Eye cyclosporin gel
An ophthalmic gel, cyclosporine technology, applied in the directions of cyclic peptide components, sensory diseases, pharmaceutical formulations, etc., can solve the problems of easy dilution of eye drops by tears, short residence time, and difficulty in passing oral drugs through the blood-eye barrier, etc. To achieve the effect of reducing gastrointestinal irritation, long residence time, and reducing other side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
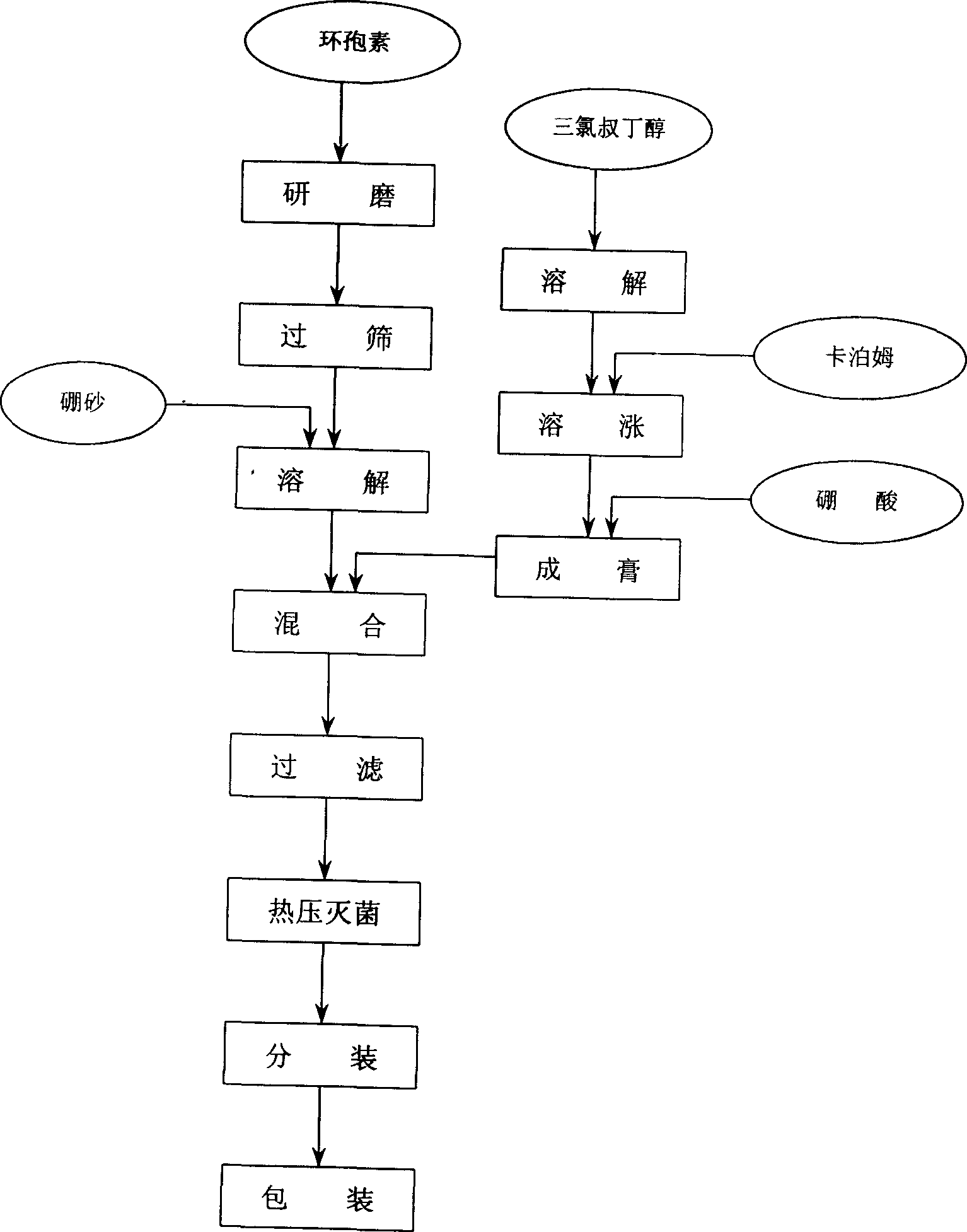
[0008] Prescription 1: Cyclosporin 2.0g
[0009] Carbomer 0.4g
[0010] Borax 1.0g
[0011] Boric acid 0.5g
[0012] Chlorobutanol 0.3g
[0013] Make 100.0g operation process:
[0014] ②. Add borax and boric acid to ① to make a transparent gel matrix.
[0015] ③. Cyclosporine is ground into fine powder, passed through a No. 9 sieve, and ground into ② in batches under heating conditions.
[0016] ④. Filter with 220-mesh sieve cloth, sterilize by autoclaving, subpackage, pack, and get ready.
[0017] Prescription 2: Cyclosporine 0.5-10.0g
[0018] Carbomer 0-4.0g
[0019] Borax 0-10.0g
[0020] Boric acid 0-5.0g
[0021] Chlorobutanol 0-3.0g
[0022] Make 100.0g operation process:
[0023] ①.Appropriate amount of water, dissolve chlorobutanol, add carbomer to dissolve, overnight.
[0024] ②. Add borax and boric acid to ① to make a transparent gel matrix.
[0025] ③. Cyclosporine is ground into fin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com