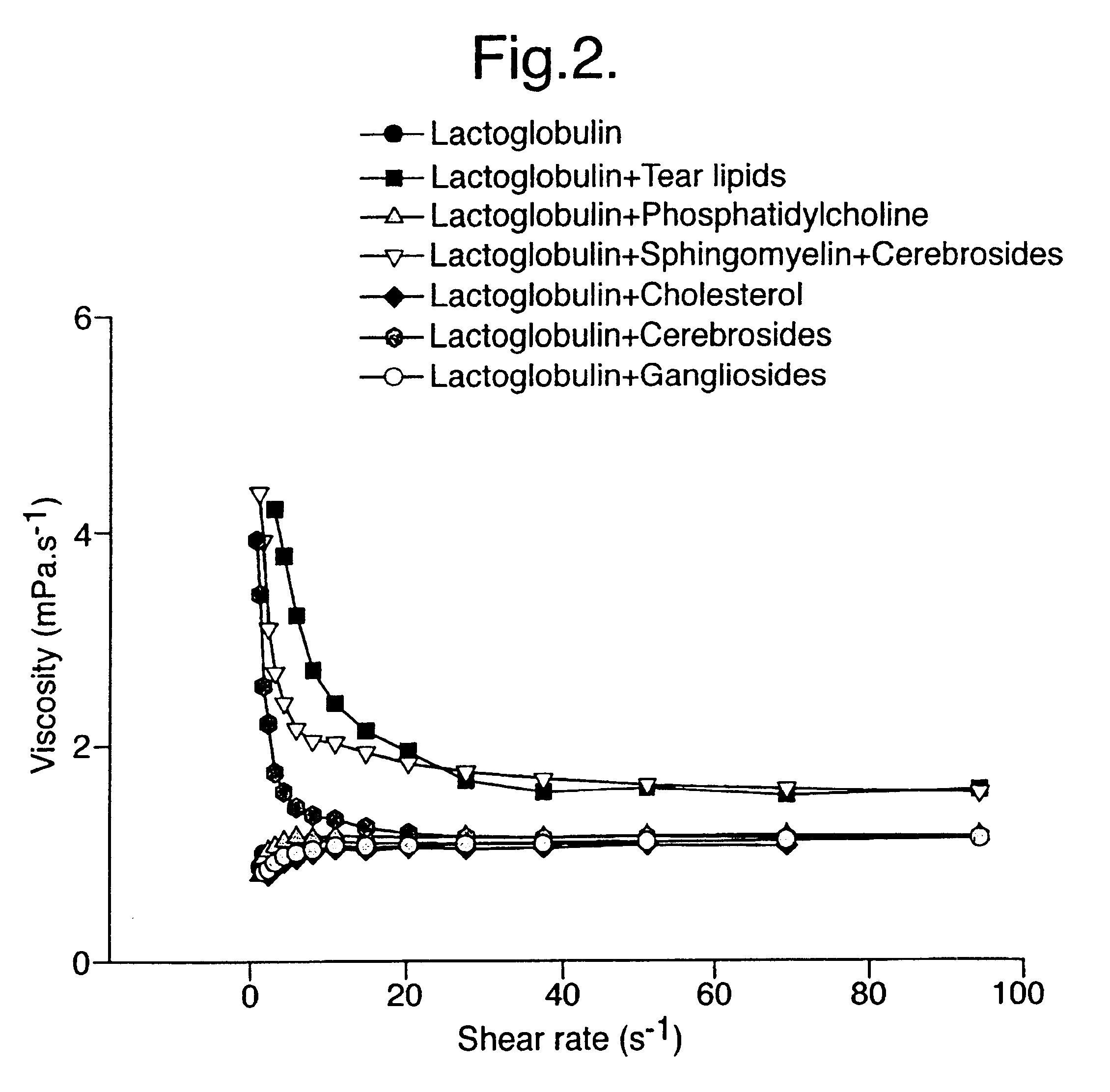
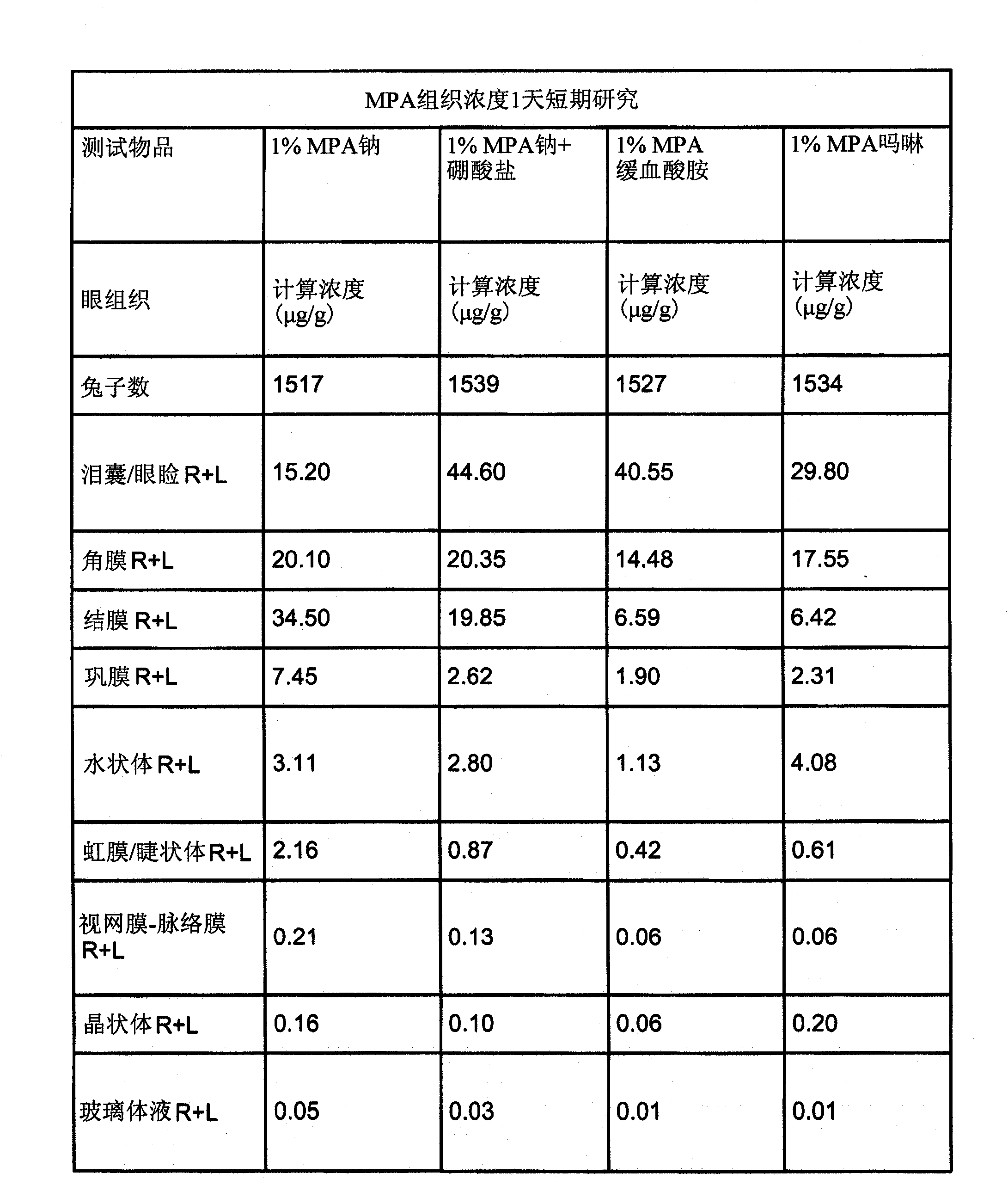
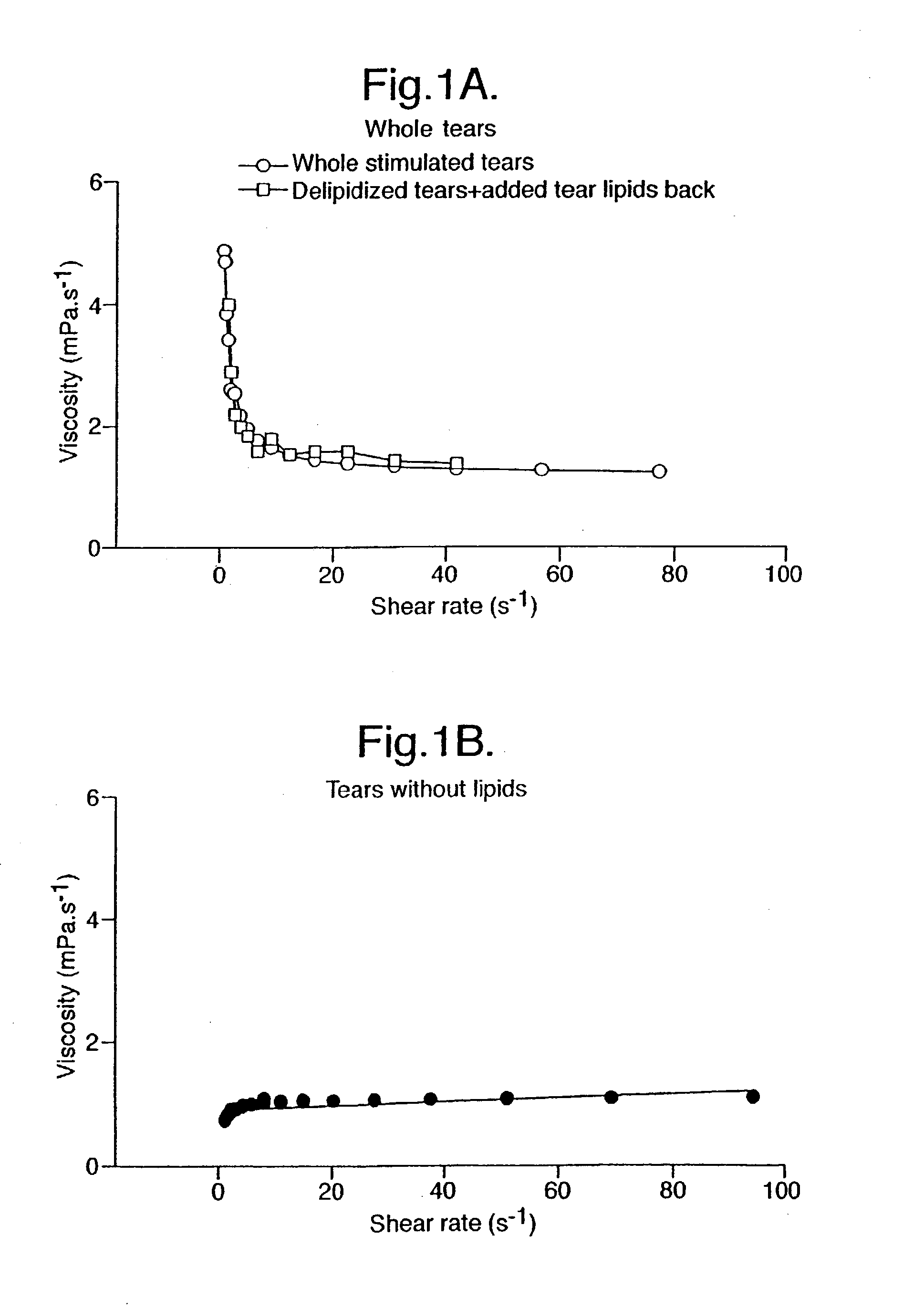
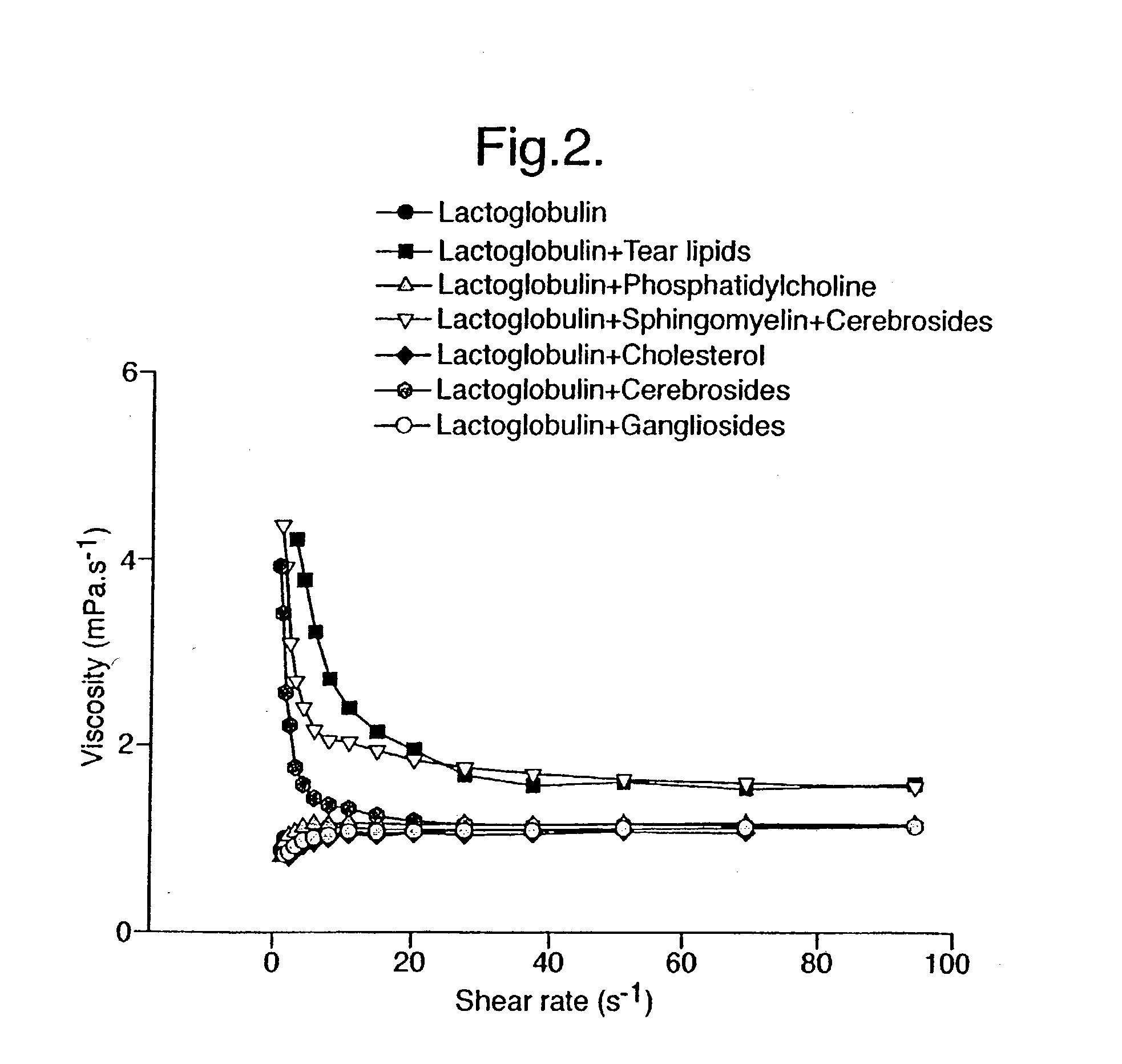
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
Pharmaceutical composition for treatment of dry eye syndrome
ActiveUS20120244177A1Efficient deliveryBetter tolerableSenses disorderAntipyreticAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel pharmaceutical compositions for the treatment of keratoconjunctivitis sicca comprising liquid vehicles which include one or more semifluorinated alkanes. The compositions incorporate an active ingredient selected from the group of macrolide immunosuppressants. They can be administered topically into the eye. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Method And Composition For The Treatment Of Moderate To Severe Keratoconjunctivitis Sicca
InactiveUS20110300097A1Ensuring structureEnsure stabilityBiocideSenses disorderKERATOCONJUNCTIVITIS SICCAKCS - Keratoconjunctivitis sicca
The present invention relates to topical ophthalmic compositions for treating or preventing epithelial lesions or ophthalmic disorders, including dry eye or keratoconjunctivitis sicca.
Owner:AL QAHTANI AHMED H
Method and composition for the treatment of moderate to severe keratoconjunctivitis sicca
InactiveUS20120141410A1Slow changeProtect drySenses disorderPeptide/protein ingredientsKCS - Keratoconjunctivitis siccaModerate to severe
Embodiments of the invention relate to compositions and methods of dry eye or keratoconjunctivitis sicca.
Owner:INVITRX
Therapeutic agent for keratoconjunctive disorders
ActiveUS20150290172A1Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyDisease
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
USE OF PITUITARY ADENYLATE CYCLASE-ACTIVATING POLYPEPTIDE (PACAP) AND PACAP ANALOGS AS ADJUNCTIVE TREATMENTS WITH INHIBITORS OF CALCINEURIN OR INHIBITORS OF THE MAMMALIAN TARGET OF RAPAMYCIN (mTOR) COMPLEXES
InactiveUS20120309683A1Protecting the major organsEffective protectionNervous disorderMetabolism disorderUveitisAutoimmune responses
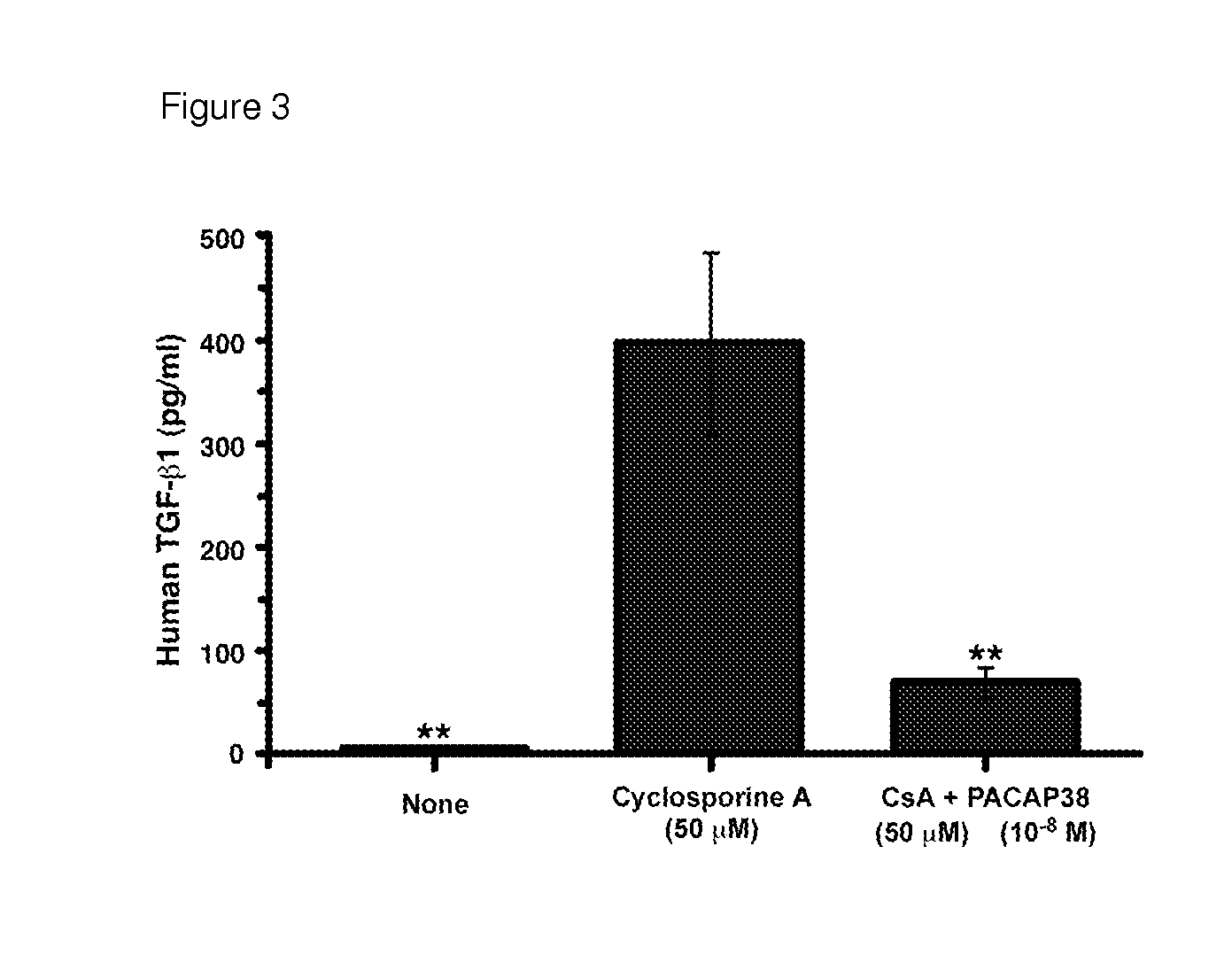
This invention relates to methods and compositions for the treatment, management, reduction, or prevention of injuries to one or more major organs of the body, e.g., the brain, heart, lung, kidneys, liver, and gastrointestinal tract, of a mammal (e.g., a human) caused by one or more calcineurin or mammalian target of rapamycin (mTOR) complex inhibitors. The methods include administering an effective amount of one or more pituitary adenylate cyclase-activating polypeptide (PACAP)-like compounds to the mammal. Combination therapy with one or more PACAP-like compounds, either alone or in combination with one or more other prophylactic / therapeutic agents, plus one or more inhibitors of either calcineurin or the mTOR complexes can be used to treat organ transplantation, autoimmune diseases, graft-versus-host disease, Behçet's disease, hematological cancers, noninfectious uveitis, sarcoidosis, tuberous sclerosis complex, acute neurological diseases, age-related neurodegenerative diseases, Huntington's disease and other CAG codon repeat expansion diseases, keratoconjunctivitis sicca, and restenosis.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
Therapeutic agent for keratoconjunctive disorders
ActiveUS9492431B2Strongly suppressing keratoconjunctive collagen contractionOrganic active ingredientsSenses disorderSuperficial punctate keratopathyInfectious Keratitis
The present invention addresses the problem of providing a novel therapeutic agent for keratoconjunctive disorders. As a means for solving the problem, a therapeutic agent for keratoconjunctive disorders which contains a RARγ agonist as an active ingredient is provided. The therapeutic agent exhibits an excellent ameliorating effect in a keratoconjunctive disorder model, and is therefore useful as a therapeutic agent for keratoconjunctive disorders such as corneal ulcer, corneal epithelial abrasion, keratitis, dry eye, conjunctivitis, chronic superficial keratitis, corneal erosion, persistent corneal disorders, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, infectious keratitis, noninfectious keratitis, infectious conjunctivitis and noninfectious conjunctivitis. The therapeutic agent is also useful as a therapeutic agent for corneal scarring and conjunctival scarring both associated with keratoconjunctive disorders.
Owner:YAMAGUCHI UNIV +1
Compositions and methods of using same for treatment of a disease or disorder of the eye and/or the adnexa of the eye
The present invention relates to compositions comprising at least one flavonoid for the treatment or amelioration of a disease or disorder of the eye and / or the adnexa of the eye in an animal subject, including a human being. More particularly, this invention relates to a composition for the treatment of conjunctivitis, keratoconjunctivitis sicca, and blepharitis. The invention furthermore relates to a pharmaceutical composition comprising at least one flavonoid, such as for example a topical formulation. The source of the flavonoids may be, but are not restricted to, flavonoids extracted from citrus plants. The compositions may furthermore optionally be used in combination with an eyecleaner or eyewash, which may comprise at least one flavonoid.
Owner:OCUMEDIC
Preventive or therapeutic agent for keratoconjunctival disorder
InactiveUS20090105313A1Improve the improvement effectGood prevention effectBiocideSenses disorderConjunctivaDisease
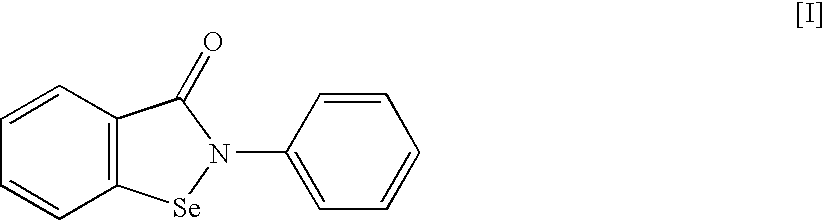
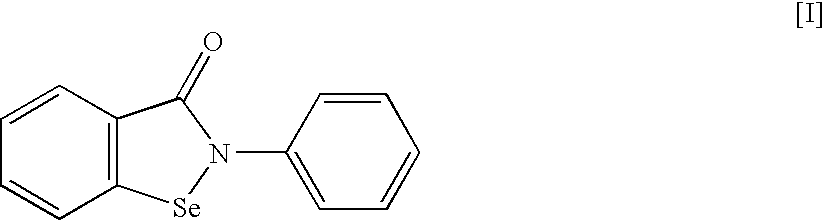
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
Method and composition for the treatment of moderate to severe keratoconjunctivitis sicca
ActiveUS20140099355A1Ensuring structureEnsure stabilityPeptide/protein ingredientsHydroxy compound active ingredientsKERATOCONJUNCTIVITIS SICCAKCS - Keratoconjunctivitis sicca
The present invention relates to topical ophthalmic compositions for treating or preventing epithelial lesions or ophthalmic disorders, including dry eye or keratoconjunctivitis sicca.
Owner:AL QAHTANI AHMED H
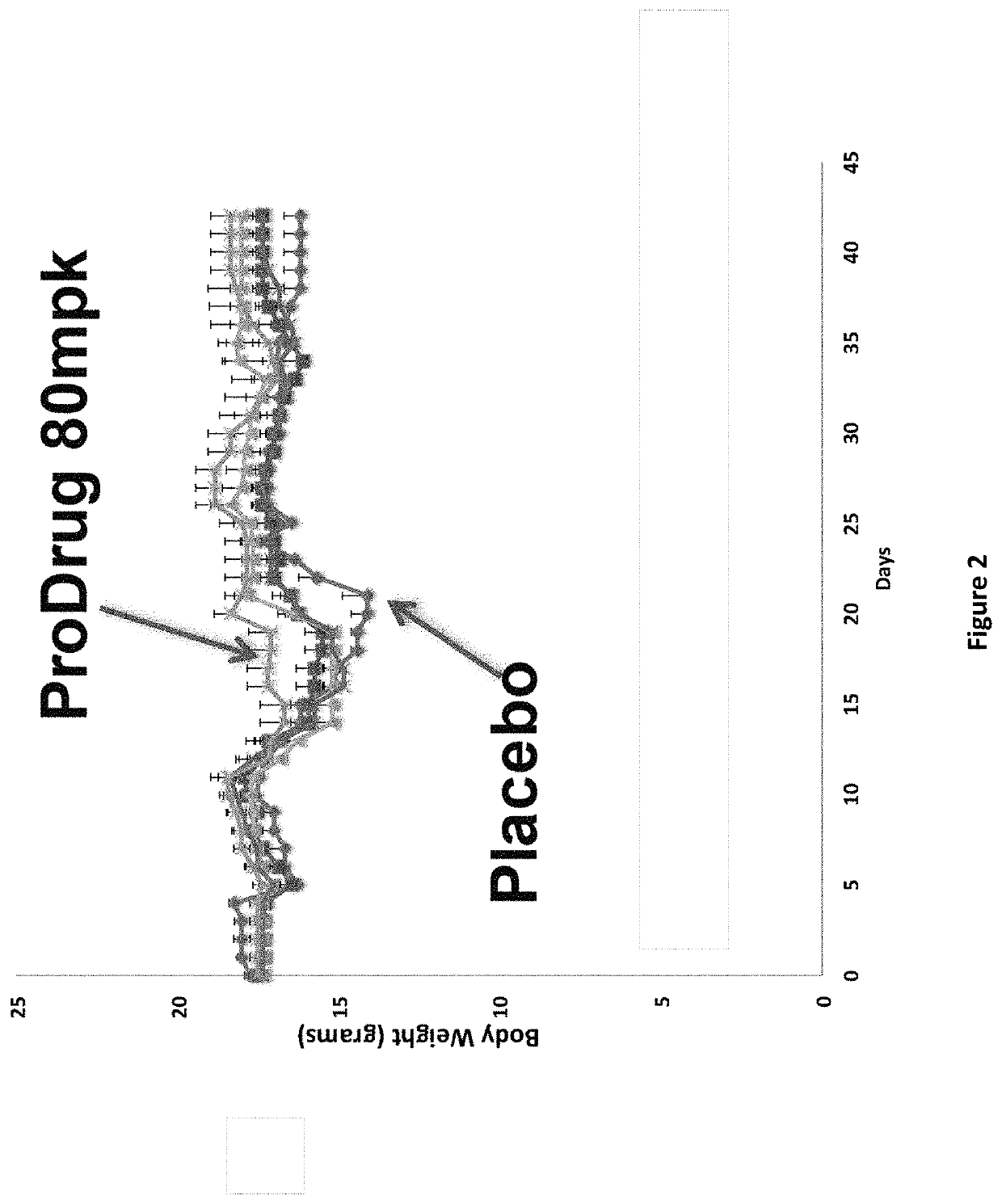
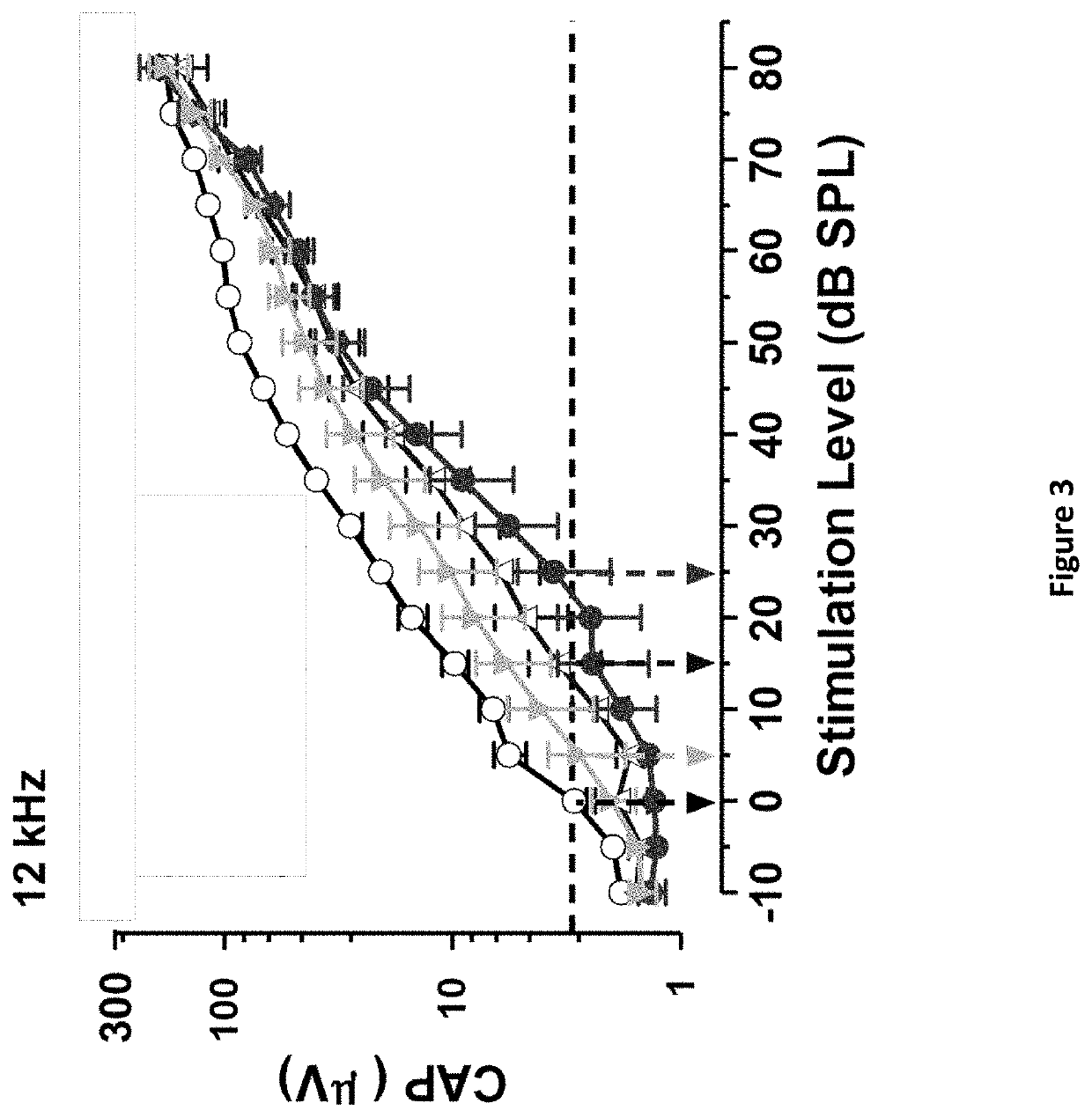
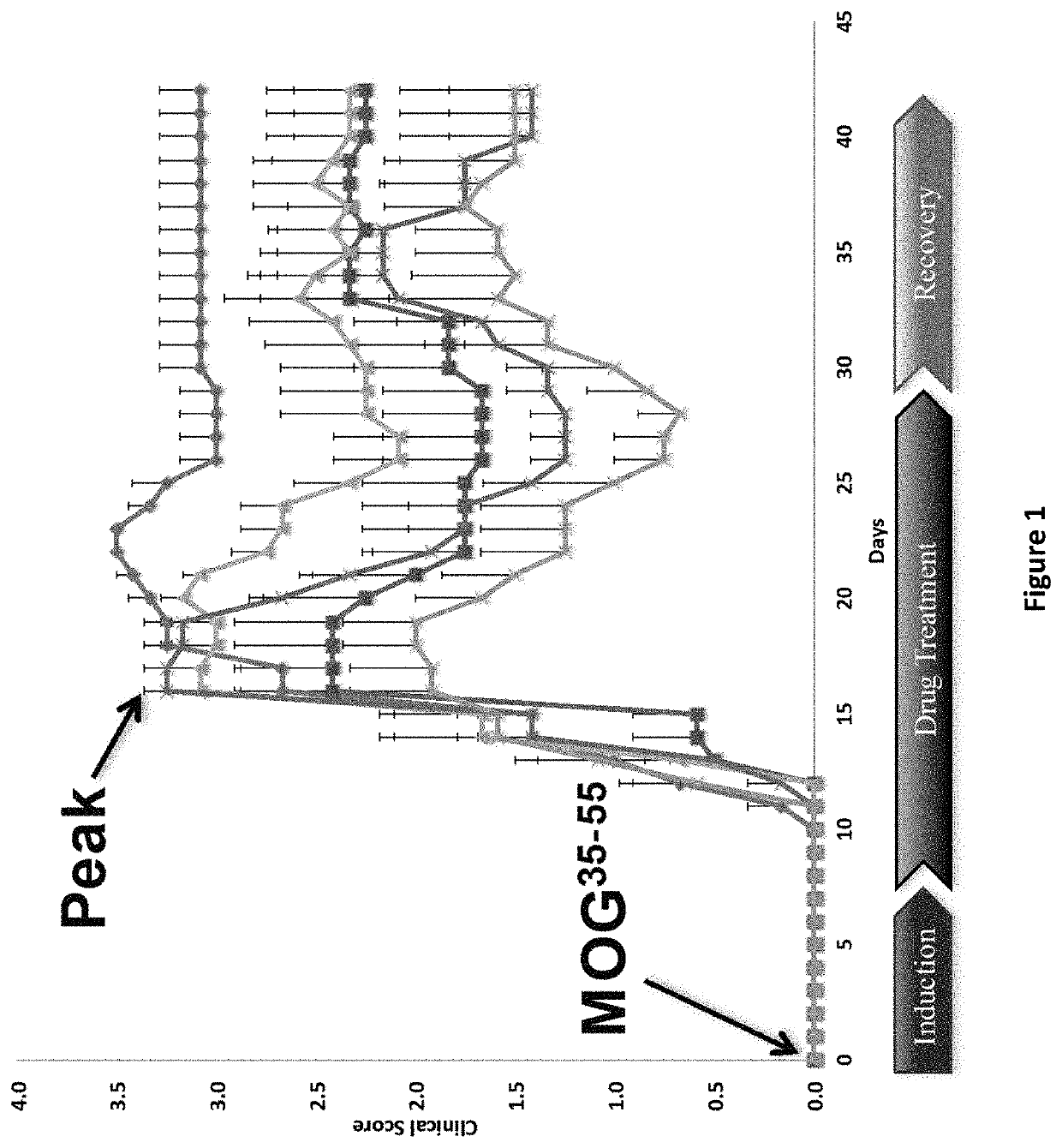
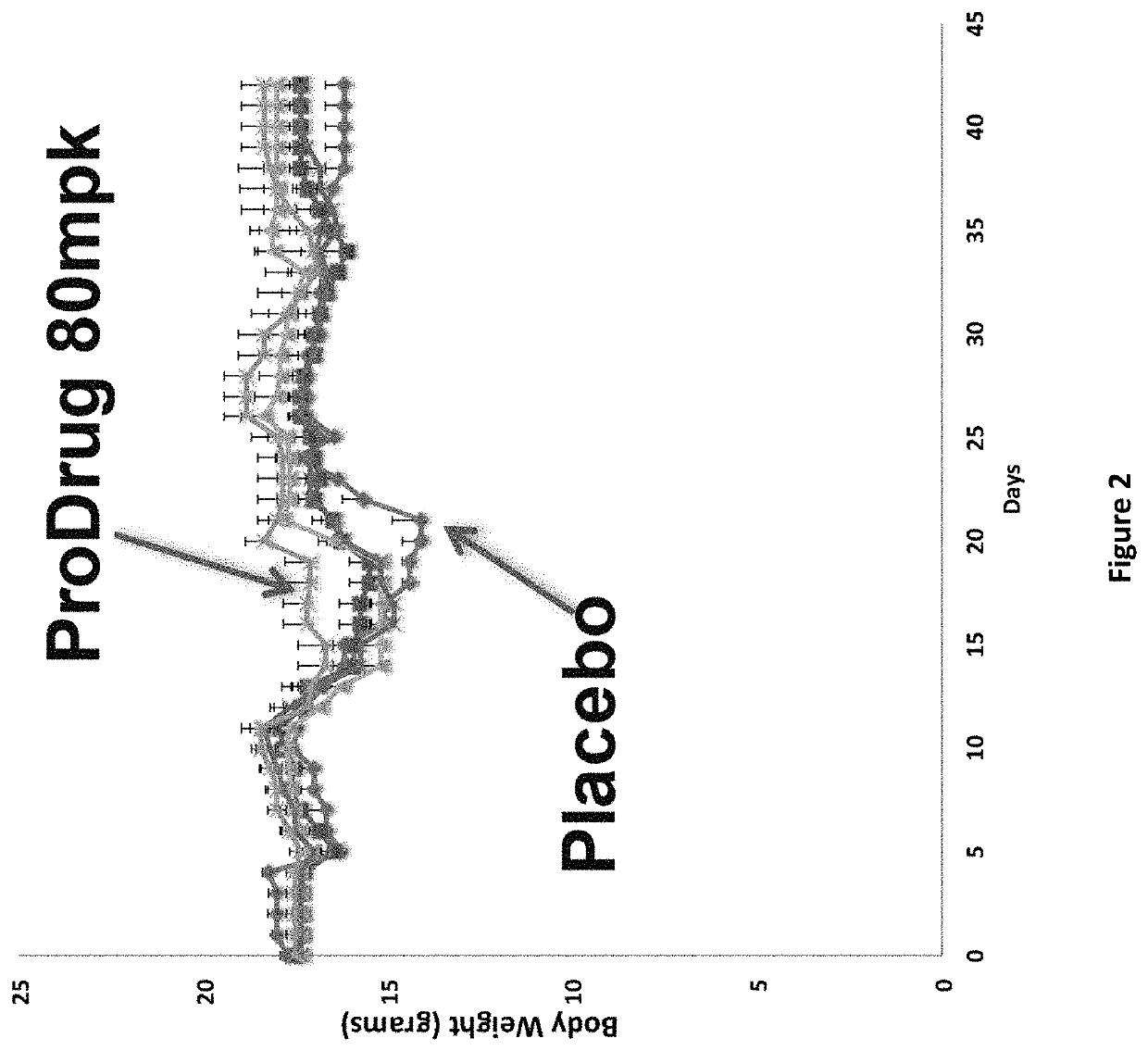
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
ActiveUS20170252347A1Good blood pressureImprove blood sugar levelsSenses disorderNervous disorderHL - Hearing lossDepressant
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Formulations for treating eye disorders
Ocular delivery of drugs to the eyes is an ongoing challenge due to the unique anatomical and physiological properties of the eye. A solution of the immunosuppressant and anti-inflammatory compound mycophenolic acid with a pH from 6.0 to 8.5 has been demonstrated to exhibit improved bioavailability when topically applied to the eye. Specifically, topical application to the eye of such a solution is effective in penetrating anterior and posterior eye structures. Said solution is effective in treating a variety of inflammatory disorders, including uveitis, allergic conjunctivits, and keratoconjunctivitis sicca.
Owner:ASPREVA INT
Artificial tear formulation
InactiveUS20040126419A1Reproduce viscosityReproduce surface tension propertyAntibacterial agentsBiocideLipid formationKERATOCONJUNCTIVITIS SICCA
Provided by the present invention are formulations suitable for application to mammalian eyes which contain a lipid binding protein and a polar lipid, present as a soluble complex in an aqueous electrolyte. The formulations described have shear-thinning (non-Newtonian viscosity) and surface tension properties to natural tears and are therefore useful as artificial tear substitutes for the treatment of dry eyes (e.g. keratoconjunctivitis sicca) and useful in ophthalmic applications in general.
Owner:ISIS INNOVATION LTD
Prophylactic or therapeutic agent for corneal/conjunctival disease
InactiveUS20100113338A1Therapy is simpleGood treatment effectSenses disorderPeptide/protein ingredientsDiseaseSuperficial punctate keratopathy
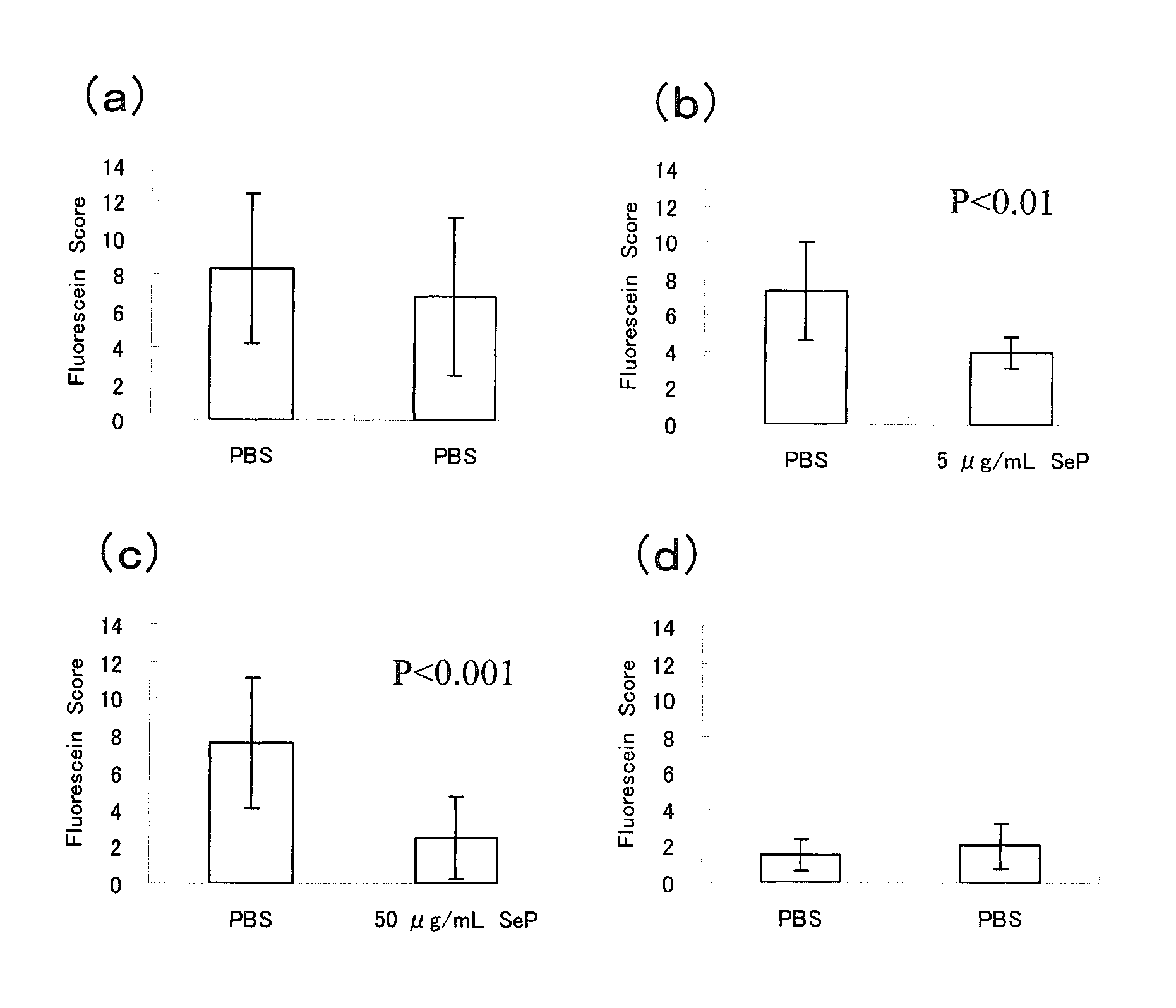
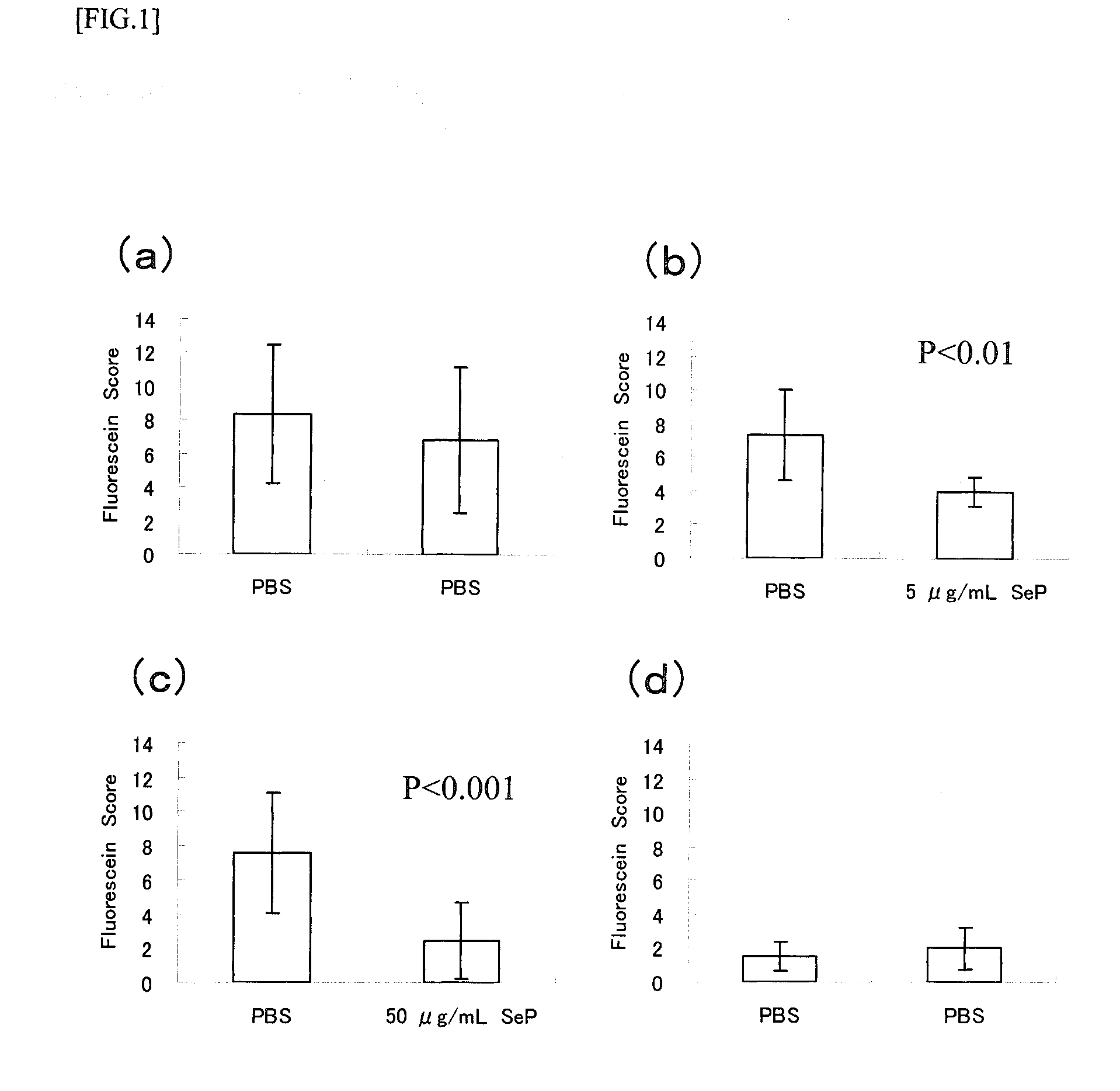
Disclosed is a novel composition for the treatment of a corneal / conjunctival disease. A prophylactic or therapeutic agent for a corneal / conjunctival disease comprising selenoprotein P as an active ingredient, more specifically a prophylactic or therapeutic agent for a corneal / conjunctival disease such as dry eye, keratoconjunctivitis sicca, superficial punctate keratopathy, corneal erosion or corneal ulcer comprising selenoprotein P as an active ingredient, particularly a prophylactic or therapeutic agent for a corneal / conjuncrtival disease such as dry eye, keratoconjunctivitis sicca, superficial punctate keratopathy, corneal erosion or corneal ulcer accompanied by a corneal / conjunctival epithelial discorder.
Owner:TSUBOTA KAZUO +2
Therapeutic agent for keratoconjunctival disorder
An object of the present invention is to discover a new medicinal use of 5-[4-[[3-methyl-4-oxo-3,4-dihydro-2-quinazolinyl]methoxy]p henylmethyl]thiazolidine-2,4-dione and N-[(4-methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine. Both of the compounds exert an excellent improving effect on corneal disorder models and is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctive epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Method for treating a keratoconjunctival disorder
An object of the present invention is to provide a new medicinal use of 2-phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof. 2-Phenyl-1,2-benzisoselenazol-3(2H)-one or a salt thereof exhibits an excellent prevention and improvement effect in corneal disorder models, and is therefore useful as a preventive or therapeutic agent for a keratoconjunctival disorder such as dry eye, superficial punctate keratopathy, corneal epithelial defects, corneal erosion, corneal ulcer, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis, filamentary keratoconjunctivitis, keratitis or conjunctivitis.
Owner:SANTEN PHARMA CO LTD
DNP and DNP Prodrug Treatment of Neuromuscular, Neurodegenerative, Autoimmune, Developmental, Traumatic Brain Injury, Concussion, Dry Eye Disease, Hearing Loss and/or Metabolic Diseases
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:BIOVENTURES LLC +1
Compositions comprising mixtures of semifluorinated alkanes
ActiveUS20180071229A1Effective timeTune viscosityHalogenated hydrocarbon active ingredientsSenses disorderAlkaneKERATOCONJUNCTIVITIS SICCA
Owner:NOVALIQ GMBH
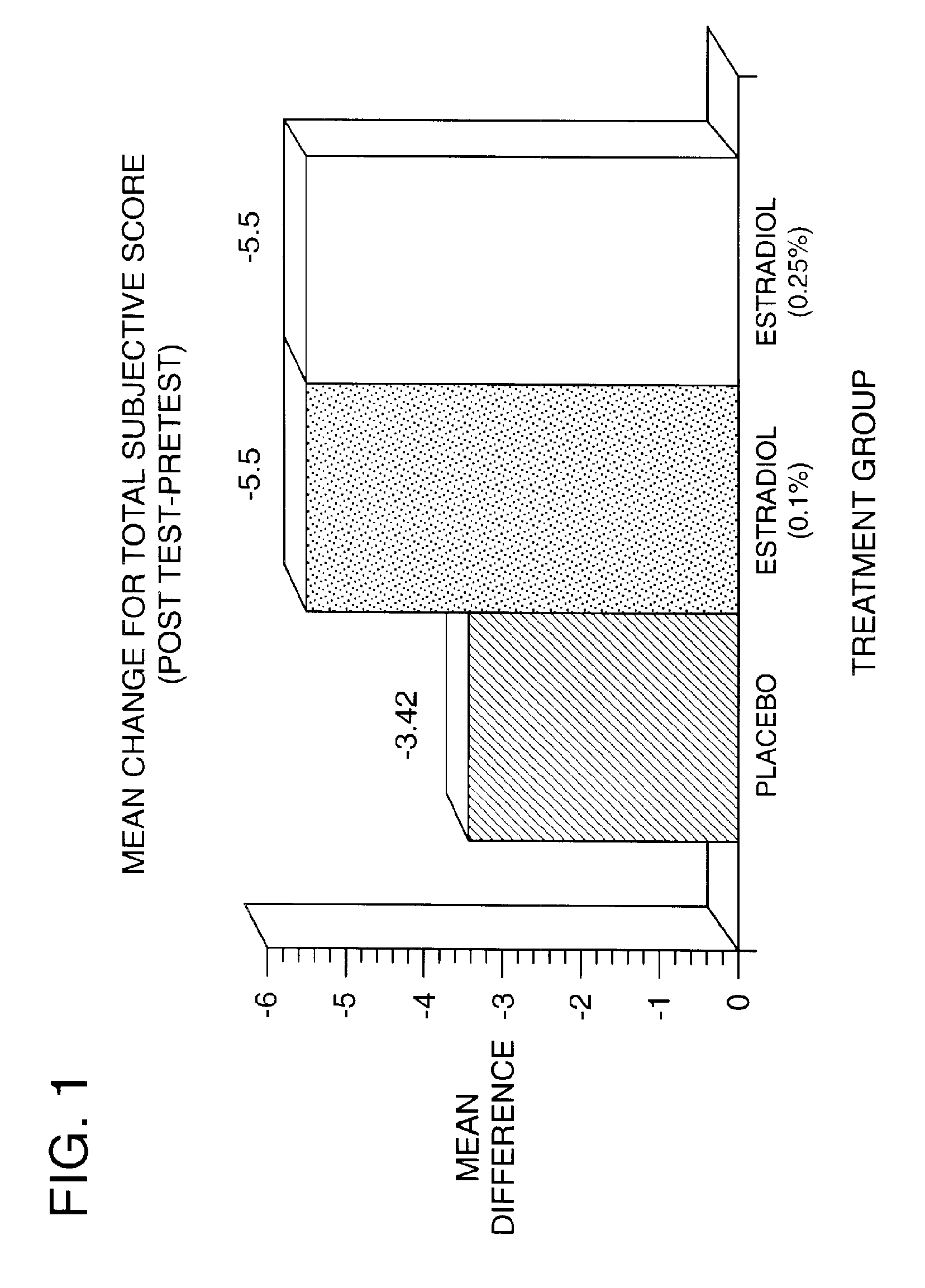
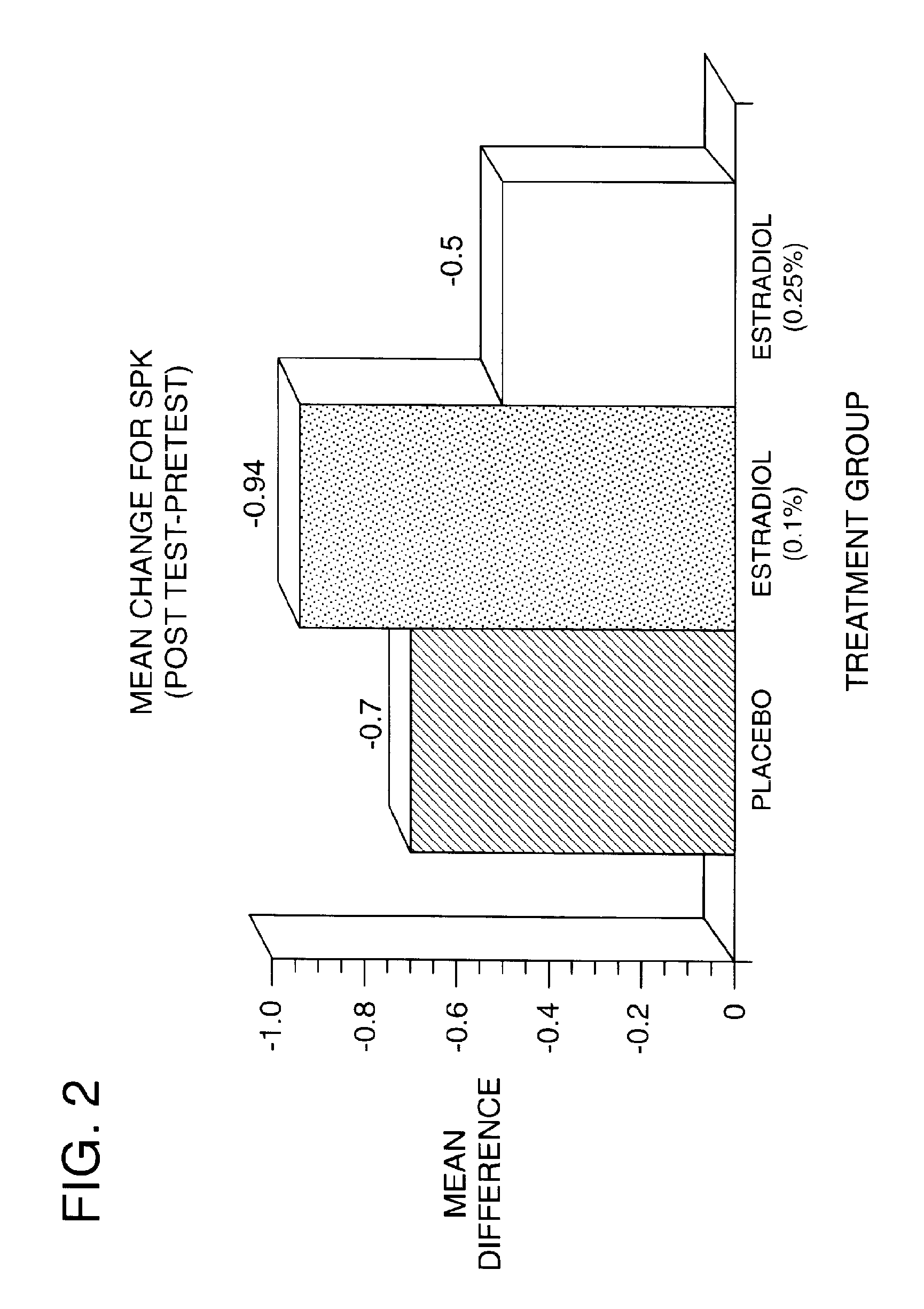
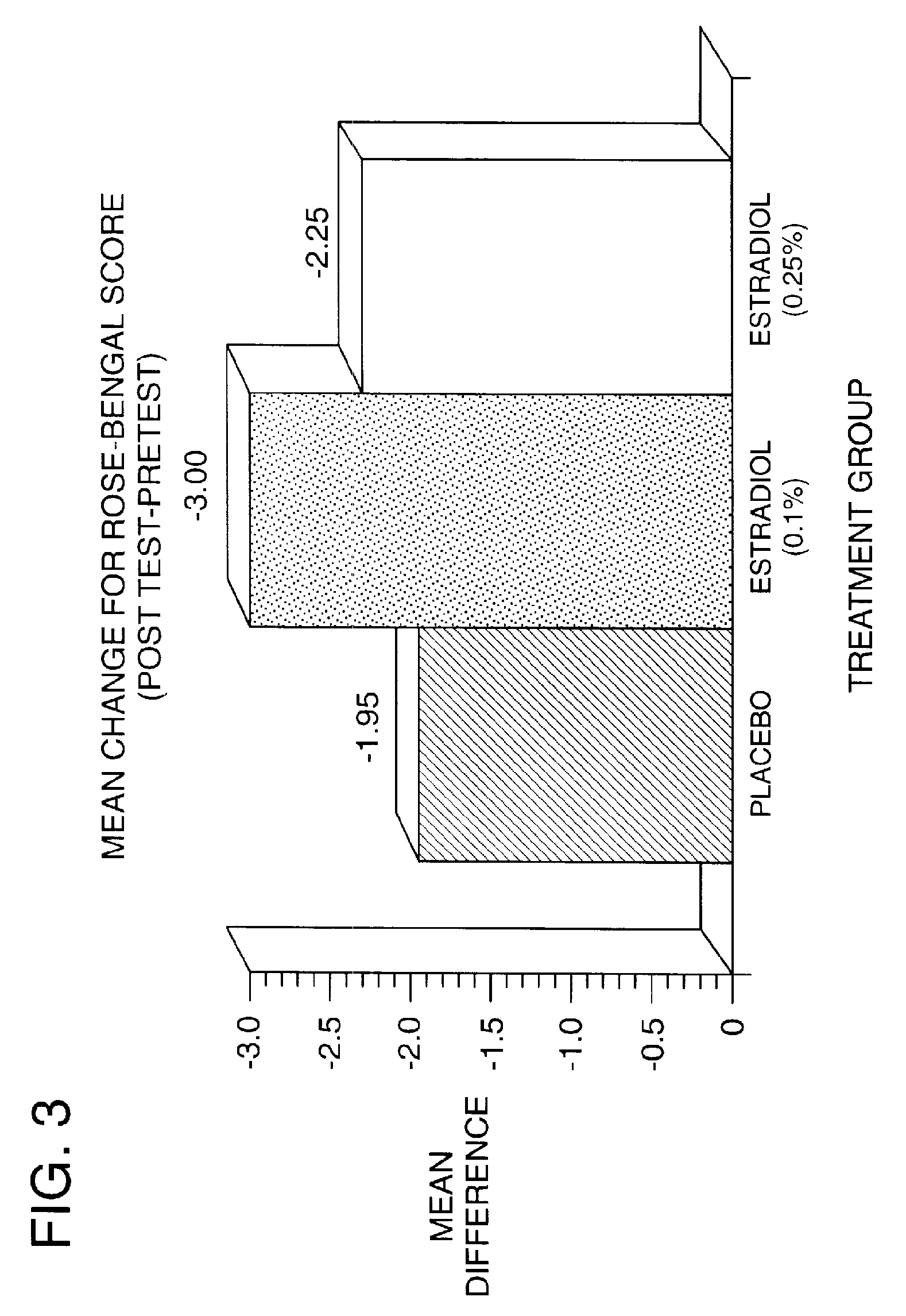
Time-release and micro-dose formulations for topical application of estrogen and estrogen analogs or other estrogen receptor modulators in the treatment of dry eye syndrome, and methods of preparation and application
ActiveUS8987241B2Alleviate dry-eye syndromeFull efficacyOrganic active ingredientsBiocide17 β estradiolGynecology
A topical application formulation of estrogen and estrogen analogs or other estrogen receptor modulators is disclosed for the treatment of primary or secondary dry eye syndrome (also known as keratoconjunctivitis sicca (KCS)). Preferred formulations include 17-β-estradiol and its derivatives in lipid, liposomes, polymers, or aqueous or non-aqueous vehicles for the topical treatment of the ocular surface tissues particularly as time-release or micro-dose formulations. These formulations may also be useful in treating other conditions where KCS may occur, such as post-operative refractive surgery and corneal transplant patients.
Owner:REDWOOD PHARMA AB
DNP and DNP prodrug treatment of neuromuscular, neurodegenerative, autoimmune, developmental, traumatic brain injury, concussion, dry eye disease, hearing loss and/or metabolic diseases
A composition and method of treatment of neuromuscular, neuromuscular degenerative, neurodegenerative, autoimmune, developmental, traumatic, hearing loss related, and / or metabolic diseases, including spinal muscular atrophy (SMA) syndrome (SMA1, SMA2, SMA3, and SMA4, also called Type I, II, III and IV), traumatic brain injury (TBI), concussion, keratoconjunctivitis sicca (Dry Eye Disease), glaucoma, Sjogren's syndrome, rheumatoid arthritis, post-LASIK surgery, anti-depressants use, Wolfram Syndrome, and Wolcott-Rallison syndrome. The composition is selected from the group consisting of 2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP, bipartite 2,3-dinitrophenol, 2,4-dinitrophenol, 2,5-dinitrophenol, 2,6-dinitrophenol, 3,4-dinitrophenol, or 3,5-dinitrophenol (2,3-DNP, 2,4-DNP, 2,5-DNP, 2,6-DNP, 3,4-DNP, or 3,5-DNP) prodrugs; Gemini prodrugs, bioprecursor molecules, and combinations thereof. A dose of the composition for treatment of neurodegenerative diseases may be from about 0.01 mg / kg of body weight to about 50 mg / kg of body weight of the patient in need of treatment. A dose of the composition for treatment of metabolic diseases may be from about 1 mg / 70 kg of body weight to about 100 mg / 70 kg of body weight of the patient in need of treatment, and a maximum dose per day is about 200 mg / 70 kg of body weight of the patient in need of treatment.
Owner:MITOCHON PHARMA INC +1
Time-release and micro-dose formulations for topical application of estrogen and estrogen analogs or other estrogen receptor modulators in the treatment of dry eye syndrome, and methods of preparation and application
ActiveUS20120164213A1Alleviate dry-eye syndromeFull efficacyBiocideOrganic active ingredients17 β estradiolKERATOCONJUNCTIVITIS SICCA
A topical application formulation of estrogen and estrogen analogs or other estrogen receptor modulators is disclosed for the treatment of primary or secondary dry eye syndrome (also known as keratoconjunctivitis sicca (KCS)). Preferred formulations include 17-β-estradiol and its derivatives in lipid, liposomes, polymers, or aqueous or non-aqueous vehicles for the topical treatment of the ocular surface tissues particularly as time-release or micro-dose formulations. These formulations may also be useful in treating other conditions where KCS may occur, such as post-operative refractive surgery and corneal transplant patients.
Owner:REDWOOD PHARMA AB
Therapeutic Agent for Keratoconjunctival Disorder
An object of the present invention is to research a new medicinal use of E-4-[4-(5-methyl-2-phenyl-4-oxazolylmethoxy) benzyloxyimino]-4-phenylbutyric acid, Z-2-[4-(5-methyl-2-phenyl-4-oxazolylmethoxy) benzyloxyimino]-2-(4-phenoxyphenyl)acetic acid, 2-[2-propyl-3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]propoxy]phenoxy] benzoic acid, 2(S)-methoxy-3-[4-[3-(4-phenoxyphenoxy)propoxy]phenyl] propionic acid, or a salt thereof. Any of the above-mentioned carboxylic acid compounds and a salt thereof exhibit an excellent improving effect on corneal disorder models and are useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis or filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Ophthalmic emulsion composition of cyclosporine
InactiveUS20160271059A1Increase tear productionCyclic peptide ingredientsEmulsion deliveryGlaucomaOcular inflammation
The invention relates to an ophthalmic emulsion composition of cyclosporine in the form of an oil-in-water emulsion. The emulsion remains stable at 25° C. for at least 6 months or at 45° C. for at least 45 days. The composition is useful for treating a dry eye condition or glaucoma, and preferably for increasing tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca.
Owner:SOMERSET THERAPEUTICS LLC
Eye Health Supplement
An eye health supplement administered to a person in order to prevent and treat eye-related conditions or diseases. The eye health supplement focuses on treating conditions and diseases, such as diabetic retinopathy, macular degeneration, hypertensive retinopathy, keratoconjunctivitis sicca, and more, and the underlying symptoms of eye-related conditions and diseases through the inclusion of a quantity of birch leaf powder, a quantity of pomegranate, a quantity of garlic bulb powder, a quantity of fenugreek powder, a quantity of curcumin, a quantity of asthaxanthin, a quantity of bilberry extract powder, and a quantity of glutathione powder. The quantity of birch leaf powder, the quantity of pomegranate, the quantity of garlic bulb powder, the quantity of fenugreek powder, the quantity of curcumin, the quantity of asthaxanthin, the quantity of bilberry extract powder, and the quantity of glutathione powder are heterogeneously mixed into a supplement mixture for oral or transdermal administration.
Owner:SAUCEDO ADAM C +1
Human corneal epithelial cell line PTA-120527
ActiveUS8877494B2Ensuring structure and stability of tear filmSlowing and preventing to ocular surfaceHydroxy compound active ingredientsPeptide/protein ingredientsKERATOCONJUNCTIVITIS SICCACorneal epithelial cell
The present invention relates to topical ophthalmic compositions for treating or preventing epithelial lesions or ophthalmic disorders, including dry eye or keratoconjunctivitis sicca.
Owner:AL QAHTANI AHMED H
Ophthalmic emulsion composition of cyclosporine
InactiveUS20160271060A1Cyclic peptide ingredientsEmulsion deliveryGlaucomaKERATOCONJUNCTIVITIS SICCA
The invention refers to an ophthalmic emulsion composition of cyclosporine in the form of an oil-in-water emulsion. The emulsion remains stable at 25° C. for at least 6 months or at 45° C. for at least 45 days. The composition is useful for treating a dry eye condition or glaucoma, and preferably for increasing the tear production in patients whose tear production is presumed to be suppressed due to ocular inflammation associated with keratoconjunctivitis sicca.
Owner:SOMERSET THERAPEUTICS LLC
Therapeutic Agent for Keratoconjunctival Disorder
InactiveUS20090270474A1Good effectEasy to optimizeOrganic active ingredientsBiocideFilamentary keratitisSuperficial punctate keratopathy
An object of the present invention is to discover a new use of eprosartan or a salt thereof. Eprosartan or a salt thereof exhibits an excellent improving effect in a corneal disorder model, and therefore is useful as a therapeutic agent for a keratoconjunctival disorder such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com